Abstract
Objective
Explore the relationship between early hypotension after ECPR and survival to hospital discharge (SHD) with favorable neurologic outcome (FNO) in children with cardiac disease.
Methods
Retrospective cohort study of patients undergoing ECPR at a single center pediatric cardiac intensive care unit. Hypotension was defined as MAP < 5th percentile for age. Primary and secondary exposure variables were presence and burden of hypotension respectively, during the first 6 h after ECPR. Our primary outcome was SHD with FNO defined by Pediatric Cerebral Performance Category score of 1–3 or no change from baseline. Secondary outcomes included acute central nervous system (CNS) injury via neuroimaging and EEG. Univariate and multivariable logistic regression analyses were performed.
Results
We analyzed 82 index ECPR events from 2010 to 2022. Hypotension was observed for at least one MAP value in 36/82 (43.9%) of the cohort. The median [IQR] burden of hypotension was 0 [0,14.3]%. Patients with SHD with FNO had shorter CPR duration, lower number of epinephrine and calcium doses, and lower maximum lactate levels when compared to patients who died or had SHD without FNO. After controlling for potential confounders, there was no association between presence of hypotension or burden of hypotension and SHD, SHD with FNO, or acute CNS injury via neuroimaging and EEG.
Conclusion
In children with cardiac disease, there was no association between early hypotension after ECPR and SHD with FNO. Multicenter studies are needed to better understand how early hypotension after ECPR affects neurologic outcomes in children with cardiac disease.
Keywords: Extracorporeal cardiopulmonary resuscitation, Hypotension, Pediatric, Cardiac arrest, Congenital heart disease, Outcome
Abbreviations
- ECMO
extracorporeal membrane oxygenation
- ECPR
extracorporeal cardiopulmonary resuscitation
- ROC
return of circulation
- PCAC
post cardiac arrest care
- CNS
central nervous system
- BP
blood pressure
- SHD
survival to hospital discharge
- FNO
favorable neurologic outcomes
- CICU
cardiac intensive care unit
- LOS
length of stay
- PCPC
Pediatric cerebral performance category
- FSS
functional status score
- SVP
single ventricle physiology.
Introduction
There is an increasing use of extracorporeal membrane oxygenation (ECMO) as a rescue therapy for failed conventional cardiopulmonary resuscitation (extracorporeal cardiopulmonary resuscitation, or ECPR), with evidence showing improved survival to hospital discharge (SHD) and favorable neurologic outcomes (FNO) compared to conventional CPR.1 Amongst children undergoing ECPR, patients with underlying cardiac disease are shown to have improved outcomes compared to those without cardiac disease, with formal advanced life support guidelines2 supporting the use of ECPR for this population in centers with ECMO expertise and equipment.3 Despite this, there continues to be a lack of evidence on post cardiac arrest care (PCAC) in children with cardiac disease after ECPR.
Key elements of PCAC after ECPR include manipulation of vasoactive medications to avoid extremes of low and high blood pressure (BP). Several studies in the post-ECMO cannulation population have demonstrated an impact of hypotension on clinical outcomes albeit few in children. In 2019, the American Heart Association published a scientific statement on pediatric PCAC that recommended avoiding hypotension.4 These recommendations were not specific to ECPR or children with cardiac disease and further highlight the lack of evidence to support optimal targets. Furthermore, many children with cardiac disease can have unique physiology, such as shunt-dependent parallel circulations, which can further complicate post-arrest BP management. No studies to date have evaluated the association of post-arrest hypotension on CNS outcomes for children with cardiac disease or in children after ECPR.
The purpose of this study is to evaluate the association between early hypotension in the post-arrest period after ECPR and outcomes of children with cardiac disease. Our hypothesis is that the presence of early hypotension, defined as the lowest MAP < 5th percentile MAP for age, during the first 6 h after ROC in patients after ECPR is associated lower chances of SHD with FNO.
Methods
Patient population
The Institutional Review Board’s approval was obtained before this study. We identified children admitted to the Children’s Medical Center Dallas pediatric cardiac intensive care unit (CICU) between November 2010 and December 2022 who had a cardiac arrest during their CICU admission. Our pediatric CICU is a 30-bed unit that admits surgical and non-surgical patients with congenital and acquired heart disease. We reviewed all CPR events to identify patients undergoing ECPR. We defined ECPR as either having active chest compressions while being cannulated onto ECMO or if cannulated onto ECMO within 20 min of return of circulation (ROSC). For this study, for patients who had multiple ECPR events during the same hospitalization, we only analyzed the first ECPR event. We excluded patients who were transferred to another hospital before hospital discharge since we did not have records to assess their outcomes as well as any repeat ECPR events during the same hospitalization.
