Abstract
Bacillus cereus strains were isolated from dried foods, which included international brands of spices from South East Asia, Mexico and India purchased from several retail stores, samples of powdered infant formula (PIF), medicated fish feed and dietary supplements. The genetic diversity of 64 strains from spices and PIF was determined using a multiplex endpoint PCR assay designed to identify hemolysin BL, nonhemolytic enterotoxin, cytotoxin K, and enterotoxin FM toxin genes. Thirteen different B. cereus toxigenic gene patterns or profiles were identified among the strains. Randomly selected B. cereus strains were sequenced and compared with reference Genomic Groups from National Center Biotechnology Information using bioinformatics tools. A comprehensive multi-loci sequence analysis (MLSA) was designed using alleles from 25 known MLST genes specifically tailored for use with whole genome assemblies. A cohort of representative genomes of strains from a few FDA regulated commodities like dry foods and medicated fish feed was used to demonstrate the utility of the 25-MLSA approach for rapid clustering and identification of Genome Groups. The analysis clustered the strains from medicated fish feed, dry foods, and dietary supplements into phylogenetically-related groups. 25-MLSA also pointed to a greater diversity of B. cereus strains from foods and feed than previously recognized. Our integrated approach of toxin gene PCR, and to our knowledge, whole genome sequencing (WGS) based sequence analysis, may be the first of its kind that demonstrates enterotoxigenic potential and genomic diversity in parallel.
Keywords: Enterotoxigenic Bacillus cereus, 25-gene MLSA, WGS, Genomic characterization
1. Introduction
Bacillus cereus, a foodborne spore-forming bacterium is a wide-spread human pathogen ubiquitous in the natural environment and found in several categories of food products. It has long been established that B. cereus can cause two types of gastrointestinal diseases, i.e., diarrheal and emetic syndromes, which result from the production of various toxins (Blakey and Priest, 1980; Ehling-Schulz et al., 2004). Both diseases can have mild clinical presentations and in most cases, are self-limiting which has contributed to under reporting (Ceuppens et al., 2013).
The virulence of B. cereus, whether intestinal or non-intestinal, is intimately associated with the production of tissue destructive/reactive proteins (Bottone, 2010). Diarrhea is caused by enterotoxins produced after ingestion of spores and/or vegetative cells, germination of spores and growth of vegetative cells in the small intestine.
Pore-forming enterotoxins: tripartite hbl enterotoxin hemolysin BL (hbl), nonhemolytic enterotoxin (nhe), a single protein enterotoxin cytotoxin K (CytK), and enterotoxin FM (entFM), are among the secreted toxins (Lund et al., 2000; Schoeni and Wong, 2005). The hbl enterotoxin, encoded by the operon hblDAC, is composed of a binding component and two hemolytic components required to maximize the hemolytic, cytotoxic, and dermo-necrotic activities of hbl (Beecher and Macmillian, 1991; Beecher et al., 1995; Heinriches et al., 1993; Ryan et al., 1997). Another three-component enterotoxin, designated nhe, consisting of nheA, nheB, and nheC, is encoded by the nheBAC operon (Granum et al., 1999). CytK is a β-barrel pore-forming toxin encoded by cytK (Lund et al., 2000; Ramarao and Sanchis, 2013) and is also widely distributed among strains. The entFM gene, known for encoding a putative cell wall peptidase, was cloned from B. cereus FM1 (Asano et al., 1997). The prevalence of these toxin genes varies among different B. cereus strains (Arnesen et al., 2008; Bartoszewicz et al., 2008; Ngamwongsatit et al., 2008).
The emetic toxin, or cereulide, a small cyclic heat-stable dodecadepsipeptide (Agata et al., 1995), is associated with the emetic syndrome. Some investigators have reported that both emetic and diarrheal outbreaks were linked to the same food and it has been reported that both nhe and cereulide can be produced by the same B. cereus strain (Kim et al., 2010).
B. cereus has been isolated from a variety of food sources including rice, potato, dried dairy products, pasta, meat products, coffee, vegetable sprouts, and spices (Egelezos et al., 2010; Fangio et al., 2010; Portnoy et al., 1976; Rajkovic et al., 2006; Samapundo et al., 2011; Wijnands et al., 2006). The Centers for Disease Control and Prevention (CDC) conducted a study between 2000 and 2008 on pathogens causing US foodborne illnesses, hospitalizations, and deaths. For B. cereus the estimated annual illnesses observed were 63,000 cases, annual hospitalizations were 20 cases, but no deaths were reported (Scallan et al., 2011).
Powers et al. (1976) reported that spices used in a large variety of food products contain a high prevalence of B. cereus (53%), confirming the role played by these additives as a common source of contamination in many countries including, Nigeria, Mexico, Denmark and France, (Antai, 1988; García et al., 2001; Van Doren et al., 2013). Added to this list are reports from Scotland of contaminated spices associated with imported ethnic foods of Chinese and Indian origin. (Candlish et al., 2001). A survey done in the UK on ready to eat foods, found spices or spice ingredients, used in food production, contained sufficient numbers of B. cereus which could potentially cause foodborne disease (Little et al., 2003). B. cereus is also known to be a common contaminant of milk (Anderson et al., 1995; Lin et al., 1998; Te Giffel et al., 1997).
Recognizing the intra-diversity found within the genus Bacillus, a strategy was developed to characterize enterotoxigenic B. cereus strains based on conventional gene-specific PCR assays combined with a comprehensive multi-locus sequence analysis scheme (25-MLSA) derived from extensive whole genome sequencing (WGS). In this study we report the prevalence of distinctly divergent B. cereus Group Genome types from different FDA-regulated food sources, medicated fish feed and dietary supplements possessing different enterotoxin gene profiles and genomic backbones.
2. Materials and methods
2.1. Isolation of B. cereus from spices and powdered infant formula (PIF)
Our analysis involved evaluating product blends from all over the world including spices originating from South East Asian, Mexican and Indian markets. Samples of whole black pepper, ground pepper, black pepper powder, paprika, and sesame seeds, along with PIF were assayed for the presence of B. cereus. Briefly, duplicate samples containing 20 g of spice or PIF were homogenized (1:10 w:v) in 180 mL Tryptic Soy Broth (TSB) or Modified Buffered Peptone Water (mBPW) (BBL, Becton Dickinson, Franklin Lakes, NJ) supplemented with polymyxin B (0.0127 mg/mL, final concentration). The 200 mL sample was divided into two 100 mL aliquots and one was heated at 56 °C for 30 min and the second one was not heated prior to plating. Using these 2 different treatment methods we devised 2 additional methods. From the heated samples described above one was directly plated onto agar plates (H-D) while the other was incubated at 30 °C, shaking overnight at 150 rpms prior to plating (H o/n). Likewise, the samples not heated were plated directly (NH-D) or plated after an overnight incubation and were designated NH-o/n.
A 10 μL aliquot from each of the samples representing the 4 treatment methods were plated directly onto Mannitol-egg yolk-polymyxin (MYP) agar (Hardy Diagnostics, Santa Maria, CA) and BACARA chromogenic agar plates (bioMérieux, Hazelwood, MO.) and incubated overnight (o/n) at 30 °C. Presumptive strains possessing typical colony morphologies were confirmed as B. cereus by plating onto 5% sheep blood agar (TSAB, Remel, KS) for demonstration of hemolytic activity. Final identities were made following the identification scheme described in the Bacteriological Analytical Manual BAM protocol (Tallent et al., 2012). Two colonies from the same agar plate were picked for analysis. For quality control purposes, B. cereus ATCC 14579 was used as a positive control for all plating and PCR experiments and B. thuringiensis ATCC 29730 was used as a negative control to differentiate B. cereus from B. thuringiensis. Frozen stocks were maintained in TSB supplemented with 50% glycerol and stored at −80 °C.
