Abstract
Cancer is one of the most prevalent diseases and the leading cause of death worldwide. Despite the improved survival rates of cancer in recent years, the current available treatments often face resistance and side effects. Drug repurposing represents a cost-effective and efficient alternative to cancer treatment. Recent studies revealed that penfluridol (PF), an antipsychotic drug, is a promising anticancer agent. In the present study, a scoping review was conducted to ascertain the anticancer properties of PF. For this, a literature search was performed using the Scopus, PubMed and Web of Science databases with the search string ‘penfluridol’ AND ‘cancer’. A total of 23 original articles with in vivo and/or in vitro studies on the effect of PF on cancer were included in the scoping review. The outcome of the analysis demonstrated the anticancer potential of PF. PF significantly inhibited cell proliferation, metastasis and invasion while inducing apoptosis and autophagy in vivo and across a spectrum of cancer cell lines, including breast, lung, pancreatic, glioblastoma, gallbladder, bladder, oesophageal, leukaemia and renal cancers. However, research on PF derivatives with high anticancer activities and reduced neurological side effects may be necessary.
Keywords: penfluridol, anticancer, antipsychotic, repurposing drug, cancer, apoptosis, autophagy
1. Introduction
Cancer is one of the most prevalent diseases and the leading cause of death worldwide. The global cancer incidence and mortality were estimated at nearly 19 million new cases and 10 million deaths in 2020, with lung, colorectal, breast and prostate cancer accounting for the majority of the diagnosed cancers worldwide (1,2). Despite the increasing survival rates of cancer patients observed in the past decades due to improved and game-changing therapies, the global incidence of cancer keeps increasing and is estimated to reach 28 million per year in 2040, leading to a physical, emotional and financial burden to the patients and their families, and a strain on resources of the public health system (2,3). The cancer burden has been attributed to the inequitable access to cancer prevention, early detection, screening and treatment of the population, particularly in low- and middle-income countries. In addition, an increase in the price of cancer drugs has been recorded in recent years. Cancer is predicted to cost $25.2 trillion to the world economy from 2020 to 2050, bringing severe financial distress to the economy, affected patients and families, and the public health system (4,5). Furthermore, highly effective treatments, including radiotherapy, chemotherapy, surgery, targeted therapy and hormonal treatments, which significantly contribute to the overall reduction of cancer mortality, recurrence and spread and increase in survival rate, have limitations in curing cancer, particularly in patients with delayed diagnoses and in patients with cancer therapy resistance, resulting in a worse survival outcome (6,7).
Drug repositioning, an approach to using approved drugs to treat diseases outside their original indication scope, has gained significant attention in recent years (8). Repurposing existing drugs reduces the exorbitant cost of developing new drugs, estimated to be between $314 million and $2.8 billion per medication in 2018. It shortens the time taken for the drug approval process, which can otherwise take 10 to 15 years to reach the market (8,9). Furthermore, repurposed drugs have a decreased likelihood of clinical failure due to adverse effects, as their safety and dosing have already received clinical approval (10). Consequently, repurposing drugs for cancer treatment represents a cost-effective method for cancer therapy.
Various antipsychotic drugs including penfluridol (PF), phenothiazine, pimozide, chlorpromazine and thioridazine have been discovered to have anticancer properties and have been identified as a promising alternative cancer treatment (11). Several studies have reported that patients taking antipsychotic drugs have a lower cancer incidence compared to the general population (12). PF is a well-established first-generation diphenylbutylpiperidine (DPBP) antipsychotic drug used to treat chronic schizophrenia and other psychotic disorders, and has been found to inhibit cancer (11,13). Indeed, PF inhibits cancer cell proliferation and induces apoptosis and autophagy in various cancer cell lines (14). PF has a half-life of 70 h, can cross the blood-brain barrier (BBB) and block the dopamine receptor (DRD2) binding sites, making it a potent drug for the treatment of brain cancers, as well as cancers with high brain metastasis ability (13,15,16). This scoping review aimed to briefly investigate the anticancer properties of PF, focusing on in vivo and in vitro studies and providing a concise overview of PF's anticancer mechanism of action.
2. Methodology
Protocol
This scoping review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist and PRISMA-ScR Tip sheets (17). The review was conducted in five stages according to Mak and Thomas's (18) steps for conducting a scoping review: i) Identify the research question; ii) identify relevant studies; iii) select the studies to be included in the review; iv) charting data; and v) collate, summarize and report results.
Identification of the research question
This review aimed to investigate the effect of PF on cancer. Following the standard recommendation for a broader research question (19), a primary research question was developed: What are the effects of PF on cancer? This research question provided sufficient literature to warrant the scoping review and envelop the studies on PF as a treatment for cancer. Hence, no further narrowing of the research question was necessary.
Identification of relevant studies
A literature search was conducted using the Scopus (https://www-scopus-com.uitm.idm.oclc.org), PubMed (https://pubmed.ncbi.nlm.nih.gov) and Web of Science (WoS; http://www-webofscience-com.uitm.idm.oclc.org) databases with the search string ‘penfluridol’ AND ‘cancer’. The search, conducted in November 2023, included no additional filters.
Study selection
Following the search, the identified records were assessed in two phases. In the first phase, one author (AAIM) evaluated the obtained articles by their titles and excluded duplicate articles, review papers and retracted papers. The same author screened the remaining journal papers based on their titles and abstracts. Any articles with no relation to the roles of PF in treating cancers according to their titles and abstracts and any conference abstracts were excluded. In the second phase, one author (AAIM) retrieved and assessed the remaining articles' full text. In the end, original research articles on the roles, functions, importance and mechanisms of PF in treating cancer written in English were retained and included in the scoping review. The second author (AAR) was consulted for their opinion on the obtained data throughout the study selection stages.
Mendeley Desktop version 1.19.8 (Elsevier) and Microsoft Excel version 16.89.1 (Microsoft Corporation) were used to sort out the literature and identify the articles' duplication by independently filtering out the articles' DOIs, titles and abstracts.
Charting the data
Microsoft Excel version 16.89.1 (Microsoft Corporation) was used to organize the data from the selected articles. Data extraction was performed following the PRISMA guidelines by (AAIM). The extracted data were categorised into author names, publication year, type of cancer, dose of PF, study design, significant findings, key findings and limitations.
Collating, summarizing and reporting of the results
The extracted data were tabulated in Microsoft Excel version 16.89.1 (Microsoft Corporation) with rows relating to the articles and columns that classify the variables and contain the relevant information. This table was used to classify and report the information collected.
3. Results
Article selection
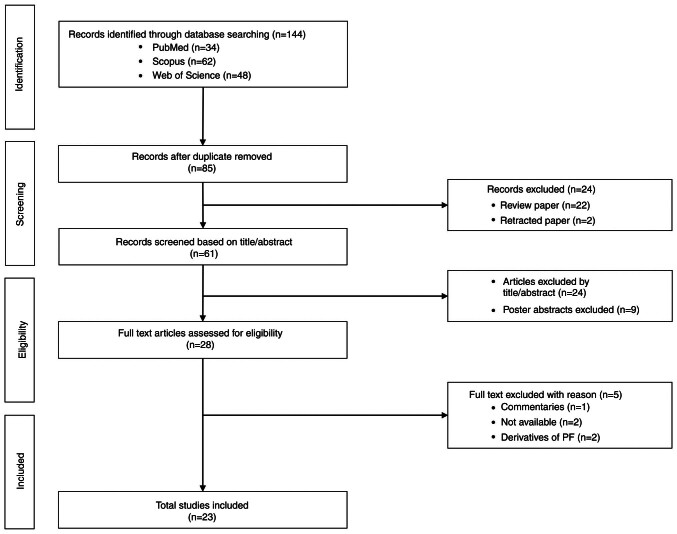
The primary search yielded 144 articles, 34 from PubMed, 62 from Scopus and 48 from WoS. A total of 59 duplicates were removed, resulting in 85 records retained and screened further. Subsequently, a total of 24 articles were eliminated due to being reviews (n=22) and retracted papers (n=2). The remaining 61 articles were screened based on title and abstract and 24 articles with no relation to the roles of PF in treating cancers and poster abstracts (n=9) were excluded. A total of 28 records were identified as eligible for further full-text assessment. Of the 28 articles, five were excluded due to lack of availability (n=2), focusing on PF derivatives (n=2) and commentaries (n=1). Finally, 23 original research studies in English were retained for this scoping review. The PRISMA flowchart on the paper selection is presented in Fig. 1.
Figure 1.
PRISMA flow diagram of the scoping review process. PF, penfluridol.
Study characteristics
The main findings are summarised and presented in Table I. Out of the 23 original articles included in this review, 4 evaluated the anticancer effect of PF on breast cancer, 4 on lung cancer, 4 on pancreatic cancer, 3 on glioblastoma, 1 on bladder cancer, 1 on gallbladder cancer, 1 on oesophageal cancer, 1 on leukaemia cancer and 1 on renal cancer. The remaining 3 articles included in this review studied the effect of PF on various types of cancer. All of the studies in this review included an in vitro model of a cell line supplemented with 0 to 40 µM PF, while most studies used an in vivo model treated with PF at 1 to 10 mg/kg.
Table I.