Routine clinical care
Since 2017, a PCAC protocol has been utilized on all patients who have CPR duration ≥ 2 min and a motor Glasgow Coma Scale ≤ 5 or change from baseline. The protocol gives guidance on various aspects of PCAC. Hemodynamic management after ECPR is focused on maximizing ECMO flow while minimizing the use of inotrope/vasopressor infusions. Screening for CNS complications includes routine EEG monitoring for the first 72 h post-arrest and daily head ultrasounds for infants. Cross sectional head imaging via CT or MRI is performed at the discretion of the clinical team. Targeted temperature management maintains goal normothermia with avoidance of fever. Blood bank practices for patients on ECMO aim to maintain a hemoglobin goal ≥ 10 g/dL.
Data collection
Study data was collected from electronic health record and managed using REDCap electronic data capture tools hosted at Children’s Medical Center Dallas.5, 6 Pre-arrest variables include: age, sex, race, weight, weight for age z score, gestational age at birth if less than or equal to 12 months of age at time of arrest, presence of single ventricle physiology (SVP, defined as patients with a mixture of systemic venous and pulmonary venous return, with total cardiac output partitioned into pulmonary and systemic blood flow),7 prior or subsequent cardiac arrest during the same hospital admission, medical vs. surgical encounter, mean vasoactive inotrope score (VIS) 2 h prior to cardiac arrest. Intra-arrest variables include: duration of CPR, number of epinephrine, calcium chloride, and sodium bicarbonate doses given, epinephrine dosing interval (calculated as number of epinephrine doses given divided by the duration of CPR), first documented rhythm, and site of ECMO cannulation (groin, neck, chest). Post-arrest variables include: duration of ECMO in days, mean VIS 6 h post-arrest, mean ECMO flow in the 6 h post-arrest, mean vasodilator score (defined as dose of nicardipine in mcg/kg/min + dose of nitroprusside in mcg/kg/min; for example if patient was on nicardipine of 2 mcg/kg/min and nitroprusside 1 mcg/kg/min then the vasodilator score is 3) 6 h post-arrest, and maximum/minimum lactate within 6 h post-arrest.
The following data was collected from the Society of Thoracic Surgeons Congenital database for surgical encounters: The Society of Thoracic Surgeons (STS)-European Association of Cardiothoracic Surgery (STAT) mortality category, cardiopulmonary bypass and cross clamp time.
Exposures:
Our primary exposure was presence of early hypotension, which was defined as the lowest documented or minimum MAP < 5th percentile for age during the first 6 h after ROC.8 The absence of hypotension was defined as no recorded MAP < 5th percentile for age. MAP values were preferentially obtained from invasive arterial BP monitoring when present. If invasive arterial BP monitoring was not present, noninvasive BP monitoring was used. All MAP values were documented at the minimum of once every hour by the bedside nurse in the electronic medical record. As an exploratory analysis, we also evaluated burden of early hypotension, which was defined by the number of MAP values < 5th percentile for age divided by total number of MAP values during the first 6 h after ROC.
Outcome measures
Our primary outcome was SHD with FNO. We defined SHD with FNO as Pediatric Cerebral Performance Score (PCPC) of 1,2, or 3, or no change in discharge and admission PCPC.9 Secondary outcomes included SHD, SHD with favorable functional status, neuroimaging injury, and electroencephalogram (EEG) abnormalities. We defined favorable functional status using Functional Status Score (FSS)10 and defined favorable functional status as a change in discharge and admission overall FSS by less than or equal to 3 or any individual domain difference of less than or equal to 2. PCPC and FSS scores were assigned independently by two of the co-authors (PY; SF). When there were discrepancies in PCPC and FSS scores, both co-authors discussed and came to a consensus on the final score. All brain MRI and CT scans taken between days 3–14 post-arrest were scored by two board certified pediatric neuroradiologists (MM; SS) using two different scoring systems: (a) the modASPECTS scoring system which has been validated in children post-arrest and (b) an ECMO scoring system which has been validated in the ECMO population.11, 12. See Supplementary Fig. 1 for ECMO scoring tool description. These scoring tools are complementary given modASPECTS is better suited for detection of ischemic changes, whereas the ECMO scoring tool incorporates hemorrhage. All EEGs during hours 25–72 h post-arrest were analyzed for qualitative analysis by a pediatric neurology fellow (TB). For every ten EEG’s analyzed, a board-certified pediatric epileptologist (DS) overread one EEG. There were no discrepancies between the two independent readers for the 1 in 10 EEG’s that were read by both raters. Each EEG was analyzed for background abnormalities (normal, mild, moderate, severe) and presence of seizures (See Supplementary Table 1, Table 2). EEG background severity classification was adapted by a study from Thorp et al.13
Table 1.
