Abstract
This study is an investigation of the impact of volatile phenols (VPs) released from burning wood during wildfires on grape composition and the resulting wines. Baseline levels of VPs in grapes and sensory differences between smoke-impacted wines and non-smoke-impacted wines were determined. The differences were related to different levels of smoke taint marker compounds in different wine matrices, using modified descriptive analysis (DA), multivariate statistics, gas chromatography mass spectrometry (GC-MS) and liquid chromatography-triple quadrupole tandem mass spectrometry (LC-QqQ-MS) of the free and total VPs, and individual bound glycosides, respectively. Across two DA panels, Cabernet Sauvignon, Cabernet Franc, Petite Verdot, Merlot, Syrah, Malbec, and Zinfandel wines made from grape originating from different areas in California were evaluated. The results show sensory differences between highly smoke-impacted and non-impacted wines with wines made from highly smoke-impacted grapes characterized as smoky, barbeque, medicinal, and having a retro-nasal ashtray character. Low smoke-impact wines based on free and total VP concentrations were not significantly different from the non-impacted wines when rated through descriptive analysis. The amount of smoke exposure was the largest contributor to smoke impact determined by sensory evaluation, but the different wine matrices from different locations and varietals also played an important role in determining the level of perceived smoke impact. The results of this study will contribute to our understanding of smoke impact and how it influences wine characteristics by relating smoke marker indicator compounds to wine sensory attributes.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77041-1.
Keywords: Smoke taint, Wine quality, Sensory evaluation, Flavor chemistry
Subject terms: Environmental sciences, Environmental chemistry, Environmental impact, Analytical chemistry
Introduction
Wildfires in California have become more frequent and destructive in recent years with some of the largest fires in California such as the Nuns fire and Atlas fires taking place in 2017, and LNU lightning complex and Grass fires which occurred in 20201–3. When wildfires occur, large amounts of pollutants are released into the air, including ash, gaseous pollutants, and other volatile organic compounds, such as volatile phenols (VPs)4. These VPs are absorbed through the grape berry skin, where they are quickly glycosylated5. The wines made from smoke-exposed grapes are often classified as smoke-impacted due to their smoky, burnt, ashy, barbeque, medicinal aromas, and retro nasal ashtray aftertaste6–10. Wines of such nature are smoke-impacted and are unsalable. The result is a large economic loss for the California wine industry, particularly in Napa Valley, in fire-affected years.
There is a family of VP marker compounds, guaiacol, o-cresol, p-cresol, m-cresol, 4-methyl-guaiacol, syringol, and 4-ethylguaiacol as identified by Parker et al.5,11. These VPs exist in both free and bound forms where the free VP binds with compounds such as sugars to form phenolic glycosides5,12,13. The presence of the ashy aftertaste can be attributed to the breakdown of the phenolic glycosides due to enzymes in the saliva14. Free VPs can be released from the bound during various winemaking stages such as grape processing, fermentation, and bottle aging14–19. This contributes to the amount of free VPs present which would be perceived as “ashy” retronasally. Current research has shown that despite being able to measure the important smoke marker compounds, it is difficult to accurately predict smoke impact in a particular wine matrix due to large variations in their baseline levels in grapes. VP biosynthesis will likely be influenced by the grape variety, and locations where is it grown6.
Wine is a complex matrix made up of water, alcohol, sugars, organic acids, amino acids, phenols, minerals, and many other compounds. All these compounds work synergistically to make a wine that is unique. Wines produced from different varieties of grapes will have different proportions of these compounds, leading to matrix differences. Similarly, wines made in different regions from the same grape variety would also have a different matrix due to the myriad of effects combining location, climate, environmental factors, and farming practices. Importantly, when understanding smoke, there may be underlying volatile phenols already present that are unique to the varietal and to the general region it is from16,20,21.
Descriptive analysis can be used as a tool to determine the level of smoke impact22,23. However, traditional descriptive analysis requires long training sessions and a dedicated panel of judges who are trained through a consensus methodology on specific attributes of wine to be able to discriminate them from each other. This is non-optimal for the industry as there is little time afforded for training, nor excess resources required for the evaluations during a short growing season. Rapid methods of descriptive analysis such as rate-all-that-apply (RATA), Flash Profile, and check-all-that-apply (CATA) have been widely adopted in the modern context as they are faster, require less resources and give similar results to classical descriptive analysis24–27.
The objective of the study was to determine the impact of smoke exposure on the sensory quality of different wine matrices as a function of variety, location, and level of smoke exposure.
Materials and methods
Grapes
During the smoke event (LNU Lightning complex fire and Grass wildfires) that occurred in the 2020 harvest in California, grapes naturally exposed to the smoke were harvested. Smoke exposure is usually measured using air sampling at the vineyard of concern. However, due to the nature of the fire event, we were not able to access the vineyard sites at the time of the event to do air sampling. Hence, Air Quality Index (AQI) was used to determine smoke exposure. In total, there were nine Cabernet Sauvignon wines from nine different sites and six commonly found red wine varieties: Cabernet Franc, Petite Verdot, Merlot, Syrah, Malbec, and Zinfandel from across California’s North Coast grape growing region. In 2021, non-impacted fruit from the same vineyard for some of the varieties were harvested to make a non-impacted control (Table 1). Also in 2021, a single lot of Cabernet Sauvignon was split into two equal lots where one lot was intentionally smoked on drying tables in a purpose-built smoking tent covered with six-mil polyethene sheeting (Frost King & Thermwell Products Co., Inc., Mahwah, NJ, USA). The grapes were smoked for one hour using a Z Grills pellet smoker (Z Grills Inc., Ontario, CA, USA) with 100 g of hickory wood pellets from Traeger (Traeger, Salt Lake City, UT, USA) to give a wine of similar matrix but with maximum smoke impact8. Hickory pellets was used as it gave a very similar VPs profile when compared to the VPs released from the ash collected in the 2020 natural fire event (data not shown). All grapes were collected with permission from commercially farmed vineyards.
Table 1.
Wine coding scheme with year, varietal, AVA, county, smoke exposure status, and smoke exposure period where AQI > 150.
| Wine name | Year | Varietal | AVA | County | Taint | Smoke exposure period | DA Panel |
|---|---|---|---|---|---|---|---|
| 20CS_A_ST | 2020 | Cabernet Sauvignon | Napa Valley | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 1 |
| 20CS_B_ST | 2020 | Cabernet Sauvignon | St. Helena | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 1 |
| 20CS_C_ST | 2020 | Cabernet Sauvignon | St. Helena | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 1 |
| 20CS_D_ST | 2020 | Cabernet Sauvignon | Russian River Valley | Sonoma | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150) | 1 |
| 20CS_E_ST | 2020 | Cabernet Sauvignon | Dry Creek Valley | Sonoma | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150 | 1 |
| 20CS_F_ST | 2020 | Cabernet Sauvignon | Oakville | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 1 |
| 20CS_G_ST | 2020 | Cabernet Sauvignon | Spring Mountain District | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 1 |
| 20CS_H_ST | 2020 | Cabernet Sauvignon | Davis (No AVA) | Yolo | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–8 Oct 2020 (3 days > 150) | 1 |
| 21CS_I_NST | 2021 | Cabernet Sauvignon | Dry Creek Valley | Sonoma | NST | 16 Aug 2021–21 Sep 2021 (2 days > 150) | 1 |
| 21CS_J_NST | 2021 | Cabernet Sauvignon | Oakville | Napa | NST | 16 Aug 2021- 21 Sep 2021 (1 days > 150) | 1 |
| 21CS_K_ST | 2021 | Cabernet Sauvignon | Oakville | Napa | ST | Intentional smoking | 1 |
| 20CF_A_ST | 2020 | Cabernet Franc | Napa Valley | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 2 |
| 20CF_B_ST | 2020 | Cabernet Franc | St. Helena | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 2 |
| 20CF_C_ST | 2020 | Cabernet Franc | Oakville | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 2 |
| 20MA_D_ST | 2020 | Malbec | Dry Creek Valley | Sonoma | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150) | 2 |
| 20ME_E_ST | 2020 | Merlot | Oakville | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 2 |
| 20ME_F_ST | 2020 | Merlot | Spring Mountain District | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 2 |
| 20PV_G_ST | 2020 | Petite Verdot | Napa Valley | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 2 |
| 20PV_H_ST | 2020 | Petite Verdot | St. Helena | Napa | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150); 17 Sep 2020–5 Oct 2020 (2 days > 150) | 2 |
| 20SY_I_ST | 2020 | Syrah | Dry Creek Valley | Sonoma | ST | 19 Aug 2020–21 Sep 2020 (5 days > 150) | 2 |
| 20ZN_J_ST | 2020 | Zinfandel | Davis (No AVA) | Yolo | ST |
19 Aug 2020- 21 Sep 2020 (5 days > 150); 17 Sep 2020–8 Oct 2020 (3 days > 150) |
2 |
| 21MA_K_NST | 2021 | Malbec | Dry Creek Valley | Sonoma | NST | 16 Aug 2021–21 Sep 2021 (2 days > 150) | 2 |
| 21SY_L_NST | 2021 | Syrah | Dry Creek Valley | Sonoma | NST | 16 Aug 2021–21 Sep 2021 (2 days > 150) | 2 |
*AQI- Air Quality Index, AVA- American Viticultural Areas.
Winemaking
Grapes were hand harvested in 2020 and 2021 from the different vineyards in California (USA) and transported to the UC Davis Teaching and Research Winery (Davis, CA, USA) for processing. Grapes, on average 120 kg per fermentation replicate, were destemmed and crushed using a Bucher Vaslin Delta E2 (Bucher Vaslin North America, Santa Rosa, CA, USA) into stainless steel vessels. 50 mg/L of sulfur dioxide (SO2) was added using a 15% potassium metabisulfite solution (Laffort, Petaluma, CA, USA). Additions were made to each vessel to adjust yeast assimilable nitrogen (YAN) to 250 mg/L, if needed, using diammonium phosphate (Laffort, Petaluma, CA, USA), and titratable acidity (TA) to 6.0 g/L, if needed, using tartaric acid (CalSoda, Rohnert Park, CA, USA). Fermentations were carried out using Saccharomyces cerevisiae strain EC1118 (Lallemand, Montreal, Canada) and inoculated according to the rehydration procedure described by the manufacturer. Fermentation temperature was controlled at 25 °C, while cap management conditions were set to one tank volume pump-over twice a day. After seven days of maceration, wines were pressed and inoculated with Oenococcus oeni VP41 to induce malolactic fermentation (MLF) (Lallemand, Montreal, Canada). MLF was considered completed when malic acid levels were under 0.2 g/L (approximately 4 weeks). Prior to bottling, all vessels of each wine were blended after evaluation for similarity and wine faults other than smoke and adjusted to 35 mg/L free SO2 using potassium metabisulfite (Laffort, Petaluma, CA, USA). Wines were rough filtered via plate and frame unit using FibraFix AF 100 depth filter sheets (Filtrox, St. Gallen, Switzerland). Subsequently, wines were sterile filtered using in-line ALpHA MF0.8-1F6RS and SteriLUX VMH0.4-1F6RS filters (Meissner, Camarillo, CA) and then bottled in antique green bottles under screw cap (Saranex liner. Amcor, Zurich, Switzerland) and stored at 14 °C until analysis.
