Abstract
In an effort to identify novel antibacterial agents, we presented two series of aminoguanidine derivatives that were designed by incorporating 1,2,4-triazol moieties. All compounds exhibited strong in vitro antibacterial activity against a variety of testing strains. Compound 5f was identified as a potent antibacterial agent with a minimum inhibitory concentration (MIC) of 2–8 µg/mL against S. aureus, E. coli, S. epidermidis, B. subtilis, C. albicans, multi-drug resistant Staphylococcus aureus and multi-drug resistant Escherichia coli and low toxicity (Hela > 100 µM). Membrane permeability and transmission electron microscopy (TEM) image studies demonstrated that compound 5f permeabilized bacterial membranes, resulting in irregular cell morphology and the rapid death of bacteria. The results of the present study suggested that aminoguanidine derivatives with 1,2,4-triazol moieties were the intriguing scaffolds for the development of bactericidal agents.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77668-0.
Keywords: Aminoguanidine derivatives; 1,2,4-triazol; Antibacterial activity; Membrane permeability; TEM
Subject terms: Microbiology, Structural biology
Introduction
Infections caused by bacterial pathogens are a major cause of morbidity and mortality worldwide1. Notorious multidrug-resistant strains, including Gram-positive bacteria methicillin-resistant Staphylococcus epidermidis (MRSE), MRSA, Vancomycin-Resistant Enterococci faecalis (VRE), and Gram-negative bacteria E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, have emerged to be the major cause of hospital and community-acquired infections2. Reports from the Center for Disease Control and Prevention (CDC) suggested that multi-drug resistant bacterial strains are affecting at least 2 million people and are responsible for approximately 23,000 deaths each year in the United States alone3. Furthermore, despite the approval of 58 new antibiotics by Food and Drug Administration (FDA) or entry into advanced clinical development (Phase II or III) since 2000, most have mainly targeted Gram-positive bacteria and only two new compounds have been used specifically against Gram-negative bacteria4. Currently, the limited treatment options with clinical antibiotics indicate that there is an urgent need to develop new antibacterial agents with an innovative and effective mechanism of action to cure drug-resistant bacterial and broad-spectrum Gram-negative antibiotics.
The bacterial membrane, a crucial structure for cell survival, has attracted quite a lot attention to develop novel antibacterial agents in the past two decades, which has become a fascinating target that fight against the problematic bacterial resistance5. A relatively smaller number of studies have described compounds targeted to the bacterial membrane and the formation and disruption of biofilm. Aminoguanidine derivatives have recently captured the attention of numerous researchers because of their diverse range of biological properties, including their antibacterial (compound A)6 and anti-inflammatory (compound B) activities7. Eissa et al. reported that a new series of synthetic compounds bearing the aminoguanidine scaffold with potent antibacterial activity against MRSA and VRSA (compound C)8. The 1,2,4-triazole and its derivatives were reported to exhibit various pharmacological activities such as antimicrobial, analgesic, anti-inflammatory, anticancer and antioxidant properties (compounds D and E)9–13. In our previous work, we have already described several synthetic modifications of the aminoguanidine molecule linked with another active part by conjugation with a new type of “double” active molecules (compound F) (Fig. 1)14,15. In view of the above considerations and as an extension of our studies on the development of antibacterial agents, it is of great interest for us to combine the aminoguanidine fragment and 1,2,4-triazol moieties to generate a new structural type of potentially antimicrobial agents (Fig. 2). The synthesized compounds were characterized and tested for their in vitro antibacterial activity. In addition, the preliminary mechanism of action of these compounds on bacterial membrane were assessed by TEM analysis.
Fig. 1.
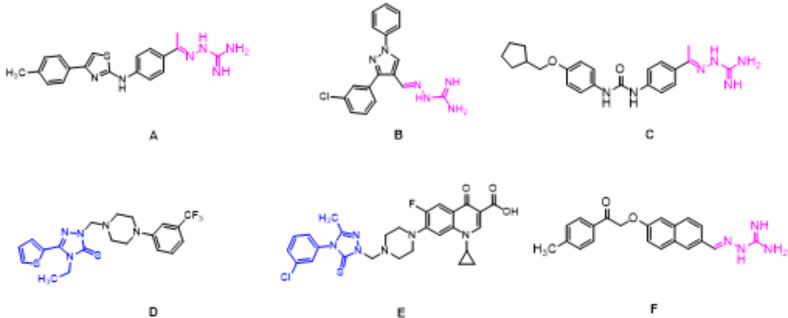
Several molecules of previously reported compounds.
Fig. 2.
Designed target compounds.
Results and discussion
Chemistry
All newly designed compounds were synthesized as depicted in Scheme 1. Regarding the partial reaction of the aromatic acid, first, the aromatic acid was formed into acid chloride under the action of thionyl chloride, which was added to the solution of thiosemicarbazone dissolved in pyridine to obtain an intermediate. Secondly, a cyclization reaction occurred under the action of a strong base of NaOH, aromatic acid triazolyl intermediate 2 was obtained16. Then reaction with 4-bromoethoxybenzaldehyde in refluxing acetone in the presence of an inorganic base (KI/K2CO3) afforded the key intermediate 3. Intermediate 7 was synthesized by reacting aniline with methyl hydrazinecarboxylate in refluxing ethanol, in the presence of triethyl orthoformate and sodium methoxide17. Then reaction with 4-bromoethoxybenzaldehyde in refluxing acetone in the presence of KI/K2CO3 afforded the key intermediate 8. The target compounds 4a-f, 5a-i and 9a-c were prepared by the reaction of intermediate 3 or 8 with aminoguanidine bicarbonate in the presence of concentrated hydrochloric acid in refluxing ethanol. The structures of the synthesized compounds were also established by 1H NMR, 13C NMR, HSQC (compounds 4f and 5f), HMBC (compounds 4f and 5f), MS and HRMS.
Scheme 1.