Characteristics of Patients with SHD with Favorable Neurologic Outcomes vs. Death or SHD with Unfavorable Neurologic Outcomes.
| Variable |
Total (N = 82) |
SHD with Favorable Neurologic Outcome (N = 35) | Death or SHD with Unfavorable Neurologic Outcome (N = 47) | P value |
|---|---|---|---|---|
| Pre-arrest | ||||
| Age (months) | 0.3[0.1,3.6] | 0.2[0.0,11.5] | 0.3[0.1,3.2] | 0.70 |
| Race (white) | 54(65.9) | 23(42.6) | 31(57.4) | 0.98 |
| female sex (%) | 35 (42.7) | 13(37.1) | 22(62.9) | 0.38 |
| Weight(kg) | 4.9[3.4,12.8] | 4.4[3.3,37.9] | 4.9[3.4,11.3] | 0.91 |
| Weight for age z score | −1.2 ± 1.8 | −0.9 ± 1.7 | −1.4 ± 1.9 | 0.32 |
| Genetic syndrome (%) | 14 (17.1) | 7(50.0) | 7(50.0) | 0.54 |
| Single ventricle (%) | 37(45.1) | 15(40.5) | 22(59.5) | 0.72 |
| Single ventricle physiology (%) | 34(41.4) | 14(41.2) | 20(58.8) | 0.82 |
| Medical encounter (%) | 29(35.3) | 13(44.8) | 16(55.2) | 0.77 |
| Gestational age at birth, if age < 12 months at time of arrest (N = 50), weeks | 38.0[36.0,39.0] | 38.0[37.0,39.0] | 39.0[35.5,39.0] | 0.83 |
| Prior or Subsequent cardiac arrest during same admission (%) | 30(36.6) | 12(40.0) | 18(60.0) | 0.71 |
| Stat Category (N = 50) | ||||
| 1,2,or 3 (%) | 9 (18) | 4(44.4) | 5(55.6) | 0.93 |
| 4 (%) | 30 | 12(40.0) | 18(60.0) | |
| 5 (%) | 11 | 4(36.4) | 7(63.6) | |
| Cardiopulmonary bypass time (N = 44), minutes | 135.0[95.0,197.0] | 157.5[101.0, 182.0] | 116.0[82.0, 197.0] | 0.43 |
| Cross clamp time (N = 44), minutes | 68.0[38.0,122.0] | 110.0[49.0,130.0] | 53.5[35.0, 84.0] | 0.08 |
| Pre-arrest VIS 2 h | 3.2[0.0,8.0] | 4.8[1.0,7.0] | 2.7[0.0,8.7] | 0.52 |
| Intra-arrest | ||||
| CPR duration (min) | 37.2 ± 19.1 | 30.3 ± 15.1 | 42.3 ± 20.3 | 0.004 |
| Number of Epinephrine doses given | 7[5,11] | 6[3,8] | 8[6,13] | 0.003 |
| Epinephrine dosing interval | ||||
| < 5 min | 47(57.3) | 18(38.3) | 29(61.7) | 0.55 |
| 5–8 min | 17(20.7) | 8(47.1) | 9(52.9) | |
| More than 8 min | 15(18.3) | 8(53.3) | 7(46.7) | |
| Calcium given (%) | 65(79.3) | 25(38.5) | 40(61.5) | 0.13 |
| Number of Calcium doses given | 1[1,3] | 1[0,2] | 2[1,3] | 0.04 |
| Sodium bicarbonate given (%) | 64(78.0) | 28(43.8) | 36(56.3) | 0.71 |
| Number of sodium bicarbonate doses given | 1[1,3] | 1[1,2] | 1[1,3] | 0.53 |
| Shockable Rhythm (%) | 17(20.7) | 9(52.9) | 8(47.1) | 0.34 |
| Post arrest | ||||
| ECMO flow (ml/kg/min) mean over 6 h (N = 79) | 123 ± 47 | 122 ± 52 | 124 ± 44 | 0.82 |
| ECMO cannulation site | ||||
| Neck | 25 (30.4 %) | 8 (32 %) | 17(68 %) | 0.20 |
| Groin | 8 (9.8 %) | 4(50 %) | 4(50 %) | 0.66 |
| Chest | 49(68.1 %) | 23(46.9 %) | 26(53.1 %) | 0.34 |
| VIS mean 6 h | 3.4[1.0,6.4] | 3.9[0.6,6.7] | 2.8[1.0,6.1] | 0.66 |
| Vasodilator score mean over 6 h | 0[0,0] | 0[0,0] | 0[0,0] | 1.00 |
| Max Lactate | 14.0 ± 5.4 | 12.5 ± 4.4 | 15.1 ± 5.9 | 0.03 |
| Min lactate | 8.0 ± 5.2 | 7.3 ± 4.0 | 8.6 ± 5.9 | 0.24 |
All variables with normalized distribution expressed as mean ± STD. All variables with non-normalized distribution expressed as median [interquartile range].
Table 2.