Chemical analysis
Free and acid-labile (total) volatile phenols
The guaiacol, creosol (4-methylguaiacol), o-cresol, phenol, 4-ethylguaiacol, p-cresol, m-cresol, 2,3-dimethoxyphenol, 4-ethylphenol, syringol and 4-methylsyringol in samples was quantified using a liquid-liquid extraction with pentane-ethyl acetate (1:1) as in Oberholster et al.18. Thereafter an Agilent 7890A gas chromatograph coupled to an Agilent 7000B triple quadrupole mass spectrometer with an MPS 2 autosampler (Gerstel, Inc., Linthicum, MD) was used with the following conditions. A DB-WAXetr (30 m length × 0.32 mm i.d. × 1.0 μm film thickness, Agilent Technologies, Santa Clara, CA, USA) column was fitted onto the gas chromatogram. The inlet temperature was held constant at 220 °C. Oven program was held at 75 °C for 1 min initially, increased to 180 °C at a rate of 15 °C/min, increased to 230 °C at a rate of 10 °C/min and held for another 1 min. Finally, temperature was increased to 250 °C at a rate of 50 °C/min and held for 3 min. Total run time was 17.4 min. The GC and MS interface was held at 220 °C. Pulsed splitless mode was used. The split vent was opened at 1 min with a flow of 50 mL/min. Helium carrier gas was used in constant flow mode at 2.0 mL/min and the electron ionization source set at 70 eV.
The source was held at 230 °C and reagent gas, helium was introduced to the source at 1 mL/min. The solvent delay was 7.5 min. Multiple reaction monitoring (MRM) quantitative and qualitative transitions and collision energies were chosen for each compound based on signal-to-noise ratios using commercially available standards (Supplementary Table S1). The dwell times were set with 15 scans over each peak to ensure quantitative peak integration. The helium quench gas and nitrogen collision gas were fixed at 2.25 mL/min and 1.5 mL/min, respectively.
Phenolic glycoside analysis
The concentrations of smoke glycosides were determined using a solid phase extraction (SPE) SPE extraction and were analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS) in accordance with the method used in Oberholster et al.18. Liquid chromatography was performed on an Agilent 1290 Infinity UHPLC (Agilent Technologies, Santa Clara, CA) equipped with a binary pump, temperature controlled autosampler, and a thermostated column compartment. The column employed for chromatographic separation was an Agilent Poroshell Bonus-RP (150 mm × 2.1 mm, 2.7 μm) fitted with a matching guard column and maintained at 40 °C. Mobile phase A was water with 10 mM ammonium formate and mobile phase B was methanol: acetonitrile (1:1) with 10 mM ammonium formate. The flow rate of the mobile phase was 0.42 mL/min. The gradient used for the separation was as follows: 0 min, 8% B; 1 min, 8% B; 6.5 min, 24.5% B; 7.5 min, 90% B; 9 min, 90% B; 10 min, 8% B. The column was equilibrated at starting conditions for two minutes before the next injection. The injection volume was 12 µL for all samples.
Tandem mass spectrometry was performed on an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA) with an Agilent JetStream electrospray source. Source conditions were sheath gas temperature 375 °C, sheath gas flow 11 L/min, drying gas temperature 250 °C, drying gas flow 12 L/min, nebulizer pressure 45 psi, capillary voltage 3500 V, and nozzle voltage 0 V. Detection of the glycosides was done using dynamic MRM. MRM transitions were determined and optimized using commercially available standards (Supplementary Table S2). Analytical grade chemicals and HPLC grade solvents were purchased from Sigma-Aldrich and Merck (Darmstadt, Germany). Calibration curves were constructed for all glycosides. Deuterated VP glycosides were used as internal standards. Deuterated standards were obtained from Toronto Research Chemicals (Toronto, Canada), C/D/N Isotopes Inc. (Quebec, Canada) and EPTES (Vevey, Switzerland).
Wine analysis
The chemical parameters of the wines were analyzed on each testing day of the descriptive analysis. The titratable acidity (TA) was measured using a Mettler-Toledo DL50 titrator (Mettler- Toledo Inc., Columbus, OH, USA); pH was measured using an Orion 5-star pH meter (Thermo Fisher Scientific, Waltham, MA, USA); alcohol content % (v/v) was measured using an alcohol analyzer (Anton Parr, Ashland, VA, USA); acetic acid, malic acid and residual sugar (RS) were determined by enzymatic analysis using the Gallery automated analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
Sensory analysis
Panel recruitment
Panelists were recruited from an in-house panel. They were initially recruited based on interest, availability, and consumption frequency of red wine (at least once a week). All panelists were screened for their ability to detect smoke using difference tests for the ashy standard diluted to varying concentrations. Panelists were all experienced at tasting red wines and in descriptive analysis (DA).
The study and recruitment were conducted in accordance to the guidelines of the Declaration of Helsinki with approval from the Institutional Review Board (IRB) of the University of California, Davis—UC Davis IRB Protocol 1288072-1. Informed consent was obtained from all participants in the study.
Modified descriptive analysis
A modified descriptive analysis method was used24. Wines were first bench-tasted by three sensory panel leaders, who generated descriptors along with reference standards. This list of descriptors and a set of reference standards were provided to the panel on the first day of training. The panel came to a consensus that standards and descriptors were sufficient in describing the set of wines28. There was a total of fifteen aroma standards, six mouthfeel/taste standards, and one ashy standard (Supplementary Table, S3). Amongst the fifteen aroma standards, seven of them were smoke-related attributes. Panelists underwent five training sessions which included familiarization with the system used, Redjade (Redjade, Redwood City, CA, USA)). All panelists were additionally screened for performance during the training sessions where underperforming panelists had additional training. Panelists were all eligible to participate in both DA panels, DA1 (n = 15), and DA2 (n = 14), with the completed training. One panelist did not complete DA2 and one panelist was dropped in each panel due to poor panel performance (i.e., inconsistency between replicates) for a final count of 14 panelists in DA1 (7 male, 7 female, ages 22–64 years old) and 13 panelists in DA2 (6 male, 7 female, ages 22–64 years old).
Training and evaluations took place in April 2022 where the DA panels DA1 and DA2 ran one after another. There was a total of six days of evaluations for each of the DA panels. Panelists evaluated six wines each day for both DA panels. Formal evaluations were held in positive pressured red-light booths, with 30 ml of wine served in a black Riedel wine glass, item number #0446 Zinfandel/Riesling (Riedel Crystal of America, Edison, NJ, USA). Each day, participants had to complete an aroma quiz before participating in the evaluations. Participants were given a code to log in to Redjade where they were prompted with a 3-digit binding code assigned to each wine. All wines were tasted in triplicate, in a randomized block design. Panelists were to first assess the aroma of the wine without tasting the wine, then to take a sip of the wine, expectorate, and evaluate the taste and mouthfeel attributes. Finally, they would take another sip and evaluate the ashy retronasal aftertaste over a thirty-second period after expectoration where they would rate the highest level of the retro nasal ashy character. The participants rated each attribute on a 15 cm unstructured line scale, with anchors at 0% (not present), 10% (low presence), 90% (very intense), 100% (max intensity). There was an enforced 2-min wait between samples to minimize carryover effects18,29. Panelist were instructed to first use the provided dextrose solution (4 g/L) to rinse their mouths between samples. Crackers and plain still mineral water were available for the panelists as well.
Ashy standard
The ashy standard was prepared as described in Fryer et al.9 with the following changes. The burnt leeks tips were crushed and mixed with 100 °C hot water (Crystal Geyser Natural Alpine Spring Water, Novato, CA, USA) for a 10% weight-by-volume solution. It was allowed to sit in a Abid clever coffee dripper (Abid Co., Ltd, Taiwan) with a coffee filter (Melitta North America, Inc, Florida, USA), for two hours at room temperature. The 10% w/v solution was filtered and diluted at a 1:5 ratio with water for the final ashy standard.
Statistical analysis
All statistical analysis was performed using R, version 4.2.1 “Funny-Looking Kid” (R Core Team, 2022) at a significance level of α = 0.05.
Descriptive data analysis
DA data was exported from Redjade, converting the position on the 15 cm line scale into scores from 0 to 100 for each attribute’s intensity ratings. A three-factor MANOVA (judge, product, replicate) was first performed across all attributes. Following, a three-factor ANOVA with two-way interactions between the panelists, replicates and products was performed to determine which attributes varied significantly among the wines. A pseudo-mixed ANOVA was then performed to determine which attributes were significant using the judge-by-product and replication-by-product interactions as the denominator when performing the test for significant product effects30. Fisher’s Least Significant Difference (LSD) test was used to compare attribute means for the significant attributes, using the “Agricolae” package.
Principal Component Analysis (PCA) biplots with 95% confidence ellipses was performed using the “SensomineR” package. Bootstrapping across the judges was used to display confidence ellipses around the products for a graphical representation of significant differences among wines31. Data was scaled to unit variance.
Multiple-factor analysis (MFA) was used to relate and compare different data sets; descriptive analysis, chemical composition, total volatile phenols, and individual bound phenolic glycosides to determine how similar wines were to each other across the different product spaces. Data was scaled to unit variance to account for scaling differences. The goodness of fit test of a MFA solution is a RV coefficient where RV values larger than 0.75 indicated strong relationships32,33. The “factomineR” package and “MFA” function were used to analyze the data and “ggplot2” was used for graphical representation.
Results
Chemical composition
The basic chemical composition of the wines is shown below in Table 2. There were significant differences across all variables measured. In particular, alcohol %, TA, and RS are deemed to have sensory implications34,35. The difference in the alcohol of the wines was driven primarily by the brix of the grapes that were harvested (Supplemental Table S4).
Table 2.
Basic chemical composition of the wines (n = 3, α ≤ 0.05).