The effect of penfluridol on cancer.
| Type of cancer | Author(s), year | Study model | Doses | Treatment duration | Findings | (Refs.) |
|---|---|---|---|---|---|---|
| Breast | Hedrick | SKBR3 & | 0.7–7.5 µM | 24 h | PF inhibited cell growth and cell migration and | (20) |
| et al 2017 | MDA-MB- | induced cell apoptosis. PF induced ROS in breast | ||||
| 231 cells | cancer cells by downregulating the expression of | |||||
| Sp transcript factor (Sp1, Sp2 and Sp4) and cMyc | ||||||
| (miR-27a and miR-20/miR-17). PF repressed the | ||||||
| expression of α5-, α6-, β1 and β4-integrin. | ||||||
| Female | 10 mg/kg/ | 19 days | PF decreased the tumour volume, inhibited tumour | |||
| athymic | day | growth and induced apoptosis. PF ROS-dependently | ||||
| nude mice | reduced the expression of Sp1, Sp3, Sp4 and Sp- | |||||
| regulated genes (α6-, α5-, β1- and β4-integrins). | ||||||
| Gupta | MCF-7, | 0-20 µM | 24-72 h | PF treatment inhibited the cell growth and colony | (21) | |
| et al 2019 | MCF-7HH, | formation of PXT-sensitive and PXT-resistant cells | ||||
| MCF-7PR, | in a concentration- and time-dependent manner. PF | |||||
| 4T1 & 4T1PR | downregulated the expression of HER2, β-catenin, | |||||
| cells | c-Myc, TCF-1, TCF-4, p-GSK3β and cyclin D1. PF | |||||
| induced apoptosis in breast cancer cells. A | ||||||
| combination of PXT and PF significantly reduced | ||||||
| the cancer cells' viability, suppressing the | ||||||
| chemoresistance markers while enhancing apoptosis. | ||||||
| Female | 10 mg/kg/ | 23 days | PF significantly suppressed the growth of paclitaxel- | |||
| Balb/c mice | day | resistant cells alone or in combination with 5 mg/kg/3 | ||||
| days PXT. PF alone and PF combined with PXT | ||||||
| inhibited HER2 and β-catenin expression and | ||||||
| increased apoptosis. | ||||||
| Ranjan | MDA-MB- | 0-20 µmol/l | 24-72 h | PF suppressed the proliferation, migration and | (22) | |
| et al 2016 | 231, 4T1 & | invasion of breast cancer. PF treatment inhibited | ||||
| HCC1806 | cells' survival and motility by reducing the | |||||
| cells | expression of integrin α6, integrin β4, FAK, paxillin, | |||||
| Rac1/2/3 and ROCK1 and inducing apoptosis. | ||||||
| Female Balb/ | 10 mg/kg | 27 days | PF suppressed tumour growth by inhibiting | |||
| c mice | β4-integrin and enhancing apoptosis in mice. | |||||
| Intracardiac or intracranial injection of breast cancer | ||||||
| cells revealed similar results. | ||||||
| Srivastava | HUVECs & | 0-5 µM | 24-48 h | Low doses of PF blocked the angiogenesis- | (23) | |
| et al 2020 | MDA-MB-23 | suppressed VEGF-induced primary endothelial cell | ||||
| cells | migration and tube formation of the cancer cells. PF | |||||
| inhibited the Src and Akt signalling pathways. | ||||||
| C57B/L6 | 1 mg/kg | 11 days | Low dose of PF blocked VEGF- and FGF-induced | |||
| mice | angiogenesis. | |||||
| Lung | Lai et al | A549 & | 0-7.5 µM | 24 h | PF inhibited lung tumour growth by reducing | (24) |
| 2022 | HCC827 | mitochondrial ATP production and downregulating | ||||
| cells | NADH levels. PF mediated the suppression of | |||||
| mitochondrial biogenesis, impeded the level | ||||||
| of PGC-1α protein and mRNA, SIRT1 and p53, and | ||||||
| induces AMPK activation. | ||||||
| NOD-SCID | 5 m/kg | 28 days | PF inhibited the growth and metastasis of lung | |||
| mice | cancer in mice. These results were enhanced in | |||||
| combination with 2DG. PF decreased the expression | ||||||
| of PGC-1α and SIRT1. | ||||||
| Xue et al | A549, H446, | 0-20 µM | 24-72 h | PF inhibited lung cancer cell growth and viability | (26) | |
| 2020 | H1993, | with G0/G1 cell cycle phase arrest by enhancing the | ||||
| SPC-A1 & | expression level of p21/p27 and decreasing the | |||||
| LL2 cells | expression levels of the cyclin-CDK complex. PF | |||||
| induced apoptosis via the mitochondria-mediated | ||||||
| intrinsic apoptosis pathway and inhibited the | ||||||
| migration and invasion of lung cancer cells. | ||||||
| Female | 10 mg/kg | 26 days | PF inhibited tumour growth in an A549 cell | |||
| Balb/c | xenograft mouse model. PF blocked the proliferation | |||||
| nude mice | and metastasis of lung cancer in mice by | |||||
| regulating the AKT and MMP signalling pathways | ||||||
| while inducing apoptosis. | ||||||
| Hung | NSCLC, | 7.5–10 µM | 24-72 h | PF suppressed cell proliferation by inducing ER | (25) | |
| et al 2019 | A549, | stress- mediated autophagosome accumulation to | ||||
| HCC827 & | deplete ATP energy. PF inhibited cell migration. | |||||
| BEAS-2B | PF induced nonapoptotic cell death by blocking | |||||
| cells | autophagic flux and autophagosome formation | |||||
| of LC3B-II protein in lung cancer cells. | ||||||
| NOD-SCID | 5-10 mg/kg | 35 days | PF inhibited the growth and the metastasis of lung | |||
| mice | cancer in mice by inducing an accumulation of | |||||
| autophagosome-related protein. | ||||||
| Hung | A549, H23, | 0-20 µM | 24-72 h | PF reduced the migration, invasion and adhesion | (27) | |
| et al 2021 | HCC827, | of LADC cells. PF inhibited MMP-12 by | ||||
| PC9, H1975, | downregulating the uPA/uPAR/TGF-β/Akt axis to | |||||
| HMEC-1 & | modulate the motility and adhesion of LADC cells. | |||||
| BEAS-2B | PF inhibited EMT byupregulating E-cadherin and | |||||
| downregulating N-cadherin. | ||||||
| Male NOD- | 2.5 µM | 5 weeks | PF suppressed lung metastasis and colony formation. | |||
| SCID mice | PF increased the expression of E-cadherin levels and | |||||
| decreased N-cadherin and MMP-12 levels in tumour | ||||||
| tissues. | ||||||
| Pancreatic | Ranjan | AsPC-1, | 0-10 µM | 24-72 h | PF induced ER stress in pancreatic cancer cells | (28) |
| et al 2017 | BxPC-3 & | characterised by the upregulation of ER stress | ||||
| Panc-1 cells | markers of BIP, CHOP and IRE1α. PF-induced ER | |||||
| stress led to autophagy. | ||||||
| Female | 10 mg/kg | 3 weeks | PF-treated mice had a high level of BIP, CHOP | |||
| athymic | and IRE1α expression in the tumour lysates and a | |||||
| nude mice | reduction of tumour mass. | |||||
| Ranjan and | BxPC-3 & | 0-20 µM | 24-72 h | PF induced apoptosis and inhibited the growth of | (29) | |
| Srivastava | AsPC-1 | pancreatic cancer cells. PF enhanced autophagy in | ||||
| 2016 | pancreatic cancer cells mediated by apoptosis. PF | |||||
| impeded the formation of lysosomes. | ||||||
| Female | 10 mg/kg | 27 & 59 | PF reduced the growth of BxPC-3 tumour xenografts | |||
| athymic | days | and the development of orthotopically implanted | ||||
| nude mice | pancreatic tumours by 80% by inducing autophagy- | |||||
| mediated apoptosis in the tumours. | ||||||
| Dandawate | AsPC-1, | 0-40 µM | 24-72 h | PF highly bound to the JAK2 domain PRLR. PF | (30) | |
| et al 2020 | BxPC-3, | suppresses pancreatic cancer cells' proliferation and | ||||
| Panc-1, | colony and spheroid formation. PF enhanced PDAC | |||||
| MiaPaCa-2, & | autophagy. The PF mechanism of inhibition of | |||||
| UNKC-6141 | PDAC cell growth did not proceed through DRD2. | |||||
| Male C57BL/6 | 5 mg/kg | 28 & 35 | PF decreased the weight of the orthotopic tumours. | |||
| days | PF significantly inhibited the tumour weight and | |||||
| mice & PDX- | volume in xenograft tumours. PF reduced PDAC | |||||
| carrying | tumour growth by inducing autophagy. | |||||
| NGS mice | ||||||
| Chien | Panc0403, | 0-10 µM | 0-36 h | PF inhibited pancreatic cancer cell proliferation and | (31) | |
| et al 2015 | SU8686, | growth and induced apoptosis. PF decreased the | ||||
| MiaPaCa2, | phosphorylation levels of SRC, AKT and p70S6K. | |||||
| Panc1, | PF induced the activation of PP2A protein | |||||
| Panc0504, | phosphatase leading to pancreatic cancer cell death. | |||||
| AsPc1, | ||||||
| Panc1005, | ||||||
| Panc0203, | ||||||
| Panc0327, | ||||||
| BxPc3 & | ||||||
| HPDE | ||||||
| Glioblastoma | Ranjan and | GBM43, | 0-20 µM | 24-72 h | PF reduced the survival rate and induced apoptosis | (32) |
| Srivastava | GBM10, | in glioblastoma cell lines. PF treatment suppressed | ||||
| 2017 | GBM44, | the phosphorylation of Akt at Ser473 and decreased | ||||
| GBM28, | the expression of GLI1, OCT4, Nanog and Sox2. | |||||
| GBM14 & | ||||||
| U251MG | ||||||
| Athymic nude | 10 mg/ | 39-54 | PF treatment inhibited the growth and reduced the | |||