2.2. B. cereus isolated from dietary supplements and medicated fish feed
Nine strains from dietary supplement samples were obtained from the National Center for Toxicological Research (NCTR). Foods included supplements: used for weight loss, to enhance athletic performance, and to reduce mental and physical fatigue. (Foster et al., 2013). One additional supplement included in this study was purchased at a local store, and is described as a fat-burner supplement containing chitosan used for weight-loss and to increase energy levels. Take note that the health benefit statements of these dietary supplements as described are based on packaging information and the statements do not reflect endorsement by the FDA.
Twenty-three strains, isolated during a study that used medicated fish feed containing the antibiotic oxytetracycline were obtained from the Center for Veterinary Medicine (CVM), FDA.
Dietary supplement and the medicated fish feed strains were inoculated onto BACARA chromogenic agar plates, and incubated (o/n) at 30 °C. Strains with typical colony morphologies were confirmed as B. cereus by plating onto 5% sheep blood agar TSAB for demonstration of hemolytic activity, and by PCR using primers specific for B. cereus and B. thuringiensis crystal toxin gene (cry).
2.3. Extraction of DNA from strains
DNA was extracted from B. cereus strains grown in 6 mL of TSB and incubated with shaking (180 rpm) at 30 °C o/n. Two mL aliquots of these cultures were pelleted (5000 ×g for 10 min.) and the supernatants removed. The cells were then suspended in 180 μL of enzymatic lysis buffer (20 mM Tris-HCl, pH 8.0, 2 mM sodium EDTA, 1.2% Triton X-100) containing 20 mg/mL lysozyme and incubated for 4 h at 37 °C. Ten microliters of RNase A (10 mg/mL) was added to each sample and incubated for 5 min. at room temperature prior to initiating the QIAcube program. Genomic DNA was isolated from the pellets of each of the 2 mL aliquots of the cultures using the robotic QIAcube workstation with its automated Qiagen DNeasy chemistry (Qiagen, Germantown, MD) for purification of DNA, following the manufacturer’s recommendations. Typically, 5–15 ng/μL of purified genomic DNA was recovered in a final elution volume of 200 μL. Genomic DNA was stored at −20 °C until needed. As an alternate method we used an UltraClean microbial DNA isolation kit (MO BIO Laboratories, Carlsbad, CA USA) according to the manufacturer’s instructions for each strain. The DNA, approximately 30–260 ng/μL, was recovered in certified DNA-free Tris buffer and again stored at −20° until needed.
2.4. PCR amplification of enterotoxin genes
Enterotoxin gene profiles were determined by using an endpoint PCR assay with primers specific for hblDAC, nheBAC, cytK, and entFM. Primer sequences were as published by Choo et al. (2007); Ngamwongsatit et al. (2008); and Thaenthanee et al. (2005). The amplification reactions were carried out in an Applied Biosystems 2720 Thermal cycler (AB, Applied Biosystems, Inc., Foster City, CA). Amplicons were separated on a 1.5% agarose gel using a 1Kb DNA molecular size standard (Invitrogen, Carlsbad, CA) to estimate sizes. B. cereus ATCC strain 14579 and B. thuringiensis ATCC strain 29730 were used as reference strains. The enterotoxin gene profiles are shown in Table 1.
Table 1.
Gene profiles of enterotoxigenic B. cereus strains isolated from spices, PIF, dietary supplements, and medicated fish feed as identified using PCR.
| Spice sample | Treatment method | Target gene |
Profile | No. of samples in each profile | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| hblD | hblA | hblC | nheB | nheA | nheC | cytK | entFM | ||||
|
| |||||||||||
| Whole black pepper | HD, NH-D, H o/n, NH o/n | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Profile I | 12 |
| H o/n, NH o/n | (−) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | Profile IV | 4 | |
| NH o/n | (−) | (−) | (−) | (−) | (+) | (−) | (+) | (−) | Profile VI | 2 | |
| Ground black pepper | HD, NH o/n | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Profile I | 4 |
| Black pepper powder | HD | (+) | (+) | (+) | (−) | (−) | (−) | (−) | (−) | Profile II | 1 |
| H o/n, NH o/n | (−) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | Profile IV | 2 | |
| Paprika | HD, NH-D, H o/n, NH o/n | (+) | (+) | (+) | (−) | (−) | (−) | (−) | (−) | Profile II | 6 |
| H o/n, NH o/n | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (+) | Profile VII | 1 | |
| Sesame seed | NH o/n | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Profile I | 1 |
| NH-D, H o/n, NH o/n | (+) | (+) | (+) | (−) | (−) | (−) | (−) | (−) | Profile II | 3 | |
| H o/n | (−) | (−) | (−) | (+) | (+) | (+) | (−) | (−) | Profile III | 1 | |
| NH o/n | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (−) | Profile V | 1 | |
| NH o/n | (−) | (−) | (−) | (−) | (+) | (−) | (+) | (−) | Profile VI | 1 | |
| NH o/n | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (+) | Profile VII | 1 | |
| Direct platinga | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Profile I | 4 | |
| PIF | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | Profile VIII | 7 | |
| Dietary supplements | Direct platinga | (−) | (−) | (−) | (+) | (+) | (+) | (−) | (+) | Profile IX | 3 |
| (−) | (−) | (−) | (+) | (−) | (+) | (−) | (+) | Profile X | 1 | ||
| (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Profile I | 3 | ||
| (−) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | Profile IV | 2 | ||
| Medicated fish feed | Direct platinga | (−) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | Profile IV | 7 |
| (−) | (−) | (−) | (+) | (+) | (+) | (−) | (+) | Profile IX | 5 | ||
| (−) | (−) | (+) | (+) | (+) | (+) | (−) | (+) | Profile XI | 2 | ||
| (−) | (−) | (+) | (+) | (+) | (+) | (+) | (+) | Profile XII | 7 | ||
| (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Profile I | 1 | ||
| (+) | (−) | (+) | (+) | (+) | (+) | (+) | (+) | Profile XIII | 1 | ||
(+) = positive by PCR; (−) = negative by PCR; HD = heated, direct plating, NH-D = Not heated, direct plating, H o/n = heated and incubated overnight, NH o/n = not heated and incubated overnight.
Direct plating on BACARA chromogenic agar (bioMérieux, Hazelwood, MO).