| Wine | % Alcohol (%v/v) | pH | TA (g/L) | Residual Sugar (g/L) | Malic Acid (mg/L) | Acetic Acid ( g/L) |
|---|---|---|---|---|---|---|
| DA 1 | ||||||
| 20CS_A_ST | 16.11 ± 0.03a | 3.70 ± 0.00d | 5.56 ± 0.06c | 0.83 ± 0.01a | 142.67 ± 24.01ab | 0.43 ± 0.06ab |
| 20CS_B_ST | 13.32 ± 0.03f | 3.99 ± 0.02a | 4.45 ± 0.02f | 0.22 ± 0.02e | 28.67 ± 49.65cd | 0.36 ± 0.04cde |
| 20CS_C_ST | 13.71 ± 0.03e | 3.47 ± 0.01e | 5.49 ± 0.11c | 0.29 ± 0.02d | 40.00 ± 36.06cd | 0.24 ± 0.04f |
| 20CS_D_ST | 11.80 ± 0.07 g | 3.92 ± 0.01ab | 5.24 ± 0.00d | 0.15 ± 0.01g | 4.00 ± 6.93d | 0.38 ± 0.04bcd |
| 20CS_E_ST | 14.13 ± 0.03d | 3.93 ± 0.01ab | 5.18 ± 0.05d | 0.25 ± 0.01e | 4.67 ± 8.08d | 0.42 ± 0.06bcd |
| 20CS_F_ST | 15.23 ± 0.01b | 3.88 ± 0.01bc | 4.91 ± 0.02e | 0.35 ± 0.00b | 77.33 ± 37.69c | 0.46 ± 0.05a |
| 20CS_G_ST | 14.63 ± 0.53c | 3.76 ± 0.19d | 5.49 ± 0.34c | 0.32 ± 0.01cd | 29.00 ± 25.71cd | 0.35 ± 0.06de |
| 20CS_H_ST | 15.25 ± 0.02b | 3.95 ± 0.06ab | 5.11 ± 0.04d | 0.34 ± 0.01bc | 24.50 ± 45.47d | 0.38 ± 0.04bcd |
| 21CS_I_NST | 14.68 ± 0.02c | 3.79 ± 0.01cd | 6.01 ± 0.06b | 0.10 ± 0.05h | 46.67 ± 49.74cd | 0.30 ± 0.01ef |
| 21CS_J_NST | 15.31 ± 0.01b | 3.49 ± 0.02e | 6.71 ± 0.07a | 0.17 ± 0.01fg | 173.67 ± 40.20a | 0.33 ± 0.01de |
| 21CS_K_ST | 15.44 ± 0.02b | 3.46 ± 0.01e | 6.82 ± 0.08a | 0.18 ± 0.01f | 85.00 ± 28.69bc | 0.34 ± 0.03de |
| DA 2 | ||||||
| 20CF_A_ST | 14.52 ± 0.53cd | 3.83 ± 0.01ab | 5.06 ± 0.08f | 0.52 ± 0ef | 6.00 ± 10.39c | 0.39 ± 0.02cdef |
| 20CF_B_ST | 14.54 ± 1.06cd | 3.96 ± 0.01a | 4.81 ± 0.05f | 0.50 ± 0.01f | 27.67 ± 30.89bc | 0.37 ± 0.13def |
| 20CF_C_ST | 15.28 ± 0.06b | 3.74 ± 0.03bc | 5.08 ± 0.05f | 0.60 ± 0.06cde | 62.67 ± 94.2abc | 0.56 ± 0.03b |
| 20MA_D_ST | 15.53 ± 0.02b | 3.63 ± 0.01 cd | 6.62 ± 0.04bc | 0.62 ± 0.00 cd | 0.00 ± 0.00c | 0.34 ± 0.02ef |
| 20ME_E_ST | 16.21 ± 0.17a | 3.52 ± 0.01def | 5.75 ± 0.07de | 1.50 ± 0.00a | 151.67 ± 13.87a | 0.49 ± 0.12bc |
| 20ME_F_ST | 14.30 ± 0.03de | 3.42 ± 0.02ef | 6.09 ± 0.06cd | 0.38 ± 0.02g | 83 ± 83.83abc | 0.24 ± 0.05g |
| 20PV_G_ST | 15.48 ± 0.26b | 3.98 ± 0.03a | 5.21 ± 0.09ef | 0.66 ± 0.00c | 0.00 ± 0.00c | 0.47 ± 0.05bcd |
| 20PV_H_ST | 15.11 ± 0.01bc | 3.97 ± 0.02a | 5.11 ± 0.04ef | 0.53 ± 0.00def | 0.00 ± 0.00c | 0.41 ± 0.03cdef |
| 20SY_I_ST | 14.90 ± 0.24bcd | 3.60 ± 0.31cde | 6.79 ± 1.34b | 0.88 ± 0.19b | 146 ± 141.71a | 0.43 ± 0.01cde |
| 20ZN_J_ST | 15.13 ± 0.03bc | 3.46 ± 0.13def | 6.62 ± 0.05bc | 0.67 ± 0.02c | 89.33 ± 36.56abc | 0.68 ± 0.11a |
| 21MA_K_NST | 15.26 ± 0.07b | 3.34 ± 0.01f | 7.84 ± 0.05a | 0.39 ± 0.01g | 0.00 ± 0.00c | 0.33 ± 0.02fg |
| 21SY_L_NST | 13.74 ± 0.34e | 3.51 ± 0.22def | 6.53 ± 0.02bc | 0.10 ± 0.02h | 115 ± 101.68ab | 0.40 ± 0.08cdef |
Fisher’s LSD for means comparison of all samples across each chemical parameter. Letters that are different indicate a significant difference, p ≤ 0.05 in each DA panel.
Free and total volatile phenols, and individual bound glycosides
The free VP, total VP, and individual bound glycoside compositions of the wines are shown in Tables 3 and 4, and 5, respectively. Across each DA panel set of wines, there were significant differences in the levels of free VPs, total VPs, and individual bound concentrations. Wines that were not smoke-tainted, 21CS_I_NST, 21CS_J_NST, 21MA_K_NST, and 21SY_L_NST had low levels of free VPs, total VPs, and individual bound glycoside concentrations in comparison to their smoke-tainted counterparts. In particular, 21CS_I_NST was significantly different from its smoked counterpart 20CS_E_ST, and 21CS_J_NST was significantly different from its smoked counterpart, 21CS_K_ST (Tables 3 and 4, and 5). The non-smoke impacted wines, in DA1, 21CS_I_NST and 21CS_J_NST, was not significantly different from each other. However, in DA 2, the 21MA_K_NST and 21SY_L_NST wines were significantly different from each other for most of the VP compounds (Tables 3 and 4, and 5) with high levels of VPs in the Syrah wine compared to the Malbec wine.
Table 3.
Free volatile phenol concentrations of the wines.
| Wine | Free_guaiacol | Free_4-methylguaiacol | Free_o-cresol | Free_phenol | Free_4-ethylguaiacol | Free_p-cresol | Free_m-cresol | Free_2,3-dimethoxyphenol | Free_4-ethylphenol | Free_syringol | Free_4-methylsyringol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DA 1 | |||||||||||
| 20CS_A_ST | 7.71 ± 0.33d | 1.06 ± 0.06e | 5.09 ± 0.19e | 16.23 ± 0.53e | 0.18 ± 0.00f | 3.26 ± 0.19c | 4.68 ± 0.21f | 1.93 ± 0.08d | 0.72 ± 0.03e | 29.41 ± 0.46d | 0.80 ± 0.07e |
| 20CS_B_ST | 7.42 ± 0.01d | 1.96 ± 0.01d | 5.08 ± 0.00e | 23.31 ± 0.06b | 0.61 ± 0.00c | 4.03 ± 0.02b | 5.35 ± 0.01e | 7.77 ± 0.02b | 1.10 ± 0.00c | 42.05 ± 0.88c | 3.93 ± 0.01c |
| 20CS_C_ST | 9.33 ± 0.04c | 2.44 ± 0.02c | 5.74 ± 0.02d | 15.97 ± 0.05e | 0.76 ± 0.00b | 3.18 ± 0.01c | 6.34 ± 0.05c | 5.56 ± 0.02c | 1.11 ± 0.01c | 45.13 ± 0.49b | 6.21 ± 0.03b |
| 20CS_D_ST | 1.92 ± 0.03f | 0.34 ± 0.00f | 1.56 ± 0.02f | 3.98 ± 0.03g | 0.08 ± 0.00g | 1.01 ± 0.01f | 1.18 ± 0.01 g | 0.45 ± 0.00e | 0.11 ± 0.01 g | 15.61 ± 1.27f | 0.27 ± 0.01ef |
| 20CS_E_ST | 33.20 ± 0.10b | 5.59 ± 0.09b | 20.93 ± 0.19b | 72.42 ± 0.19a | 0.44 ± 0.00d | 16.7 ± 0.11a | 22.17 ± 0.17b | 19.05 ± 0.24a | 2.92 ± 0.03a | 18.85 ± 0.05e | 1.80 ± 0.02d |
| 20CS_F_ST | 2.37 ± 0.05ef | 0.33 ± 0.01f | 1.31 ± 0.04f | 3.12 ± 0.12h | 0.12 ± 0.02 g | 0.94 ± 0.11fg | 0.95 ± 0.03gh | 0.00 ± 0.00f | 0.12 ± 0.02 fg | 18.53 ± 1.12e | 0.20 ± 0.03f |
| 20CS_G_ST | 9.77 ± 0.19c | 1.85 ± 0.03d | 6.41 ± 0.17c | 18.60 ± 0.25d | 0.32 ± 0.02e | 2.84 ± 0.04d | 5.78 ± 0.11d | 5.58 ± 0.13c | 0.68 ± 0.00e | 12.51 ± 0.17g | 1.52 ± 0.18d |
| 20CS_H_ST | 2.49 ± 0.03e | 0.30 ± 0.02fg | 1.35 ± 0.04f | 8.05 ± 0.18f | 0.16 ± 0.00f | 2.25 ± 0.16e | 0.80 ± 0.04 h | 0.12 ± 0.01f | 0.78 ± 0.04d | 16.04 ± 0.17f | 0.28 ± 0.00ef |
| 21CS_I_NST | 0.78 ± 0.02g | 0.11 ± 0.00gh | 0.56 ± 0.02g | 2.92 ± 0.23h | 0.02 ± 0.00h | 0.79 ± 0.01g | 0.43 ± 0.02i | 0.00 ± 0.00f | 0.16 ± 0.01f | 11.35 ± 0.40g | 0.00 ± 0.00f |
| 21CS_J_NST | 0.79 ± 0.01g | 0.10 ± 0.00h | 0.62 ± 0.02g | 1.65 ± 0.15i | 0.00 ± 0.00h | 0.40 ± 0.01h | 0.46 ± 0.03i | 0.00 ± 0.00f | 0.16 ± 0.01f | 8.01 ± 0.20h | 0.00 ± 0.00f |
| 21CS_K_ST | 81.45 ± 0.73a | 31.47 ± 0.28a | 22.31 ± 0.29a | 20.52 ± 0.10c | 5.49 ± 0.05a | 0.37 ± 0.01h | 34.76 ± 0.40a | 0.00 ± 0.00f | 2.65 ± 0.04b | 138.9 ± 1.34a | 77.57 ± 0.91a |
| DA 2 | |||||||||||
| 20CF_A_ST | 7.19 ± 0.35h | 1.75 ± 0.13g | 3.46 ± 0.14f | 9.24 ± 0.31 g | 0.09 ± 0.00g | 4.34 ± 0.28c | 3.78 ± 0.24d | 0.02 ± 0.00f | 0.86 ± 0.08e | 38.63 ± 3.22d | 0.42 ± 0.00e |
| 20CF_B_ST | 14.02 ± 0.63e | 5.15 ± 0.24c | 6.82 ± 0.30c | 26.70 ± 1.8b | 1.05 ± 0.03b | 10.15 ± 0.97a | 11.79 ± 0.84a | 0.16 ± 0.00e | 2.18 ± 0.16a | 55.10 ± 6.79c | 3.71 ± 0.20a |
| 20CF_C_ST | 2.34 ± 0.05i | 0.32 ± 0.00hi | 1.30 ± 0.04g | 3.04 ± 0.04hi | 0.10 ± 0.00 g | 0.87 ± 0.00fg | 0.94 ± 0.03e | 0.00 ± 0.00f | 0.10 ± 0.00 h | 19.22 ± 0.69gh | 0.04 ± 0.04fg |
| 20MA_D_ST | 19.97 ± 0.68d | 5.74 ± 0.11b | 9.24 ± 0.29a | 25.17 ± 0.52c | 0.43 ± 0.01c | 6.5 ± 0.22b | 9.35 ± 0.20b | 0.4 ± 0.01c | 1.55 ± 0.04c | 21.85 ± 0.78fg | 0.92 ± 0.04c |
| 20ME_E_ST | 1.61 ± 0.12j | 0.14 ± 0.01i | 0.53 ± 0.01i | 1.90 ± 0.09i | 0.10 ± 0.02g | 0.51 ± 0.05 g | 0.69 ± 0.02e | 0.00 ± 0.00f | 0.12 ± 0.00 h | 27.09 ± 1.72ef | 0.13 ± 0.00f |
| 20ME_F_ST | 10.20 ± 0.13g | 2.90 ± 0.02f | 4.14 ± 0.05e | 11.03 ± 0.14f | 0.27 ± 0.01d | 2.6 ± 0.03e | 4.93 ± 0.03c | 0.32 ± 0.01d | 0.57 ± 0.01 g | 7.62 ± 0.25i | 0.70 ± 0.02d |
| 20PV_G_ST | 25.31 ± 0.50c | 4.13 ± 0.07e | 5.79 ± 0.32d | 12.88 ± 0.58e | 0.25 ± 0.01d | 3.47 ± 0.33d | 5.34 ± 0.24c | 2.09 ± 0.11a | 0.70 ± 0.05f | 108.26 ± 2.62a | 0.72 ± 0.02d |
| 20PV_H_ST | 47.27 ± 0.58a | 14.06 ± 0.18a | 9.23 ± 0.07a | 21.71 ± 0.05d | 1.72 ± 0.02a | 4.76 ± 0.05c | 11.26 ± 0.03a | 1.91 ± 0.03b | 1.70 ± 0.02b | 81.84 ± 7.80b | 2.98 ± 0.02b |
| 20SY_I_ST | 34.56 ± 0.95b | 4.63 ± 0.07d | 8.82 ± 0.25b | 33.62 ± 0.71a | 0.21 ± 0.00e | 5.93 ± 0.19b | 9.53 ± 0.20b | 0.41 ± 0.02c | 1.27 ± 0.03d | 29.29 ± 0.39e | 0.88 ± 0.03c |
| 20ZN_J_ST | 1.91 ± 0.08ij | 0.34 ± 0.01h | 0.28 ± 0.01i | 3.88 ± 0.04 h | 0.17 ± 0.01f | 1.18 ± 0.08f | 0.81 ± 0.04e | 0.00 ± 0.00f | 0.63 ± 0.03 fg | 7.42 ± 0.20i | 0.20 ± 0.00f |
| 21MA_K_NST | 1.38 ± 0.02j | 0.19 ± 0.00hi | 0.52 ± 0.01i | 2.49 ± 0.35i | 0.03 ± 0.00h | 0.57 ± 0.03fg | 0.48 ± 0.01e | 0.00 ± 0.00f | 0.22 ± 0.00h | 11.74 ± 0.28hi | 0.00 ± 0.00g |
| 21SY_L_NST | 12.23 ± 0.07f | 0.15 ± 0.00i | 0.91 ± 0.02 h | 3.11 ± 0.13hi | 0.00 ± 0.00h | 0.84 ± 0.01fg | 0.39 ± 0.01e | 0.00 ± 0.00f | 0.22 ± 0.01h | 9.01 ± 0.03i | 0.00 ± 0.00g |
All concentrations are in µg/L (n = 3).