| mice | kg/day | volume of days in vivo glioblastoma tumour models. | ||||
| PF reduced pAkt, GL1 and OCT4 and enhanced | ||||||
| apoptosis. | ||||||
| Kim et al | CSC2, X01, | 0-20 µM | 24-72 h | PF suppressed the growth of GSCs in a dose- and | (33) | |
| 2019 | 0315, 528NS, | time- dependent manner. PF suppressed the stemness | ||||
| 83NS, U87MG | of GSCs and inhibited their sphere-forming ability | |||||
| & T98G cells | and invasiveness. PF inhibited the EMT potential | |||||
| in human GSCs. | ||||||
| Female Balb/c | 0.8 | 60 days | PF decreased invasion and tumour volume in | |||
| nude mice | mg/kg/ | orthotopic xenografts by reducing GLI1, uPAR, | ||||
| week | SOX2 and vimentin expression. With a | |||||
| combination of PF and TMZ, the tumour | ||||||
| disappeared in the mice. | ||||||
| Ranjan | Female | 10 mg/kg | 40-48 | PF treatment suppressed glioblastoma tumour | (34) | |
| et al 2017 | athymic | days | growth by reducing murine myeloid-derived | |||
| nude mice & | suppressor cells (MDSC). PF enhanced splenic cell | |||||
| female SCID- | proliferation, increased M1 macrophages and | |||||
| NOD mice | suppressed T-regulatory cells and inflammation in | |||||
| the tumour. | ||||||
| Gallbladder | Hu et al | EH-GB1, | 0-10 µM | 24-72 h | PF inhibited the proliferation and migration of | (35) |
| 2022 | GBC-SD & | gallbladder cancer (GBC) cells and enhanced | ||||
| SGC-996 | apoptosis. PF treatment caused the activation | |||||
| cells | of AMPK/PFKB3-mediated glycolysis in GBCs, | |||||
| and a combination of PF and 2-DG or AMPK | ||||||
| inhibitor CC improved the anticancer effect of PF. | ||||||
| Old female | 10 mg/kg | 15 days | PF suppressed lung tumour growth. PF promoted | |||
| nude mice | apoptosis and the activation of AMPK/PFKFB3 | |||||
| signaling. | ||||||
| Bladder | van der | UCB & | 0-100 µM | 0-40 h | PF inhibited the cell viability and clonogenicity | (36) |
| Horst et al | UM-UC- | of the UCB cell line. PF induced lysosomal | ||||
| 2020 | 3luc2 cells | leakage and destabilized lysosomal structures in | ||||
| human UCB cells, resulting in the redistribution | ||||||
| of phosphatidylserine from the internal to the | ||||||
| external membrane surface, an early indicator | ||||||
| of apoptosis. | ||||||
| Female Balb/c | 130 µg/kg | 29 days | PF significantly decreased tumour growth, | |||
| nude mice | per week | invasion and metastasis in mice. | ||||
| Oesophageal | Zheng | KYSE30, | 0-10 µmol/l | 24-72 h | PF inhibited proliferation and colony formation | (37) |
| et al 2020 | KYSE150 & | and enhanced the apoptosis of the esophageal | ||||
| KYDEE270 | squamous cell carcinoma (ESCC) cells. The | |||||
| inhibition of the cells was mediated by | ||||||
| AMPK/FOXO3a/BIM signalling. PF | ||||||
| inhibited glycolysis. | ||||||
| Female Balb/c | 9-18 mg/kg | 42 days | PF decreased tumour volume and cell | |||
| nude mice | proliferation and induced apoptosis in tumour | |||||
| xenograft mice. PF inhibited glycolysis. | ||||||
| Leukaemia | Wu et al | HL-60, U937 | 7.5–20 µM | 24-72 h | PF inhibited the proliferation of AML cells. | (38) |
| 2019 | & MV4-11 | PF induced apoptosis by activating PP2A to | ||||
| cell lines | suppress Akt and MAPK activities. PF | |||||
| with FLT3-WT | triggered autophagic responses by inducing | |||||
| or FLT3-ITD | the elevation of intracellular ROS levels. | |||||
| Renal | Tung | 786-O, | 0-25 µM | 24-72 h | PF decreased the proliferation and colony | (39) |
| et al 2022 | Caki-1, A498 | formation of RCC cells. PF induced autophagy | ||||
| & ACHN cells | through the ER stress-mediated UPR, leading | |||||
| to apoptosis. PF reduced stemness and sphere | ||||||
| formation in RCC cells. | ||||||
| NOD-scid | 5 µM | 5 weeks | PF reduced the tumour volume and growth by | |||
| IL2Rγ | suppressing the GLI1 and proliferation | |||||
| (NSG) mice | index Ki-67. | |||||
| Diverse | Wu et al | B16, LL/2, | 0-9 µmol/l | 24-72 h | PF inhibited the proliferation of melanoma, | (40) |
| 2014 | CT26 & | lung carcinoma, colon carcinoma and breast | ||||
| 4T1 cells | cancer cells in a time-dependent manner. PF | |||||
| increased the accumulation of unesterified | ||||||
| cholesterol in the cancer cells. | ||||||
| Female | 0.06–0.12 mg/ | 45 days | PF suppressed lung, colon, melanoma and | |||
| C57BL/6 | week | breast tumour growth, and tumour weight of | ||||
| mice & | the mice. PF decreased the total cholesterol | |||||
| Balb/c mice | in the tumour tissue of the mice. | |||||
| Varalda | HCT116, | 10-160 µmol/l | 24-72 h | PF inhibited the proliferation of colorectal | (14) | |
| et al 2020 | SW620, | and breast cancer and glioblastoma cells. | ||||
| MCF7, MDA- | PF reduced MCF7 and HCT116 cell motility | |||||
| MB-231, | and migration. PF induced mitochondrial | |||||
| U87 & U251 | and lysosomal membrane alteration and | |||||
| cells | phospholipid aggregation in cancer cells. PF | |||||
| suppressed the mTOR pathway, induced | ||||||
| by AMPK activation and autophagy. | ||||||
| Du et al | HeLa | 5 µmol/l | 24 h | PF has high radiosensitivity activity. PF | (41) | |
| 2018 | inhibited the repair of DSBs post-IR in HeLa | |||||
| cells by reducing NHEJ repair and preventing | ||||||
| the activation of DNA-PKcs. |
AML, acute myeloid leukaemia; AMPK, AMP-activated protein kinase; AKT, serine/threonine-specific protein kinase 1; ATP, adenosine triphosphate; BAX, BCL-2-associated X; BCL-2, B-cell leukemia/lymphoma 2; BIM, BCL-2-interacting mediator; BIP, binding protein; CC, compound C; CCL4, C-C motif chemokine ligand 4; CHOP, C/EBP homologous protein; CNS, central nervous system; DPBP, diphenylbutylpiperidine; EMT, epithelial-mesenchymal transition; ER, endoplasmic reticulum; ESCC, esophageal squamous cell carcinoma; FAK, focal adhesion kinase; GBC, gallbladder cancer; GLI1, glioma-associated oncogene homolog 1; GSC, glioma-sphere forming cells; GSH, glutathione; GSK, glycogen synthase kinase; IR, ionizing radiation; IRE1α, inositol requiring 1α; KRT, keratine; LADC, lung adenocarcinoma; LC3, light chain; LLC, Lewis lung carcinoma; MAPK, mitogen-activated protein kinase; MDSC, myeloid-derived suppressor cells; miR, microRNA; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; NHEJ, non-homologous end joining; NSCLC, non-small-cell lung cancer; OCTA, optical coherence tomography angiography; OXPHOS, oxidative phosphorylation; PARP, poly-ADP ribose polymerase; PDAC, pancreatic ductal adenocarcinoma; PF, penfluridol; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3; PP2A, protein phosphatase 2A; PRLR, prolactin receptor; PTX, paclitaxel; PUMA, p53-upregulated modulator of apoptosis; RCC, renal cell carcinoma; ROCK1, Rho-associated coiled-coil containing protein kinase 1; ROS, reactive oxygen species; Sp, specificity protein; TCF, transcription factor; UCB, urothelial carcinoma of the bladder; UPR, unfolded protein response; uPAR, urokinase plasminogen activator receptors; VEGF, vascular endothelial growth factor; 2-DG, 2-deoxy-D-glucose.
Synthesis of the results
Breast cancer
PF was found to exert its anticancer properties on breast cancer through multiple pathways. PF inhibits cell proliferation in breast cancer cells (20–23). For instance, in the relevant studies, PF successfully reduced the tumour volume and weight in the in vivo model of breast cancer. Furthermore, PF was found to reduce cell migration through reactive oxygen species (ROS) (20), the integrin pathway (22) and the VEGF pathway (23). In addition, PF was capable of inducing apoptosis in breast cancer cells (20–22). While Hedrick et al (20) imparted that the anticancer properties of PF in breast cancer are ROS-dependent, Gupta et al (21) revealed that PF downregulates the expression of chemoresistance markers in paclitaxel (PXT)-resistant breast cancer, leading to apoptosis. In fact, cotreatment of PF with glutathione attenuated the effect of PF on breast cancer (20), and the combination of PF and PXT decreased the expression of human EGFR 2 (HER2), β-catenin and cyclin D, simultaneously enhancing apoptosis (21). It is worth mentioning that chronic administration of PF failed to elicit any significant toxic or behavioural side effects in mice (22) and that low concentrations of PF can block angiogenesis in vitro and in vivo (23).