2.5. Whole genome sequencing of B. cereus isolates from food and feeds samples
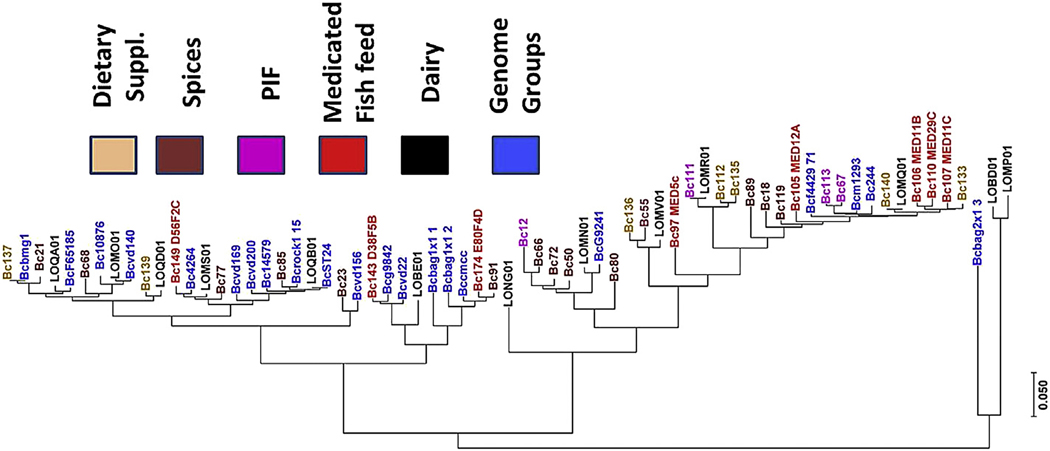
A total of 66 B. cereus genomes were used for comparative genomic analyses. This includes (Fig. 1) thirty-two genomes from the B. cereus strains isolated from spices, PIF, dietary supplements, and medicated fish feed samples (this study); genomes from 13 B. cereus strains previously recovered from dairy (Kovac et al., 2016), and 21 ‘Genome Groups’ genomes from the National Center Biotechnology Information (NCBI) databases which grouped B. cereus genomes by their nucleotide relatedness. At the beginning of this study, these 21 annotated genomes were part of a larger set of Genome Groups designated by NCBI based on nucleotide relatedness among B. cereus genomes. When this manuscript was prepared for submission, these Genome Groups were incorporated into a routine phylogenetic analysis to create NCBI Genome Tree reports (https://www.ncbi.nlm.nih.gov/genome/tree/157, last accessed 2/1/2018). The GenBank sequences listed in the Table 2 were part of the original NCBI Genome Groups. Library preparations and WGS were completed for 32 strains from various dried foods, dietary supplements and animal feed using Nextera XT Library Kit and MiSeq platform respectively (Illumina, San Diego, CA, USA). De novo assembly of 500 cycle, paired-end read datasets was routinely carried out using the recommended workflow on CLC Genomics Work bench version 8.0 (CLC bio, Aarhus, Denmark).
Fig. 1.
Phylogenetic analysis using the 25-gene MLSA strategy with 66 B. cereus genomes. The tree was developed by comparing 25 loci from these genomes with B. cereus 14579. A 66-genome data matrix was constructed using these alleles which contained 2179 base positions. This allelic data matrix was then subjected to phylogenetic analysis using the Neighbor-Joining algorithm in MEGA7 (Kumar et al., 2016). The sources of the isolates are color coded.
Table 2.
WGS assembly and annotations of the B. cereus strains and reference genomes used in this study.
| Source | Biosample sccession | Strain | Assembly name | NCBI GenBank accession | No. of CDSa | STb |
|---|---|---|---|---|---|---|
|
| ||||||
| Spice# | SAMN05415284 | MOD1_Bc18 | Bc18 | GCA_001913615 | 5654 | NDc |
| SAMN05415285 | MOD1_Bc21 | Bc21 | GCA_001913455 | 6353 | 13 | |
| SAMN05415286 | MOD1_Bc23 | Bc23 | GCA_001913405 | 5729 | ND | |
| SAMN05608038 | MOD1_Bc50 | Bc50 | GCA_001913375 | 5535 | ND | |
| SAMN05608072 | MOD1_Bc55 | Bc55 | GCA_001913485 | 5012 | 1295 | |
| SAMN05608045 | MOD1_Bc66 | Bc66 | GCA_001913385 | 5568 | ND | |
| SAMN05608063 | MOD1_Bc68 | Bc68 | GCA_001913475 | 5655 | Newd | |
| SAMN05608041 | MOD1_Bc72 | Bc72 | GCA_001913395 | 5473 | ND | |
| SAMN05608061 | MOD1_Bc77 | Bc77 | GCA_001913625 | 5267 | New | |
| SAMN05608083 | MOD1_Bc80 | Bc80 | GCA_001913315 | 5538 | ND | |
| SAMN05415288 | MOD1_Bc85 | Bc85 | GCA_001913635 | 5470 | ND | |
| SAMN05608078 | MOD1_Bc89 | Bc89 | GCA_001913305 | 5201 | ND | |
| SAMN05608051 | MOD1_Bc119 | Bc119 | GCA_001913295 | 5359 | ND | |
| Medicated animal feed# | SAMN05901947 | MOD1_Bc97 | Bc97_MED5c | GCA_001982885 | 6267 | 869 |
| SAMN05901945 | MOD1_Bc105 | Bc105_MED12A | GCA_001982925 | 5831 | 205 | |
| SAMN05901944 | MOD1_Bc106 | Bc106_MED11B | GCA_001982935 | 5773 | ND | |
| SAMN05901942 | MOD1_Bc107 | Bc107_MED11C | GCA_001982805 | 5909 | ND | |
| SAMN05901943 | MOD1_Bc110 | Bc110_MED29C | GCA_001982815 | 5689 | 1065 | |
| Hybrid tilapia# (medicated) | SAMN05901949 | MOD1_Bc143 | Bc143 | NCBI is Processing | 5919 | 265 |
| SAMN05905663 | MOD1_Bc149 | Bc149 | GCA_001982855 | 5328 | ND | |
| SAMN05901941 | MOD1_Bc174 | Bc174 | GCA_001940365 | 6268 | ND | |
| Dietary supplements# | SAMN05415710 | MOD1_Bc133 | Bc133 | GCA_001913535 | 5498 | New |
| SAMN05608070 | MOD1_Bc135 | Bc135 | GCA_001913545 | 5559 | 120 | |
| SAMN05608069 | MOD1_Bc136 | Bc136 | GCA_001913645 | 5485 | 90 | |
| SAMN05608068 | MOD1_Bc137 | Bc137 | GCA_001913555 | 6642 | 90 | |
| SAMN05608066 | MOD1_Bc139 | Bc139 | GCA_001913575 | 5633 | 8 | |
| SAMN05608065 | MOD1_Bc140 | Bc140 | GCA_001719025 | 5474 | 1084 | |
| Powdered infant formula# | SAMN05415728 | MOD1_Bc12* | Bc12 | GCA_002021385 | 5698 | New |
| SAMN05415720 | MOD1_Bc67* | Bc67 | GCA_001901815 | 6078 | 205 | |
| SAMN05415740 | MOD1_Bc111* | Bc111 | GCA_001901895 | 5686 | 32 | |
| SAMN05415756 | MOD1_Bc112* | Bc112 | GCA_001901905 | 5628 | 127 | |
| SAMN05415765 | MOD1_Bc113* | Bc113 | GCA_001901885 | 5682 | 205 | |
| NCBI genome groups† | SAMN00715886 SAMN02469355 |
ATCC 10876 LCT-BC244 |
Bc10876 Bc244 |
GCA_000160895
GCA_000256545 |
||
| SAMN02604059 | B4264 | Bc4264 | GCA_000021205 | |||
| SAMN02603340 | ATCC 14579 | Bc14579 | GCA_000007825 | |||
| SAMN02596802 | BAG1X1–1 | Bcbag1×1_1 | GCA_000399005 | |||
| SAMN02596803 | BAG1X1–2 | Bcbag1×1_2 | GCA_000291035 | |||
| SAMN02596813 | BAG2X1–3 | Bcbag2×1_3 | GCA_000291515 | |||
| SAMN02596828 | BMG1.7 | Bcbmg1 | GCA_000399345 | |||
| SAMN03457174 | CMCC P0011 | Bccmcc | GCA_001635955 | |||
| SAMN03325933 | F4429–71 | Bcf4429_71 | GCA_001044635 | |||
| SAMN00727647 | F65185 | BcF65185 | GCA_000161315 | |||
| SAMN02435890 | G9241 | BcG9241 | GCA_000832805 | |||