Fisher’s LSD was used to determine differences between each column for each DA across wines.
Table 4.
Acid-labile (total) volatile phenol concentrations of wines as determined by acid hydrolysis.
| Wine | Total_guaiacol | Total_4-methylguaiacol | Total_o-cresol | Total_phenol | Total_4-ethylguaiacol | Total_p-cresol | Total_m-cresol | Total_2,3-dimethoxyphenol | Total_4-ethylphenol | Total_syringol | Total_4-methylsyringol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DA 1 | |||||||||||
| 20CS_A_ST | 16.88 ± 0.98f | 3.03 ± 0.19e | 7.65 ± 0.38e | 25.02 ± 1.42de | 0.55 ± 0.04g | 7.40 ± 0.38cd | 9.14 ± 0.52e | 2.80 ± 0.45e | 4.94 ± 0.40e | 73.3 ± 4.90e | 17.99 ± 2.49f |
| 20CS_B_ST | 23.74 ± 0.69e | 6.54 ± 0.34d | 10.48 ± 0.55d | 40.25 ± 2.15c | 1.31 ± 0.07d | 8.62 ± 0.40c | 13.47 ± 0.70d | 30.79 ± 1.30b | 10.5 ± 1.26d | 104.96 ± 7.87d | 25.68 ± 0.17e |
| 20CS_C_ST | 40.97 ± 0.85c | 14.34 ± 0.83c | 18.63 ± 1.12c | 51.72 ± 3.64b | 2.09 ± 0.09b | 11.9 ± 0.59b | 26.65 ± 1.31c | 25.11 ± 0.25c | 17.5 ± 2.16b | 158.92 ± 5.50c | 57.11 ± 0.31d |
| 20CS_D_ST | 9.23 ± 0.29g | 2.26 ± 0.03ef | 4.30 ± 0.15f | 15.86 ± 0.88f | 0.79 ± 0.03f | 5.53 ± 0.03e | 4.59 ± 0.08f | 1.43 ± 0.06f | 8.56 ± 0.23d | 45.03 ± 1.47f | 3.44 ± 0.10g |
| 20CS_E_ST | 84.38 ± 0.85b | 19.66 ± 0.82b | 33.42 ± 1.23b | 112.16 ± 3.32a | 1.30 ± 0.04d | 33.45 ± 1.43a | 47.76 ± 2.07b | 31.88 ± 0.44a | 14.14 ± 1.39c | 415.38 ± 2.77a | 211.29 ± 0.45a |
| 20CS_F_ST | 3.85 ± 0.13i | 0.70 ± 0.03f | 1.72 ± 0.12g | 3.16 ± 0.04g | 0.25 ± 0.00 h | 1.51 ± 0.03f | 1.65 ± 0.12g | 0.00 ± 0.00g | 1.42 ± 0.05f | 39.65 ± 0.08f | 1.17 ± 0.06h |
| 20CS_G_ST | 31.75 ± 2.25d | 7.97 ± 0.69d | 9.30 ± 0.66de | 28.01 ± 1.76de | 0.95 ± 0.08e | 8.38 ± 0.76cd | 11.56 ± 0.97de | 8.87 ± 0.72d | 3.80 ± 0.56ef | 109.66 ± 0.44d | 72.73 ± 0.17c |
| 20CS_H_ST | 6.02 ± 0.61h | 1.45 ± 0.20ef | 3.23 ± 0.44fg | 23.77 ± 1.41e | 1.79 ± 0.12c | 7.31 ± 0.09d | 3.81 ± 0.18fg | 0.43 ± 0.02 fg | 19.56 ± 1.3b | 21.67 ± 0.54g | 1.44 ± 0.10h |
| 21CS_I_NST | 6.60 ± 0.60h | 0.94 ± 0.02f | 2.56 ± 0.24g | 25.45 ± 0.14de | 0.58 ± 0.01g | 4.45 ± 0.41e | 3.52 ± 0.26fg | 0.00 ± 0.00g | 17.54 ± 0.20b | 22.76 ± 0.84g | 0.32 ± 0.00h |
| 21CS_J_NST | 6.98 ± 0.37h | 1.07 ± 0.11f | 2.98 ± 0.18fg | 29.34 ± 0.11d | 0.92 ± 0.09ef | 4.49 ± 0.32e | 4.20 ± 0.20fg | 0.00 ± 0.00g | 24.38 ± 3.53a | 20.62 ± 0.19g | 0.53 ± 0.05h |
| 21CS_K_ST | 96.8 ± 1.37a | 53.26 ± 2.15a | 39.28 ± 1.72a | 50.03 ± 3.05b | 6.82 ± 0.14a | 5.00 ± 0.59e | 65.36 ± 3.56a | 0.00 ± 0.00g | 18.19 ± 1.51b | 181.27 ± 1.8b | 88.64 ± 0.48b |
| DA 2 | |||||||||||
| 20CF_A_ST | 27.54 ± 0.32g | 9.56 ± 0.50e | 10.25 ± 0.46d | 36.56 ± 2.81de | 1.70 ± 0.12d | 12.40 ± 0.59c | 12.64 ± 0.43d | 0.48 ± 0.00g | 32.76 ± 4.05a | 128.35 ± 5.35d | 25.39 ± 0.04e |
| 20CF_B_ST | 54.39 ± 1.15f | 23.85 ± 1.90d | 16.06 ± 1.29b | 45.12 ± 1.82b | 2.60 ± 0.08b | 19.05 ± 0.91b | 26.78 ± 0.96b | 1.12 ± 0.11f | 12.36 ± 0.84b | 196.88 ± 11.43b | 77.05 ± 1.16b |
| 20CF_C_ST | 4.15 ± 0.43i | 0.77 ± 0.10f | 1.72 ± 0.11h | 3.16 ± 0.04g | 0.25 ± 0.00g | 1.51 ± 0.03h | 1.63 ± 0.10g | 0.00 ± 0.00h | 1.45 ± 0.10e | 69.32 ± 4.53e | 1.23 ± 0.14h |
| 20MA_D_ST | 88.77 ± 0.61d | 37.92 ± 0.18b | 21.45 ± 0.36a | 63.32 ± 0.62a | 2.17 ± 0.02c | 25.42 ± 0.17a | 29.30 ± 0.40a | 1.83 ± 0.03e | 12.43 ± 0.11b | 153.57 ± 1.70c | 54.85 ± 0.63d |
| 20ME_E_ST | 6.55 ± 0.14hi | 1.40 ± 0.03f | 1.63 ± 0.06h | 4.21 ± 0.15g | 0.33 ± 0.01g | 2.90 ± 0.07gh | 2.02 ± 0.07g | 0.00 ± 0.00h | 1.62 ± 0.17e | 49.14 ± 70ef | 1.56 ± 0.11h |
| 20ME_F_ST | 96.44 ± 5.87c | 37.98 ± 3.63b | 13.64 ± 1.67c | 39.93 ± 4.55cd | 2.10 ± 0.11c | 17.88 ± 2.23b | 22.67 ± 2.41c | 2.31 ± 0.13c | 4.84 ± 0.32de | 180.18 ± 3.17b | 134.3 ± 2.30a |
| 20PV_G_ST | 52.00 ± 0.89f | 11.00 ± 0.89e | 8.57 ± 0.63e | 20.35 ± 1.41f | 0.74 ± 0.09f | 7.03 ± 1.00e | 10.54 ± 0.83e | 4.68 ± 0.13b | 5.51 ± 0.38d | 192.08 ± 11.24b | 16.66 ± 2.21f |
| 20PV_H_ST | 115.79 ± 2.09b | 44.57 ± 2.05a | 15.04 ± 0.68bc | 34.43 ± 1.88e | 3.74 ± 0.07a | 10.26 ± 0.70d | 21.94 ± 0.92c | 6.29 ± 0.15a | 8.41 ± 1.06 cd | 222.74 ± 38.71a | 75.56 ± 0.62b |
| 20SY_I_ST | 171.54 ± 5.65a | 34.7 ± 1.94c | 21.41 ± 0.99a | 62.59 ± 1.94a | 1.04 ± 0.03e | 25.28 ± 1.61a | 28.21 ± 1.40ab | 2.00 ± 0.04d | 11.28 ± 0.88bc | 148.29 ± 0.13cd | 60.50 ± 0.10c |
| 20ZN_J_ST | 7.54 ± 0.23hi | 2.05 ± 0.05f | 4.36 ± 0.26g | 16.05 ± 0.57f | 1.85 ± 0.24d | 7.21 ± 0.27e | 5.99 ± 0.15f | 0.00 ± 0.00h | 10.62 ± 1.44bc | 12.44 ± 0.27 g | 3.92 ± 0.24g |
| 21MA_K_NST | 10.79 ± 0.47h | 2.79 ± 0.14f | 3.50 ± 0.21g | 41.72 ± 4.61bc | 0.99 ± 0.07e | 6.76 ± 0.45ef | 6.45 ± 0.30f | 0.00 ± 0.00h | 31.07 ± 3.47a | 26.52 ± 0.32fg | 0.62 ± 0.01h |
| 21SY_L_NST | 82.28 ± 1.59e | 2.49 ± 0.12f | 6.87 ± 0.41f | 32.03 ± 2.98e | 0.56 ± 0.05f | 4.82 ± 0.37fg | 4.91 ± 0.12f | 0.00 ± 0.00h | 29.08 ± 3.01a | 20.35 ± 0.11g | 0.70 ± 0.02h |
All concentrations are in µg/L.