Synthetic scheme for the compounds 4a-f, 5a-i and 9a-c. Reagents and conditions: a SOCl2, DCM/DMF, reflux, 4 h; Pyridine, rt, 16 h; NaOH/H2O, reflux, 3 h; b EtOH, MeONa, reflux, 48 h; c K2CO3, KI, acetone, reflux, 6 h; d aminoguanidine hicarbonate, EtOH, 50ºC, reflux, 10–12 h.
Antibacterial activity
The antibacterial efficacy of these compounds was assessed in a suitable culture medium and expressed as the MIC18,19. The antibacterial activity of the synthesized compounds was evaluated in comparison to moxifoxacin, gatifoxacin, tetracycline and fluconazole. Generally, all compounds exhibited good antibacterial activity against a wide spectrum of drug-sensitive bacteria such as S. aureus ATCC25923, S. epidermidis 102,555, B. subtilis CMCC63501, E. coli ATCC25922, C. albicans 7535, multi-drug resistant Staphylococcus aureus ATCC 43,300 and multi-drug resistant Escherichia coli ATCC BAA-196, and the results are shown in Tables 1 and 2. In general, all compounds showed good activity against all the bacteria tested.
Table 1.
MICa (µg/mL) of small molecules 4a-f, 5a-i and 9a-c against drug-sensitive bacteria.
| Compound | S. epidermidis b | S. aureus c | B. subtilis d | E. coli e | C. albicans f |
|---|---|---|---|---|---|
| 4a | 32 | 16 | 16 | 16 | 16 |
| 4b | 16 | 16 | 8 | 16 | 16 |
| 4c | 8 | 8 | 8 | 8 | 8 |
| 4d | 8 | 4 | 2 | 16 | 4 |
| 4e | 16 | 16 | 8 | 8 | 16 |
| 4f | 4 | 4 | 4 | 8 | 4 |
| 5a | 16 | 64 | 16 | 16 | 32 |
| 5b | 8 | 16 | 16 | 16 | 16 |
| 5c | 8 | 8 | 4 | 8 | 8 |
| 5d | 8 | 8 | 8 | 8 | 8 |
| 5e | 16 | 16 | 16 | 16 | 16 |
| 5f | 4 | 4 | 2 | 2 | 4 |
| 5g | 16 | 32 | 32 | 32 | 32 |
| 5h | 8 | 8 | 4 | 8 | 8 |
| 5i | 8 | 8 | 8 | 8 | 8 |
| 5j | 32 | 16 | 16 | 32 | 16 |
| 9a | 64 | 64 | 64 | 32 | 64 |
| 9b | 32 | 32 | 32 | 8 | 64 |
| 9c | 32 | 64 | 64 | 8 | 64 |
| Moxifoxacin | 2 | 0.5 | 1 | 1 | n.d |
| Gatifoxacin | 2 | 0.5 | 2 | 1 | n.d |
| tetracycline | 32 | 32 | 32 | 32 | n.d |
| fluconazole | n.d | n.d | n.d | n.d | 1 |
nd not determined. aMICs were determined by micro broth dilution method for microdilution plates. bS. epidermidis 102,555. cS. aureus ATCC 25,923. dB. subtilis CMCC 63,501. eE. coli ATCC 25,922. fCandida albicans 7535.
Table 2.
Antibacterial activity and cytotoxicity for 5f.
| Test organisms | 5f | Moxifoxacin | Gatifoxacin | |
|---|---|---|---|---|
| MIC (µg/mL) | S. aureus ATCC 43,300b | 8 | 1 | 1 |
| E. Coli ATCC BAA-196c | 4 | 1 | 1 | |
| IC50a (µM) | HeLad | > 100 |
aIC50 is defined as the concentration to inhibit the cell growth by 50%. bmulti-drug resistant Staphylococcus aureus ATCC 43,300; cmulti-drug resistant Escherichia coli ATCC BAA-196; dHuman cervical cells.
Compounds 4f and 5f were found to be the most potent compounds against S. epidermidis, with a MIC of 4 µg/mL in comparion to the positive controls moxifoxacin and gatifoxacin. Compounds 4d and 5f (MIC = 2 µg/mL) displayed equal activity against B. subtilis compared with the commercial drug gatifoxacin (MIC = 2 µg/mL). Against the E. coli ATCC25922, compound 5f had the highest activity, with a MIC value of 2 µg/mL, and this was slightly lower than gatifoxacin (MIC = 1 µg/mL) and moxifoxacin (MIC = 1 µg/mL). Furthermore, in terms of its activity towards the fungus C. albicans 7535, compounds 4d, 4f and 5f (MIC = 4 µg/mL) displayed the strongest activity in the current study, which were lower than that of fluconazole (MIC = 1 µg/mL). Bacterial resistance against most antibiotics presents a serious problem20. Thus, the propensity of compound to inhibit bacterial resistance is an important property. Molecule 5f to suppress the development of resistance was tested. In this trial, compound 5f had excellent inhibitory activity against the multi-drug resistant E. coli ATCC BAA-196 and S. aureus ATCC 43,300, with a MIC of 4 and 8 µg/mL, respectively (Table 2).
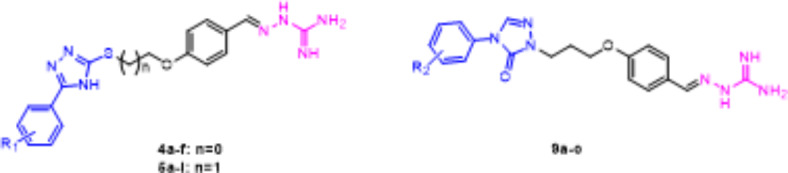
Compound 5f infiltrated sterile paper plate, and 1% DMSO infiltrated sterile paper plates was used as the control group. The cultures were incubated at 35 °C for 18 h. The inhibition zone (DIZ) diameter was subsequently measured. The disc diffusion assay was performed, with compound 5f presenting antibacterial activity (Fig. 3). Analyzing the results, it was verified that compound 5f inhibited the bacterial growth in S. aureus and E. coli strains.
Fig. 3.
(A) DIZ of S.aureus treated by Gatifoxacin, 5f and 1% DMSO respectively; (B) DIZ of E. coli treated by Gatifoxacin, 5f and 1% DMSO respectively.