| SAMN02604060 | G9842 | Bcg9842 | GCA_000021305 | |||
| SAMN00717290 | m1293 | Bcm1293 | GCA_000003645 | |||
| SAMN00727711 | Rock1–15 | Bcrock1_15 | GCA_000161175 | |||
| SAMN00727632 | BDRD-ST24 | BcST24 | GCA_000161055 | |||
| SAMN02596867 | VD140 | Bcvd140 | GCA_000399545 | |||
| SAMN02596871 | VD156 | Bcvd156 | GCA_000290775 | |||
| SAMN02596873 | VD169 | Bcvd169 | GCA_000290735 | |||
| SAMN02596876 | VD200 | Bcvd200 | GCA_000290715 | |||
| SAMN02596796 | VD022 | Bcvd22 | GCA_000290955 | |||
| Dairy isolate genomes‡ | SAMN03800014 | FSL H7–0926 | LOBD01 | GCA_001584065 | ||
| SAMN03800017 | FSL K6–0040 | LOBE01 | GCA_001584085 | |||
| SAMN03800019 | FSL K6–0067 | LOMN01 | GCA_001583925 | |||
| SAMN03800021 | FSL K6–0073 | LOMO01 | GCA_001583935 | |||
| SAMN03800022 | FSL K6–0267 | LOMP01 | GCA_001583955 | |||
| SAMN03800024 | FSL W8–0003 | LOMQ01 | GCA_001583705 | |||
| SAMN03800025 | FSL W8–0050 | LOMR01 | GCA_001584005 | |||
| SAMN03800027 | FSL W8–0268 | LOMS01 | GCA_001583745 | |||
| SAMN03800031 | FSL W8–0523 | LOMV01 | GCA_001583765 | |||
| SAMN03800023 | FSL M8–0117 | LONG01 | GCA_001583975 | |||
| SAMN03800032 | FSL W8–0640 | LOQA01 | GCA_001583875 | |||
| SAMN03800033 | FSL W8–0824 | LOQB01 | GCA_001583865 | |||
| SAMN03800035 | FSL W8–0767 | LOQD01 | GCA_001583845 | |||
CDS Coding DNA Sequences.
ST Sequence Types, MLST.
ND Not determined.
All ND isolates are non-typable because of one or more of the following reasons:
allele not found in the B. cereus MLST database
locus not found in the WGS assembly
nucleotide profile could not be determined for the sequence in the query
alleles not recognized by the B. cereus MLST database.
New ST types are
as recognized by the database
alleles in the query sequence determined to be eligible for a ST category by the authors.
WGS assembly from this work originally reported in Carter et al. Genome Announcements (2017).
NCBI Genome group strains were obtained from https://www.ncbi.nlm.nih.gov/genome/genomes/157.
Dairy isolate genome IDs were obtained from Kovac et al. BMC Genomics (2016) and downloaded from NCBI.
The 32 genomes published from this study.
2.6. Comparative genome analyses of 66 B. cereus genomes
Sequence analysis was completed on all 66 B. cereus genomes (Table 2) using different approaches. PCR reactions as described by Priest et al. (2004) were routinely performed to generate MLST loci sequences which were queried against the SuperCAT database (Tourasse and Kolstø, 2008). The known and new sequence types (STs) are tabulated in Table 2. Initially, a local BLAST database of these B. cereus genomes (Altschul et al., 1990) was employed for querying using in-house perl scripts.
This seven-gene approach (Priest et al., 2004) was expanded to include additional loci available on the SuperCAT database and derived from the scheme by Helgason et al. (2004), Sorokin et al. (2006), Ko et al. (2004) and Tourasse et al. (2006). Based on these five published schemes outlined in the B. cereus SuperCAT database a non-redundant, comprehensive multi-loci sequence typing scheme (25-MLSA) was developed to be used with WGS datasets from this and subsequent studies. Nucleotide sequences of 25 markers in this list were obtained from B. cereus 14579 (GenBank Accession (GCA_000007825) as the reference strain. These 25-MLSA loci were used to query the local BLAST database to identify alleles across the genomes. The resulting “25-MLSA”-based allelic data matrix was used for phylogenetic analysis.
B. cereus ATCC 14579 was independently annotated using RAST (Aziz et al., 2008), resulting in 5538 coding DNA sequences (CDS). Using the local B. cereus genome database, we had created, 4430 out of 5538 B. cereus 14579 genes were identified to have homologs in all the 66 genomes and were designated as “conserved genes”. This list of conserved genes was used to identify alleles across the 66 genomes. A large data matrix comprised of thousands of alleles from these 4430 “conserved genes” in these 66 genomes was generated. We randomly selected 1000 loci for phylogenetic analysis.
A whole genome SNP analysis was carried out using the kSNP3 program (Gardner et al., 2015). kSNP3 software generates a unique list of sequences of pre-determined virtual fragments called k-mers (k = random size) from the query genomes. A specific in-build algorithm was applied to determine the optimal k-mer size to be used based on the input genomes. In our genome-wide analysis, a k-mer size of 19 was chosen by the kSNP3 program. High quality alleles in these k-mers in all the queried genomes are generated and the resulting data matrix file with these alleles is used for downstream analysis. The resultant “whole genome” SNP data matrix was analyzed for phylogenetic relationship.
The data matrices resulting from the three genome-based analytical methods described above, viz. 25 MLSA, conserved genes-based and whole genome SNP were used for phylogenetic analysis in MEGA7 suite (Kumar et al., 2016). The Neighbor-Joining algorithm was used to build trees with bootstrap values using the utilities provided in MEGA7 suite. Splitstree analysis (Huson and Bryant, 2006) was used to compare phylogenetic relatedness among the strains as identified by 25- MLSA and kSNP3 analyses when needed.