Fisher’s LSD was used to determine differences between each column for each DA across wines.
Table 5.
Individual bound glycoside concentrations of wines.
| Wine | Guaiacol gentiobioside | Syringol gentiobioside | Guaiacol glucoside | Phenol rutinoside | Guaiacol rutinoside | 4-Methylsyringol gentiobioside | Cresol rutinoside | 4-Methylguaiacol rutinoside |
|---|---|---|---|---|---|---|---|---|
| DA 1 | ||||||||
| 20CS_A_ST | 0.55 ± 0.02e | 35.58 ± 0.24d | 6.16 ± 0.17f | 15.62 ± 0.4d | 5.58 ± 0.20d | 4.70 ± 0.05d | 7.75 ± 0.01e | 3.10 ± 0.04e |
| 20CS_B_ST | 0.84 ± 0.04d | 26.21 ± 0.34e | 17.21 ± 0.26e | 15.90 ± 0.30d | 5.67 ± 0.25d | 2.82 ± 0.13e | 9.5 ± 0.11d | 3.69 ± 0.04d |
| 20CS_C_ST | 3.62 ± 0.07b | 78.36 ± 0.25c | 38.22 ± 0.41a | 27.02 ± 0.32c | 14.78 ± 0.27c | 11.85 ± 0.12c | 15.01 ± 0.45c | 10.22 ± 0.13c |
| 20CS_D_ST | 0.06 ± 0.00f | 7.42 ± 0.10f | 1.57 ± 0.01 g | 4.68 ± 0.12f | 1.42 ± 0.03e | 0.63 ± 0.02f | 3.80 ± 0.12 g | 1.05 ± 0.01 g |
| 20CS_E_ST | 3.03 ± 0.07c | 448.29 ± 2.77a | 33.68 ± 0.16b | 53.51 ± 0.37a | 32.84 ± 0.13a | 48.61 ± 0.46a | 42.49 ± 0.39a | 20.88 ± 0.24a |
| 20CS_F_ST | 0.04 ± 0.00f | 2.41 ± 0.06 h | 0.74 ± 0.01hi | 3.24 ± 0.11 g | 0.68 ± 0.03f | 0.10 ± 0.00 g | 1.43 ± 0.03 h | 0.13 ± 0.00i |
| 20CS_G_ST | 3.92 ± 0.11a | 105.06 ± 1.09b | 23.92 ± 0.19c | 39.86 ± 0.30b | 25.30 ± 0.20b | 18.65 ± 0.6b | 27.35 ± 0.55b | 14.00 ± 0.19b |
| 20CS_H_ST | 0.09 ± 0.00f | 4.49 ± 0.08 g | 0.99 ± 0.06 h | 8.68 ± 0.33e | 1.46 ± 0.10e | 0.29 ± 0.01 fg | 4.72 ± 0.05f | 0.47 ± 0.03 h |
| 21CS_I_NST | 0.02 ± 0.00f | 1.34 ± 0.07 h | 0.55 ± 0.00i | 1.28 ± 0.00i | 0.32 ± 0.01 g | 0.11 ± 0.01 g | 1.24 ± 0.07 h | 0.50 ± 0.03 h |
| 21CS_J_NST | 0.06 ± 0.01f | 2.21 ± 0.04 h | 0.40 ± 0.02i | 2.25 ± 0.06 h | 0.53 ± 0.03 fg | 0.16 ± 0.01 g | 1.12 ± 0.03 h | 0.31 ± 0.01hi |
| 21CS_K_ST | 0.59 ± 0.01e | 7.71 ± 0.08f | 21.9 ± 0.30d | 2.37 ± 0.06 h | 0.71 ± 0.01f | 2.67 ± 0.04e | 1.48 ± 0.02 h | 1.30 ± 0.02f |
| DA 2 | ||||||||
| 20CF_A_ST | 2.60 ± 0.23f | 36.53 ± 1.67e | 11.47 ± 0.27 h | 3.24 ± 0.06 h | 1.39 ± 0.09hi | 5.01 ± 0.34f | 4.36 ± 0.18 g | 2.17 ± 0.04 g |
| 20CF_B_ST | 6.68 ± 0.35c | 84.95 ± 3.84d | 42.96 ± 0.07e | 5.25 ± 0.34 g | 2.01 ± 0.01 g | 14.08 ± 1.00d | 11.08 ± 0.25e | 3.98 ± 0.06f |
| 20CF_C_ST | 0.45 ± 0.02 h | 4.82 ± 0.02 g | 2.04 ± 0.05j | 1.54 ± 0.11i | 0.42 ± 0.02k | 0.19 ± 0.00i | 1.78 ± 0.13i | 0.50 ± 0.03j |
| 20MA_D_ST | 3.90 ± 0.03e | 110.03 ± 1.49b | 48.34 ± 0.14d | 41.25 ± 0.57c | 20.83 ± 0.28c | 17.74 ± 0.17c | 29.97 ± 0.47c | 18.61 ± 0.22c |
| 20ME_E_ST | 0.42 ± 0.04 h | 2.80 ± 0.07 g | 2.45 ± 0.06j | 3.38 ± 0.15 h | 0.95 ± 0.05j | 0.24 ± 0.02i | 3.27 ± 0.23 h | 1.23 ± 0.01i |
| 20ME_F_ST | 50.0 ± 0.02a | 224.12 ± 2.35a | 66.04 ± 1.16b | 42.81 ± 0.59b | 21.70 ± 0.48b | 45.1 ± 0.94a | 52.45 ± 0.54b | 29.22 ± 0.15b |
| 20PV_G_ST | 1.06 ± 0.07 g | 37.90 ± 1.53e | 18.42 ± 0.32 g | 9.81 ± 0.15f | 8.70 ± 0.21e | 2.81 ± 0.12 g | 8.26 ± 0.29f | 8.14 ± 0.02e |
| 20PV_H_ST | 3.65 ± 0.1e | 82.44 ± 0.88d | 52.60 ± 0.6c | 12.31 ± 0.24e | 13.67 ± 0.28d | 12.84 ± 0.50e | 14.58 ± 0.30d | 15.69 ± 0.12d |
| 20SY_I_ST | 27.21 ± 0.34b | 104.58 ± 0.77c | 121.40 ± 0.59a | 65.24 ± 1.11a | 40.27 ± 0.06a | 22.98 ± 0.20b | 59.67 ± 0.89a | 34.89 ± 0.24a |
| 20ZN_J_ST | 1.28 ± 0.03 g | 8.27 ± 0.17f | 3.85 ± 0.04i | 17.32 ± 0.83d | 1.75 ± 0.05gh | 1.44 ± 0.06 h | 7.86 ± 0.20f | 1.15 ± 0.02i |
| 21MA_K_NST | 0.19 ± 0.01 h | 2.96 ± 0.04 g | 1.07 ± 0.03k | 1.85 ± 0.10i | 1.28 ± 0.02ij | 0.19 ± 0.01i | 2.30 ± 0.02i | 2.06 ± 0.06gh |
| 21SY_L_NST | 4.94 ± 0.04d | 2.69 ± 0.02 g | 31.48 ± 0.16f | 1.27 ± 0.13i | 7.35 ± 0.15f | 0.28 ± 0.00i | 2.34 ± 0.09i | 1.89 ± 0.04 h |
All concentrations are in µg/L.
Fisher’s LSD was used to determine differences between each column for each DA across wines.
Sensory profile
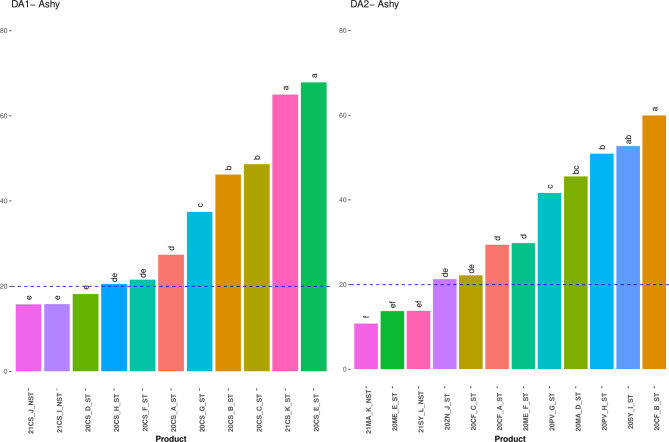
Trained panelists evaluated across two panels (DA1, n = 14 judges, DA2, n = 13 judges) fifteen aroma, six taste/mouthfeel and one ashy retronasal attribute for a total of 22 attributes. In DA1, the wines differed significantly across twelve attributes and in DA 2 across nine attributes, as analyzed through a pseudo-mixed model ANOVA. The attribute means and Fisher’s Least Significant Difference (LSD) are shown in Tables 6 and 7, respectively. The “ashy” attribute can be defined as an indicator of the amount of smoke taint as it is the single characteristic unique to smoke-tainted wines5,14. Here, the NST wines were rated as having a low level of ashy (Fig. 1). Based on the NST data, a level up to 20 out of a 100 is generally seen as no smoke taint. The low ashy rating could have been due to cross-over effects between the samples. This was seen before in studies by Oberholster et al. and Fryer et al.18,29.
Table 6.
DA 1 sensory attribute overall means with Fisher’s LSD.