Association of Presence of Hypotension vs. Primary and Secondary Outcomes.
| Outcomes |
Yes hypotension (MAP < 5th percentile) (N = 36) |
No Hypotension (MAP ≥ 5th percentile) (N = 46) |
P value | Unadjusted Odds Ratio [95 % CI] | Adjusted Odds Ratio* [95 %CI] | P value |
|---|---|---|---|---|---|---|
| SHD | 16(44.4) | 21(45.7) | 0.91 | 0.95[0.39,2.29] | 1.35[0.48,3.31] | 0.57 |
| SHD with FNO | 16(44.4) | 19(41.3) | 0.78 | 1.14[0.47,2.74] | 1.83[0.629,5.31] | 0.27 |
| SHD with favorable functional status | 9(25) | 8(17.4) | 0.40 | 1.58[0.54,4.63] | 2.60[0.72, 9.47] | 0.15 |
| ECMO score ≥ 5# | 9 (75 %) | 16 (66.7 %) | 0.61 | 1.50[0.32,7.12] | 0.69[0.09,5.44] | 0.73 |
| modASPECTS score ≥ 5# | 7 (58.3 %) | 6 (25 %) | 0.049 | 4.2[0.96,18.3] | 3.29[0.58,18.6] | 0.18 |
| Seizure Yes | 4 (25 %) | 6 (25 %) | 1 | 1[0.23,4.31] | 0.87[0.15,5.14] | 0.88 |
| Background EEG severity moderate or severe | 12 (75 %) | 15 (62.5 %) | 0.41 | 1.80[0.44,7.31] | 0.88[0.13,5.8] | 0.89 |
*adjusted for CPR duration, number of intra-arrest epinephrine doses given, number of intra-arrest calcium doses given, and maximum lactate in the first 6 h.
# N = 36 with neuroimaging.
♢ N = 40 with EEG.
Abbreviations: SHD = survival to hospital discharge, FNO = favorable neurologic outcome, EEG = electroencephalogram, ECMO = extracorporeal membrane oxygenation
Statistical analysis
Categorical variables are presented as counts and percentages. Differences in categorical variables between patients with favorable and death/unfavorable neurologic outcome were tested by Chi Square test. Mean and STD or medians and interquartile range were used to describe continuous variables. Differences in continuous variables were tested by Wilcoxon and t-test. The association between presence of hypotension and burden of hypotension with primary and secondary outcomes were completed with univariate logistic regression and multivariable logistic regression model. Covariates for the multivariable logistic regression model were chosen if they had a p value < 0.1. Collinearity was assessed by the variance inflation factor. We also performed a subgroup analysis on patients with SVP. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). All statistical tests were two-sided, with P < 0.05 considered significant.
Results
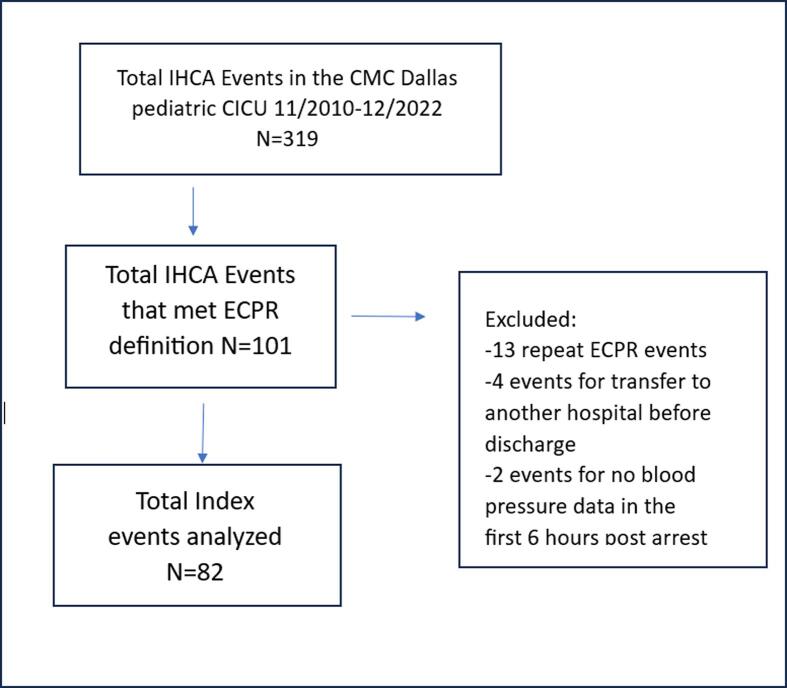
There were 82 index ECPR events that met inclusion and exclusion criteria during the study period that were analyzed (See Fig. 1 for flow diagram of data analysis). Table 1 displays the pre-, intra- and post-arrest characteristics and outcomes for patients with SHD with FNO compared to those who died or had SHD without FNO. Of the 82 patients, 37 (45 %) had SHD, 35 (42.6 %) had SHD with FNO. Compared to patients who died or had SHD without FNO, those with SHD with FNO had shorter CPR duration, lower number of epinephrine and calcium doses used intra-arrest, and lower maximum lactate levels within 6 h post-arrest. Characteristics of survivors vs. non-survivors showed similar results (Supplementary Table 3).