3. Results and discussion
3.1. Prevalence of B. cereus enterotoxins genes in spices and PIF isolates
Strains were identified by the presence of hemolytic, lecithinase and β-glucosidase activities and absence of the Bacillus thuringiensis insecticidal crystal toxin, cry gene. Sixty-four B. cereus isolates obtained from paprika, several grades of black pepper (whole black pepper, ground black pepper, and black pepper powder), sesame seeds, and PIF were analyzed for enterotoxin gene prevalence and distribution. The enterotoxins primarily associated with foodborne illness and targeted in this study included hemolysin, hbl (hblDAC), nonhemolytic hemolysin, nhe (nheBAC), cytotoxin K (cytK), and enterotoxin FM (entFM). The hemolysin hbl was the most prevalent toxin gene observed in our isolates (Table 1). In contrast, Hansen and Hendriksen (2001) and Guinebretière et al. (2002) found isolates with only nhe present (63%) and isolates with both nhe and hbl (37%) present in clinical and food-borne B. cereus isolates, respectively. In a study by Ankolekar et al. (2009) it was reported that 55 (59%) of 94 isolates produced both the nhe and hbl toxins, whereas 89 (95%) were positive for either enterotoxin. In our analysis some of the isolates possessed hblDAC genes without the presence of the nheBAC genes. Interestingly, Granum (2001) reported that the hbl operon is only present in about 50% of foodborne B. cereus isolates, while nhe operon is present in almost 100% of the strains. These findings are supported by similar results described by Arnesen et al. (2008) and Moravek et al. (2006). Together, these results suggest that toxin gene prevalence and distribution among B. cereus strains varies greatly. Furthermore, according to Agaisse et al. (1999), Gohar et al. (2002), and Lindbäck et al. (2004), expression of both hbl and nhe is positively regulated by the pleiotropic regulator PlcR. Later Lindbäck et al. (2010) reported that both nheB and nheC are required for membrane binding and complex formation, and nheA triggers the cytotoxicity. Guinebretière et al. (2010) and Ngamwongsatit et al. (2008) reported that all B. cereus strains possess the nhe genes, even though highly divergent nucleotide sequence variations may exist. Biochemical and genetic characterization of several similar strains led to a recent designation of a new species, Bacillus cytotoxicus (Guinebretière et al., 2013), which features an unusual nhe operon, encoding the components of nhe. The role that nhe plays in the virulence of some of these distinct strains is mainly attributed to the greater cytotoxic activity of the pore-forming cytotoxin K (Fagerlund et al., 2004, 2007) which is thought to cause lysis of small intestine epithelial cells, resulting in diarrhea (Hardy et al., 2001; Lund et al., 2000). Gene profile results of the B. cereus strains isolated from spices and PIF are summarized in Table 1.
We identified eight different toxin gene profiles (I-VIII) among 51 positive strains isolated from spices and PIF. The most prevalent toxigenic gene profile included the presence of hblDAC, nheBAC, cytK and EntFM genes, under profile I. Twenty-one (21/51, 41%) spice and PIF strains were classified in this group. Some of the strains were obtained from different areas of a sample, (i.e., subs, taken from the top, middle or bottom). Ten (20%) of the 51 samples tested possessed hblDAC toxin genes. (Profile II).
It should be noted that in some cases, direct plating versus an overnight enrichment, as well as, heating versus not heating of the same sample, led to the isolation of strains with different toxin genotypes. Nonetheless, these results suggest that these samples possessed a mixed population of strains with varying genotypes, supporting the hypothesis proposed by others (Ehling-Schulz and Messelhäusser, 2013) that certain characteristics, i.e. toxin genes, are more strain-specific. These results clearly emphasize the need for further surveillance studies to understand the enterotoxigenic diversity of B. cereus.
Toxin gene profile IV included six strains which possessed nheBAC, cytK and entFM toxin genes. These strains were all recovered from whole black pepper and black pepper powder originating from two different manufacturers. One possible explanation for this could be these two manufacturers bought their raw material from the same supplier. It is interesting to note that many of the strains taken from the whole black pepper samples fell under three different toxigenic profiles and were obtained using various treatment regimens. Six out of seven strains from paprika were positive for hblDAC toxin genes (toxin profile II). Strains obtained from the sesame seed samples also showed a diverse toxigenic genotype pattern consisting of six different profiles (I–II and V–VII, Table 1). One sesame seed strain was found to only possess the nheBAC gene cluster (toxin profile III). Hansen and Hendriksen (2001) reported that enterotoxin proteins produced by strains in which specific nhe or hbl genes were not detected may be related to gene sequence polymorphisms. We also observed that many of our strains lacked one or more of these genes.
Toxigenic B. cereus was present in several PIF samples as well. The incidence of B. cereus spores found in dried milk products, which was recently reported (Merzougui et al., 2014) confirms earlier findings by Bartoszewicz et al., 2008; of high contamination levels of this micro-organism in PIF. The incidence of B. cereus in infant food alone has been reported to be as high as 54% of 261 samples distributed in 17 countries. (Becker et al., 1994). Two different gene prevalence patterns emerged from the PIF strains investigated in the present study. Four of the 11 PIF strains (36%) possessed all the toxin genes. Most of the PIF strains however, (7 of the 11 or 64%), had a toxin gene profile that included hblDAC, nheBAC and entFM, but lacked cytK.
The finding of cytK in the majority of spice, dietary supplements, and medicated feed strains supports the results of Guinebretière et al. (2002) and Oltuszak-Walczak and Walczak (2013) who reported that this gene is widely distributed among strains belonging to several B. cereus Genome Groups. Likewise, other reports describe the presence of cytK in numerous Bacillus species as being more of a strain-specific rather than a species-specific attribute (Ehling-Schulz and Messelhäusser, 2013).
3.2. Prevalence of B. cereus enterotoxin genes in dietary supplement strains and medicated fish feed
All the dietary supplement strains had the nheBAC toxin gene cluster and entFM (representing toxin profiles I, IV and IX) except for one strain lacking the nheA toxin gene (toxin profile X). Three strains, out of nine (33%), possessed all the toxin genes, which included hblDAC, nheBAC, cytK and entFM (toxin profile I). Only five of the dietary supplement strains carried the cytK gene.
A total of 23 medicated fish feed strains were analyzed for the presence of B. cereus enterotoxin genes. Among the strains, there were six toxin gene profiles observed (1, IV, IX, XI–XIII). Toxin genes nheBAC and entFM were present in all the strains.
Sixteen of the 23 strains (70%) were positive for the cytK gene. Two strains were positive for both the hblD and hblC genes, whereas the frequency of having hblC gene was 48% among these strains (11 of 23); occurring under 4 different profiles (profiles I, XI, XII, XIII). Notably only 1 medicated fish feed strain was positive for all eight B. cereus enterotoxin genes (hblDAC, nheBAC, cytK and entFM, profile I).
3.3. Phylogenetic analysis and whole genome sequencing
As part of the food safety surveillance program, we routinely analyze food samples for the presence of B. cereus. Many of these strains from the B. cereus complex have been sequenced as part of the FDA GenomeTrakr project (Allard et al., 2016) and we have included 32 of these genomes in this study. Whole genome assemblies of strains from spices, PIF, dietary supplements and medicated fish feed samples were generated as described. A single local database consisting of genomes from 32 of our strains, 13 dairy genomes (Kovac et al., 2016) and 21 NCBI Genome Groups was used in the subsequent analysis. Table 2 shows the sequence types of 32 of our strains generated using the seven house-keeping genes described by Priest et al. (2004). It has been reported (Ceuppens et al., 2013; Priest et al., 2004) that the B. cereus complex has significant nucleotide diversity among strains with expanding allelic information as new genomes are sequenced. In our hands, extensive sampling of diverse food sources has a high potential for finding previously unidentified sequence-types. For example, in our dataset of 32 samples, we describe four new STs while 13 of the strains did not fit any of the known sequence types.
It was not surprising the 7-loci MLST scheme initially used was in-adequate to capture this diversity. At least five MLST schemes have been reported for the B. cereus group.