| Wine | Red fruit | Dark fruit | Alcohol hotness | Medicinal/Brett | Liquid smoke | Sweet BBQ | Tar | Viscosity | Drying | Sweet | Sour | Ashy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Significant attributes | ||||||||||||
| 20CS_A_ST | 14.64 bc | 18.02 abc | 56.93 a | 17.43 bcd | 11.62 cd | 11.55 bcd | 8.48 d | 28.19 ab | 48.05 ef | 24.86 a | 44.976 b | 27.38 de |
| 20CS_B_ST | 14.93 bc | 19.74 abc | 47.52 bcde | 21.62 abc | 16.76 bc | 16.74 b | 10.26 cd | 27.24 ab | 50.86 de | 16.81 bc | 36.93 c | 46.24 bc |
| 20CS_C_ST | 16.79 abc | 16.10 bcd | 43.29 cde | 25.64 ab | 20.31 b | 15.14 bc | 15.91 bc | 21.55 ab | 64.17 ab | 12.88 c | 43.67 bc | 48.64 b |
| 20CS_D_ST | 23.72 a | 18.64 abc | 38.76 e | 12.02 d | 3.57 e | 8.91 cd | 4.31 d | 19.79 b | 66.48 a | 10.95 c | 38.86 bc | 18.19 ef |
| 20CS_E_ST | 11.38 c | 13.19 cd | 41.14 de | 27.74 a | 33.31 a | 28.24 a | 17.33 ab | 22.57 ab | 59.41 abcd | 12.36 c | 40.57 bc | 67.86 a |
| 20CS_F_ST | 25.07 a | 24.12 a | 53.60 ab | 11.64 d | 3.93 e | 5.07 d | 4.64 d | 29.24 ab | 51.41 cde | 21.52 ab | 42.91 bc | 21.55 ef |
| 20CS_G_ST | 20.36 ab | 21.05 ab | 50.33 abc | 13.38 cd | 7.67 de | 8.57 cd | 7.79 d | 23.38 ab | 60.60 ab | 16.36 bc | 46.10 ab | 37.45 cd |
| 20CS_H_ST | 22.39 ab | 17.20 abcd | 49.10 abcd | 16.93 bcd | 10.12 cde | 7.49 d | 6.83 d | 30.33 a | 41.20 f | 24.21 ab | 42.74 bc | 20.50 ef |
| 21CS_I_NST | 22.45 ab | 18.19 abc | 49.26 abcd | 14.69 cd | 3.88 e | 7.31 d | 8.12 d | 25.81 ab | 60.41 abc | 13.43 c | 53.57 a | 15.79 f |
| 21CS_J_NST | 24.38 a | 19.31 abc | 51.17 abc | 15.64 cd | 4.33 e | 8.98 cd | 8.07 d | 26.60 ab | 55.21 bcde | 16.95 bc | 52.95 a | 15.76 f |
| 21CS_K_ST | 14.52 bc | 9.43 d | 43.43 cde | 29.10 a | 38.05 a | 30.17 a | 23.50 a | 28.91 ab | 50.48 de | 16.81 bc | 53.24 a | 65.00 a |
| Wine | Cooked Fruit | Dried Fruit | Bell Pepper | Spice | Cigarette Smoke | Musty | Menthol | Solvent | Alcohol Hotness- Mouthfeel | Bitter |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-significant attributes | ||||||||||
| 20CS_A_ST | 15.33 | 17.60 | 5.26 | 10.29 | 6.88 | 17.50 | 18.88 | 33.60 | 56.43 | 45.31 |
| 20CS_B_ST | 16.43 | 17.67 | 17.50 | 8.79 | 18.36 | 17.45 | 17.33 | 30.10 | 44.14 | 46.79 |
| 20CS_C_ST | 16.10 | 17.40 | 12.12 | 7.57 | 16.12 | 21.26 | 16.26 | 27.90 | 44.88 | 43.64 |
| 20CS_D_ST | 27.26 | 21.50 | 9.71 | 7.76 | 5.55 | 15.95 | 17.29 | 25.07 | 37.57 | 37.90 |
| 20CS_E_ST | 11.79 | 10.57 | 5.95 | 5.76 | 24.57 | 27.64 | 15.69 | 24.00 | 49.45 | 46.55 |
| 20CS_F_ST | 27.00 | 20.90 | 7.74 | 11.64 | 5.02 | 11.05 | 16.69 | 33.76 | 56.50 | 46.21 |
| 20CS_G_ST | 21.76 | 21.31 | 6.69 | 8.88 | 8.36 | 17.79 | 18.93 | 32.24 | 52.83 | 45.19 |
| 20CS_H_ST | 24.32 | 14.46 | 9.17 | 9.83 | 12.24 | 18.29 | 17.32 | 29.75 | 53.81 | 44.37 |
| 21CS_I_NST | 26.05 | 19.93 | 8.36 | 10.50 | 4.71 | 17.05 | 16.71 | 36.74 | 54.12 | 40.17 |
| 21CS_J_NST | 22.07 | 17.76 | 8.55 | 8.60 | 5.48 | 18.48 | 20.17 | 31.24 | 51.24 | 43.48 |
| 21CS_K_ST | 8.83 | 13.33 | 4.14 | 6.88 | 25.48 | 20.07 | 14.45 | 27.55 | 52.83 | 43.50 |
Fisher’s LSD was used to determine differences between each column for the significant attributes.
Table 7.
DA 2 sensory attribute overall means with Fisher’s LSD.
| Wine | Red fruit | Bell pepper | Liquid smoke | Sweet BBQ | Solvent | Viscosity | Drying | Bitter | Ashy |
|---|---|---|---|---|---|---|---|---|---|
| Significant attributes | |||||||||
| 20CF_A_ST | 11.85 de | 21.39 a | 13.46 c | 9.49 de | 22.70 a | 26.97 a | 51.26 def | 51.33 a | 29.41 d |
| 20CF_B_ST | 11.51 de | 9.62 b | 17.51 bc | 20.82 bc | 21.41 a | 27.95 a | 46.28 f | 52.56 a | 59.92 a |
| 20CF_C_ST | 16.92 bcde | 22.13 a | 3.80 d | 2.95 e | 31.49 a | 24.95 a | 61.30 ab | 50.82 a | 22.21 de |
| 20MA_D_ST | 11.82 de | 6.05 b | 25.03 a | 25.36 b | 22.46 a | 26.18 a | 61.13 abc | 50.90 a | 45.56 bc |
| 20ME_E_ST | 21.41 abc | 11.33 b | 3.36 d | 6.03 e | 28.18 a | 25.26 a | 63.59 ab | 44.87 a | 13.72 ef |
| 20ME_F_ST | 17.44 bcde | 5.64 b | 4.23 d | 7.87 e | 29.44 a | 21.13 a | 68.26 a | 48.13 a | 29.82 d |
| 20PV_G_ST | 10.10 de | 6.67 b | 14.56 c | 25.67 b | 29.13 a | 30.15 a | 56.46 bcde | 50.00 a | 41.62 c |
| 20PV_H_ST | 13.97 cde | 5.95 b | 16.90 bc | 16.62 cd | 23.82 a | 24.51 a | 58.28 bcd | 47.69 a | 50.90 abc |
| 20SY_I_ST | 9.51 e | 8.44 b | 23.10 ab | 44.80 a | 22.10 a | 25.85 a | 52.82 cdef | 43.97 a | 52.74 ab |
| 20ZN_J_ST | 18.49 abcd | 8.03 b | 4.97 d | 6.87 e | 25.87 a | 28.59 a | 48.62 ef | 44.31 a | 21.28 def |
| 21MA_K_NST | 26.15 a | 5.97 b | 4.95 d | 6.21 e | 28.39 a | 19.67 a | 67.39 a | 45.03 a | 10.80 f |
| 21SY_L_NST | 23.03 ab | 7.13 b | 3.92 d | 4.41 e | 31.51 a | 22.77 a | 52.61 def | 44.31 a | 13.82 ef |
| Wine | Dark fruit | Cooked fruit | Dried fruit | Spice | Alcohol hotness | Cigarette smoke | Musty | Medicinal/Brett | Menthol | Tar | Alcohol hotness- mouthfeel | Sweet | Sour |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-significant attributes | |||||||||||||
| 20CF_A_ST | 20.46 | 8.87 | 16.77 | 8.59 | 50.79 | 12.92 | 23.77 | 18.49 | 17.79 | 7.05 | 56.74 | 19.36 | 47.85 |
| 20CF_B_ST | 19.26 | 13.28 | 20.74 | 8.41 | 50.64 | 17.79 | 21.08 | 21.05 | 14.18 | 8.05 | 55.77 | 19.82 | 40.85 |
| 20CF_C_ST | 18.00 | 17.44 | 18 | 6.18 | 49.03 | 5.64 | 16.87 | 10.97 | 18.95 | 5.08 | 57.74 | 19.15 | 44.31 |
| 20MA_D_ST | 15.72 | 15.18 | 17.13 | 10.13 | 50.64 | 13.33 | 17.85 | 15.69 | 14.82 | 9.72 | 56.38 | 16.97 | 53.00 |
| 20ME_E_ST | 18.08 | 17.08 | 18.08 | 8.28 | 49.92 | 7.21 | 13.10 | 15.59 | 20.64 | 6.33 | 50.36 | 16.79 | 55.15 |
| 20ME_F_ST | 22.36 | 23.72 | 17.56 | 9.79 | 54.72 | 2.74 | 12.44 | 9.62 | 18.97 | 6.08 | 62.69 | 21.05 | 53.03 |
| 20PV_G_ST | 16.21 | 12.15 | 22.51 | 9.26 | 53.08 | 11.92 | 23.18 | 19.49 | 15.03 | 8.41 | 61.59 | 19.9 | 48.13 |
| 20PV_H_ST | 15.18 | 13.26 | 19.46 | 7.26 | 49.36 | 15.44 | 23.13 | 21.36 | 19.46 | 6.77 | 54.62 | 19.41 | 45.41 |
| 20SY_I_ST | 11.67 | 11.13 | 20.54 | 11.08 | 46.05 | 14.85 | 17.90 | 16.79 | 17.28 | 8.28 | 53.41 | 19.79 | 63.33 |
| 20ZN_J_ST | 20.44 | 19.41 | 21.05 | 10.87 | 51.00 | 7.51 | 20.03 | 14.44 | 17.08 | 4.36 | 52.82 | 19.51 | 53.74 |
| 21MA_K_NST | 18.54 | 23.67 | 18.28 | 10.97 | 51.31 | 5.26 | 13.49 | 10.13 | 17.87 | 2.21 | 54.87 | 14.97 | 63.21 |
| 21SY_L_NST | 20.64 | 24.05 | 19.82 | 12.77 | 48.97 | 4.08 | 15.38 | 14.62 | 15.08 | 5.56 | 51.51 | 17.49 | 60.38 |
Fisher’s LSD was used to determine differences between each column for the significant attributes.
Fig. 1.
Mean attribute intensities of “Ashy” attribute in DA1 and DA2 wines, with Fisher’s LSD (α = 0.05).
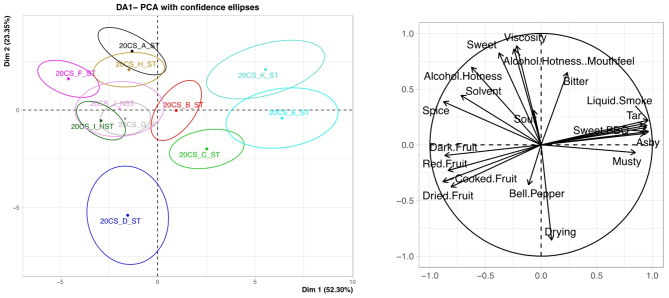
A PCA with confidence ellipses around the wine is shown in Fig. 2 for DA1. All attributes were considered. Overlapping circles mean that the wines are not significantly different from each other at a 95% confidence interval. Across PC1 (52.30%) and PC2 (23.35%) which explains 75.65% of the data, there is a clear separation of the wines across PC1. 20CS_F_ST, 21CS_I_NST, 21CS_J_NST and 20CS_G_ST are not significantly different from each other, these wines were “dark fruit”, “cooked fruit”, “red fruit”, and “dried fruit” driven. 20CS_A_ST and 20CS_H_ST are not significantly different from each other and were “sweet”, “viscosity”, “alcohol hotness” driven. 20CS_E_ST and 21CS_K_ST are not significantly different from each other, with these wines being, “musty”, “sweet BBQ”, “tar”, “ashy” and “liquid smoke” driven. 20CS_D_ST is significantly different from the rest of the wines with it being “drying” driven, confirmed by the mean values (Table 6).
Fig. 2.
Score plot and loadings plot of DA1 with confidence ellipses around wines at 95% CI.
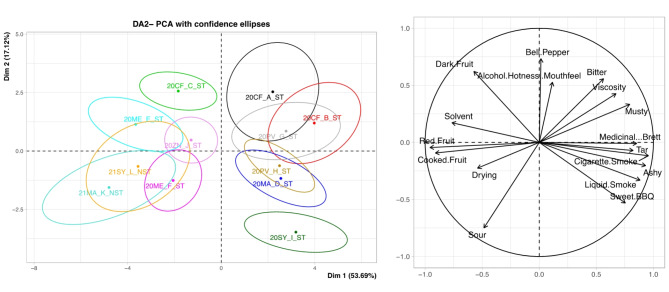
For DA2 in Fig. 3, across PC1 (53.69%) and PC2 (17.12%) which explains 70.81% of the data, there is a clear separation of the wines across PC1. 20ME_F_ST, 21MA_K_NST and 21SY_L_NST are not significantly different from each other, these wines were “cooked fruit”, “red fruit”, “drying” and “sour” driven. 21SY_L_NST, 20ZN_J_ST, and 20ME_E_ST are not significantly different from each other and were “dark fruit”, “solvent”, and “earthy” driven. 20CF_A_ST, 20CF_B_ST, 20MA_D_ST and 20PV_H_ST are not significantly different from each other, with these wines being, “musty”, “bitter”, “tar”, “cigarette smoke”, “ashy” and “liquid smoke” driven. 20SY_I_ST and 20CF_C_ST are significantly different from the rest of the wines with different smoke attributes and bell pepper attributes respectively. 20SY_I_ST had a high mean score for “sweet BBQ” at 44.80 while 20CF_C_ST had a mean score for “bell pepper” at 21.39. 20CF_C_ST differed from 20CF_A_ST and 20CF_B_ST as it had a higher “red fruit” score and lower smoke-related attribute mean scores (Table 7).