Lung cancer
Studies on PF in the treatment of lung cancer revealed that PF inhibits lung tumour growth by inducing mitochondrial ATP energy loss in vivo and in vitro (24). Similar results were obtained pertaining to autophagy (25). PF inhibited lung cancer proliferation, migration and metastasis in vivo and in vitro by regulating the AKT and MMP signalling pathway (26,27). PF suppressed MMPs and epithelial-mesenchymal transition (EMT) required for cancer cell motility and adhesion (27). Induced G0/G1 phase arrest, ROS and endoplasmic reticulum (ER) stress, as well as loss of the mitochondrial membrane potential (ΔΨm), were noted in PF-treated lung cancer (25,26). On the other hand, PF in combination with glycolysis inhibitor 2-deoxy-D-glucose synergistically enhanced the inhibitory effect of PF on lung cancer cell growth and the total amount of mitochondria (24). No side effects were noted in the mice used in these studies.
Pancreatic cancer
In pancreatic cancer, PF was reported to induce autophagy (28–30) and apoptosis (29,31) in in vitro and in vivo models. PF inhibited the proliferation and colony formation of pancreatic cancer cells (28–31) through the suppression of prolactin receptor (PRLR) signalling (30) and the activation of protein phosphatase 2A (PP2A) protein phosphatase (31). A study by Ranjan & Srivastava (29) revealed that PF induced autophagy-mediated apoptosis in pancreatic cancer. However, while PF exerts its antipsychotic activity by blocking the DRD2 receptors, the antiproliferative activity of PF in pancreatic ductal adenocarcinoma is not related to DRD2 and does not trigger protein degradation (30).
Glioblastoma
In glioblastoma, PF appeared to reduce the growth of glioblastoma cells and a tumour model through the apoptosis pathway (32). In addition, GLA1 was downregulated in a glioblastoma cancer model treated with PF (32,33). On the other hand, Ranjan et al (34) showed that PF treatment suppresses glioblastoma growth through immune regulation, while Kim et al (33) denoted that it is by reducing the sphere formation, stemness and invasiveness of the cells. Of note, a combination of PF and temozolomide (TMZ) produced maximal antiproliferative and antitumor effects in in vivo and in vitro models (33).
Other cancers
PF inhibited the cell proliferation and colony formation of esophageal squamous cell carcinoma (ESCC) cells (37), renal cell carcinoma (RCC) cell lines (39), acute myeloid leukaemia (AML) cells (38), gallbladder cancer (35), bladder cancer (36), colorectal and breast cancer, glioblastomas, lung cancer and melanoma cells (14,40). For instance, PF reduces glycolysis and promotes cell apoptosis (35,36). Apoptosis was induced in cancer cells through repressed glycolysis (37) and activation of PP2A (38) in ESCC and AML cells, respectively. Combining 2-deoxy-D-glucose (2-DG) or AMP-activated protein kinase (AMPK) inhibitor Compound C (CC) significantly improved PF's anticancer effect in gallbladder cancer (35). Furthermore, PF enhanced autophagy in cancer cells by upregulating ER stress and ROS (38,39). It is worth highlighting that the anticancer activities of PF on RCC partially occurred through DRD2 (39) and that PF suppresses the mTOR pathway in various cell lines (14). Besides that, in vivo studies uncovered that PF effectively prolongs the survival rate of mice bearing lung tumours and enhances the decrease of total cholesterol in tumour tissues with no significant difference in the serum cholesterol levels of the PF-treated mice (40). No side effects to the vital organs of the PF-treated mice were noted despite the decrease in tumour growth and volume in vivo (37,39). In addition, PF-treated urothelial carcinoma of the bladder (UCB) orthotopic xenograft mice exhibited normal murine urothelium, epithelial tissue integrity, proliferation index (proliferating cell nuclear antigen), viable epithelial cell numbers and fragmented keratin (36). In the meantime, extensive research by Du et al (41) unveiled that PF has high radiosensitizing activity and can suppress the activity of non-homologous end joining (NHEJ).
4. Discussion
PF
PF {4-(4-chloro-α,α,α,-trifluoro-m-tolyl)-1-[4,4-bis-(p-fluorophenyl)-butyl]-4-piperidinol} is a DPBP, first-generation antipsychotic drug used to treat chronic schizophrenia, Tourette's syndrome, acute psychosis and other psychotic disorders discovered by Janssen Pharmaceutical in 1968 (15,42,43). PF is a long-lasting neuroleptics drug orally administered once a week with a half-life of 66 h and a terminal plasma half-life of 199 h (44). Indeed, PF is highly lipophilic and is deposited in the adipose tissue after absorption in the gastrointestinal tract, which heightens its prolonged duration of action, since PF is gradually released from these reservoirs (15,44). PF is excreted via urine and faeces (45). The maximal weekly dose of PF treatment for patients with chronic schizophrenia varies between 60 and 140 mg (46,47). According to Andrade (48), long half-life antipsychotic drugs significantly reduce the withdrawal or discontinuation syndrome of the drug but can be disadvantageous in case of toxicity or side effects. For instance, PF treatment fosters various adverse effects, including depression, agitation, drowsiness, tachycardia, hypotension, insomnia and neuroleptic malignant syndrome (49). Therefore, a low dose of PF treatment is recommended.
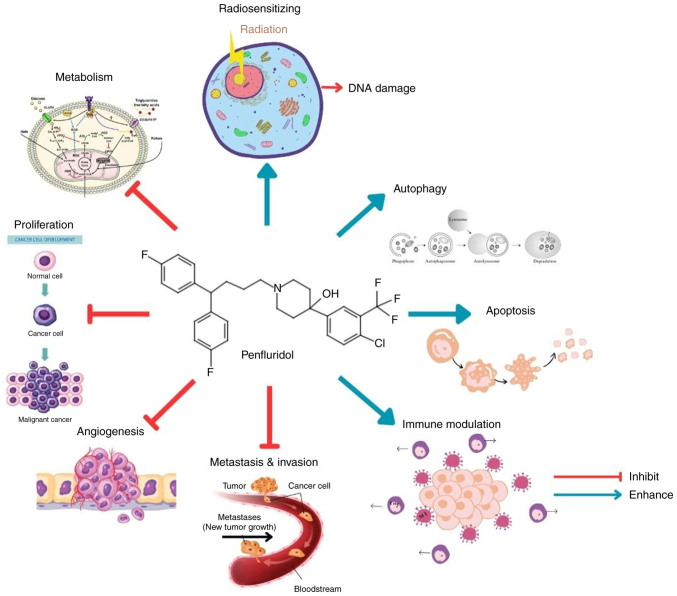
PF is capable of penetrating the BBB, making it a promising option for the treatment of brain-metastasized cancers (33). Airoldi et al (43) revealed that despite the distribution of PF in the adipose tissue being higher, PF remains concentrated in the brain with the highest concentration recorded in the hemisphere part of the brain 1 h after the injection and the mesencephalon 24 h after the injection of 0.5 mg/kg PF in rats. PF acts by blocking the dopamine receptor, particularly DRD2, where it binds with a high affinity, leading to the inhibition of dopaminergic neurotransmission (13,15,16). A former study by Enyeart et al (50) revealed that the PF also blocks the T-type and L-type calcium channels at low concentrations. Additionally, Vlachos et al (12) have reported that patients taking antipsychotic drugs have a lower cancer incidence compared to the general population (12). Henceforth, examining the anticancer properties of PF may offer valuable insight. Fig. 2 summarises the anticancer activities of PF.
Figure 2.
Anticancer properties of penfluridol. The figure was created using certain elements designed by brgfx/Freepik.
The anticancer properties of PF
PF was found to inhibit cell proliferation in a dose- and time-dependent manner in all the cancer cells reported in this review (14,20–41). For instance, <10 µmol/l of PF induced 50% cell death in various cancer cell lines (14), while <2.5 µM of PF inhibited the proliferation and colony formation of lung and renal cancer cells (25,39). Meanwhile, 0.12 mg/week of PF remarkably prolonged the survival time in the LL/2 in vivo cancer model (40), indicating that a low concentration of PF can provide anticancer properties. The antiproliferative effects of PF are not mediated by major neuroreceptor systems (14), but instead by interfering with the mechanism involved in cancer growth. Indeed, PF induced cell death by downregulating the cell growth proteins (cyclin D and MYC) and expression of the G2-M phase of cell cycle arrest protein cyclin B1 and p21 in pancreatic cells (31). The regulatory protein of the transition between the G2 to M phase has a significant role in the proliferation of the tumour and inhibition of these proteins led to cell-cycle arrest and eventually apoptosis (51,52). In addition, PF activates PP2A, mediating apoptosis and cell death (31,38). PP2A is a positive regulator of apoptosis and activation of PP2A dephosphosphorylates and inactivates anti-apoptotic Bcl-2, inducing apoptosis (53).