Fig. 1.
Flow diagram for data analysis. Abbreviations: IHCA- in hospital cardiac arrest, CMC-Children's Medical Center, CICU-cardiac intensive care unit, ECPR-extracorporeal cardiopulmonary resuscitation.
Table 3.
Burden of Hypotension vs. Primary and Secondary Outcomes.
| Outcomes | Unadjusted Odds Ratio [95 % CI] | Adjusted Odds Ratio* [95 %CI] | P value |
|---|---|---|---|
| SHD | 1.00[0.98,1.01] | 1.00[0.99,1.02] | 0.85 |
| SHD with FNO | 1.00[0.98,1.01] | 1.01[0.99,1.02] | 0.59 |
| SHD with favorable functional status | 1.01[0.99,1.02] | 1.01[0.99,1.03] | 0.18 |
| ECMO score ≥ 5# | 0.99[0.96,1.03] | 0.97[0.92,1.01] | 0.16 |
| modASPECTS score ≥ 5# | 1.02[0.99,1.06] | 1.02[0.98,1.06] | 0.44 |
| Seizure Yes♢ | 0.96[0.90,1.03] | 0.96 [0.88, 1.02] | 0.17 |
| Background EEG severity moderate or severe♢ | 1.02[0.99,1.05] | 1.00[0.95,1.04] | 0.83 |
*adjusted for CPR duration, number of intra-arrest epinephrine doses given, number of intra-arrest calcium doses given, and maximum lactate in the first 6 h.
# N = 36 with neuroimaging.
♢N = 40 with EEG.
Abbreviations: SHD = survival to hospital discharge, FNO = favorable neurologic outcome, EEG = electroencephalogram, ECMO = extracorporeal membrane oxygenation.
Table 2 illustrates the association of presence of hypotension vs. outcomes. During the first 6 h after ROC, 36/82 (43.9 %) patients had hypotension. Of the 82 patients, 35 (42.6 %) had SHD with FNO, 17 (20.7 %) had SHD with favorable functional status, 40 (48.8 %) had EEG and 36 (43.9 %) had neuroimaging available for analysis. Of the 40 patients who had EEG for analysis, 10 (25 %) patients had seizures and 27 (67.5 %) patients had moderate or severe EEG background. Of the 36 patients who had neuroimaging, 25(75 %) patients had ECMO score ≥ 5 and 13 (36.1 %) had a modASPECTS score ≥ 5. There was no difference in rates of SHD with FNO, SHD with favorable functional status, or SHD in patients with and without the presence of hypotension. There was no difference in the rates of acute CNS injury defined by seizures, background EEG severity of moderate or severe, or neuroimaging injury defined by ECMO scores. Patients with hypotension vs. no hypotension had higher rates of neuroimaging injury