Abstract
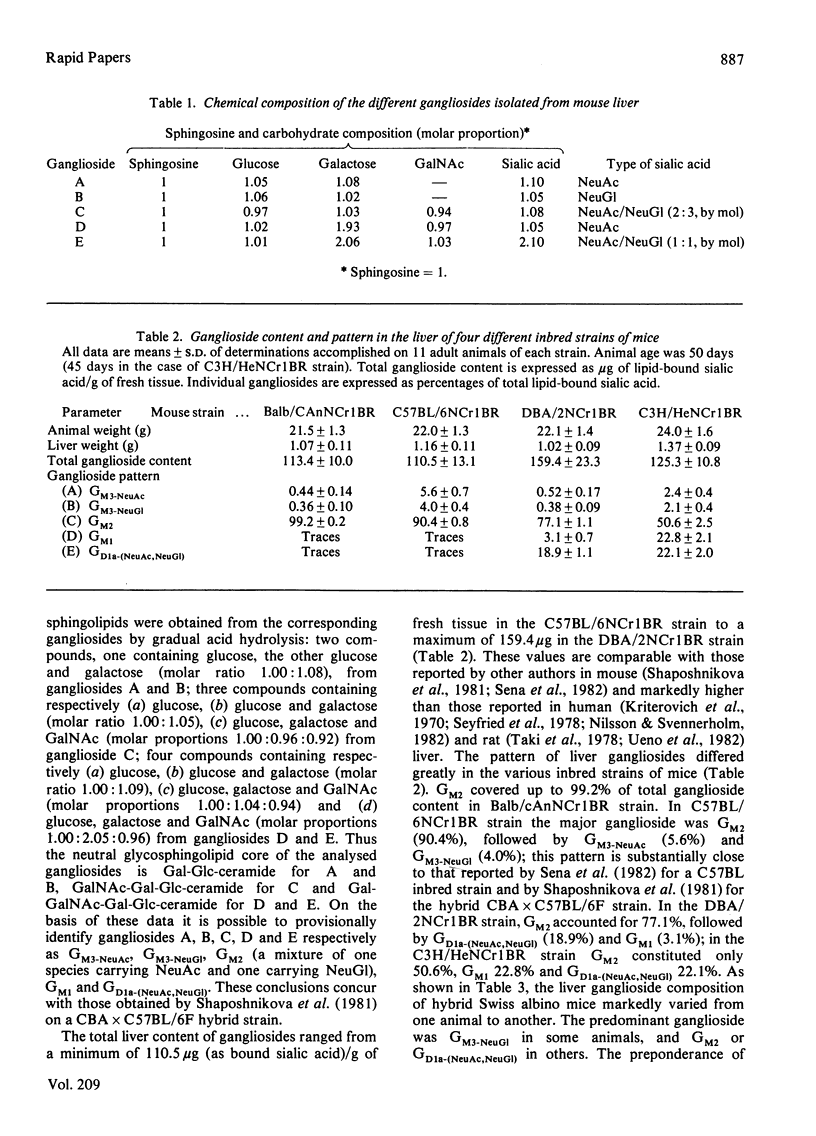
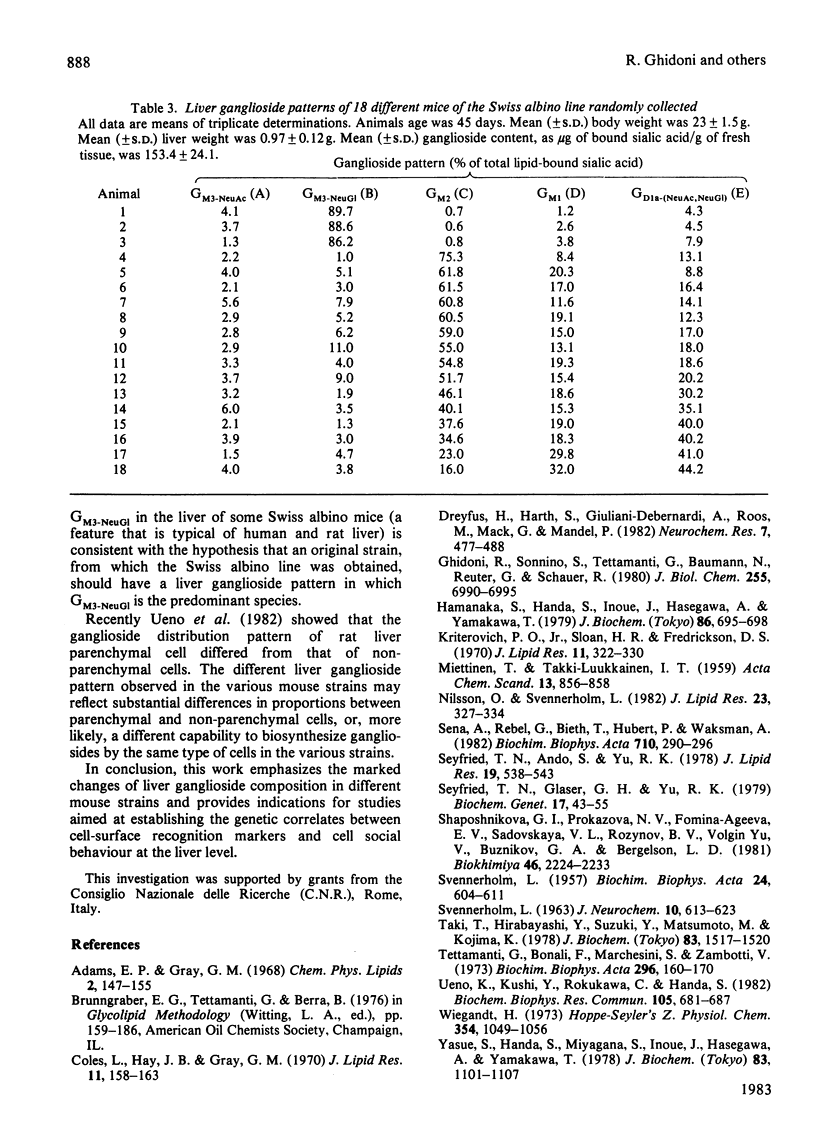
The ganglioside patterns in the liver of different inbred and hybrid strains of mice were investigated. The inbred strains were Balb/cAnNCr1BR, C57BL/6NCr1BR, DBA/2NCr1BR. C3H/HeNCr1BR; the hybrid strain was the Swiss albino. The following major gangliosides were found to be present in mouse liver: GM3-NeuAc; GM3-NeuGl, GM2 [a mixture of one species carrying N-acetylneuraminic acid (NeuAc) and one carrying N-glycollylneuraminic acid (NeuGl)], GM1 and GD1a-(NeuAc,NeuGl). The qualitative and quantitative patterns of liver gangliosides were markedly different in the various inbred strains of mice; in Balb/cAnNCr1BR strain, ganglioside GM2 was preponderant (99.2% of total ganglioside content); in C57BL/6NCr1BR, the major ganglioside was GM2 (90.4%), followed by GM3-NeuAc (5.6%) and GM3-NeuGl (4.0%); in DBA/2NCr1BR, GM2 accounted for 77.1%, GD1a-(NeuAc,NeuGl) 18.9% and GM1 3.1% of gangliosides; in C3H/HeNCr1BR, GM2 constituted 50.6%, GM1 22.8% and GD1a-(NeuAc,NeuGl) 22.1%. In the hybrid Swiss albino mice, liver ganglioside composition markedly varied from one animal to another, GM3-NeuGl, GM2 and GD1a-(NeuAc,NeuGl) being the predominant gangliosides in the various cases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. P., Gray G. M. The carbohydrate structures of the neutral ceramide glycolipids in kidneys of different mouse strains with special reference to the ceramide dihexosides. Chem Phys Lipids. 1968 Jun;2(2):147–155. doi: 10.1016/0009-3084(68)90018-2. [DOI] [PubMed] [Google Scholar]