Fig. 3.
Score plot and loadings plot of DA2 with confidence ellipses around wines at 95% CI.
Combining sensory and compositional data
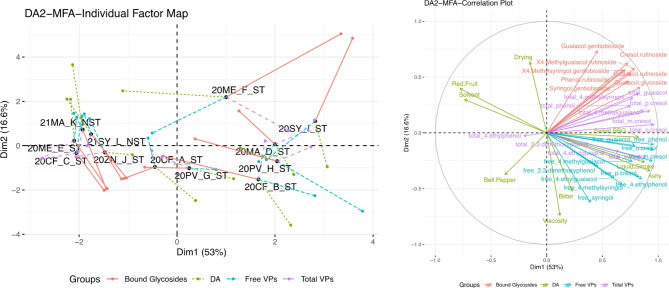
Multiple factor analysis was used to relate sensory data to the chemical data of the wines. The free VPs, total VPs, individual bound glycosides, and significant attributes of the sensory data were analyzed using the MFA. Figure 4 shows the MFA of DA1. Wines were distinguished in the individual factor map similarly to the PCA in Fig. 2. The first two dimensions explain a total of 81.4% of the data with 56.4% across Dim1 and 25.0% across Dim2. In the loading plot, it is observed that the smoke-related DA attributes were driven by mainly free and total VPs. This was further confirmed by RV coefficients, where large RV coefficients indicate a good fit of the data. Between the DA and total VP, it has a RV coefficient of 0.857, DA and free VP at 0.758, and DA and bound glycosides at 0.404. However, the overall fit based on RV coefficients of the DA, total VPs, free VPs, and bound glycosides to the consensus positions were at 0.884, 0.972, 0.895, and 0.687. This indicates a good overall fit of the data. Certain VPs “free o-cresol”, “total guaiacol”, “free 4-ethylphenol”, “free m-cresol”, “total o-cresol”, and “total m-cresol” drive the smoke-related attributes, “tar”, “sweet BBQ”, “liquid smoke” and “ashy”. High smoke-impacted wines such as 21CS_K_ST and 20CS_E_ST were more smoke-driven sensorially by attributes (“tar”, “sweet BBQ”, and “ashy”), and chemically by free VPs, total VPs and bound glycosides. Contrary, low/no impact wines such as “20CS_F_ST” and “21CS_J_NST” were fruit-driven by the terms “red fruit” and “dark fruit” and was not driven by free VPs, total VPs and bound glycosides.
Fig. 4.
Multiple factor analysis of DA1 data, relating individual bound glycosides, significant attributes from the DA, free VPs, and total VPs. Individual factor map and loading plots are shown here.
Figure 5 shows the MFA of DA2. The first two dimensions explain a total of 69.6% of the overall data with 53.0% across Dim1 and 16.6% across Dim2. The overall spread of the wines in the individual factor map of the MFA is similar to the PCA biplot in Fig. 3. Here it was observed that the free VPs are the main drivers of the DA attributes. The RV coefficients for the DA and total VPs was 0.591, DA and free VPs was 0.674, and DA and bound glycosides was 0.313. Importantly, RV coefficients of the DA, total VPs, free VPs, and bound glycosides to the MFA consensus positions are at 0.805, 0.923, 0.829, and 0.681. When comparing the RV with the overall MFA plot, the total VPs are a better explanation for the DA attributes. This is seen when “total 2,3-dimethoxyphenol”, “total syringol”, “total o-cresol”, “total p-cresol”, “total m-cresol”, and “total ethylguaiacol” are key drivers of “liquid smoke”, “sweet BBQ”, and “ashy” attributes. Additionally, free VPs such as “free o-cresol”, “free m-cresol”, “free phenol”, and “free guaiacol” also contributed to the smoke DA attributes “liquid smoke”, “ashy”, and “sweet BBQ” which was seen in the relatively high RV coefficient values. Higher impacted wines, 20SY_I_ST, 20MA_D_ST, 20PV_H_ST and 20CF_B_ST were driven by more smoke-related DA attributes as well as the total and free VP amounts. Low/no impact wines such as 21MA_K_NST and 21SY_L_NST were “red fruit” and “dark fruit” driven overall.
Fig. 5.
Multiple factor analysis of DA2 data, relating individual bound glycosides, significant attributes from the DA, free VPs, and total VPs. Individual factor map and loading plots are shown here.
Discussion
There were significant differences in the basic wine chemical analysis, specifically the alcohol %, TA, and RS of the different wines which can have sensory implications34,35. However, “alcohol hotness”, “sweet”, and “sour” descriptor terms were significant only in DA1, determined by the pseudo-mixed ANOVA model (Fig. 4). When the DA1 data was normalized against the “alcohol hotness”, “sour” and “sweet” terms, it did not give a different result. Hence for the rest of the analysis, the effect of alcohol %, TA and RS was not considered, and the original data was used without normalization.
The determination of smoke impact could be quantified in the following ways, chemical analysis of smoke marker compounds, sensory analysis or a combination of both. Chemical analysis of smoke marker compounds in the free VP, total VP and the individual bound glycosides would give us an idea of the compositional impact on smoke. However, sensory analysis would need to be done on the wine to determine if there is indeed a smoke impact. In our experiment, there were significant chemical differences among the non-impacted and low-impacted, and highly impacted wines. The smoke impact can be determined using a trained descriptive analysis panel. The wines that were highly smoke impacted as determined by high values of VPs and the individual bound glycosides also were described by the sensory panel to be “ashy”, “smoky”, “sweet BBQ”. These wines lacked overall flavor intensity with a decrease in attributes such as “red fruit”, “dark fruit” and “jam fruit”.
In general, the impact of smoke on the wines were largely separated (> 50%) along PC1 for all the analyses presented. In the PCA plots shown in Figs. 2 and 3, wines that had higher levels of smoke marker compounds were grouped together and the non-tainted or those with low concentrations of smoke marker compounds were on the opposite ends of the PCA8. The wines that had higher smoke impact determined by higher concentrations of smoke marker compounds were described as more smoky and ashy. Contrary, wines that were not smoke-impacted or low impacted as determined by low concentrations of smoke marker compounds were more fruity and less ashy. Among these two groups of wines, the low smoke impacted and non-impacted wines, the wines that had low smoke impact were less fruity compared to the non-impacted wines. This could be attributed to the fruity aromas masking the smoky aromas and vice versa, which was seen in previous studies where low levels of smoke reduced the perception of fruit attributes in a wine7,17,36. The low smoke-impacted wines also had lower “ashy” scored when compared to the high smoke-impacted wines. The concentration of the free and total (free + bound) VP were the main drivers of the smoke-related attributes and it was confirmed using the MFA (Fig. 4), mean sensory values (Table 6), the total VP (Table 4), and individual bound glycoside (Table 5) values.
VP glycosides are non-volatile precursors and odorless. However, glycosidic bonds are released through fermentation, during wine storage, and potentially in the mouth through bacteria or enzymatic hydrolysis14. These events can release VPs and give rise to the smoky related characters, in particular the “ashy” term14. Acid hydrolysis was used to cleave the acid labile compounds to give a quantitative measure of the total VP concentrations which include both the free VP and acid labile forms. Other research pointed to the individual bound forms as the main driver of smoke19,37. However, in these other studies a much larger set of phenolic glycosides were measured. This study showed that the amount of total VPs (Table 4) was reflective of the smoke impact seen in the wines (Tables 6 and 7) as seen with the high RV coefficients between the total VP and the DA which represents a good relation between the two data sets.
Across the free VP, total VP and individual bound glycosides, there were differences in the non-impacted samples across varietals such as between the Malbec and Syrah sample. However, there were no significant differences in the non-impacted Cabernet Sauvignon sample across different sites. This could indicate that baseline levels of the VPs and individual bound glycosides would be consistent across each varietal. When compared to the study done by Crews et al.20, the sum of glycosides was below 6 µg/L for baseline samples, which is consistent with the findings in this study for the non-impacted Cabernet Sauvignon tested. Syrah is a varietal that is known to have naturally elevated levels of VPs and the individual bound glycosides. These elevated values makes it significantly different from other non-impacted varietals. This was seen in the values for 21SY_L_NST being significantly different from 21MA_K_NST for the different VPs and individual bound glycosides (Tables 3, 4 and 5). It This shows that different varieties can have different baseline levels of VPs present in them naturally.
There was a difference between the “ashy” scores across different wine varietals and across locations for the same varietal. The variations could be attributed to concentration differences of free VPs, total VPs, and bound glycosides which vary naturally across different varietals and the amount of smoke exposure based on location as seen in Tables 1, 3 and 4, and 541–43. Across all the wines evaluated, wines from Dry Creek Valley and St. Helena generally had the highest score for “ashy”. Within those locations, Cabernet Sauvignon wines had the highest score for “ashy”. Next when looking at the different varietals, wines made from Dry Creek Valley (20MA_D_ST and 20SY_I_ST) were highly smoke impacted and St. Helena (20CF_B_ST and 20PV_H_ST) were smoke-impacted at a medium level. It was observed that even with different varietals, the extent of smoke impact varied mainly by the location of the grapes as the location determined the amount of smoke exposure and VPs absorbed by the grapes. The varietal was a secondary effect. In Oakville where the smoke impact was relatively low, the “ashy” rating across varietals were similar ( 20CS_F_ST, 20_CF_C_ST, and 20ME_E_ST). The wines all had relatively low “ashy scores” due to lower grape smoke exposure compared to Dry Creek Valley and St. Helena. The wines from Dry Creek Valley and St. Helena also had the highest concentrations of free VPs, total VPs, and bound glycosides confirming that these marker compounds are driving smoke taint character in wine, confirming previous findings12,21,41. When comparing the smoke-exposed wines to their non-smoke counterparts, the Syrah (21SY_L_NST & 20SY_I_ST), Malbec (21MS_K_NST & 20MA_D_ST) and Cabernet Sauvignon (21CS_I_NST & 20CS_E_ST, 21CS_J_NST & 21CS_K_ST (intentional smoked)) wines, there was large differences in smoke marker compound levels which correlated with ashy scores and overall smoke impact. In comparing the smoke-exposed wines’ total VPs to some of the known threshold values guaiacol (23 µg/L), m-cresol (20 µg/L), o-cresol (62 µg/L), and p-cresol (64 µg/L) reported by Parker et al.5, some of the smoke-exposed wine has lower total VP values than the threshold values yet they are still perceived as smoky and ashy. This suggests a synergistic impact among VPs as discussed by McKay et al.42. Ultimately, with the data captured, it is not just the duration of fruit smoke exposure that is important but the proximity of the vineyard in relation to the smoke event as that determines the amount of VPs present in the smoke43. VPs in the gas phase have short lifetimes and break down within a few hours in the atmosphere43. It will be of great benefit if the released VPs in the smoke can be measured and related to grape and wine composition. However, this will most likely only be possible under controlled smoke situations as access to active wildfire zones are restricted41.
