Abstract
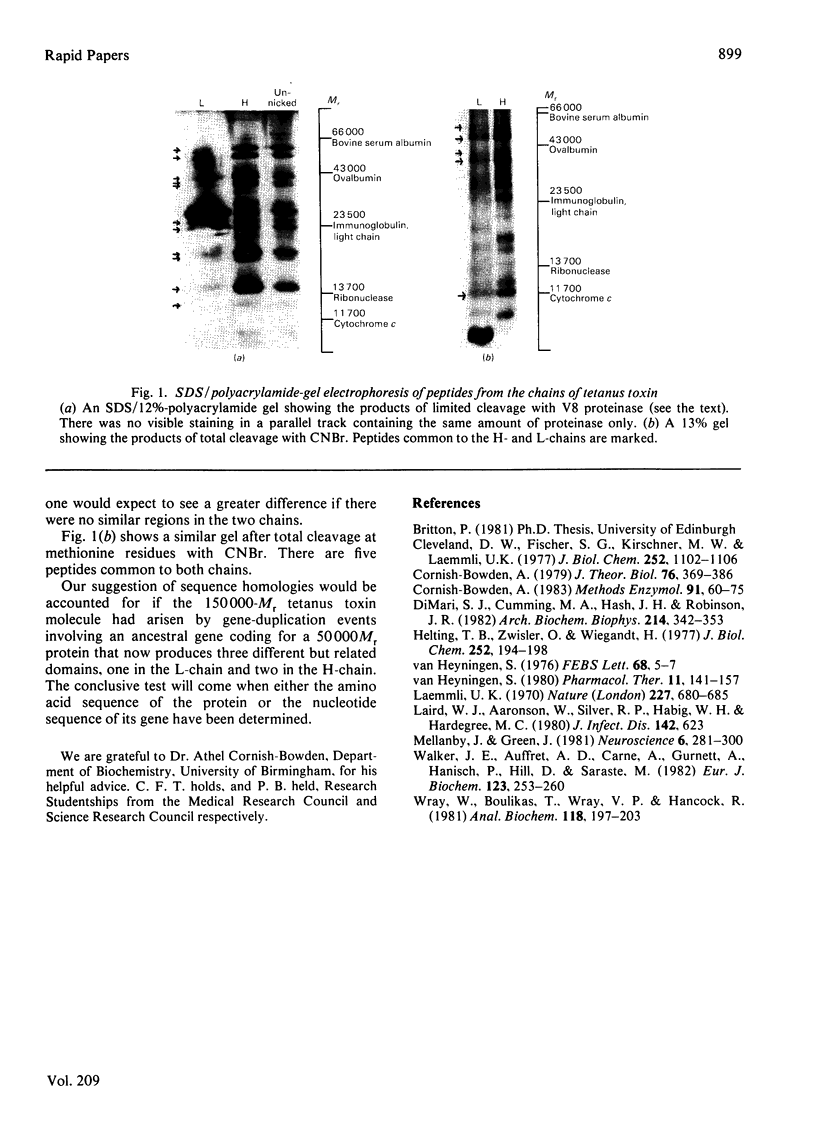
Quantitative comparison of the amino acid compositions of the heavy and light chains of tetanus toxin by the method of Cornish-Bowden [(1983) Methods Enzymol. 91, 60-75)] suggests strongly that there is sequence homology between the two chains and that the heavy chain has two similar halves. Examination (by electrophoresis in polyacrylamide gels in the presence of sodium dodecyl sulphate) of peptides produced from the chains by proteolytic cleavage supports this idea.