Simple structure–activity relationships (SARs) could be obtained from Table 1. Aminoguanidine scaffold was discovered to contribute to the accumulation of compounds in Gram-negative E. coli. This finding is consistent with the results of a series of aminoguanidine derivatives previously reported7. The antimicrobial activity was only slightly affected by the introduction of various substituted benzyl groups with the exception of compounds 4f and 5f, where the introduction of a trifluoromethyl group to the benzene ring led to increase in the activity. Furthermore, the antibacterial activity was insignificantly impacted by the varying carbon chain lengths, as unclear SARs were observed for the 4 and 5 series.
In order to determine the safety profile, the highly active compound 5f was further evaluated for its toxicity against the human cervical cell (HeLa) line using the colorimetric cell proliferation MTT assay21. The cytotoxicity of compound 5f was found to be weak (IC50 > 100 µM), as demonstrated in Table 2. The finding suggested that the promising antibacterial activity was unrelated to its toxicity, but rather to the mechanism of action, which remained unknown. Compound 5f was more effective against E. coli than against S. aureus. Consequently, we conducted additional in-depth experiments to investigate the antibacterial mechanism of compound 5f in relation to E. coli.
Membrane permeability assays
The disruption of membrane integrity is an important step of molecule because it can lead to the leakage of intracellular materials22. To determine the physicochemical factors that influence membrane permeabilization, we performed both outer- and inner-membrane permeabilization assays for compound 5f.
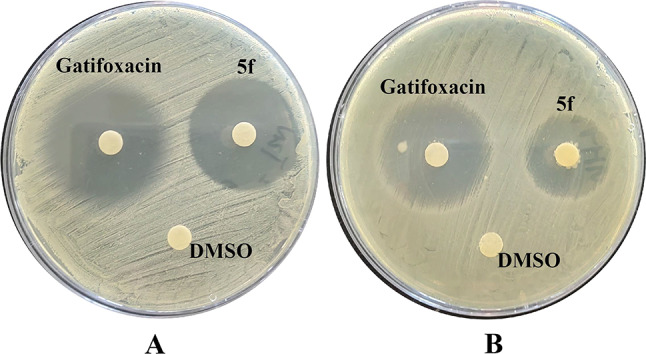
The outer membrane changes were measured using 1-N-phenylnaphthylamine (NPN) dye23. NPN is a hydrophobic fluorescent agent normally used for emission of weak fluorescence in aqueous solution but it emits strong fluorescence upon entering to a hydrophobic medium. As shown in Fig. 4A, compound 5f was able to permeabilize the outer membrane of E. coli ATCC25922 at concentrations from 1 to 64 µg/mL in a concentration-dependent manner. It indicated that 5f could disrupt the outer membrane architecture in E. coli.
Fig. 4.
(A) the outer membrane probed with NPN; (B) permeability of the inner membrane probed with PI.
The inner-membrane permeabilization was studied spectroscopically by measuring the uptake of fluorescent probe propidium iodide (PI)24. The findings of inner membrane permeability assays revealed that compound 5f increased the inner membrane permeability, as evidenced by the dose-dependent uptake of PI (Fig. 4B). Collectively, these data suggested that compound 5f disrupted the membrane of E. coli. Mechanistic studies suggest that the antibacterial mechanism of action of compound 5f permeabilized the inner/outer membrane, leading to bacterial death.
TEM was subsequently employed to visualize bacterial cell morphology at a high resolution and to provide direct evidence of the effects of compound 5f on the membrane. Untreated E. coli ATCC 25,922 possessed a lipid bilayer membrane. The following treatment with 5f at 1 × MIC resulted in extensive membrane damage, as illustrated by the appearance of lysed cells and the release of cell contents (Fig. 5). These findings suggested that 5f might attack the bacterial cell membrane and cause the membrane damage. The consequent increased membrane permeability will then allow 5f to enter the cells.
Fig. 5.

Transmission electron micrographs of (A) untreated E. coli ATCC 25,922; (B) treated with 5f. The arrows mark areas of membrane damage and cell content release. Scale bar 500 nm.
Conclusions
In conclusion, a novel class of aminoguanidine derivatives was synthesized and tested for their in vitro antibacterial activity. The results suggested that these compounds exhibited a significant antibacterial activity against the majority of the testing strains, including the drug-resistant strains. Compound 5f possessed the strongest potential as a therapeutic agent, with an MIC of 2–4 µg/mL against selected bacterial strains. Furthermore, compound 5f was also demonstrated to be minimally cytotoxic to HeLa cell lines. Membrane permeability and TEM image studies indicated that compound 5f permeabilized bacterial membranes, leading to irregular cell morphology and the rapid death of bacteria. These findings reveal that compound 5f is a promising lead for further development.
Experimental section
All of the solvents were reagent grade and dried prior to use. Melting points were determined in open capillaries and were uncorrected. 1H, 13C NMR, HSQC and HMBC spectra (see the Supporting Information, Figure S1-S57) were recorded on Bruker 400 MHz by making a solution of samples in the DMSO-d6 as solvent using tetramethylsilane (TMS) as the internal standard. The following abbreviations were used to describe peak patterns: s = singlet, d = doublet, t = triplet, q = quadruplet, m = multiplet. Coupling constants (J) were expressed in hertz unit (Hz). High Resolution Mass Spectrometry was measured on a Thermo Scientific LTQ Orbitrap XL spectrometer. Mass spectra were measured on an MALDI-TOF (Shimadzu, Japan).
Preparation of intermediate 2 and 3
Intermediate 2 was synthesized according to the literature25. Intermediate 2 (10 mmol) and 4-bromoethoxybenzaldehyde (10 mmol) in refluxing acetone (25 mL) in the presence of KI/K2CO3 was stirred for 6 h. After reaction was completed, the solvent was removed by evaporation and extracted with ethyl acetate. The combined organic layers were dried with Na2SO4, filtered. The residue was subjected to column chromatography (petroleum ether/ethyl acetate 5:1) to afford intermediate 3.
Preparation of intermediate 7 and 8
Intermediate 7 was synthesized according to the literature26. Intermediate 8 was synthesized according to the procedure for the preparation of intermediate 3.