(Tourasse and Kolstø, 2008). SuperCAT database (http://mlstoslo.uio.no/, last accessed 2/1/2018) exploits high powered computing using supertree reconstruction algorithms (Biniinda-Edmonda, 2004) by combining MLST typing from each of these schemes routinely for hundreds of genomes. We adapted this integrated approach in a different way by combining loci from different schemes to type the B. cereus isolates from food and feed. This approach is more of an expanded MLST approach with 25 unique and tested housekeeping genes instead of seven. The comprehensive 25-gene MLSA strategy was designed using unique sequences derived from B. cereus 14579 genome, as described earlier. With the 66-genome local database, alleles located in 2179 base positions were detected. This allelic data matrix was subject to phylogenetic analysis in MEGA7 and a high-resolution cladogram was generated using Neighbor-Joining algorithm (Supplemental Fig. 1). The bootstrap test scores pointed to the confidence in the cladistics relationship among the strains after testing the reproducibility of the analysis 500 times. Strains from foods and feed clustered with dairy and other Genome Groups intermittently. The larger cluster consisted of 16 of the 21 Genome Groups with only a handful of the genomes from the strains used in this study grouping in this cluster. Most of these clustered in a second clade (bottom clade in the figure) suggesting a deeper intra-species genomic diversity than what has been previously recognized. It is clear from this analysis (Fig. 1) that food and feed strains from this study represent unreported phylogenetically-related clusters within the B. cereus Genomic Group species complex. By anchoring unknown strains from our samples against the NCBI Genome Groups, we could compare them with other B. cereus strains from many kinds of FDA-regulated commodities like spices, PIF, dietary supplements, dairy and medicated fish feed. Public health agencies like FDA and CDC are relying more on WGS based approaches for typing and source tracking (Allard et al., 2016). The 25-MLSA approach enables rapid typing and identification of B. cereus complex isolates before submission to the above GenomeTrakr project for public use. To evaluate the robustness of our comprehensive 25- MLSA approach, we used two different bioinformatic tools. The first approach created a list of 4430 conserved gene sequences from a reference genome (in this case 25 loci based on the B. cereus 14579 genome) and contained variant alleles in more than 120,000 positions across the 66 genomes. We randomly selected 1000 genes out of these 4430 query loci alleles for further analysis with MEGA7 suite. The phylogenetic tree from this analysis (Supplemental Fig. 1) showed a high degree of congruency when compared with the phylogenic clusters generated by 25-MLSA (Fig. 1)
The second approach consisted of detecting single nucleotide polymorphisms (SNPs) across the 66 genomes using kSNP3 (Gardner et al., 2015), a widely used whole genome SNP finding tool. This analysis resulted in a large data set of SNPs present in 11,000 positions across 66 genomes which were used to build a similar phylogenetic tree (Supplemental Fig. 2). When the two trees generated from conserved genes and the kSNP3 tools were compared in splitstree, no significant anomalies were observed (data not shown). When compared with the 25-MLSA-based tree as shown in Fig. 1, these two approaches produced highly similar cladograms. It is interesting to note the trees from 79% of the coding genes and the whole genome based k-mers showed indistinguishable phylogenetic relationship among the isolates, and these two trees were highly congruent with the 25-MLSA approach developed for this study.
The results from the two alternative methods confirmed that our approach of using 25-MLSA with 25 housekeeping genes was sufficient to capture the intra-species genomic diversity of B. cereus strains. These analyses pointed to a highly similar genomic backbone with significant and comparable intra-species nucleotide divergence deep enough to be captured singly by the 25-MLSA approach described in this work.
The close phylogenetic alignment of Genome Groups in the upper cluster (Fig. 1) also points to previously unrecognized deep intra-species divergence among the food and feed strains. This work highlights the necessity of extensive sequencing of B. cereus strains from foods, feed and associated environments to fill this gap. In contrast, nine dairy strains are distributed almost evenly (Fig. 1) within different Genome Groups in the tree.
The elevated level of genetic relatedness among the members of the B. cereus complex has been confirmed by comparative genomics (Tourasse et al., 2006). The existence of high levels of synteny and conserved genome sequences point to a closely related group of organisms. By increasing the number of loci from seven or nine to 25 under the 25-MLSA approach described here, the intra-species sequence divergence among the clades of B. cereus strains was captured. The result of whole genome SNP profiling using kSNP3 and the alleles from 1000 conserved coding genes highlights the limitations of quantifying nucleotide differences as seen by 7-gene MLST. It is important here to consider plasmid-borne genes and genes associated with mobile elements in the genomes for strain differentiation as suggested previously (Rasko et al. (2005). The genotyping strategy confirmed the presence of the enterotoxin genes, while also elucidating strain-level genotypic differences.
4. Conclusion
Our approach, consisting of parallel bioinformatics, comparative genomics and endpoint PCR analyses of enterotoxin genes, represents a comprehensive strategy for characterizing B. cereus enterotoxigenic strains from dried foods and feed. The results of our toxigenic profiles have led us to speculate that certain profiles may be specific for certain types of food. Since B. cereus enterotoxin genes are known to be strain specific, certain commodities may allow for colonization of particular strains which possess a specific toxin genotype. However, more isolates from these same commodities and other commodities should be studied before this can be definitively answered.
With WGS becoming a routine technique for food safety surveillance studies of cases and outbreaks, the genomic data are now being used extensively for source tracking and identification of foodborne pathogens (Toro et al., 2016; Allard et al., 2016) In the present study, we chose 25 loci used in 5 different MLST schemes that were applied by SuperCAT database for its supertree reconstruction to create a comprehensive 25-MLSA method. This was an expanded MLST-like method necessary to capture emergent and expanding allelic variations among B. cereus complex isolates. Predictably, the 25-MLSA based phylogenetic analysis, separated 45 B. cereus genomes from spices, PIF, dietary supplements, dairy and medicated animal feed samples into distinct groups that ignored food sources. In addition, this method seamlessly sorted these isolates into 21 anchors that NCBI had identified as genomically distinct groups among the growing number of B. cereus genomes (totaling 66). This robust method was further evaluated against two computation-intensive bioinformatic tools for validation.
Thus, we suggest that our 25-MLSA approach provides a simple, but rapid tool, to investigate WGS datasets from uncharacterized B. cereus strains for comparison with established genomes. By confirming the high level of similarity in the genomic backbone with specific but discrete intra-species divergence, and combining WGS-based 25-MLSA approach with specific endpoint PCR analysis, our study recognizes the use of B. cereus toxin genes as an important component in the toolbox for food safety investigations associated with B. cereus.
Whole genome sequencing has become an integral and helpful part of our agency’s routine workflow in responding to foodborne microbial contamination events.
The current approach of analyzing WGS assemblies with our comprehensive 25- MLSA approach could easily be extended to include selected plasmid-borne genes and virulence factors. This study establishes a powerful platform for further genomics research of the phylogenetically diverse B. cereus group, a prerequisite towards development of future countermeasures against this important foodborne pathogen.
Supplementary Material
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijfoodmicro.2018.06.016.