On the other hand, PF increased ROS levels in lung cancer cells, leading to cell death (26). ROS functions as a double-edged sword in cancer cells and can act as a tumour-promoting or suppressing agent (54,55). Cancer cells possess elevated levels of ROS in comparison to normal cells due to their fast proliferation and alteration in cellular metabolism, particularly the reprogrammed metabolism to aerobic glycolysis (56,57). Moderate levels of ROS are necessary for cancer cell proliferation, invasion, migration and angiogenesis, while excessively elevated ROS increase cancer cell damage and death (58,59). Indeed, PF induced ROS production triggering autophagy in AML cells (38). Furthermore, Hedrick et al (20) revealed that PF enhances ROS production, leading to the downregulation of cMyc expression and decreased expression of Sp1, Sp3, Sp4 and Sp-regulated genes in vitro and in an in vivo model of breast cancer, hence inhibiting cell viability and inducing apoptosis. The specificity proteins Sp and the multifaceted oncogene c-Myc are essential regulators of cellular proliferation, metabolism, metastasis and apoptosis, which, when upregulated, conduce to the progression of cancer (60–63). Furthermore, Sp regulates the activation or repression of integrins, transmembrane receptors which partake in cancer development, progression, invasion, metastasis and resistance to therapy (64–67). Ranjan et al (22) showed that PF reduced the expression of α6, α4, αv, β4, β1 and β3 integrins, as well as the downstream effector molecules of integrin signalling, including focal adhesion kinase (FAK), phosphorylated paxillin and paxillin in breast cancer (22). Desgrosellier & Cheresh (68) revealed that integrin signalling plays a significant role in cancer migration, proliferation and invasion and inhibition of integrins lead to the repression of tumour invasion and growth.
Apart from that, PF enhances energy loss. For instance, according to Lai et al (24), PF inhibits non-small cell lung cancer (NSCLC) cell growth by increasing ATP energy loss through the suppression of mitochondrial ATP production activity and biogenesis. Similarly, suppression of ATP increased the formation and accumulation of autophagosomes in NSCLC, leading to death (25). Indeed, cancer cells require a considerable energy supply for their metabolism (69). This phenomenon known as the Warburg effect is marked by an elevated glycolysis in the cancer cells, including aerobic glycolysis to meet the energy needs of the cells to grow (70). Several studies have shown that targeting the metabolism of cancer cells impacts the growth of the cancer and inhibition of the glycolysis reduced the biosynthesis of ATP, a constituent of DNA, RNA and phospholipids (71), thus suppressing cancer cell growth. PF inhibited ESCC cell proliferation by repressing glycolysis through AMPK signalling (37). The activation of AMPK/forkhead box (FOX)O3a/BCL-2-interacting mediator (BIM) signalling in an oesophageal cell line induced apoptosis (37). In addition, Hu et al (35) reported that the combination of glycolytic inhibitor 2-DG or AMPK inhibitor CC with PF treatment significantly inhibited the proliferation of gallbladder cancer. Furthermore, PF dysregulated cholesterol homeostasis by enhancing the accumulation of unesterified cholesterol in LL/2, 4T1, B16 and CT26 cancer cells and decreased the total cholesterol in tumour tissues of PF-treated mice (40). Cholesterol plays a significant role in tumour cell growth (72). Henceforth, PF has potential anti-metabolic properties for the treatment of cancer.
Apoptosis and autophagy, programmed cell death pathways crucial in maintaining cellular and organismal homeostasis, are the main mechanisms researched in cancer (73,74). PF was found to enhance apoptosis by inducing nuclear fragmentation, poly-ADP ribose polymerase cleavage, caspase-3 activation and condensed nuclear chromatin (21,29,38). Similarly, PF upregulated the expression levels of the proapoptotic proteins BIM, BAX and p53-upregulated modulator of apoptosis (PUMA) and downregulated the antiapoptotic gene Bcl-2 in pancreatic cancer (31). Then again, PF induced G0/G1 phase arrest and, vehemently, cell apoptosis in lung cancer via the mitochondrial apoptotic pathway (26). For instance, Xue et al (26) reported that PF increased the loss of ΔΨm. Thus, PF induces tumour suppression through apoptosis. Despite that, Hung et al (25) remarked that PF's antiproliferative effect on NSCLC cells is independent of apoptosis. In fact, instead of the apoptotic characteristics, formation of autophagosome protein light chain (LC)3B-II and expression of autophagosome markers autophagy-related protein 5, Beclin-1 and p62 were recorded in PF-treated NSCLC cells, implying that PF enhances cell death through autophagy (25,73,75,76). Indeed, PF promoted an increase in LC3-II and p62 protein expression, phospholipid aggregates and lysosomal membrane damage and consequently cell death in various cell lines (14,29,30). In addition, in in vivo and in vitro studies of PF-treated lung and pancreatic cancer, an increase in ER stress was observed, which stimulated autophagy, noticeable by the overexpression of binding protein, C/EBP homologous protein (CHOP) and inositol-requiring 1α, increased unfolded protein response (UPR) signals and activation of p38/MAPK (25,28). Pre-treatment of the pancreatic cells with pharmacological inhibitors such as sodium phenylbutyrate and mithramycin or silencing of CHOP, followed by PF treatment, considerably suppressed autophagy, revealing that PF-induced enhancement of ER stress leads to autophagy in pancreatic cancer (28). Of note, blocking autophagy with pharmacological inhibitors significantly increases PF-induced apoptosis in AML cells (38). However, treatment of BxPC-3 and AsPC-1 with autophagy inhibitors and LC3B silencing followed by PF treatment resulted in a significant reduction of apoptosis, suggesting that PF induces autophagy-mediated apoptosis in pancreatic cancer (29). Similar results were obtained in RCC cell lines, where PF induced autophagy-mediated apoptosis through the upregulation of ER stress-mediated UPR (39). Therefore, PF enhances autophagy and autophagy-mediated apoptosis in cancer.
In addition, PF constrained migration, adhesion, invasion and metastasis in cancer cells (27,36). Metastasis, a hallmark of cancer-cell migration from their initial site, is a crucial factor of cancer therapy failure associated with an unfavourable prognosis and cancer-associated death (77). PF treatment significantly reduced cell migration and invasion by reducing the expression of phosphorylated Rac family small GTPase 1 (Rac1), Rac1/2/3 and Rho-associated coiled-coil containing protein kinase 1 proteins in triple-negative breast cancer cells (22). Furthermore, Kim et al (33) revealed that PF inhibited stemness by reducing the expression of SOX2, Nestin and optical coherence tomography angiography and invasiveness by decreasing the expression of integrin α6 and urokinase plasminogen activator receptor in glioma-sphere forming cells. In addition, PF reduced the potential for EMT, associated with cancer migration, invasion, stemness and metastasis, of glioblastoma cancer models by downregulating the expression of EMT markers (vimentin, Zeb 1, N-cadherin, Snail and Slug) (33,78,79). Besides that, PF reduced the expression of glioma-associated oncogene homolog 1 and OCT4 in several glioblastoma cell lines (32) and reduced lung cancer migration in vivo and in vitro by blocking the FAK-related migration signalling pathway and regulating the AKT and MMP signalling pathway (26). Despite PF's ability to constrain the mobility of bladder cancer cells, no notable effects were registered in the urothelium and epithelial tissue of UCB orthotopic xenograft mice (36). Therefore, PF exhibits anti-migration and anti-metastasis properties without any side effects.
PF significantly inhibits angiogenesis, a mechanism by which tumours acquire new nutrients for cell metabolism, leading to cancer growth (80). Research by Srivastava et al (23) revealed that PF inhibits angiogenesis in in vitro and in vivo models of triple-negative breast cancer by suppressing VEGF-induced Src and Akt activation to prevent VEGF activity (23). VEGF is a major angiogenic agent in tumours that can initiate the growth and metastasis of tumours (81). Furthermore, PF increased the immune surveillance in glioblastoma cancer (33). Indeed, PF reduced the number of regulatory T cells (Treg), suppressed the expression of FoxP3 and CD4 by Tregs and increased M1 macrophages (increase of CD86 and IL-12 expression), but also inhibited tumour inflammation markers [C-C motif chemokine ligand 4 (CCL4) and IFNγ] responsible for tumour progression, as observed in PF-treated mouse glioblastoma cancer model (33,34). CCL4 has been identified as a marker of tumour proliferation in RCC and is associated with immune checkpoint genes, including lymphocyte activating 3, cytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein 1 in RCC (82). Hence, the reduction of CCL4 in glioblastoma by PF indicates that PF suppresses the progression and immunity of the cancer cells. Therefore, PF can impede angiogenesis in tumours and modulate the immune system.
Lastly, PF considerably downregulated the expression of the chemoresistance markers HER2, β-catenin, cyclin D1 and c-Myc in PXT-resistant breast cancer (21). Indeed, chemoresistance promoted cancer relapses and metastasis, thus leading to a more aggressive form of cancer (83,84). Gupta et al (21) revealed that PF reduced the tumour volume in PXT-resistant breast cancer female Balb/c mice by 40%, but the combination of PF and PXT induced apoptosis and decreased the expression of HER2, β-catenin and cyclin D1. Moreover, Du et al (41) unveiled that PF has radiosensitizing activity and can suppress the NHEJ activity/repair of the ionizing radiation (IR)-induced DNA double-strand breaks (DSBs). Radiosensitizers heighten the lethal effect of radiotherapy on tumours by boosting DNA damage and free radical production without affecting normal tissue (85) and PF impaired the IR-induced DNA damage repair, enhanced the formation of DSB markers (γ-H2AX and tumour protein 53 binding protein 1 foci) and inhibited the repair of DSB post-IR in HeLa cells, thus promoting the cytotoxicity of IR in HeLa cells (41). Taken together, it can be suggested that PF is likely an excellent chemo-radiotherapeutic agent.