General procedure for the preparation of compounds 4a-f, 5a-i and 9a-c
A mixture of the intermediate 3 or 8 (5 mmol) and aminoguanidine hydrochloride (5 mmol) in ethanol (25 mL) in the presence of 5 drops of concentrated hydrochloric acid was stirred at 50ºC for 10–12 h. The solvent was then removed under reduced pressure. Finally, the product was purified by using silica gel chromatography (CH2Cl2–MeOH 10:1).
(E)-2-(4-(2-((5-Phenyl-4 H-1,2,4-triazol-3-yl)thio)ethoxy)benzylidene)hydrazine-1-carboximidamide (4a) White solid. Yield (0.13 g, 58%), m.p. 199–200oC. 1H NMR (400 MHz, DMSO) δ 14.46 (s, 1H, NH), 11.97 (s, 1H, NH), 8.12 (s, 1H, CH = N), 8.00 (d, J = 7.7 Hz, 2 H, Ar–H), 7.80 (d, J = 8.8 Hz, 3 H, Ar–H), 7.72 (s, 2 H, NH2), 7.56–7.45 (m, 3 H, Ar–H and NH), 7.06 (d, J = 8.8 Hz, 2 H, Ar–H), 4.38 (t, J = 6.5 Hz, 2 H, CH2), 3.56 (t, J = 6.4 Hz, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.46, 155.86, 147.02, 130.42, 129.77 (3 C), 129.43 (3 C), 126.81, 126.48 (3 C), 115.23 (2 C), 67.17, 30.89. HRMS (ESI) m/z calcd for C18H20N7OS+ [M + H]+: 382.14446, found: 382.14374. (see the Supporting Information, Figure S1-S3).
(E)-2-(4-(2-((5-(4-Fluorophenyl)-4 H-1,2,4-triazol-3-yl)thio)ethoxy)benzylidene)hydrazine-1-carboximidamide (4b) White solid. Yield (0.13 g, 58%), m.p. 129–130oC. 1H NMR (400 MHz, DMSO) δ 14.70 (s, 1H, NH), 11.93 (s, 1H, NH), 8.12 (s, 1H, CH = N), 8.09–8.01 (m, 2 H, Ar–H), 7.78 (t, J = 14.8 Hz, 6 H, Ar–H and NH), 7.36 (s, 2 H, NH2), 7.05 (d, J = 8.5 Hz, 2 H, Ar–H), 4.37 (t, J = 6.4 Hz, 2 H, CH2), 3.56 (t, J = 6.4 Hz, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.45, 155.87 (2 C), 147.03 (2 C), 129.78 (4 C), 126.80, 115.23 (4 C), 67.16 (2 C), 49.05. HRMS (ESI) m/z calcd for C18H19FN7OS+ [M + H]+: 400.13503, found: 400.13467. (see the Supporting Information, Figure S4-S6).
(E)-2-(4-(2-((5-(4-Chlorophenyl)-4 H-1,2,4-triazol-3-yl)thio)ethoxy)benzylidene)hydrazine-1-carboximidamide (4c) White solid. Yield (0.12 g, 54%), m.p. 186–188oC. 1H NMR (400 MHz, DMSO) δ 14.63 (s, 1H, NH), 11.97 (s, 1H, NH), 8.12 (s, 1H, CH = N), 8.02 (d, J = 7.9 Hz, 2 H, Ar–H), 7.89–7.43 (m, 8 H, Ar–H and NH), 7.05 (d, J = 8.4 Hz, 2 H, Ar–H), 4.37 (t, J = 6.2 Hz, 2 H, CH2), 3.58 (t, J = 6.4 Hz, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.43, 155.89, 147.00 (2 C), 129.77 (3 C), 129.52 (3 C), 128.21 (3 C), 126.81, 115.23 (3 C), 67.15. HRMS (ESI) m/z calcd for C18H19ClN7OS+ [M + H]+: 416.10548, found: 416.10501. (see the Supporting Information, Figure S7-S9).
(E)-2-(4-(2-((5-(4-Bromophenyl)-4 H-1,2,4-triazol-3-yl)thio)ethoxy)benzylidene)hydrazine-1-carboximidamide (4d) White solid. Yield (0.12 g, 54%), m.p. 98–100oC. 1H NMR (400 MHz, DMSO) δ 14.53 (s, 1H, NH), 11.86 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.96–7.89 (m, 2 H, Ar–H), 7.75 (m, 7 H, Ar–H and NH), 7.04 (d, J = 7.9 Hz, 2 H, Ar–H), 4.36 (t, J = 6.2 Hz, 2 H, CH2), 3.57 (t, J = 6.0 Hz, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.43, 155.86, 147.02 (2 C), 132.44 (3 C), 129.78 (3 C), 128.41 (3 C), 126.81, 115.23 (3 C), 67.14. MS (MALDI-TOF) m/z 460 [M + H]+. (see the Supporting Information, Figure S10-S12).
(E)-2-(4-(2-((5-(P-tolyl)-4 H-1,2,4-triazol-3-yl)thio)ethoxy)benzylidene)hydrazine-1-carboximidamide (4e) White solid. Yield (0.13 g, 56%), m.p. 74–76oC. 1H NMR (400 MHz, DMSO) δ 14.60 (s, 1H, NH), 11.98 (s, 1H, NH), 8.12 (s, 1H, CH = N), 7.96–7.66 (m, 7 H, Ar–H and NH), 7.33 (s, 2 H, NH2), 7.06 (d, J = 8.2 Hz, 2 H), 4.37 (t, J = 6.3 Hz, 2 H, CH2), 3.53 (t, J = 6.0 Hz, 2 H, CH2), 2.36 (s, 3 H, CH3). 13C NMR (101 MHz, DMSO) δ 160.48, 155.92 (2 C), 147.02 (2 C), 129.77 (4 C), 126.69 (2 C), 115.25 (3 C), 67.20 (2 C), 21.44 (2 C). HRMS (ESI) m/z calcd for C19H22N7OS+ [M + H]+: 396.16011, found: 396.15952. (see the Supporting Information, Figure S13-S15).