- Coles L., Hay J. B., Gray G. M. Factors affecting the glycosphingolipid composition of murine tissues. J Lipid Res. 1970 Mar;11(2):158–163. [PubMed] [Google Scholar]
- Dreyfus H., Harth S., Giuliani-Debernardi A., Roos M., Mack G., Mandel P. Gangliosides in various brain areas of three inbred strains of mice. Neurochem Res. 1982 Apr;7(4):477–488. doi: 10.1007/BF00965499. [DOI] [PubMed] [Google Scholar]
- Ghidoni R., Sonnino S., Tettamanti G., Baumann N., Reuter G., Schauer R. Isolation and characterization of a trisialoganglioside from mouse brain, containing 9-O-acetyl-N-acetylneuraminic acid. J Biol Chem. 1980 Jul 25;255(14):6990–6995. [PubMed] [Google Scholar]
- Hamanaka S., Handa S., Inoue J., Hasegawa A., Yamakawa T. Occurrence of hematoside with two moles of N-acetyl-neuraminic acid in a certain breed of Persian cat. J Biochem. 1979 Sep;86(3):695–698. doi: 10.1093/oxfordjournals.jbchem.a132573. [DOI] [PubMed] [Google Scholar]
- Kwiterovich P. O., Jr, Sloan H. R., Fredrickson D. S. Glycolipids and other lipid constituents of normal human liver. J Lipid Res. 1970 Jul;11(4):322–330. [PubMed] [Google Scholar]
- Nilsson O., Svennerholm L. Characterization and quantitative determination of gangliosides and neutral glycosphingolipids in human liver. J Lipid Res. 1982 Feb;23(2):327–334. [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Sena A., Rebel G., Bieth R., Hubert P., Waksman A. Lipid composition in liver and brain of genetically obese (ob/ob), heterozygote (ob/+)and normal (+/+) mice. Biochim Biophys Acta. 1982 Mar 12;710(3):290–296. doi: 10.1016/0005-2760(82)90111-4. [DOI] [PubMed] [Google Scholar]
- Seyfried T. N., Ando S., Yu R. K. Isolation and characterization of human liver hematoside. J Lipid Res. 1978 Jul;19(5):538–543. [PubMed] [Google Scholar]