Overall, PF exhibits several anticancer effects in various cancer cell lines. PF affects the proliferation of cancer cells, provides radiosensitizing activity, induces apoptosis and autophagy, inhibits the invasion, metastasis and angiogenesis of cancer, and reduces the immunosuppressive effect of cancer cells.
PF treatment induced apoptosis via the mitochondrial apoptotic pathway in lung cancer and via the suppression of Akt in glioblastoma. In addition, PF instigated apoptosis through nuclear fragmentation, PARP cleavage and caspase 3 activation in leukaemia and through the activation of the BIM, BAX and PUMA proapoptotic proteins in pancreatic cancer. PF inhibited cell proliferation in lung cancer via mitochondrial ATP energy loss and the inhibition of glycolysis, which consequently led to autophagy and cell death. Similarly, in gallbladder, bladder and oesophageal cancer, PF promoted apoptosis via inhibition of glycolysis. However, in pancreatic cancer, PF induced autophagy via induction of ER stress but suppressed the mTOR pathway in numerous cancer cell lines. Upregulation of ROS by PF has been associated with the ability of PF to enhance autophagy in renal cancer and leukaemia. However, ROS led to the downregulation of cMyc and Sp resulting in apoptosis in breast cancer. PF inhibited cell migration via integrin and the VEGF pathway in breast cancer. PF induced cell death via the activation of PP2A and PRLR signalling in pancreatic cancer but PP2A in leukaemia cells. PF modulated the immune system in glioblastoma cells by reducing the number of Tregs and increasing M1 macrophages. In HeLa cells, PF was able to reduce DNA repair by inhibiting the activity of NHEJ.
5. Limitation of PF and future direction
The dosage of PF as an anticancer drug exceeds the clinical therapeutic range of PF as an antipsychotic. Indeed, the 10 mg/kg dose treatment of PF regularly used in in vivo studies, including in chronic treatment, has successfully reduced the tumour size and weight without triggering any major side effects, as no significant changes in the body weight of the mice and clinical chemical blood analysis parameters were observed (29), and this 10 mg/kg dose of PF is equivalent to the dose of 0.83 mg/kg in humans. Thus, for a person weighing 60 kg, the human equivalent dose of PF would be ~50 mg (21). However, most of the available studies indicate that mice are given PF treatment daily, which exceeds the maximum weekly dose of PF for chronic schizophrenia patients, which varies between 60 and 140 mg (46,47). Of note, this high dose may cause various adverse effects (49). In fact, since PF interacts extensively with most G-protein coupled receptors at levels consistent with the recommended anticancer dosage, which is 50 mg per day for humans, the off-target central nervous system (CNS) activity of PF as an anticancer drug will exacerbate the drug's neurological side effects (86). Therefore, studying other mechanisms of PF delivery to targeted sites, including the use of nanotechnology, could potentially reduce the CNS side effects of the drug.
Nonetheless, a study by Kim et al (33) on an orthotopic mouse glioblastoma model revealed that a lower dose of 0.8 mg/week of PF, which is equivalent to 4 mg/week in an adult patient with a body weight of 60 kg, showed effective antitumor activity. In this study, PF significantly reduced the proliferation, invasion, migration and stemness of glioblastoma cells, as well as the tumour size and invasion in vivo, indicating that a lower dose of PF has a potent anticancer effect (33). Similarly, 0.12 mg/week of PF resulted in significant inhibition of the tumour and prolonged the survival time in an LL/2 lung cancer in vivo model (40). Furthermore, Kim et al (33) demonstrated that a combination of 0.8 mg/kg of PF with TMZ provides better anticancer properties and prolonged survival in the mouse model. Hence, a lower dose of PF alone or in combination with other chemotherapeutic drugs may be considered for future clinical and in vivo studies.
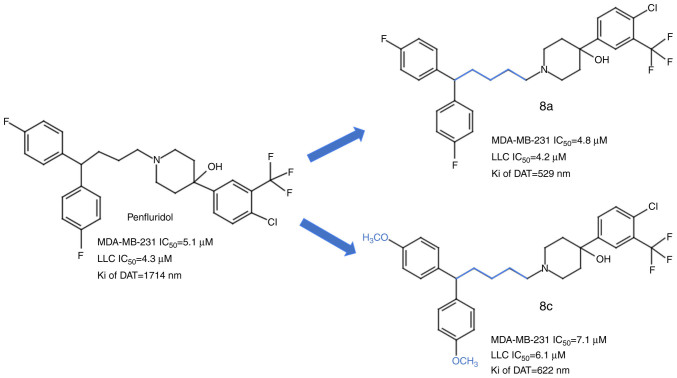
In addition, Ashraf-Uz-Zaman et al (86,87) suggested the usage of PF derivatives with enhanced anticancer activity and a weakened antipsychotic effect. Their study identified 2 PF analogue compounds with less affinity for the CNS and greater anticancer properties than PF (87) (Fig. 3). These compounds, which harbour modifications of PF in the spacer linker by the addition of one carbon in the chain and the introduction of a functional methoxy group (8c), displayed no toxicity in mice and successfully inhibited the cell proliferation of the metastatic triple-negative breast cancer cell line MDA-MB-231 and the lung carcinoma cell line LLC (87). A further study by Ashraf-Uz-Zaman et al (86) identified other PF derivatives with the ability to induce apoptosis in the MDA-MB-231 cell line without affecting the cell cycle. Nonetheless, these compounds have yet to be tested and compared with PF in various cancers. Therefore, more thorough research needs to be done on the PF and PF derivatives to validate them as a clinical therapeutic option for cancer.
Figure 3.
PF analogues with anticancer effect against human triple-negative breast cancer MDA-MB-231 and LLC cell lines. The blue colour in compounds 8a and 8c represents the modification made to PF. 8a: Chain elongation by adding one carbon; 8c: Chain elongation by adding one carbon and introducing the methoxy group. Taken and modified from Ashraf-Uz-Zaman et al (87). IC50, half maximal inhibitory concentration; Ki of DAT, inhibitory constant of the dopamine transporter. PF, penfluridol; LLC, Lewis lung carcinoma.
6. Conclusion
This scoping review provides an overview of the anticancer properties of PF in various cancers. It is crucial to investigate potential cost- and time-effective drugs for managing cancer. Based on the available studies, repurposing PF for cancer treatment has promising effects, particularly in cancer with low survival rates and high resistance to the current treatment. PF inhibited the growth, metastasis and migration of various cancers, including glioblastoma, as well as breast, lung, pancreatic, renal, bladder and oesophageal cancer in vitro and in vivo through distinctive mechanisms. Less than 10 µM of PF can provide anticancer activity in vitro. Nonetheless, the in vivo dose of PF exceeds the dose required for antipsychotic treatment; therefore, the subsequent development of PF derivatives with elevated anticancer and reduced antipsychotic activities was deemed necessary. In addition, despite the approval of PF as a valuable anticancer agent, dose-related side effects in the clinical setting and in other types of cancer have yet to be studied. Henceforth, extensive research on the effect of PF on other cancers and in patients needs to be performed. In addition, this scoping review focused on original articles published and indexed in PubMed, Scopus and WoS before November 2023 in English. Therefore, non-indexed, grey literature, studies in other languages and review papers were overlooked. Furthermore, only articles focusing on PF were considered and no clinical studies were included in this review. Thus, future scoping reviews on the anticancer properties of PF should include PF derivatives and clinical studies when available.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by a grant from the Ministry of Higher Education Malaysia (grant no. FRGS/1/2023/SKK10/UITM/02/1).
Availability of data and materials
Not applicable.
Authors' contributions
AAIM contributed to the conceptualization, methodology, validation, formal analysis, data collection, visualisation, writing of the original draft and reviewing and editing. AAR contributed to the conceptualization of the study, reviewed and edited the manuscript as well as supervised the preparation of and validated the manuscript. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Authors' information
Asma Ali Ibrahim Mze (ORCID no. 0009-0002-1848-6160); Amirah Abdul Rahman (ORCID no. 0000-0002-3566-1787).