(E)-2-(4-(2-((5-(4-(Trifluoromethyl)phenyl)-4 H-1,2,4-triazol-3-yl)thio)ethoxy)benzylidene)hydrazine-1-carboximidamide (4f) White solid. Yield (0.11 g, 52%), m.p. 168–170oC. 1H NMR (400 MHz, DMSO) δ 14.77 (s, 1H, NH), 11.92 (s, 1H, NH), 8.21 (d, J = 8.0 Hz, 2 H, Ar–H), 8.12 (s, 1H, CH = N), 7.88 (d, J = 5.4 Hz, 2 H, Ar–H), 7.80 (d, J = 8.8 Hz, 3 H, Ar–H and NH), 7.69 (s, 2 H, NH2), 7.05 (d, J = 8.6 Hz, 2 H, Ar–H), 4.39 (t, J = 6.3 Hz, 2 H, CH2), 3.62 (t, J = 6.0 Hz, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.40, 155.84, 147.00 (2 C), 129.78 (3 C), 127.19 (2 C), 126.82, 126.40 (4 C), 115.21 (3 C), 67.12 (2 C). HRMS (ESI) m/z calcd for C19H19F3N7OS+ [M + H]+: 450.13184, found: 450.13141. By 2D NMR including HSQC and HMBC spectra, we determined the chemical bonding connections in compound 4f and further confirmed its structure (see the Supporting Information, Figure S16-S20).
(E)-2-(4-(3-((5-Phenyl-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5a) White solid. Yield (0.12 g, 52%), m.p. 80–82oC. 1H NMR (400 MHz, DMSO) δ 14.48 (s, 1H, NH), 12.00 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.98 (d, J = 7.9 Hz, 2 H, Ar–H), 7.79 (d, J = 8.8 Hz, 3 H, Ar–H), 7.70–7.66 (m, 1H, Ar–H), 7.53–7.43 (m, 3 H, Ar–H and NH), 7.01 (d, J = 8.8 Hz, 2 H, Ar–H), 4.18–4.13 (m, 2 H, CH2), 3.18 (s, 2 H, CH2), 2.23–2.14 (m, 2 H, CH2). HRMS (ESI) m/z calcd for C19H22N7OS+ [M + H]+: 396.16011, found: 396.15952. (see the Supporting Information, Figure S21, S22).
(E)-2-(4-(3-((5-(4-Fluorophenyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5b) White solid. Yield (0.11 g, 50%), m.p. 78–79oC. 1H NMR (400 MHz, DMSO) δ 14.46 (s, 1H, NH), 11.91 (s, 1H, NH), 8.11 (s, 1H, CH = N), 8.02 (s, 2 H, Ar–H), 7.79 (d, J = 8.7 Hz, 5 H, Ar–H and NH), 7.34 (s, 2 H, NH2), 7.01 (d, J = 8.8 Hz, 2 H, Ar–H), 4.17 (t, J = 6.0 Hz, 2 H, CH2), 3.35 (s, 2 H, CH2), 2.22–2.13 (m, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.82, 155.83, 147.07, 129.74 (4 C), 129.27 (4 C), 126.53, 115.13 (3 C), 66.52 (2 C), 29.43 (2 C). HRMS (ESI) m/z calcd for C19H21FN7OS+ [M + H]+: 414.15068, found: 414.14993. (see the Supporting Information, Figure S23-S25).
(E)-2-(4-(3-((5-(4-Chlorophenyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5c) White solid. Yield (0.11 g, 48%), m.p. 100–101oC. 1H NMR (400 MHz, DMSO) δ 14.53 (s, 1H, NH), 11.92 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.97 (d, J = 8.3 Hz, 2 H, Ar–H), 7.79 (d, J = 8.6 Hz, 5 H, Ar–H and NH), 7.55 (s, 2 H, NH2), 7.01 (d, J = 8.6 Hz, 2 H, Ar–H), 4.17 (t, J = 5.8 Hz, 2 H, CH2), 3.35 (s, 2 H, CH2), 2.24–2.13 (m, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.81, 155.84, 147.06 (2 C), 129.74 (3 C), 128.13 (4 C), 126.54, 115.13 (3 C), 66.49 (2 C), 29.44 (2 C). MS (MALDI-TOF) m/z 430 [M + H]+. (see the Supporting Information, Figure S26-S28).
(E)-2-(4-(3-((5-(4-Bromophenyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5d) White solid. Yield (0.11 g, 52%), m.p. 181–183oC. 1H NMR (400 MHz, DMSO) δ 14.53 (s, 1H, NH), 11.91 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.90 (d, J = 7.5 Hz, 2 H, Ar–H), 7.82–7.49 (m, 7 H, Ar–H and NH), 7.01 (d, J = 8.7 Hz, 2 H, Ar–H), 4.22–4.12 (m, 2 H, CH2), 3.36 (s, 2 H, CH2), 2.18–2.12 (m, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.81, 155.84, 147.06, 132.43 (4 C), 129.74 (3 C), 126.54, 115.13 (3 C), 66.50 (2 C), 29.44 (2 C). HRMS (ESI) m/z calcd for C19H21BrN7OS+ [M + H]+: 474.07062, found: 474.07022. (see the Supporting Information, Figure S29-S31).
(E)-2-(4-(3-((5-(P-tolyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5e) White solid. Yield (0.12 g, 54%), m.p. 68–70oC. 1H NMR (400 MHz, DMSO) δ 14.41 (s, 1H, NH), 11.98 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.77 (d, J = 7.6 Hz, 6 H, Ar–H and NH), 7.30 (d, J = 7.8 Hz, 2 H, Ar–H), 7.01 (d, J = 8.5 Hz, 2 H, Ar–H), 4.17 (t, J = 5.9 Hz, 2 H, CH2), 3.36 (s, 2 H, CH2), 2.35 (s, 3 H, CH3), 2.23–2.10 (m, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.82, 155.91, 147.03, 129.92 (3 C), 129.73 (3 C), 126.41 (4 C), 115.14 (3 C), 66.57, 29.45 (2 C), 21.42. HRMS (ESI) m/z calcd for C20H24N7OS+ [M + H]+: 410.17576, found: 410.17511. (see the Supporting Information, Figure S32-S34).