Conclusion
The overall smoke impact on the wines was driven primarily by the location (origin) of the grapes, including proximity of the vineyard to the smoke event. The varietal impact of the grapes on smoke expression is small when compared to the location. Fresh smoke contains the most VPs and thus vineyards in close proximity to fires were exposed to more VPs in the atmosphere leading to more absorption. However, it is known that topography is another important factor to consider when determining risk from nearby fires44. Furthermore the direction and speed of air currents can have a large impact45. The wind could be blowing the smoke in the opposite direction from a vineyard, hence even in close proximity, these grapes may not be smoke impacted46. A modified descriptive analysis is a good rapid tool that can be used with minimal training to train a panel of relative experience to agree on a set of sensory terms to describe a product. However, additional studies are needed to determine smoke threshold levels in the different grape and wine matrixes both from a VP standpoint and a sensory standpoint.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Arpa Boghozian, Fermin Ramirez, Matharin Lee and Leticia Chacon Rodriguez for their support in data collection.
Author contributions
Conceptualization, A.O, L.X.L and C.M.P. Methodology, A.O, J.X.G, I.A.P, L.X.L, C.M.P. and L.L. Formal Analysis, L.X.L, C.M.P, I.A.P, I.A.P, Y.W and B.N. Writing: L.X.L, C.M.P, I.A.P, J.X.G, and A.O. Supervision: A.O and J.X.G. Project Administration: A.O. Funding acquisition: A.O. All authors have read and agreed to published version of manuscript.
Funding
This research was funded by “Jackson Family Wines” and “United States Department of Agriculture- Agricultural Research Service (USDA-ARS)”.
Data availability
The datasets used and/or analysed during the current study is available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of California, Davis (protocol code 1288072-1, 26 June 2018).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ca.gov. Incident Archive. https://www.fire.ca.gov/incidents/2020/ (2020).
- 2.Li, Y. et al. Dominance of wildfires Impact on Air Quality exceedances during the 2020 record-breaking wildfire season in the United States. Geophys. Res. Lett. 48, 45 (2021).
- 3.Rosenthal, A., Stover, E. & Haar, R. J. Health and social impacts of California wildfires and the deficiencies in current recovery resources: an exploratory qualitative study of systems-level issues. PLoS One 16, 7856 (2021). [DOI] [PMC free article] [PubMed]
- 4.Simoneit, B. R. T. Biomass burning—a review of organic tracers for smoke from incomplete combustion. Appl. Geochem. 17, 129–162 (2002). [Google Scholar]
- 5.Parker, M. et al. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 60, 2629–2637 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Krstic, M. P., Johnson, D. L. & Herderich, M. J. Review of smoke taint in wine: smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust J. Grape Wine Res. 21, 537–553 (2015). [Google Scholar]
- 7.Ristic, R. et al. Impact of grapevine exposure to smoke on vine physiology and the composition and sensory properties of wine. Theor. Exp. Plant. Physiol. 28, 67–83 (2016). [Google Scholar]
- 8.Kennison, R., Wilkinson, K. L., Williams, K. G., Smith, H. H., Gibberd, R. & J. & Smoke-derived taint in wine: Effect of Postharvest smoke exposure of grapes on the Chemical Composition and sensory characteristics of wine. J. Agric. Food Chem. 55, 10897–10901 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Fryer, J. A. & Tomasino, E. Analysis of retronasal flavor alterations in smoke-affected wines and the efficacy of various inter-stimulus rinse protocols in clearing smoke-related attributes. Beverages 8, 4586 (2022).
- 10.Summerson, V., Viejo, C. G., Pang, A., Torrico, D. D. & Fuentes, S. Review of the effects of grapevine smoke exposure and technologies to assess smoke contamination and taint in grapes and wine. Beverages 7, 1–21. 10.3390/beverages7010007 (2021).
- 11.Parker, M. et al. Factors contributing to Interindividual Variation in retronasal odor perception from Aroma glycosides: the role of odorant sensory detection threshold, oral microbiota, and hydrolysis in saliva. J. Agric. Food Chem. 68, 10299–10309 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Jiang, W., Parker, M., Hayasaka, Y., Simos, C. & Herderich, M. Compositional changes in grapes and leaves as a consequence of smoke exposure of vineyards from multiple bushfires across a ripening season. Molecules 26, 7456 (2021). [DOI] [PMC free article] [PubMed]
- 13.Oberholster et al. Grape smoke exposure risk assessment: wine matrix impact on smoke marker compound smoke expression. BIO Web Conf. 56, 02039 (2023). [Google Scholar]
- 14.Mayr, C. M. et al. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wines. J. Agric. Food Chem. 62, 2327–2336 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Caffrey, A. et al. Changes in smoke-taint volatile-phenol glycosides in wildfire smoke-exposed cabernet sauvignon grapes throughout winemaking. Am. J. Enol. Vitic. 70, 373–381 (2019). [Google Scholar]
- 16.Hayasaka, Y. et al. Glycosylation of smoke-derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. J. Agric. Food Chem. 58, 10989–10998 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Ristic, R., Van Der Hulst, L., Capone, D. L. & Wilkinson, K. L. Impact of Bottle Aging on smoke-tainted wines from different grape cultivars. J. Agric. Food Chem. 65, 4146–4152 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Oberholster, A. et al. Investigation of different Winemaking protocols to mitigate smoke taint character in wine. Molecules 27, 1732 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitmore, B. A. et al. Glycosidically-bound volatile phenols linked to smoke taint: Stability during fermentation with different yeasts and in finished wine. Molecules 26, 478 (2021). [DOI] [PMC free article] [PubMed]
- 20.Crews, P. et al. Natural product phenolic diglycosides created from wildfires, defining their impact on california and oregon grapes and wines. J. Nat. Prod. 85, 547–561. 10.1021/acs.jnatprod.2c00028 (2022). [DOI] [PMC free article] [PubMed]
- 21.Hayasaka, Y. et al. Assessing the impact of smoke exposure in grapes: development and validation of a HPLC-MS/MS method for the quantitative analysis of smoke-derived phenolic glycosides in grapes and wine. J. Agric. Food Chem. 61, 25–33 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Lawless, H. T. & Heymann, H. Descriptive analysis. In Sensory Evaluation of Food: Principles and Practices (eds. Lawless, H. T. & Heymann, H.) 341–378 (Springer US, 1999). 10.1007/978-1-4615-7843-7_10. [Google Scholar]
- 23.Bilogrevic, E. et al. Consumer response to wine made from smoke-affected grapes. OENO One 57, 417–430 (2023). [Google Scholar]
- 24.Nishida, M., Lestringant, P., Cantu, A. & Heymann, H. Comparing classical descriptive analysis with modified descriptive analysis, modified rate-all-that-apply, and modified check-all-that-apply. J. Sens. Stud.. 10.1111/joss.12684 (2021).
- 25.Danner, L. et al. Comparison of rate-all-that-apply and descriptive analysis for the sensory profiling of wine. Am. J. Enol. Vitic. 69, 12–21 (2018). [Google Scholar]
- 26.Muñoz, A. M., Kemp, S. E., Hollowood, T. & Hort, J. Comparison of descriptive analysis methods. In Descriptive Anal. Sens. Evaluation 681–709 (Wiley, 2017). 10.1002/9781118991657.ch20.
- 27.Liu, J., Bredie, W. L. P., Sherman, E., Harbertson, J. F. & Heymann, H. Comparison of rapid descriptive sensory methodologies: free-choice profiling, Flash Profile and modified Flash Profile. Food Res. Int. 106, 892–900 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Chambers, E. Consensus methods for descriptive analysis. In Descriptive Anal. Sens. Evaluation 211–236 (Wiley, 2017). 10.1002/9781118991657.CH6.
- 29.Fryer, J. A., Collins, T. S. & Tomasino, E. Evaluation of different interstimulus rinse protocols on smoke attribute perception in wildfire-affected wines. Molecules 26, 745 (2021). [DOI] [PMC free article] [PubMed]
- 30.Simons, T. et al. Chemical and sensory analysis of commercial Navel oranges in California. NPJ Sci. Food 3, 1–11 (2019). [DOI] [PMC free article] [PubMed]
- 31.Husson, F., Lê, S. & Pagès, J. Confidence ellipse for the sensory profiles obtained by principal component analysis. Food Qual. Prefer 16, 245–250 (2005). [Google Scholar]
- 32.Escofier, B. & Pagb, J. Multiple factor analysis (AFMULT package). Comput. Stat. Data Anal. 18, 121 (1994). [Google Scholar]
- 33.Bécue-Bertaut, M. & Pagès, J. Multiple factor analysis and clustering of a mixture of quantitative, categorical and frequency data. Comput. Stat. Data Anal. 52, 3255–3268 (2008). [Google Scholar]
- 34.King, E. S., Dunn, R. L. & Heymann, H. The influence of alcohol on the sensory perception of red wines. Food Qual. Prefer 28, 235–243 (2013). [Google Scholar]
- 35.Fontoin, H., Saucier, C., Teissedre, P. L. & Glories, Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Prefer 19, 286–291 (2008). [Google Scholar]
- 36.McKay, M., Bauer, F. F., Panzeri, V. & Buica, A. Investigation of olfactory interactions of low levels of five off-flavour causing compounds in a red wine matrix. Food Res. Int. 128, 478 (2020). [DOI] [PubMed]
- 37.Favell, J. W. et al. Correlating sensory assessment of smoke-tainted wines with inter-laboratory study consensus values for volatile phenols. Molecules 27, 452 (2022). [DOI] [PMC free article] [PubMed]
- 38.Runnebaum, R., Arvik, T. & Merrell, C. UC Davis UC Davis Previously Published Works Title Understanding Smoke Taint Results: Pinot Noir Baseline Concentrations of Smoke Taint Markers across Five Vintages Publication Date (2023).
- 39.Kelly, D. & Zerihun, A. The effect of phenol composition on the sensory profile of smoke affected wines. Molecules 20, 9536–9549 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennison, K. R., Wilkinson, K. L., Pollnitz, A. P., Williams, H. G. & Gibberd, M. R. Effect of timing and duration of grapevine exposure to smoke on the composition and sensory properties of wine. Aust J. Grape Wine Res. 15, 228–237 (2009). [Google Scholar]
- 41.Ugliano, M. et al. Consumer response to wine made from smoke-affected grapes. OENO One 57, 417–430 (2023). [Google Scholar]
- 42.McKay, M., Bauer, F. F., Panzeri, V., Mokwena, L. & Buica, A. Potentially smoke tainted red wines: volatile phenols and aroma attributes. S. Afr. J. Enol. Vitic. 40, 7489 (2019).
- 43.Sun, Y., Xu, F., Li, X., Zhang, Q. & Gu, Y. Mechanisms and kinetic studies of OH-initiated atmospheric oxidation of methoxyphenols in the presence of O2 and NOx. Phys. Chem. Chem. Phys. 21, 21856–21866 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Liu, Y. et al. Smoke Plume Dynamics (Springer, 2022). 10.1007/978-3-030-87045-4_4.
- 45.Kochanski, A. K. et al. Modeling wildfire smoke feedback mechanisms using a coupled fire-atmosphere Model with a radiatively active Aerosol Scheme. J. Geophys. Res.: Atmos. 124, 9099–9116 (2019). [Google Scholar]
- 46.Pamela Kan-Rice. Where there is fire, is there smoke flavor in winegrapes? UC ANR News (2019). https://ucanr.edu/News/?routeName=newsstory&postnum=39034.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study is available from the corresponding author on request.