defined by modASPECTS scores. Patients with neuroimaging had longer CPR duration and higher maximum lactate levels that were more similar to patients with death or SHD without FNO compared to those with SHD with FNO (Supplementary Table 6). ECMO cannulation site was not associated with presence of neuroimaging injury (Supplementary Table 7). After controlling for CPR duration, maximum lactate level, number of intra-arrest epinephrine and calcium doses given, presence of hypotension was not associated with any outcomes, including neuroimaging injury defined by modASPECTS scores.
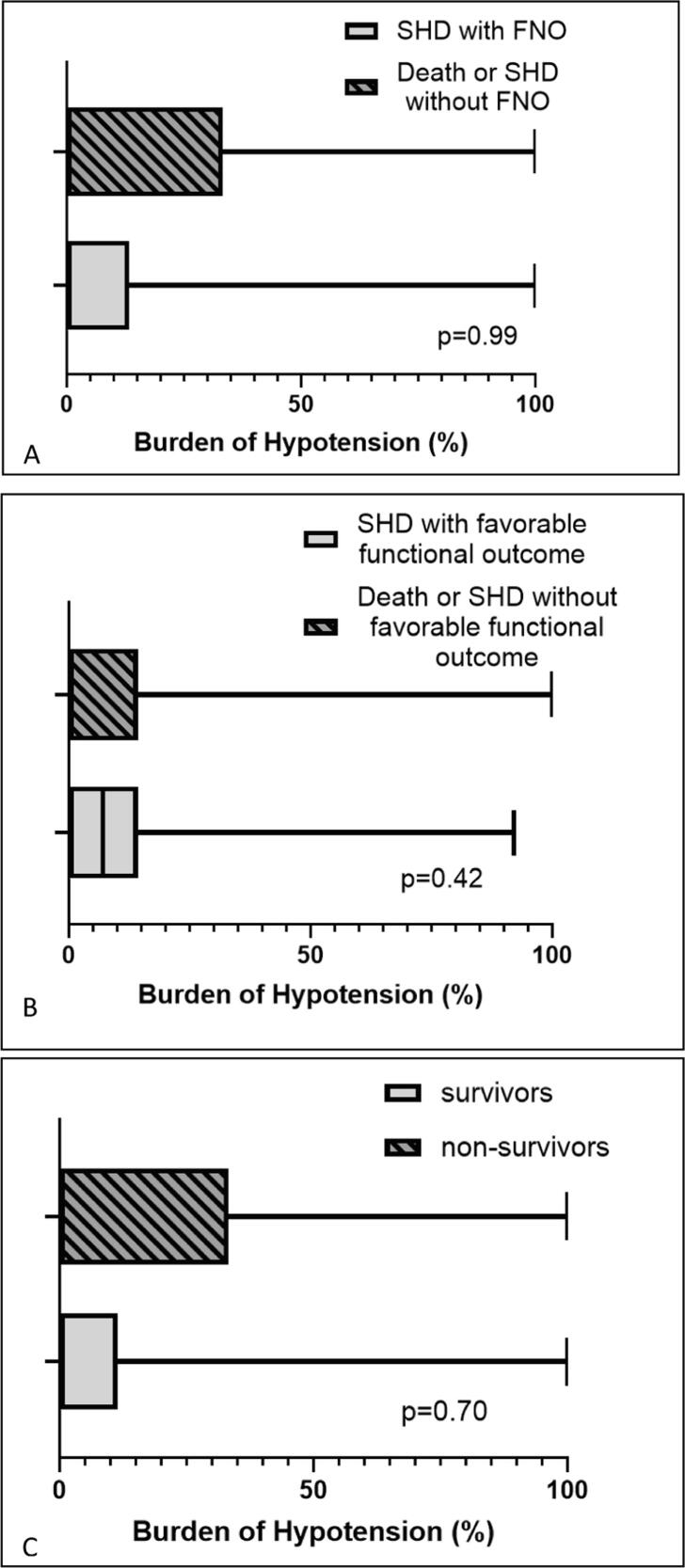
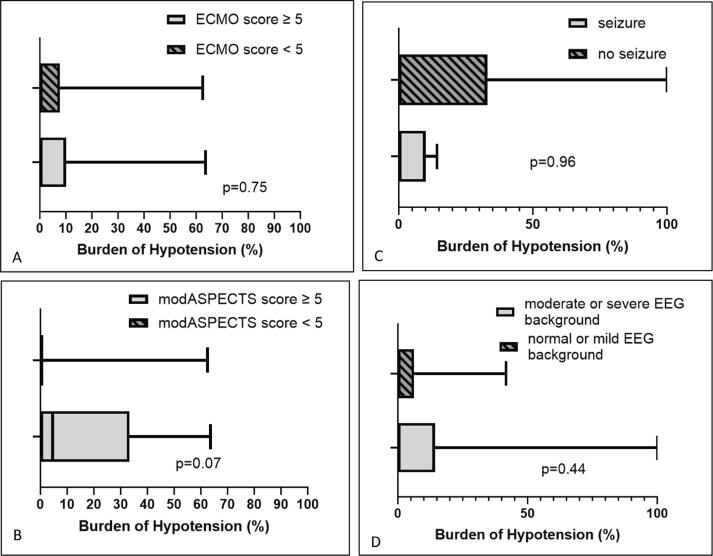
Fig. 2 displays burden of hypotension vs. various outcomes at hospital discharge. Fig. 3 displays burden of hypotension vs. various acute CNS injury outcomes. Of the 82 patients, the median [IQR] of burden of hypotension was 0[0,14.3]%. There was no difference in the burden of hypotension for various outcomes. Table 3 shows the results of univariate and multivariable logistic regression analyses for burden of hypotension vs. various outcomes. After controlling for CPR duration, maximum lactate level, and number of intra-arrest epinephrine and calcium doses given, burden of hypotension was not associated with any outcomes.
Fig. 2.
Burden of hypotension for various outcomes at hospital discharge: (A) SHD with favorable neurologic outcome vs. death/SHD unfavorable neurologic outcome, (B) SHD with favorable functional status vs. death/SHD unfavorable functional status, and (C) survivors vs. non-survivors. Abbreviations: SHD = survival to hospital discharge.
Fig. 3.
Burden of hypotension for various acute neurologic injury outcomes: (A) neuroimaging ECMO score ≥ 5 vs. < 5, (B) neuroimaging modASPECTS score ≥ 5 or < 5, (C) presence of seizures, (D) moderate or severe vs. normal or mild EEG background.
A subgroup analysis was performed for patients with SVP (Supplementary Tables 4 and 5). Given the small number of SVP patients in our cohort (N = 34), only a univariate analysis was completed. Results were similar to the larger cohort in that no association was found between presence of hypotension or burden of hypotension and any outcomes.