(E)-2-(4-(3-((5-(4-(Trifluoromethyl)phenyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5f) White solid. Yield (0.12 g, 54%), m.p. 181–183oC. 1H NMR (400 MHz, DMSO) δ 14.68 (s, 1H, NH), 11.90 (s, 1H, NH), 8.13 (m, 3 H, CH = N and Ar–H), 7.77 (t, J = 13.9 Hz, 6 H, Ar–H), 7.01 (d, J = 7.8 Hz, 2 H, Ar–H), 4.18 (t, J = 5.3 Hz, 2 H, CH2), 3.34 (s, 2 H, CH2), 2.25–2.13 (m, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.81, 155.86, 147.05, 129.74 (3 C), 126.40 (7 C), 115.14 (3 C), 66.46 (2 C), 29.46 (2 C). HRMS (ESI) m/z calcd for C20H21F3N7OS+ [M + H]+: 464.14749, found: 464.14679. By 2D NMR including HSQC and HMBC spectra, we determined the chemical bonding connections in compound 5f and further confirmed its structure (see the Supporting Information, Figure S35-S39).
(E)-2-(4-(3-((5-(4-Methoxyphenyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5 g) White solid. Yield (0.11 g, 48%), m.p. 94–95oC. 1H NMR (400 MHz, DMSO) δ 14.27 (s, 1H, NH), 11.84 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.89 (d, J = 7.7 Hz, 2 H, Ar–H), 7.8–7.61 (m, 4 H, Ar–H and NH), 7.03 (d, J = 8.6 Hz, 4 H, Ar–H), 4.17 (s, 2 H, CH2), 3.81 (s, 3 H, OCH3), 3.33 (s, 2 H, CH2), 2.17 (s, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 161.01, 155.82, 147.11, 129.75 (3 C), 128.05 (3 C), 115.14 (3 C), 114.80 (3 C), 66.58, 55.78 (2 C), 29.46 (2 C). HRMS (ESI) m/z calcd for C20H24N7O2S+ [M + H]+: 426.17067, found: 426.16992. (see the Supporting Information, Figure S40-S42).
(E)-2-(4-(3-((5-(3-Chlorophenyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5 h) White solid. Yield (0.12 g, 52%), m.p. 168–169oC. 1H NMR (400 MHz, DMSO) δ 14.38 (s, 1H, NH), 12.10 (s, 1H, NH), 8.10 (s, 1H, CH = N), 7.99 (s, 1H, Ar–H), 7.93 (d, J = 8.0, 1H, Ar–H), 7.84–7.61 (m, 5 H, Ar–H and NH), 7.52 (dd, J = 3.7, 1.6 Hz, 2 H, Ar–H), 4.16 (q, J = 6.4 Hz, 2 H, CH2), 3.33 (d, J = 7.0 Hz, 2 H, CH2), 2.18 (s, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.81, 155.86, 147.07 (2 C), 134.10, 131.37 (2 C), 129.73 (3 C), 126.54, 124.98 (2 C), 115.12 (3 C), 66.50 (2 C), 29.45 (2 C). HRMS (ESI) m/z calcd for C19H21ClN7OS+ [M + H]+: 430.12113, found: 430.12054. (see the Supporting Information, Figure S43-S45).
(E)-2-(4-(3-((5-(M-tolyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5i) White solid. Yield (0.12 g, 52%), m.p. 68–70oC. 1H NMR (400 MHz, DMSO) δ 14.50 (s, 1H, NH), 11.89 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.85–7.67 (m, 6 H, Ar–H), 7.34 (d, J = 17.1 Hz, 2 H, Ar–H), 7.01 (s, 2 H, NH2), 4.16 (d, J = 5.5 Hz, 2 H, CH2), 3.35 (s, 2 H, CH2), 2.37 (s, 3 H, CH3), 2.24–2.12 (m, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.84, 155.82, 147.09 (2 C), 129.74 (4 C), 123.62 (2 C), 115.13 (4 C), 66.57 (2 C), 29.42 (2 C), 21.45 (2 C). HRMS (ESI) m/z calcd for C20H24N7OS+ [M + H]+: 410.17576, found: 410.17511. (see the Supporting Information, Figure S46-S48).
(E)-2-(4-(3-((5-(3-Methoxyphenyl)-4 H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)hydrazine-1-carboximidamide (5j) White solid. Yield (0.13 g, 56%), m.p. 88–90oC. 1H NMR (400 MHz, DMSO) δ 14.54 (s, 1H, NH), 11.89 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.87–7.77 (m, 4 H, Ar–H), 7.56 (d, J = 7.7 Hz, 2 H, Ar–H), 7.41 (s, 1H, Ar–H), 7.02 (t, J = 9.1 Hz, 3 H, Ar–H and NH), 4.17 (t, J = 6.0 Hz, 2 H, CH2), 3.82 (s, 3 H, OCH3), 3.31 (s, 2 H, CH2), 2.21–2.13 (m, 2 H, CH2). 13C NMR (101 MHz, DMSO) δ 160.84, 160.03, 155.83, 147.10 (2 C), 129.74 (3 C), 126.53, 118.79 (2 C), 115.14 (3 C), 66.58 (2 C), 55.76 (2 C), 29.42 (2 C). HRMS (ESI) m/z calcd for C20H24N7O2S+ [M + H]+: 426.17067, found: 426.16986. (see the Supporting Information, Figure S49-S51).
(E)-2-(4-(3-(4-(4-Fluorophenyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl)propoxy)benzylidene)hydrazine-1-carboximidamide (9a) White solid. Yield (0.11 g, 50%), m.p. 181–183oC. 1H NMR (400 MHz, DMSO) δ 11.87 (s, 1H, NH), 8.46 (s, 1H, NH), 8.10 (s, 1H, CH = N), 7.85–7.59 (m, 7 H, Ar–H and NH), 7.36 (t, J = 8.8 Hz, 2 H, Ar–H), 6.98 (d, J = 8.7 Hz, 2 H, Ar–H), 4.12 (t, J = 5.9 Hz, 2 H, CH2), 3.94 (t, J = 6.7 Hz, 2 H, CH2), 2.16–2.08 (m, 2 H, CH2). HRMS (ESI) m/z calcd for C19H21FN7O2+ [M + H]+: 398.17353, found: 398.17282. (see the Supporting Information, Figure S52, S53).