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021 Apr 5; doi: 10.1002/ijc.33588. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO), corp-author Cancer. WHO; Geneva: 2022. [Google Scholar]
- 4.Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, Wang Z, Li W, Geldsetzer P, Bärnighausen T, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9:465–472. doi: 10.1001/jamaoncol.2022.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon N, Stemmer SM, Greenberg D, Goldstein DA. Trajectories of injectable cancer drug costs after launch in the United States. J Clin Oncol. 2018;36:319–325. doi: 10.1200/JCO.2016.72.2124. [DOI] [PubMed] [Google Scholar]
- 6.Hendouei N, Saghafi F, Shadfar F, Hosseinimehr SJ. Molecular mechanisms of anti-psychotic drugs for improvement of cancer treatment. Eur J Pharmacol. 2019;856:172402. doi: 10.1016/j.ejphar.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Qu LG, Brand NR, Chao A, Ilbawi AM. Interventions addressing barriers to delayed cancer diagnosis in low- and middle-income countries: A systematic review. Oncologist. 2020;25:e1382–e1395. doi: 10.1634/theoncologist.2019-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda T, Tsuruda Y, Matsumoto Y, Uchida H, Nakayama KI, Mimori K. Drug repositioning in cancer: The current situation in Japan. Cancer Sci. 2020;111:1039–1046. doi: 10.1111/cas.14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009–2018. JAMA. 2020;323:844–853. doi: 10.1001/jama.2020.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low ZY, Farouk IA, Lal SK. Drug Repositioning: New approaches and future prospects for life-debilitating diseases and the COVID-19 pandemic outbreak. Viruses. 2020;12:1058. doi: 10.3390/v12091058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JS. Treatment of cancer with antipsychotic medications: Pushing the boundaries of schizophrenia and cancer. Neurosci Biobehav Rev. 2022;141:104809. doi: 10.1016/j.neubiorev.2022.104809. [DOI] [PubMed] [Google Scholar]
- 12.Vlachos N, Lampros M, Voulgaris S, Alexiou GA. Repurposing antipsychotics for cancer treatment. Biomedicines. 2021;9:1785. doi: 10.3390/biomedicines9121785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw V, Srivastava S, Srivastava SK. Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin Cancer Biol. 2021;68:75–83. doi: 10.1016/j.semcancer.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varalda M, Antona A, Bettio V, Vachamaram A, Yellenki V, Massarotti A, Baldanzi G, Capello D. Psychotropic drugs show anticancer activity by disrupting mitochondrial and lysosomal function. Front Oncol. 2020;10:562196. doi: 10.3389/fonc.2020.562196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soares BG, Lima MS. Penfluridol for schizophrenia. Cochrane Database Syst Rev. 2006;2006:CD002923. doi: 10.1002/14651858.CD002923.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Chokhawala K, Lee S. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL: 2023. Antipsychotic medications. [Google Scholar]
- 17.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 18.Mak S, Thomas A. Steps for conducting a scoping review. J Grad Med Educ. 2022;14:565–567. doi: 10.4300/JGME-D-22-00621.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arksey H, O'Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 20.Hedrick E, Li XX, Safe S. Penfluridol represses integrin expression in breast cancer through induction of reactive oxygen species and downregulation of Sp transcription factors. Mol Cancer Ther. 2017;16:205–216. doi: 10.1158/1535-7163.MCT-16-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta N, Gupta P, Srivastava S. Penfluridol overcomes paclitaxel resistance in metastatic breast cancer. Sci Rep. 2019;9:5066. doi: 10.1038/s41598-019-41632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjan A, Gupta P, Srivastava SK. Penfluridol: An antipsychotic agent suppresses metastatic tumor growth in triple-negative breast cancer by inhibiting integrin signaling axis. Cancer Res. 2016;76:877–890. doi: 10.1158/0008-5472.CAN-15-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava S, Zahra FT, Gupta N, Tullar PE, Srivastava SK, Mikelis CM. Low Dose of Penfluridol Inhibits VEGF-Induced Angiogenesis. Int J Mol Sci. 2020;21:755. doi: 10.3390/ijms21030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai TC, Lee YL, Lee WJ, Hung WY, Cheng GZ, Chen JQ, Hsiao M, Chien MH, Chang JH. Synergistic tumor inhibition via energy elimination by repurposing penfluridol and 2-Deoxy-D-Glucose in lung cancer. Cancers (Basel) 2022;14:2750. doi: 10.3390/cancers14112750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung WY, Chang JH, Cheng Y, Cheng GZ, Huang HC, Hsiao M, Chung CL, Lee WJ, Chien MH. Autophagosome accumulation-mediated ATP energy deprivation induced by penfluridol triggers nonapoptotic cell death of lung cancer via activating unfolded protein response. Cell Death Dis. 2019;10:538. doi: 10.1038/s41419-019-1785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue Q, Liu Z, Feng Z, Xu Y, Zuo W, Wang Q, Gao T, Zeng J, Hu X, Jia F, et al. Penfluridol: An antipsychotic agent suppresses lung cancer cell growth and metastasis by inducing G0/G1 arrest and apoptosis. Biomed Pharmacother. 2020;121:109598. doi: 10.1016/j.biopha.2019.109598. [DOI] [PubMed] [Google Scholar]
- 27.Hung WY, Lee WJ, Cheng GZ, Tsai CH, Yang YC, Lai TC, Chen JQ, Chung CL, Chang JH, Chien MH. Blocking MMP-12-modulated epithelial-mesenchymal transition by repurposing penfluridol restrains lung adenocarcinoma metastasis via uPA/uPAR/TGF-β/Akt pathway. Cell Oncol (Dordr) 2021;44:1087–1103. doi: 10.1007/s13402-021-00620-1. [DOI] [PubMed] [Google Scholar]
- 28.Ranjan A, German N, Mikelis C, Srivenugopal K, Srivastava SK. Penfluridol induces endoplasmic reticulum stress leading to autophagy in pancreatic cancer. Tumour Biol. 2017;39:1010428317705517. doi: 10.1177/1010428317705517. [DOI] [PubMed] [Google Scholar]
- 29.Ranjan A, Srivastava SK. Penfluridol suppresses pancreatic tumor growth by autophagy-mediated apoptosis. Sci Rep. 2016;6:26165. doi: 10.1038/srep26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dandawate P, Kaushik G, Ghosh C, Standing D, Ali Sayed AA, Choudhury S, Subramaniam D, Manzardo A, Banerjee T, Santra S, et al. Diphenylbutylpiperidine Antipsychotic Drugs Inhibit Prolactin Receptor Signaling to Reduce Growth of Pancreatic Ductal Adenocarcinoma in Mice. Gastroenterology. 2020;158:1433–1449.e27. doi: 10.1053/j.gastro.2019.11.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chien W, Sun QY, Lee KL, Ding LW, Wuensche P, Torres-Fernandez LA, Tan SZ, Tokatly I, Zaiden N, Poellinger L, et al. Activation of protein phosphatase 2A tumor suppressor as potential treatment of pancreatic cancer. Mol Oncol. 2015;9:889–905. doi: 10.1016/j.molonc.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranjan A, Srivastava SK. Penfluridol suppresses glioblastoma tumor growth by Akt-mediated inhibition of GLI1. Oncotarget. 2017;8:32960–32976. doi: 10.18632/oncotarget.16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H, Chong K, Ryu BK, Park KJ, Yu MO, Lee J, Chung S, Choi S, Park MJ, Chung YG, Kang SH. Repurposing penfluridol in combination with temozolomide for the treatment of glioblastoma. Cancers (Basel) 2019;11:1310. doi: 10.3390/cancers11091310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranjan A, Wright S, Srivastava SK. Immune consequences of penfluridol treatment associated with inhibition of glioblastoma tumor growth. Oncotarget. 2017;8:47632–47641. doi: 10.18632/oncotarget.17425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu J, Cao J, Jin R, Zhang B, Topatana W, Juengpanich S, Li S, Chen T, Lu Z, Cai X, Chen M. Inhibition of AMPK/PFKFB3 mediated glycolysis synergizes with penfluridol to suppress gallbladder cancer growth. Cell Commun Signal. 2022;20:105. doi: 10.1186/s12964-022-00882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Horst G, van de Merbel AF, Ruigrok E, van der Mark MH, Ploeg E, Appelman L, Tvingsholm S, Jäätelä M, van Uhm J, Kruithof-de Julio M, et al. Cationic amphiphilic drugs as potential anticancer therapy for bladder cancer. Mol Oncol. 2020;14:3121–3134. doi: 10.1002/1878-0261.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng C, Yu X, Liang Y, Zhu Y, He Y, Liao L, Wang D, Yang Y, Yin X, Li A, et al. Targeting PFKL with penfluridol inhibits glycolysis and suppresses esophageal cancer tumorigenesis in an AMPK/FOXO3a/BIM-dependent manner. Acta Pharm Sin B. 2022;12:1271–1287. doi: 10.1016/j.apsb.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu SY, Wen YC, Ku CC, Yang YC, Chow JM, Yang SF, Lee WJ, Chien MH. Penfluridol triggers cytoprotective autophagy and cellular apoptosis through ROS induction and activation of the PP2A-modulated MAPK pathway in acute myeloid leukemia with different FLT3 statuses. J Biomed Sci. 2019;26:63. doi: 10.1186/s12929-019-0557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tung MC, Lin YW, Lee WJ, Wen YC, Liu YC, Chen JQ, Hsiao M, Yang YC, Chien MH. Targeting DRD2 by the antipsychotic drug, penfluridol, retards growth of renal cell carcinoma via inducing stemness inhibition and autophagy-mediated apoptosis. Cell Death Dis. 2022;13:400. doi: 10.1038/s41419-022-04828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu LL, Liu YY, Li ZX, Zhao Q, Wang X, Yu Y, Wang YY, Wang YQ, Luo F. Anti-tumor effects of penfluridol through dysregulation of cholesterol homeostasis. Asian Pac J Cancer Prev. 2014;15:489–494. doi: 10.7314/APJCP.2014.15.1.489. [DOI] [PubMed] [Google Scholar]