Discussion
In this single center retrospective cohort study of children admitted to a CICU after ECPR, we found no association between our primary exposure variable of presence of hypotension within 6 h of ROC and SHD with FNO. Similarly, no associations were observed for our secondary outcomes of SHD with favorable functional status or other markers of acute CNS injury such as presence of seizure, abnormal background EEG, or neuroimaging injury defined by ECMO scores. We found an initial association between presence of hypotension and neuroimaging injury defined by modASPECTS scores yet this association was not proven after controlling for potential confounding factors. Similar results were found for burden of hypotension.
While multiple studies have shown an association between hypotension and mortality after pediatric cardiac arrest,14, 15, 16, 17 there has only been one prior study that has included children after ECPR by Topjian et al.18 This secondary analysis of the THAPCA-IH trial showed that while non ECMO patients who had early hypotension were less likely to have SHD, it was not associated with SHD for ECMO patients. While the results of our study are consistent with the findings by Topjian et al, it is unclear why the presence of hypotension is not associated with mortality in ECPR patients. CNS injury that occurs during cardiac arrest can differ from patient to patient, further compounded by the heterogeneity seen across age groups and underlying cardiovascular malformations. In addition, ECMO patients have other risk factors that can alter their risk of CNS injury, such as the need for anticoagulation which in the setting of hypertension, can heighten the risk of hemorrhage stroke. In addition, there is evidence that the non-pulsatile flow from ECMO can cause impaired cerebral autoregulation by causing endothelial dysfunction.19 Our cohort was similar to the ECMO group analyzed in the study by Topjian et al. in that both studies had cohorts with high percentage of pre-existing cardiac disease and young age. This could potentially explain why there a lack of association with hypotension was seen with different survival outcomes. Nearly half of our cohort had SVP, a population known to be sensitive to high afterload. Our subgroup analysis of patients with SVP showed similar results to the larger cohort, however the analysis was limited by use of a univariate analysis given small numbers in the SVP subgroup. The median age of our cohort at the time of arrest was 0.3 months. Given limitations on the neurologic exam of young infants (i.e. a seemingly normal exam can be associated with abnormal MRI findings), it is possible that if we had completed long term neurodevelopmental testing on these children, CNS deficits may start to present. In our study, assessments were performed at hospital discharge using PCPC and FSS. These tools have the advantage of being quick to administer and not relying on subjective assessments. Although validated for short-term outcomes, they require estimation, especially in younger children, and pose challenges when extracting information from charts in a retrospective study. FSS and PCPC are rudimentary measures and are not age-validated. Future studies using more sensitive assessments may be indicated to elucidate the correlations between MAP and long term neurodevelopmental outcomes.
Our study has several strengths. Most pediatric studies evaluating the association between post-arrest BP and outcomes at hospital discharge have focused on more global outcomes such as SHD and SHD with FNO. While PCPC is a measure of global cognitive impairment, it does not assess more granular cognitive and functional status changes that FSS is able to evaluate. Although some may consider our single center study a limitation, we consider it to be a strength since it has allowed us to analyze similar practice patterns across the cohort and obtain more granular data, such as measures of acute CNS injury such as EEG and neuroimaging results. As such, results of this single center study may not be applicable to all centers. Prior studies have shown that an increased burden of hypotension in the post-arrest period to be associated with mortality in children,20, 21 similar to the results of our study. As opposed to the study by Liu et al. which used high resolution blood pressure waveform data to calculate burden of hypotension, our study was limited by the use of hourly EHR blood pressure data.21 Future studies should use high resolution blood pressure waveform data to evaluate the association of burden of hypotension with neurologic outcomes in children with heart disease after ECPR. Additionally, our study period spans a period of 12 years. We did not explore a time-based analysis to account for practice changes over time however future studies should address this concern. As a retrospective study, the assessments of neurologic and functional outcomes were limited by chart review. Future studies should address these limitations by prospectively evaluating neurologic and functional outcomes by caregiver interviews. Our study sought to evaluate post-arrest BP targets in children with cardiac disease after ECPR, and thus it is unclear how to apply these results to children with cardiac disease who did not undergo ECPR. Future studies should address how post-arrest hypotension affects children with cardiac disease after conventional CPR.
Conclusion
In this retrospective observational study of children admitted to a single center pediatric CICU, after controlling for potential confounders, we found no association between presence of early hypotension after ECPR and our primary outcome of SHD with FNO. Additionally, we did not find any association with our secondary exposure of burden of hypotension and SHD with FNO. Multicenter studies that incorporate post arrest neuroimaging and long-term neurodevelopmental outcomes are needed to better our understanding of how blood pressure management in the post-ECPR period affects neurologic outcomes for children with cardiac disease.