(E)-2-(4-(3-(5-Oxo-4-(p-tolyl)-4,5-dihydro-1H-1,2,4-triazol-1-yl)propoxy)benzylidene)hydrazine-1-carboximidamide (9b) White solid. Yield (0.11 g, 48%), m.p. 131–133oC. 1H NMR (400 MHz, DMSO) δ 11.88 (s, 1H, NH), 8.43 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.87–7.67 (m, 7 H, Ar–H and NH), 7.30 (d, J = 8.0 Hz, 2 H, Ar–H), 6.98 (d, J = 8.6 Hz, 2 H, Ar–H), 4.11 (t, J = 5.5 Hz, 2 H, CH2), 3.93 (t, J = 6.5 Hz, 2 H, CH2), 2.30 (t, J = 6.4 Hz, 3 H, CH3), 2.23–2.09 (m, 2 H, CH2). HRMS (ESI) m/z calcd for C20H24N7O2+ [M + H]+: 394.19860, found: 394.19803. (see the Supporting Information, Figure S54, S55).
(E)-2-(4-(3-(4-(4-Methoxyphenyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl)propoxy)benzylidene)hydrazine-1-carboximidamide (9c) White solid. Yield (0.12 g, 52%), m.p. 51–53oC. 1H NMR (400 MHz, DMSO) δ 11.83 (s, 1H, NH), 8.37 (s, 1H, NH), 8.11 (s, 1H, CH = N), 7.86–7.67 (m, 7 H, Ar–H and NH), 7.02–6.96 (m, 4 H, Ar–H), 4.12 (t, J = 5.7 Hz, 2 H, CH2), 3.93 (t, J = 6.6 Hz, 2 H, CH2), 3.79 (s, 3 H), 2.21–2.11 (m, 2 H, CH2). MS (MALDI-TOF) m/z 410 [M + H]+. (see the Supporting Information, Figure S56, S57).
In vitro inhibition of bacterial and fungal growth
The micro-organisms used in the present study were S. aureus ATCC25923, S. epidermidis 102,555, B. subtilis CMCC63501, E. coli ATCC25922 and C. albicans 7535. The strains of multidrug-resistant clinical isolates were multi-drug resistant Staphylococcus aureus ATCC 43,300 and multi-drug resistant Escherichia coli ATCC BAA-196. Test bacteria were grown to mid-log phase in Muellere-Hinton broth (MHB) and diluted 1000-fold in the same medium. The bacteria of 105 CFU/mL were inoculated into MHB and dispensed at 0.2 mL/well in a 96-well microtiter plate. Test compounds were dissolved in DMSO, the final concentration of which did not exceed 0.05%. A two-fold serial dilution technique was used to obtain final concentrations of 64–0.25 µg/mL. Bacteria growth was determined by measuring the absorption at 650 nm using a microtiter enzyme-linked immunosorbent assay (ELISA) reader.
Outer membrane permeability assay
The outer membrane permeability was examined using fluorescent dye NPN according to the method mentioned previously27. Briefly, E. coli ATCC25922 cells were harvested, washed three times, and resuspended in 5 mM HEPES buffer (pH 7.4, containing 5 mM glucose). Then, 10 µM NPN was added to the suspension, and incubated at room temperature in the dark for 30 min. The fluorescence intensities of the samples were measured with an F4500 fluorescence spectrophotometer.
Inner membrane permeability assay
E. coli ATCC25922 cell was harvested, washed and resuspended in PBS buffer at pH 7.4 to obtain an OD600 of 0.5, followed by the addition of 10 nmol/L of PI in the presence of 5f. The excitation wavelength was 535 nm, and the emission wavelength was 617 nm. The uptake of PI was measured by the increase in fluorescence of PI for 10 min as a measure of inner-membrane permeabilization.
Transmission electron microscopy
The intracellular changes in E. coli cells induced by 5f was determined by TEM according a previously described method28. The E. coli treated with 5f was streaked on slides and washed with PBS 3 times, fixed with 2.5% glutaraldehyde for 12 h and 1% osmium for 4 h, then gradient elution was performed with different concentrations of acetone, embedded in epoxy resin polymer. Finally, the sections were coated and stained with 2% bisoxyl acetate and lead citrate, and the bacterial samples were observed by using TEM. The sections were examined with a HT7700 high resolution transmission electron microscope (HITACHI Company, Japan) at 80 kV.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Natural Science Foundation of Jilin Province (No. 20230204068YY); the Department of Education of Jilin Province (No. JJKH20210494KJ) and the Studentsʼ Program for Innovation and Entrepreneurship Training (No. X202313706057).
Author contributions
Zhang TY and Zhang HM designed the experiments and critically revised the manuscript. Bai XQ performed the bioactivity assay and wrote the article. Wang JH and Jiao FT participated the experiments, and analyzed the data.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hongmei Zhang, Email: hongmeizhang_1981@163.com.
Tianyi Zhang, Email: tianyizhang@126.com.