References
- Agaisse H, Gominet M, Økstad O, Kolstø A, Lereclus D, 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Agata N, Mori M, Ohta M, Suwan S, Ohtani I, Isobe M, 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129, 17–20. [DOI] [PubMed] [Google Scholar]
- Allard M, Strain E, Melka D, Bunning K, Musser S, Brown E, Timme R, 2016. Practical value of food pathogen traceability through building a whole genome sequencing network and database. J. Clin. Microbiol. 54, 1975–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S, Gish W, Miller W, Myers E, Lipman D, 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Anderson A, Ronner U, Granum P, 1995. What problems does the food industry have with the spore-forming pathogens Bacillus cereus and Clostridium perfringens? Int. J. Food Microbiol. 28, 145–155. [DOI] [PubMed] [Google Scholar]
- Ankolekar C, Rahmati T, Labbé R, 2009. Detection of toxigenic Bacillus cereus and Bacillus thuringiensis spores in U.S. rice. Int. J. Food Microbiol. 128, 460–466. [DOI] [PubMed] [Google Scholar]
- Antai S, 1988. Study of the Bacillus flora of Nigerian spices. Int. J. Food Microbiol. 6, 259–261. [DOI] [PubMed] [Google Scholar]
- Arnesen S, Fagerlund A, Granum P, 2008. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32, 579–606. [DOI] [PubMed] [Google Scholar]
- Asano SI, Nukumizu Y, Bando H, Iizuka T, Yamamoto T, 1997. Cloning of novel enterotoxin genes from Bacillus cereus and Bacillus thuringiensis. Appl. Environ. Microbiol. 63, 1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz R, Bartels D, Best A, DeJongh M, Disz T, Edwards R, Formsma K, Gerdes S, Glass E, Kubal M, Meyer F, Olssen G, Olson R, Osterman A, Overbeek R, McNeil L, Paarmann D, Paczian T, Parrello B, Pusch G, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zangnitko O, 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewicz M, Hansen B, Swiecicka I, 2008. The members of the Bacillus cereus group are commonly present contaminates of fresh and heat-treated milk. Food Microbiol. 25, 588–596. [DOI] [PubMed] [Google Scholar]
- Becker H, Schaller G, Wiese W, Terplan G, 1994. Bacillus cereus in infant foods and dried milk products. Int. J. Food Microbiol. 23, 1–15. [DOI] [PubMed] [Google Scholar]
- Beecher D, Macmillian J, 1991. Characterization of the components of hemolysin BL from Bacillus cereus. Infect. Immun. 59, 1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher D, Schoeni J, Wong A, 1995. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 63, 4423–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biniinda-Edmonda O, 2004. The evolution of supertrees. Trends Ecol. Evol. 19, 315–322. [DOI] [PubMed] [Google Scholar]
- Blakey L, Priest F, 1980. The occurrence of Bacillus cereus in some dried foods including pulses and cereals. J. Appl. Bacteriol. 48, 297–302. [DOI] [PubMed] [Google Scholar]
- Bottone E, 2010. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 23, 382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candlish A, Pearson S, Aidoo K, Smith J, Kelly B, Irvine H, 2001. A survey of ethnic foods for microbial quality and aflatoxin content. Food Addit. Contam. 18, 129–136. [DOI] [PubMed] [Google Scholar]
- Ceuppens S, Boon N, Uyttendaele M, 2013. Diversity of Bacillus cereus group strains is reflected in their broad range of pathogenicity and diverse ecological lifestyles. FEMS Microbiol. Ecol. 84, 433–450. [DOI] [PubMed] [Google Scholar]
- Choo E, Jang S, Kim K, Lee K, Heu S, Ryu S, 2007. Prevalence and genetic diversity of Bacillus cereus in dried red pepper in Korea. J. Food Prot. 70, 917–922. [DOI] [PubMed] [Google Scholar]
- Egelezos S, Huang B, Dykes G, Fegan N, 2010. The prevalence and concentration of Bacillus cereus in retail food products in Brisbane, Australia. Foodborne Pathog. Dis. 7, 867–870. [DOI] [PubMed] [Google Scholar]
- Ehling-Schulz M, Messelhäusser U, 2013. Bacillus “next generation” diagnostics: moving from detection toward subtyping and risk-related strain profiling. Front. Microbiol. 4, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling-Schulz M, Fricker M, Scherer S, 2004. Identification of emetic toxin producing Bacillus cereus strains by a novel molecular assay. FEMS Microbiol. Lett. 232, 189–195. [DOI] [PubMed] [Google Scholar]
- Fagerlund A, Ween O, Lund T, Hardy S, Granum P, 2004. Genetic and functional analysis of the cytK family of genes in Bacillus cereus. Microbiology 150, 2689–2697. [DOI] [PubMed] [Google Scholar]
- Fagerlund A, Brillard J, Fürst R, Guinebretière M, Granum P, 2007. Toxin production in a rare and genetically remote cluster of strains of the Bacillus cereus group. BMC Microbiol. 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangio M, Roura S, Fritz R, 2010. Isolation and identification of Bacillus spp. and related genera from different starchy foods. J. Food Sci. 75, M218–M221. [DOI] [PubMed] [Google Scholar]
- Foster L, Allan M, Khan A, Moore P, Williams D, Hubbard M, Dixon L, Gurley B, 2013. Multiple dosing of Ephedra-free dietary supplements: hemodynamic, electrocardiographic, and bacterial contamination effects. Clin. Pharmacol. Ther. 93, 267–274. [DOI] [PubMed] [Google Scholar]
- García S, Iracheta F, Galván F, Heredia N, 2001. Microbiological survey of retail herbs and spices from Mexican markets. J. Food Prot. 64, 99–103. [DOI] [PubMed] [Google Scholar]
- Gardner S, Slezak T, Hall B, 2015. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics 31, 2877–2878. [DOI] [PubMed] [Google Scholar]
- Gohar M, Økstad O, Gilois N, Sanchis V, Kolstø A, Lereclus D, 2002. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2, 784–791. [DOI] [PubMed] [Google Scholar]
- Granum PE, 2001. Bacillus cereus. In: Doyle MP (Ed.), Food Microbiology: Fundamentals and Frontiers, 2nd ed. ASM Press, Washington, DC, pp. 373–381. [Google Scholar]
- Granum P, O’Sullivan K, Lund T, 1999. The sequence of the non-haemolytic enterotoxin operon from Bacillus cereus. FEMS Microbiol. Lett. 177, 225–229. [DOI] [PubMed] [Google Scholar]
- Guinebretière M, Broussolle V, Nguyen-The C, 2002. Enterotoxigenic profiles of food poisoning and food-borne Bacillus cereus strains. J. Clin. Microbiol. 40, 3053–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinebretière M, Velge P, Couvert O, Carlin F, Debuyser M, Nguyen-The C, 2010. Ability of Bacillus cereus group strains to cause food poisoning varies according to phylogenetic affiliation (groups I to VII) rather than species affiliation. J. Clin. Microbiol. 48, 3388–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinebretière M, Auger S, Galleron N, Contzen M, De Sarrau B, De Buyser M, Lamberet G, Fagerlund A, Granum P, Lereclus D, De Vos P, Nguyen-The C, Sorokin A, 2013. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus Group occasionally associated with food poisoning. Int. J. Syst. Evol. Microbiol. 63, 31–40. [DOI] [PubMed] [Google Scholar]
- Hansen B, Hendriksen N, 2001. Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl. Environ. Microbiol. 67, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, Lund T, Granum P, 2001. CytK toxin of Bacillus cereus forms pores in planar lipid bilayers and is cytotoxic to intestinal epithelia. FEMS Microbiol. Lett. 197, 47–51. [DOI] [PubMed] [Google Scholar]