CRediT authorship contribution statement
Priscilla Yu: Writing – original draft, Visualization, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Sierra Foster: Writing – review & editing, Investigation. Xilong Li: Writing – review & editing, Formal analysis, Data curation. Priya Bhaskar: Writing – review & editing. Michael Morriss: Writing – review & editing, Methodology, Investigation. Sumit Singh: Writing – review & editing, Methodology, Investigation. Tyler Burr: Writing – review & editing, Conceptualization. Deepa Sirsi: Writing – review & editing, Methodology, Investigation. Lakshmi Raman: Writing – review & editing, Supervision, Methodology. Javier J. Lasa: Writing – review & editing, Supervision, Methodology.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Lakshmi Raman reports a relationship with The University of Texas Southwestern Medical Center that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Ron Reeder for his help with the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2024.100808.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lasa J.J., et al. Extracorporeal Cardiopulmonary Resuscitation (E-CPR) During Pediatric In-Hospital Cardiopulmonary Arrest Is Associated With Improved Survival to Discharge: A Report from the American Heart Association's Get With The Guidelines-Resuscitation (GWTG-R) Registry. Circulation. 2016;133(2):165–176. doi: 10.1161/CIRCULATIONAHA.115.016082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bembea M.M., et al. Outcomes After Extracorporeal Cardiopulmonary Resuscitation of Pediatric In-Hospital Cardiac Arrest: A Report From the Get With the Guidelines-Resuscitation and the Extracorporeal Life Support Organization Registries. Crit Care Med. 2019;47(4):e278–e285. doi: 10.1097/CCM.0000000000003622. [DOI] [PubMed] [Google Scholar]
- 3.Topjian, A.A., et al., Part 4: Pediatric Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation, 2020. 142(16_suppl_2): p. S469-S523. [DOI] [PubMed]
- 4.Topjian A.A., et al. Pediatric Post-Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation. 2019;140(6):e194–e233. doi: 10.1161/CIR.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 5.Harris P.A., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris P.A., et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz S.M., et al. Single-ventricle physiology. Crit Care Clin. 2003;19(3):393–411. doi: 10.1016/s0749-0704(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 8.Roberts J.S., Yanay O., Barry D. Age-Based Percentiles of Measured Mean Arterial Pressure in Pediatric Patients in a Hospital Setting. Pediatr Crit Care Med. 2020;21(9):e759–e768. doi: 10.1097/PCC.0000000000002495. [DOI] [PubMed] [Google Scholar]
- 9.Fiser D.H., et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28(7):2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 10.Pollack M.M., et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124(1):e18–e28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor G.A., et al. Neurologic status in infants treated with extracorporeal membrane oxygenation: correlation of imaging findings with developmental outcome. Radiology. 1987;165(3):679–682. doi: 10.1148/radiology.165.3.3317500. [DOI] [PubMed] [Google Scholar]
- 12.Kirschen M.P., et al. Association of MRI Brain Injury With Outcome After Pediatric Out-of-Hospital Cardiac Arrest. Neurology. 2021;96(5):e719–e731. doi: 10.1212/WNL.0000000000011217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tharp B.R., Laboyrie P.M. The incidence of EEG abnormalities and outcome of infants paralyzed with neuromuscular blocking agents. Crit Care Med. 1983;11(12):926–929. doi: 10.1097/00003246-198312000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Topjian A.A., et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med. 2014;42(6):1518–1523. doi: 10.1097/CCM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner M.M., et al. Identification of post-cardiac arrest blood pressure thresholds associated with outcomes in children: an ICU-Resuscitation study. Crit Care. 2023;27(1):388. doi: 10.1186/s13054-023-04662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ushpol A., et al. Association of blood pressure with neurologic outcome at hospital discharge after pediatric cardiac arrest resuscitation. Resuscitation. 2024;194 doi: 10.1016/j.resuscitation.2023.110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topjian A.A., et al. Association of Early Postresuscitation Hypotension With Survival to Discharge After Targeted Temperature Management for Pediatric Out-of-Hospital Cardiac Arrest: Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2018;172(2):143–153. doi: 10.1001/jamapediatrics.2017.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topjian A.A., et al. The association of early post-resuscitation hypotension with discharge survival following targeted temperature management for pediatric in-hospital cardiac arrest. Resuscitation. 2019;141:24–34. doi: 10.1016/j.resuscitation.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingyinn M., et al. Altered cerebrovascular responses after exposure to venoarterial extracorporeal membrane oxygenation: role of the nitric oxide pathway. Pediatr Crit Care Med. 2006;7(4):368–373. doi: 10.1097/01.PCC.0000225372.38460.12. [DOI] [PubMed] [Google Scholar]
- 20.Laverriere E.K., et al. Association of Duration of Hypotension With Survival After Pediatric Cardiac Arrest. Pediatr Crit Care Med. 2020;21(2):143–149. doi: 10.1097/PCC.0000000000002119. [DOI] [PubMed] [Google Scholar]
- 21.Liu R., et al. Association of Postarrest Hypotension Burden With Unfavorable Neurologic Outcome After Pediatric Cardiac Arrest. Crit Care Med. 2024;52(9):1402–1413. doi: 10.1097/CCM.0000000000006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.