References
- 1.Moore, B. S., Carter, G. T. & Brönstrup, M. Are natural products the solution to antimicrobial resistance? Nat. Prod. Rep.34, 685–686. 10.1039/c7np90026k (2017). [DOI] [PubMed] [Google Scholar]
- 2.Marr, A. K., Gooderham, W. J. & Hancock, R. E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol.6, 468–472. 10.1016/j.coph.2006.04.006 (2006). [DOI] [PubMed] [Google Scholar]
- 3.https://www.cdc.gov/drugresistance/index.html
- 4.Liu, Y. H. et al. Synthesis and structure-activity relationship of novel bisindole amidines active against MDR gram-positive and gram-negative bacteria. Eur. J. Med. Chem.150, 771–782. 10.1016/j.ejmech.2018.03.031 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature. 415, 389–395. 10.1038/415389a (2002). [DOI] [PubMed] [Google Scholar]
- 6.Shahin, I. G. et al. Evaluation of N-phenyl-2-aminothiazoles for treatment of multi-drug resistant and intracellular Staphylococcus aureus infections. Eur. J. Med. Chem.202, 112497. 10.1016/j.ejmech.2020.112497 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Li, Y. R. et al. Synthesis and biological evaluation of 1,3-diaryl pyrazole derivatives as potential antibacterial and anti-inflammatory agents. Bioorg. Med. Chem. Lett.25, 5052–5057. 10.1016/j.bmcl.2015.10.028 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Eissa, I. H. et al. Diphenylurea derivatives for combating methicillin- and vancomycin-resistant Staphylococcus aureus. Eur. J. Med. Chem.130, 73–85. 10.1016/j.ejmech.2017.02.044 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Koparir, M. et al. Synthesis and biological activities of some novel aminomethyl derivatives of 4-substituted-5-(2-thienyl)-2,4-dihydro-3H-1,2,4-triazole-3-thiones. Eur. J. Med. Chem.63, 340–346. 10.1016/j.ejmech.2013.02.025 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Padmavathi, V., Thriveni, P., Reddy, G. S. & Deepti, D. Synthesis and antimicrobial activity of novel sulfone-linked bis heterocycles. Eur. J. Med. Chem.43, 917–924. 10.1016/j.ejmech.2007.06.011 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Amir, M., Kumar, H. & Javed, S. A. Condensed bridgehead nitrogen heterocyclic system: Synthesis and pharmacological activities of 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives of ibuprofen and biphenyl-4-yloxy acetic acid. Eur. J. Med. Chem.43, 2056–2066. 10.1016/j.ejmech.2007.09.025 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Sztanke, K. et al. determination of the lipophilicity, anticancer and antimicrobial properties of some fused 1,2,4-triazole derivatives. Eur. J. Med. Chem.43, 404–419. 10.1016/j.ejmech.2007.03.033 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Kus, C. et al. Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3(4H)-thione derivatives of benzimidazole class. Bioorg. Med. Chem.16, 4294–4303. 10.1016/j.bmc.2008.02.077 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Zhang, T. Y. et al. Synthesis and antimicrobial evaluation of aminoguanidine and 3-amino-1,2,4-triazole derivatives as potential antibacterial agents. Lett. Drug Des. Discov. 13, 1063–1075. 10.2174/1570180813666160819151239 (2016). [Google Scholar]
- 15.Wei, Z. Y. et al. Synthesis and biological evaluation of chalcone derivatives containing aminoguanidine or acylhydrazone moieties. Bioorg. Med. Chem. Lett.26, 5920–5925. 10.1016/j.bmcl.2016.11.001 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Xiao, S. N. et al. Novel panaxadiol triazole derivatives induce apoptosis in HepG-2 cells through the mitochondrial pathway. Bioorg. Chem.102, 104078. 10.1016/j.bioorg.2020.104078 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Chi, K. Q. et al. Design, synthesis, and evaluation of novel ursolic acid derivatives as HIF-1α inhibitors with anticancer potential. Bioorg. Chem.75, 157–169. 10.1016/j.bioorg.2017.09.013 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Guan, D. L. et al. Sulfonium, an underestimated moiety for structural modification, alters antibacterial profile of vancomycin against multidrug-resistant bacteria. Angew Chem. Int. Ed. Engl.58, 6678–6682. 10.1002/anie.201902210 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Zhang, T. Y. et al. Design, synthesis and evaluation of dihydrotriazine derivatives-bearing 5-aryloxypyrazole moieties as antibacterial agents. Mol. Divers.25, 861–876. 10.1007/s11030-020-10071-9 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Song, M. X. et al. Synthesis, antimicrobial and cytotoxic activities, and molecular docking studies of N-arylsulfonylindoles containing an aminoguanidine, a semicarbazide, and a thiosemicarbazide moiety. Eur. J. Med. Chem.166, 108–118. 10.1016/j.ejmech.2019.01.038 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Shen, Q. K., Deng, H., Wang, S. B., Tian, Y. S. & Quan, Z. S. Synthesis, and evaluation of in vitro and in vivo anticancer activity of 14-substituted oridonin analogs: A novel and potent cell cycle arrest and apoptosis inducer through the p53-MDM2 pathway. Eur. J. Med. Chem.173, 15–31. 10.1016/j.ejmech.2019.04.005 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Song, M. R. et al. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol.5, 1040–1050. 10.1038/s41564-020-0723-z (2020). [DOI] [PubMed] [Google Scholar]
- 23.Jia, B. Y. et al. High cell selectivity and bactericidal mechanism of symmetric peptides centered on d-Pro-Gly cairs. Int. J. Mol. Sci.21, 1140. 10.3390/ijms21031140 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoque, J. et al. Cleavable cationic antibacterial amphiphiles: Synthesis, mechanism of action, and cytotoxicities. Langmuir. 28, 12225–12234. 10.1021/la302303d (2020). [DOI] [PubMed] [Google Scholar]
- 25.Wei, Z. Y. et al. Design, synthesis, and negative inotropic evaluation of 4-phenyl-1H-1,2,4-triazol-5(4H)-one derivatives containing triazole or piperazine moieties. Chem. Biol. Drug Des.89, 47–60. 10.1111/cbdd.12828 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Shang, F. F. et al. Discovery of triazolyl derivatives of cucurbitacin B targeting IGF2BP1 against non-small cell lung cancer. J. Med. Chem.66, 12931–12949. 10.1021/acs.jmedchem.3c00872 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Je, J. Y. & Kim, S. K. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J. Agric. Food Chem.54, 6629–6633. 10.1021/jf061310p (2006). [DOI] [PubMed] [Google Scholar]
- 28.Dai, J. K., Dan, W. J., Li, N. & Wang, J. R. Computer-aided drug discovery: Novel 3,9-disubstituted eudistomin U derivatives as potent antibacterial agents. Eur. J. Med. Chem.157, 333–338. 10.1016/j.ejmech.2018.08.001 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.