- 41.Du J, Shang J, Chen F, Zhang Y, Yin N, Xie T, Zhang H, Yu J, Liu F. A CRISPR/Cas9-Based screening for non-homologous end joining inhibitors reveals ouabain and penfluridol as radiosensitizers. Mol Cancer Ther. 2018;17:419–431. doi: 10.1158/1535-7163.MCT-17-0090. [DOI] [PubMed] [Google Scholar]
- 42.Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM, Verbruggen FJ, Van Nueten JM, Schaper WK. The pharmacology of penfluridol (R 16341) a new potent and orally long-acting neuroleptic drug. Eur J Pharmacol. 1970;11:139–154. doi: 10.1016/0014-2999(70)90043-9. [DOI] [PubMed] [Google Scholar]
- 43.Airoldi L, Marcucci F, Mussini E, Garattini S. Distribution of penfluridol in rats and mice. Eur J Pharmacol. 1974;25:291–295. doi: 10.1016/0014-2999(74)90257-X. [DOI] [PubMed] [Google Scholar]
- 44.Andrade C. Psychotropic drugs with long half-lives: Implications for drug discontinuation, occasional missed doses, dosing interval, and pregnancy planning. J Clin Psychiatry. 2022;83:22f14593. doi: 10.4088/JCP.22f14593. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharyya R, Bhadra R, Roy U, Bhattacharyya S, Pal J, Saha SS. Resurgence of penfluridol: Merits and demerits. East J Psychiatry. 2015;18:23–29. doi: 10.5005/EJP-18-1-23. [DOI] [Google Scholar]
- 46.Nikvarz N, Vahedian M, Khalili N. Chlorpromazine versus penfluridol for schizophrenia. Cochrane database Syst Rev. 2017;9:CD011831. doi: 10.1002/14651858.CD011831.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang RI, Larson C, Treul SJ. Study of penfluridol and chlorpromazine in the treatment of chronic schizophrenia. J Clin Pharmacol. 1982;22:236–242. doi: 10.1002/j.1552-4604.1982.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 48.Andrade C. The practical importance of half-life in psychopharmacology. J Clin Psychiatry. 2022;83:22f14584. doi: 10.4088/JCP.22f14584. [DOI] [PubMed] [Google Scholar]
- 49.Clarke Z. Elsevier; New York, NY: 2007. Penfluridol; pp. 1–4. [Google Scholar]
- 50.Enyeart JJ, Biagi BA, Day RN, Sheu SS, Maurer RA. Blockade of low and high threshold Ca2+ channels by diphenylbutylpiperidine antipsychotics linked to inhibition of prolactin gene expression. J Biol Chem. 1990;265:16373–16379. doi: 10.1016/S0021-9258(17)46233-8. [DOI] [PubMed] [Google Scholar]
- 51.Cabrera M, Gomez N, Remes Lenicov F, Echeverría E, Shayo C, Moglioni A, Fernández N, Davio C. G2/M cell cycle arrest and tumor selective apoptosis of acute leukemia cells by a promising benzophenone thiosemicarbazone compound. PLoS One. 2015;10:e0136878. doi: 10.1371/journal.pone.0136878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbas T, Dutta A. p21 in cancer: Intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boudreau RT, Conrad DM, Hoskin DW. Apoptosis induced by protein phosphatase 2A (PP2A) inhibition in T leukemia cells is negatively regulated by PP2A-associated p38 mitogen-activated protein kinase. Cell Signal. 2007;19:139–151. doi: 10.1016/j.cellsig.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura H, Takada K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021;112:3945–3952. doi: 10.1111/cas.15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, Liang X. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res. 2018;37:266. doi: 10.1186/s13046-018-0909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah MA, Rogoff HA. Implications of reactive oxygen species on cancer formation and its treatment. Semin Oncol. 2021;48:238–245. doi: 10.1053/j.seminoncol.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Singh R, Manna PP. Reactive oxygen species in cancer progression and its role in therapeutics. Explor Med. 2022;3:43–57. doi: 10.37349/emed.2022.00073. [DOI] [Google Scholar]
- 58.Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp Mol Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SJ, Kim HS, Seo YR. Understanding of ROS-Inducing strategy in anticancer therapy. Oxid Med Cell Longev. 2019;2019:5381692. doi: 10.1155/2019/5381692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res. 2012;18:5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao FY, Li XT, Xu K, Wang RT, Guan XX. c-MYC mediates the crosstalk between breast cancer cells and tumor microenvironment. Cell Commun Signal. 2023;21:28. doi: 10.1186/s12964-023-01043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Safe S. Specificity Proteins (Sp) and Cancer. Int J Mol Sci. 2023;24:5164. doi: 10.3390/ijms24065164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vellingiri B, Iyer M, Devi Subramaniam M, Jayaramayya K, Siama Z, Giridharan B, Narayanasamy A, Abdal Dayem A, Cho SG. Understanding the role of the transcription factor sp1 in ovarian cancer: From theory to practice. Int J Mol Sci. 2020;21:1153. doi: 10.3390/ijms21031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dufour S, Broders-Bondon F, Bondurand N. Academic Press; Boston, MA: 2015. Chapter 13 - β1-Integrin Function and Interplay during Enteric Nervous System Development; pp. 153–166. [Google Scholar]
- 65.Bergonzini C, Kroese K, Zweemer AJM, Danen EHJ. Targeting integrins for cancer therapy-disappointments and opportunities. Front cell Dev Biol. 2022;10:863850. doi: 10.3389/fcell.2022.863850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valdembri D, Serini G. The roles of integrins in cancer. Fac Rev. 2021;10:45. doi: 10.12703/r/10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yousefi H, Vatanmakanian M, Mahdiannasser M, Mashouri L, Alahari NV, Monjezi MR, Ilbeigi S, Alahari SK. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene. 2021;40:1043–1063. doi: 10.1038/s41388-020-01588-2. [DOI] [PubMed] [Google Scholar]
- 68.Desgrosellier JS, Cheresh DA. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim SY. Cancer energy metabolism: Shutting power off cancer factory. Biomol Ther (Seoul) 2018;26:39–44. doi: 10.4062/biomolther.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chelakkot C, Chelakkot VS, Shin Y, Song K. Modulating glycolysis to improve cancer therapy. Int J Mol Sci. 2023;24:2606. doi: 10.3390/ijms24032606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fadaka A, Ajiboye B, Ojo O, Adewale O, Olayide I, Emuowhochere R. Biology of glucose metabolization in cancer cells. J Oncol Sci. 2017;3:45–51. doi: 10.1016/j.jons.2017.06.002. [DOI] [Google Scholar]
- 72.Lu J, Chen S, Bai X, Liao M, Qiu Y, Zheng LL, Yu H. Targeting cholesterol metabolism in Cancer: From molecular mechanisms to therapeutic implications. Biochem Pharmacol. 2023;218:115907. doi: 10.1016/j.bcp.2023.115907. [DOI] [PubMed] [Google Scholar]
- 73.Fan YJ, Zong WX. The cellular decision between apoptosis and autophagy. Chin J Cancer. 2013;32:121–129. doi: 10.5732/cjc.012.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das S, Shukla N, Singh SS, Kushwaha S, Shrivastava R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis. 2021;26:512–533. doi: 10.1007/s10495-021-01687-9. [DOI] [PubMed] [Google Scholar]
- 75.Mulcahy Levy JM, Thorburn A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020;27:843–857. doi: 10.1038/s41418-019-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koukourakis MI, Kalamida D, Giatromanolaki A, Zois CE, Sivridis E, Pouliliou S, Mitrakas A, Gatter KC, Harris AL. Autophagosome Proteins LC3A, LC3B and LC3C have distinct subcellular distribution kinetics and expression in cancer cell lines. PLoS One. 2015;10:e0137675. doi: 10.1371/journal.pone.0137675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ribatti D, Tamma R, Annese T. Epithelial-Mesenchymal transition in cancer: A historical overview. Transl Oncol. 2020;13:100773. doi: 10.1016/j.tranon.2020.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15:129. doi: 10.1186/s13045-022-01347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8:198. doi: 10.1038/s41392-023-01460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y, Cao Y. The impact of VEGF on cancer metastasis and systemic disease. Semin Cancer Biol. 2022;86((Pt 3)):251–261. doi: 10.1016/j.semcancer.2022.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Zhang M, Wang L, Li J, Yang T, Shao Q, Liang X, Ma M, Zhang N, Jing M, et al. Identification of CCL4 as an immune-related prognostic biomarker associated with tumor proliferation and the tumor microenvironment in clear cell renal cell carcinoma. Front Oncol. 2021;11:694664. doi: 10.3389/fonc.2021.694664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rezayatmand H, Razmkhah M, Razeghian-Jahromi I. Drug resistance in cancer therapy: The Pandora's Box of cancer stem cells. Stem Cell Res Ther. 2022;13:181. doi: 10.1186/s13287-022-02856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gong L, Zhang Y, Liu C, Zhang M, Han S. Application of radiosensitizers in cancer radiotherapy. Int J Nanomedicine. 2021;16:1083–1102. doi: 10.2147/IJN.S352169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ashraf-Uz-Zaman M, Shahi S, Akwii R, Sajib MS, Farshbaf MJ, Kallem RR, Putnam W, Wang W, Zhang R, Alvina K, et al. Design, synthesis and structure-activity relationship study of novel urea compounds as FGFR1 inhibitors to treat metastatic triple-negative breast cancer. Eur J Med Chem. 2021;209:112866. doi: 10.1016/j.ejmech.2020.112866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ashraf-Uz-Zaman M, Sajib MS, Cucullo L, Mikelis CM, German NA. Analogs of penfluridol as chemotherapeutic agents with reduced central nervous system activity. Bioorg Med Chem Lett. 2018;28:3652–3657. doi: 10.1016/j.bmcl.2018.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.