Full text
PDF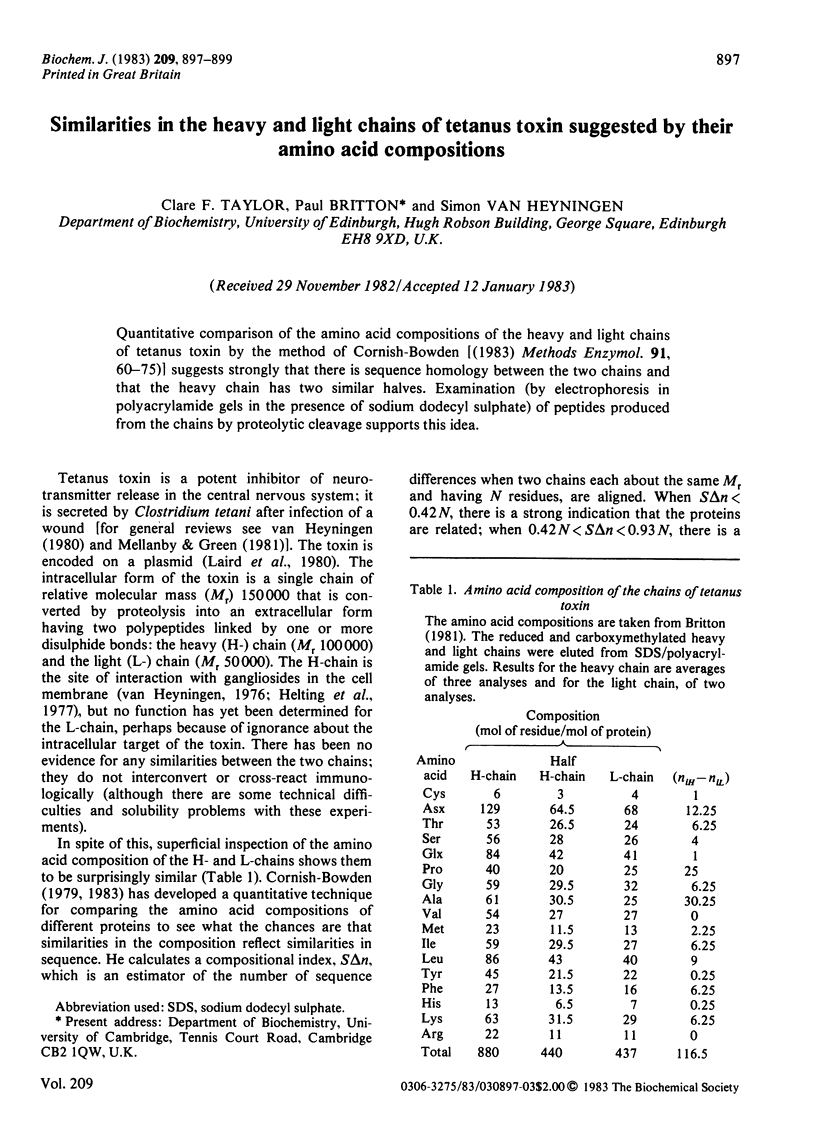

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cornish-Bowden A. How reliably do amino acid composition comparisons predict sequence similarities between proteins? J Theor Biol. 1979 Feb 21;76(4):369–386. doi: 10.1016/0022-5193(79)90007-9. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. Relating proteins by amino acid composition. Methods Enzymol. 1983;91:60–75. doi: 10.1016/s0076-6879(83)91011-x. [DOI] [PubMed] [Google Scholar]
- DiMari S. J., Cumming M. A., Hash J. H., Robinson J. P. Purification of tetanus toxin and its peptide components by preparative polyacrylamide gel electrophoresis. Arch Biochem Biophys. 1982 Mar;214(1):342–353. doi: 10.1016/0003-9861(82)90039-x. [DOI] [PubMed] [Google Scholar]
- Helting T. B., Zwisler O., Wiegandt H. Structure of tetanus toxin. II. Toxin binding to ganglioside. J Biol Chem. 1977 Jan 10;252(1):194–198. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laird W. J., Aaronson W., Silver R. P., Habig W. H., Hardegree M. C. Plasmid-associated toxigenicity in Clostridium tetani. J Infect Dis. 1980 Oct;142(4):623–623. doi: 10.1093/infdis/142.4.623. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Auffret A. D., Carne A., Gurnett A., Hanisch P., Hill D., Saraste M. Solid-phase sequence analysis of polypeptides eluted from polyacrylamide gels. An aid to interpretation of DNA sequences exemplified by the Escherichia coli unc operon and bacteriophage lambda. Eur J Biochem. 1982 Apr 1;123(2):253–260. doi: 10.1111/j.1432-1033.1982.tb19761.x. [DOI] [PubMed] [Google Scholar]
- van Heyningen S. Binding of ganglioside by the chains of tetanus toxin. FEBS Lett. 1976 Sep 15;68(1):5–7. doi: 10.1016/0014-5793(76)80391-2. [DOI] [PubMed] [Google Scholar]