- Heinriches J, Beecher D, MacMillan J, Zilinskas B, 1993. Molecular cloning and characterization of the hblA gene encoding the B component of hemolysin BL from Bacillus cereus. J. Bacteriol. 175, 6760–6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason E, Tourasse N, Meisal R, Caugant D, Kolstø A, 2004. Multilocus sequence typing scheme for bacteria of the Bacillus cereus group. Appl. Environ. Microbiol. 70, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D, Bryant D, 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267. [DOI] [PubMed] [Google Scholar]
- Kim B, Kim M, Kim H, Seo K, Park Y, Choi N, Oh D, 2010. Emetic toxin producing Bacillus cereus Korean isolates contain genes encoding diarrheal-related enterotoxins. Int. J. Food Microbiol. 144, 182–186. [DOI] [PubMed] [Google Scholar]
- Ko K, Kim W, Kim M, Kim W, Chung S, Kim I, Kook Y, 2004. Population structure of the Bacillus cereus group as determined by sequence analysis of six housekeeping genes and the plcR gene. Infect. Immun. 72, 5253–5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac J, Miller R, Carroll L, Kent D, Jian J, Beno S, Wiedmann M, 2016. Production of hemolysin BL by Bacillus cereus group isolates of dairy origin is associated with whole-genome phylogenetic clade. BMC Genomics 17, 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K, 2016. MEFA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Schraft H, Odumeru J, Griffiths M, 1998. Identification of contamination sources of Bacillus cereus in pasteurized milk. Int. J. Food Microbiol. 43, 159–171. [DOI] [PubMed] [Google Scholar]
- Lindbäck T, Fagerlund A, RØdland M, Granum P, 2004. Characterization of Bacillus cereus Nhe enterotoxin. Microbiology 150, 3959–3967. [DOI] [PubMed] [Google Scholar]
- Lindbäck T, Hardy S, Dietrich R, Sødring M, Didier A, Moravek M, Fagerlund A, Bock S, Nielsen C, Casteel M, Granum P, Märtlbauer E, 2010. Cytotoxicity of the Bacillus cereus Nhe enterotoxin requires specific binding order of its three exoprotein components. Infect. Immun. 78, 3813–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C, Omotoye R, Mitchell R, 2003. The microbiological quality of ready-to-eat foods with added spices. Int. J. Environ. Health Res. 13, 31–42. [DOI] [PubMed] [Google Scholar]
- Lund T, De Buyser ML, Granum PE, 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38, 254–261. [DOI] [PubMed] [Google Scholar]
- Merzougui S, Lkhider M, Grosset N, Gautier M, Cohen N, 2014. Prevalence, PFGE typing, and antibiotic resistance of Bacillus cereus group isolated from food in Morocco. Foodborne Pathog. Dis. 11, 145–149. [DOI] [PubMed] [Google Scholar]
- Moravek M, Dietrich R, Buerk C, Broussolle V, Guinebretière M, Granum P, Nguyen-The C, Märtlbauer E, 2006. Determination of the toxic potential of Bacillus cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 257, 293–298. [DOI] [PubMed] [Google Scholar]
- Ngamwongsatit P, Buasri W, Pianariyanon P, Pulsrikarn C, Ohba M, Assavanig A, Panbangred W, 2008. Broad distribution of enterotoxin genes (hblCDA, nheABC, cytK, and entFM) among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Int. J. Food Microbiol. 121, 352–356. [DOI] [PubMed] [Google Scholar]
- Oltuszak-Walczak E, Walczak P, 2013. PCR detection of cytK gene in Bacillus cereus group strains isolated from food samples. J. Microbiol. Methods 95, 295–301. [DOI] [PubMed] [Google Scholar]
- Portnoy B, Goepfert J, Harmon S, 1976. An outbreak of Bacillus cereus food poisoning resulting from contaminated vegetable sprouts. Am. J. Epidemiol. 103, 589–594. [DOI] [PubMed] [Google Scholar]
- Powers M, Latt G, Brown T, 1976. Incidence and levels of Bacillus cereus in processed spices. J. Milk Food Technol. 39, 668–670. [Google Scholar]
- Priest F, Barker M, Baillie L, Holmes E, Maiden M, 2004. Population structure and evolution of the Bacillus cereus group. J. Bacteriol. 186, 7959–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovic A, Uyttendaele M, Ombregt S, Jaaskelainen E, Salkinoja-Salonen M, Debevere J, 2006. Influence of type of food on the kinetics and overall production of Bacillus cereus emetic toxin. J. Food Prot. 69, 847–852. [DOI] [PubMed] [Google Scholar]
- Ramarao N, Sanchis V, 2013. The pore-forming haemolysins of Bacillus cereus: a review. Toxins 5, 1119–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko D, Altherr M, Han C, Ravel J, 2005. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 29, 303–329. [DOI] [PubMed] [Google Scholar]
- Ryan P, Macmillan J, Zilinskas B, 1997. Molecular cloning and characterization of the genes encoding the L1 and L2 components of hemolysin BL from Bacillus cereus. J. Bacteriol. 79, 2551–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samapundo S, Everaert H, Wandutu J, Rajkovic A, Uyttendaele M, Devlieghere F, 2011. The influence of headspace and dissolved oxygen level on growth and haemolytic BL enterotoxin production of psychrotolerant Bacillus weihenstephanensis isolate on potato based ready–to-eat food products. Food Microbiol. 28, 298–304. [DOI] [PubMed] [Google Scholar]
- Scallan E, Hoekstra R, Angulo F, Tauxe R, Widdowson M, Roy S, Jones J, Griffin P, 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeni J, Wong A, 2005. Bacillus cereus food poisoning and its toxins. J. Food Prot. 68, 636–648. [DOI] [PubMed] [Google Scholar]
- Sorokin A, Candelon B, Guilloux K, Galleron N, Wackerow-Kouzova N, Ehrlich S, Bourgeuet D, Sanchis V, 2006. Multiple-locus sequence typing analysis of Bacillus cereus and Bacillus thuringiensis reveals separate clustering and a distinct population structure of psychrotrophic strains. Appl. Environ. Microbiol. 72, 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallent S, Rhodehamel E, Harmon S, Bennett R, 2012. BAM: Bacillus cereus. In: Bacteriological Analytical Manual Chapter. 14. [Google Scholar]
- Te Giffel M, Beumer R, Granum P, Rombouts F, 1997. Isolation and characterization of Bacillus cereus from pasteurized milk in household refrigerators in the Netherlands. Int. J. Food Microbiol. 34, 307–318. [DOI] [PubMed] [Google Scholar]
- Thaenthanee S, Wong A, Panbangred W, 2005. Phenotypic and genotypic comparisons reveal a broad distribution and heterogeneity of hemolysin BL genes among Bacillus cereus isolates. Int. J. Food Microbiol. 105, 203–212. [DOI] [PubMed] [Google Scholar]
- Toro M, Retamal P, Ayers S, Barreto M, Allard M, Brown E, Gonzalez-Escalona N, 2016. Whole-genome sequencing analysis of Salmonella enterica serovar Enteritidis isolates in Chile provides insights into possible transmission between gulls, poultry, and humans. Appl. Environ. Microbiol. 82, 6223–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourasse N, Kolstø A, 2008. SuperCAT: a supertree database for combined and integrative multilocus sequence typing analysis of the Bacillus cereus group of bacteria (including B. cereus, B. anthracis and B. thuringiensis). Nucleic Acids Res. 36, D461–D468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourasse N, Helgason E, Økstad O, Hegna I, Kolstø A, 2006. The Bacillus cereus group: novel aspects of population structure and genome dynamics. J. Appl. Microbiol. 101, 579–593. [DOI] [PubMed] [Google Scholar]
- Van Doren J, Neil K, Parish M, Gieraltowski L, Gould L, Gombas K, 2013. Foodborne illness outbreaks from microbial contaminants in spices, 1973–2010. Food Microbiol. 36, 456–464. [DOI] [PubMed] [Google Scholar]
- Wijnands L, Dufrenne J, Rombouts F, in’tVeld P, van Leusden F, 2006. Prevalence of potentially pathogenic Bacillus cereus in food commodities in the Netherlands. J. Food Prot. 69, 2587–2594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.