Abstract
Myeloperoxidase and eosinophil peroxidase have been isolated from outdated human blood. Peroxidase activity was extracted from washed leucocytes using 0.5 M-CaCl2 and the extract further purified by chromatography on concanavalin A--Sepharose, phenyl-Sepharose and finally by gel filtration. The final enzyme preparations were highly purified according to spectral and gel-electrophoretic criteria. Under reducing and denaturing conditions on polyacrylamide-gel electrophoresis myeloperoxidase gave rise to bands of Mr 57 000, 39 000 and 15 500, whereas the eosinophil enzyme yielded bands of Mr 50 000 and 15 500. Both enzymes were very resistant to denaturation either by the chaotropic agents urea and guanidinium chloride or by elevated temperatures. Spectral properties of the native and reduced forms of the enzymes are reported.
Full text
PDF
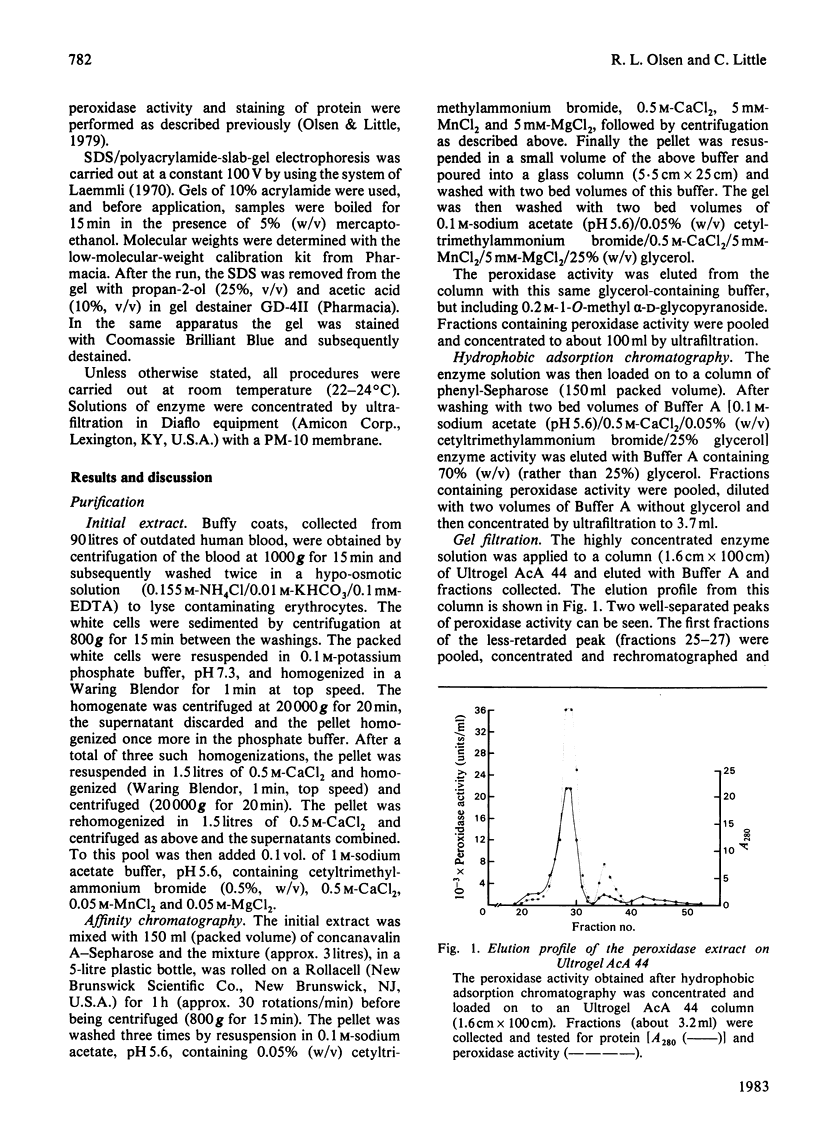
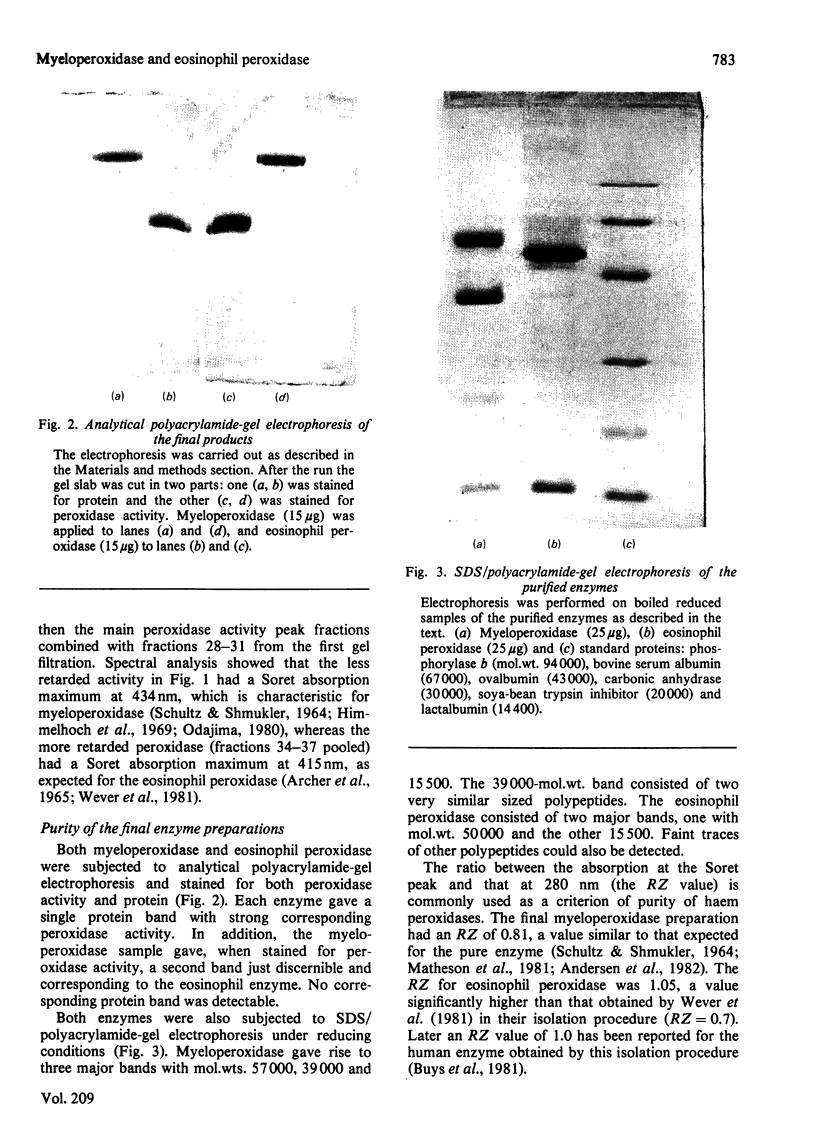
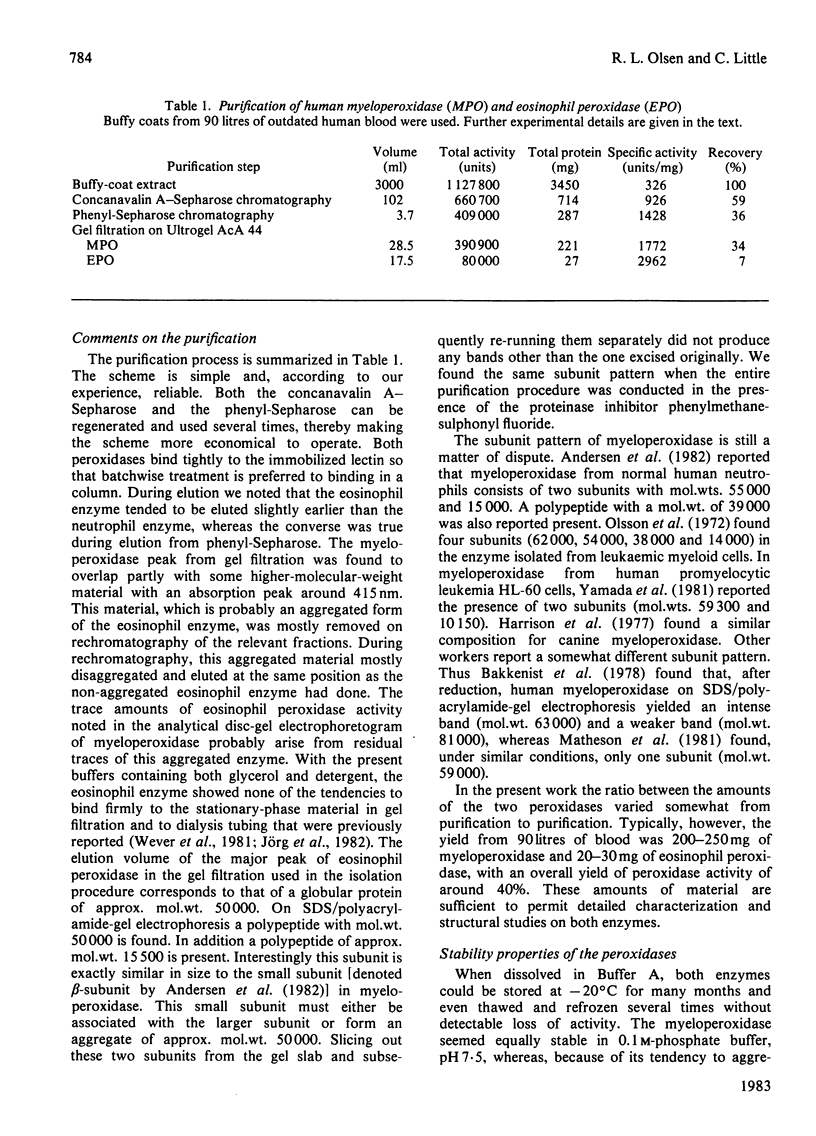