- Seyfried T. N., Glaser G. H., Yu R. K. Genetic variability for regional brain gangliosides in five strains of young mice. Biochem Genet. 1979 Feb;17(1-2):43–55. doi: 10.1007/BF00484473. [DOI] [PubMed] [Google Scholar]
- Shaposhnikova G. I., Prokazova N. V., Sadovskaia V. L., Rozynov B. V., Volgin Iu V. Gangliozidy pecheni i astsitnoi gepatomy 22a myshei. Biokhimiia. 1981 Dec;46(12):2224–2233. [PubMed] [Google Scholar]
- Taki T., Hirabayashi Y., Suzuki Y., Matsumoto M., Kojima K. Comparative study of glycolipid compositions of plasma membranes among two types of rat ascites hepatoma and normal rat liver. J Biochem. 1978 May;83(5):1517–1520. doi: 10.1093/oxfordjournals.jbchem.a132062. [DOI] [PubMed] [Google Scholar]
- Tettamanti G., Bonali F., Marchesini S., Zambotti V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim Biophys Acta. 1973 Jan 19;296(1):160–170. doi: 10.1016/0005-2760(73)90055-6. [DOI] [PubMed] [Google Scholar]
- Ueno K., Kushi Y., Rokukawa C., Handa S. Distribution of gangliosides in parenchymal and non-parenchymal cells of rat liver. Biochem Biophys Res Commun. 1982 Mar 30;105(2):681–687. doi: 10.1016/0006-291x(82)91488-7. [DOI] [PubMed] [Google Scholar]
- Wiegandt H. Gangliosides of extraneural organs. Hoppe Seylers Z Physiol Chem. 1973 Sep;354(9):1049–1056. doi: 10.1515/bchm2.1973.354.2.1049. [DOI] [PubMed] [Google Scholar]
- Yasue S., Handa S., Miyagawa S., Inoue J., Hasegawa A., Yamakawa T. Difference in form of sialic acid in red blood cell glycolipids of different breeds of dogs. J Biochem. 1978 Apr;83(4):1101–1107. doi: 10.1093/oxfordjournals.jbchem.a131999. [DOI] [PubMed] [Google Scholar]