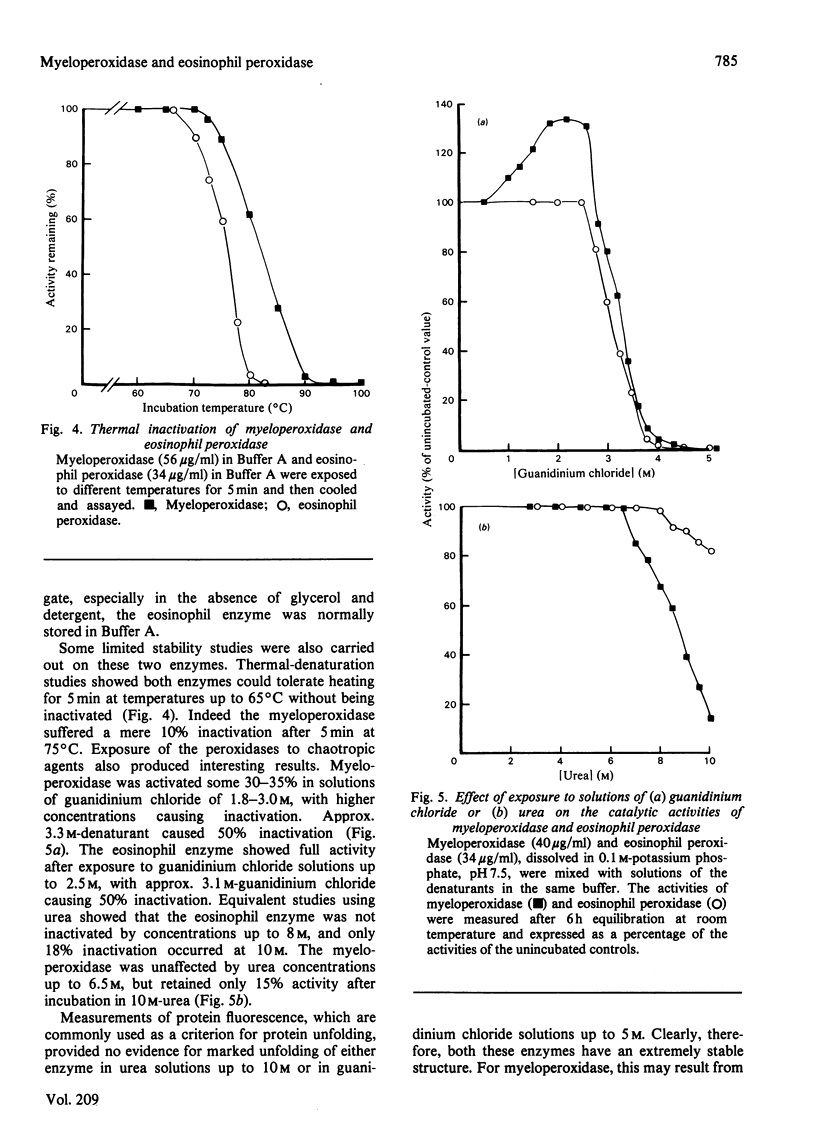
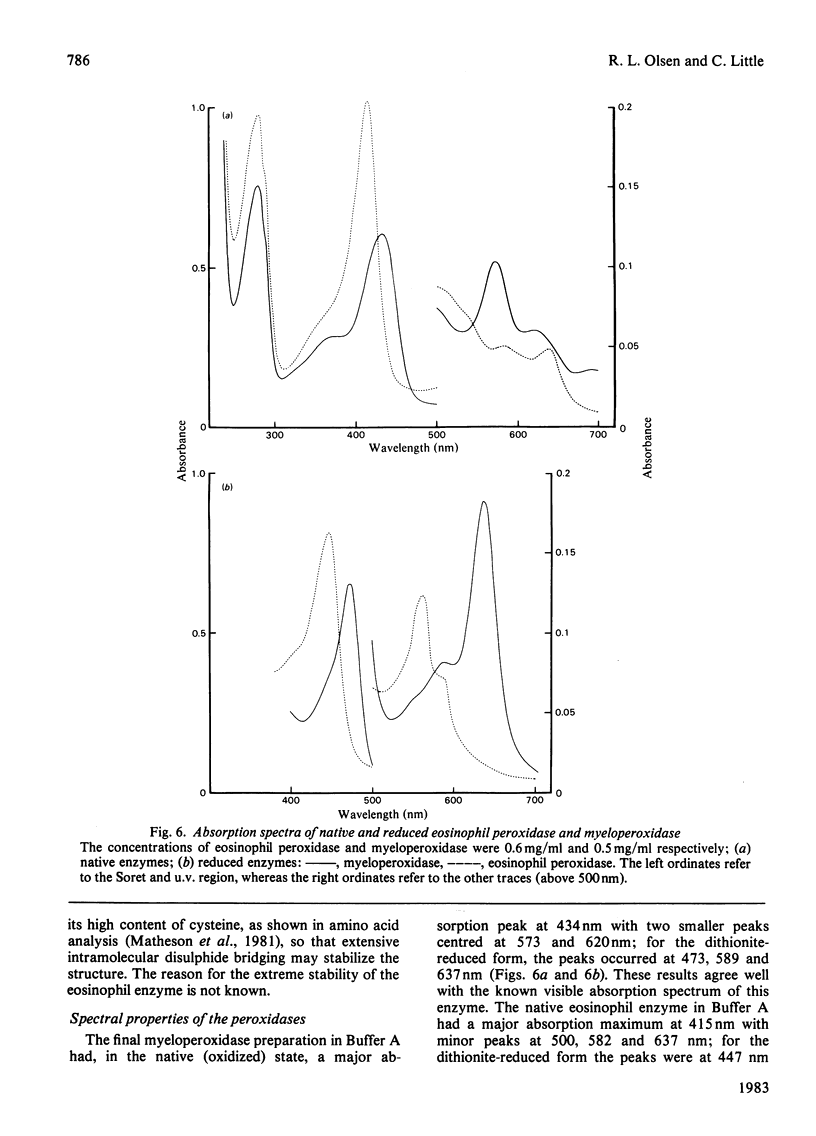

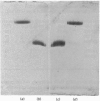
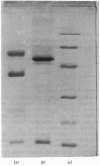
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHER G. T., AIR G., JACKAS M., MORELL D. B. STUDIES ON RAT EOSINOPHIL PEROXIDASE. Biochim Biophys Acta. 1965 Apr 26;99:96–101. doi: 10.1016/s0926-6593(65)80010-8. [DOI] [PubMed] [Google Scholar]
- Andersen M. R., Atkin C. L., Eyre H. J. Intact form of myeloperoxidase from normal human neutrophils. Arch Biochem Biophys. 1982 Mar;214(1):273–283. doi: 10.1016/0003-9861(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1979 Oct;64(4):913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desser R. K., Himmelhoch S. R., Evans W. H., Januska M., Mage M., Shelton E. Guinea pig heterophil and eosinophil peroxidase. Arch Biochem Biophys. 1972 Feb;148(2):452–465. doi: 10.1016/0003-9861(72)90164-6. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A., Haudenschild C. C. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J Clin Invest. 1980 Nov;66(5):908–917. doi: 10.1172/JCI109958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Pabalan S., Schultz J. The subunit structure of crystalline canine myeloperoxidase. Biochim Biophys Acta. 1977 Aug 23;493(2):247–259. doi: 10.1016/0005-2795(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Jörg A., Pasquier J. M., Klebanoff S. J. Purification of horse eosinophil peroxidase. Biochim Biophys Acta. 1982 Feb 18;701(2):185–191. doi: 10.1016/0167-4838(82)90112-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Odajima T. Myeloperoxidase of the leukocyte of normal blood. Nature of the prosthetic group of myeloperoxidase. J Biochem. 1980 Feb;87(2):379–391. doi: 10.1093/oxfordjournals.jbchem.a132758. [DOI] [PubMed] [Google Scholar]
- Olsen R. L., Flatmark T., Little C. Spectral properties of the oestrogen-induced rat uterus peroxidase II and some of its derivatives. Biochem J. 1982 Jan 1;201(1):91–94. doi: 10.1042/bj2010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. L., Little C. Comparative studies on oestrogen-induced rat uterus peroxidase and rat eosinophil peroxidase. Biochem J. 1982 Dec 1;207(3):613–616. doi: 10.1042/bj2070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. L., Little C. The peroxidase activity of rat uterus. Eur J Biochem. 1979 Nov;101(2):333–339. doi: 10.1111/j.1432-1033.1979.tb19725.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- SCHULTZ J., SHMUKLER H. W. MYELOPEROXIDASE OF THE LEUCOCYTE OF NORMAL HUMAN BLOOD. II. ISOLATION, SPECTROPHOTOMETRY, AND AMINO ACID ANALYSIS. Biochemistry. 1964 Sep;3:1234–1238. doi: 10.1021/bi00897a009. [DOI] [PubMed] [Google Scholar]
- Stelmaszyńska T., Zgliczyński J. M. Myeloperoxidase of human neutrophilic granulocytes as chlorinating enzyme. Eur J Biochem. 1974 Jun 1;45(1):305–312. doi: 10.1111/j.1432-1033.1974.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Wever R., Plat H., Hamers M. N. Human eosinophil peroxidase: a novel isolation procedure, spectral properties and chlorinating activity. FEBS Lett. 1981 Jan 26;123(2):327–331. doi: 10.1016/0014-5793(81)80320-1. [DOI] [PubMed] [Google Scholar]
- Wever R., Plat H. Spectral properties of myeloperoxidase and its ligand complexes. Biochim Biophys Acta. 1981 Oct 13;661(2):235–239. doi: 10.1016/0005-2744(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Yamada M., Mori M., Sugimura T. Purification and characterization of small molecular weight myeloperoxidase from human promyelocytic leukemia HL-60 cells. Biochemistry. 1981 Feb 17;20(4):766–771. doi: 10.1021/bi00507a018. [DOI] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T., Ostrowiski W., Naskalski J., Sznajd J. Myeloperoxidase of human leukaemic leucocytes. Oxidation of amino acids in the presence of hydrogen peroxide. Eur J Biochem. 1968 May;4(4):540–547. doi: 10.1111/j.1432-1033.1968.tb00246.x. [DOI] [PubMed] [Google Scholar]