Abstract
Covalent organic frameworks have emerged as a thriving family in the realm of photocatalysis recently, yet with concerns about their high exciton dissociation energy and sluggish charge transfer. Herein, a strategy to enhance the built-in electric field of series β-keto-enamine-based covalent organic frameworks by ionic polarization method is proposed. The ionic polarization is achieved through a distinctive post-synthetic quaternization reaction which can endow the covalent organic frameworks with separated charge centers comprising cationic skeleton and iodide counter-anions. The stronger built-in electric field generated between their cationic framework and iodide anions promotes charge transfer and exciton dissociation efficiency. Moreover, the introduced iodide anions not only serve as reaction centers with lowered H* formation energy barrier, but also act as electron extractant suppressing the recombination of electron-hole pairs. Therefore, the photocatalytic performance of the covalent organic frameworks shows notable improvement, among which the CH3I-TpPa-1 can deliver an high H2 production rate up to 9.21 mmol g−1 h−1 without any co-catalysts, representing a 42-fold increase compared to TpPa-1, being comparable to or possibly exceeding the current covalent organic framework photocatalysts with the addition of Pt co-catalysts.
Subject terms: Photocatalysis, Photocatalysis, Hydrogen energy
Covalent organic frameworks (COFs) show great promise in photocatalysis but are limited by slow charge transfer. Here, the authors report a strategy to enhance the built-in electric field of COFs via ionic polarization, resulting in a hydrogen evolution rate of 9.21 mmol g-¹ h-¹ without Pt co-catalysts.
Introduction
Converting solar energy to “solar fuels” has been recognized as one of the most promising solutions for addressing the global energy and environmental crisis. Photocatalytic water splitting stands out among these solutions, since it harnesses renewable resources to yields green hydrogen product which possesses the advantages of green, sustainable and high energy density1–3. Despite decades of investigation, the performance of most photocatalysts still falls short in real-world applications. Developing highly efficient and stable photocatalysts to promote water splitting remains at the forefront of our agenda.
Covalent organic frameworks (COFs), a kind of porous crystalline materials, are assembled by the condensation of organic building units through covalent bond4–6. Compared with traditional semiconductors, COFs display incomparable advantages in photocatalysis, including large specific surface area, excellent optical properties, high thermal/chemical stability, and functional designability7–9. However, it’s important to note that there are also some drawbacks potentially impeding their photocatalytic efficiency, such as high exciton dissociation energy and sluggish charge transfer. To overcome these issues, some strategies, such as heterojunction construction10–13, defect engineering14,15, metal doping16–19, and donor-acceptor system construction20–23, have been proposed. Nevertheless, these methods remain unsatisfactory due to inherent structural limitations, which contribute to the requirement for expensive Pt co-catalysts. Actually, the reliance on expensive co-catalysts extends beyond COFs and presents a universal challenge in the field of photocatalysis, stemming from the scarcity of active sites for hydrogen evolution through H* formation24,25.
Recently, polarization strategy has been proven to be an alternative method to enhance the charge separation/migration properties of the photocatalysts26–30. From a perspective of mechanism, polarization can enhance the dipole moment of the photocatalysts, and cause a change in the distribution of positive and negative charges on/in the surface/bulk of the materials, thus establishing a built-in electric field (BIEF) to provide a strong and persistent driving force for promoting the electron-hole pairs separation. Moreover, the enhancement of the dipole moment can also lower the exciton binding energy of the metal-free organic polymers31–34. However, the polarization strategy has rarely been employed for COFs materials due to the difficulty in polarizing. Yet, we notice that endowing COFs materials with an ionic skeleton can not only improve electronic conductivity and charge transfer but also theoretically alter their positive and negative charge distribution and implant large numbers of polar sites into the frameworks, thereby achieving ionic polarization35–37. Besides, ionic polarization may also open up new possibilities for new active sites for H* formation, potentially reducing the necessity for Pt co-catalysts. However, due to steric hindrance effects and the electrostatic repulsion of ionic building units, it is difficult to directly synthesize COFs with ionized frameworks using traditional bottom-up synthetic methods38–40.
Among various different kinds of COFs, β-keto-enamine-based COFs have several advantages in photocatalysis. At first, they exhibit high structural stability due to the irreversible keto-enamine linkages, which contribute to their resistance against harsh chemical environments41,42. Besides, β-keto-enamine-based COFs also have strong visible-light absorption capabilities and efficient charge separation, making them highly effective in visible-light photocatalysis43,44 In addition, following the transformation of C = N linkage into the C-N bond in keto-enamine-based COFs, post-synthetic quaternization reaction can take place at the N sites of C-N bond, resulting in the formation of ammonium groups, thus endowing COFs with cationic frameworks to achieve ionic polarization.
Herein, we explore the possibility of using post modification approach to endow β-keto-enamine-based COFs with ionic structure. A series of β-keto-enamine-based COFs are successfully endowed with cationic frameworks by a well-designed post-synthetic quaternization reaction. As a result of the enhanced BIEF induced by ionic polarization, the migration/separation efficiency of photogenerated electron-hole pairs can be significantly improved. It can also enhance the dipole moment of COFs, thus lowering the exciton dissociation energy. Moreover, it is found that the incorporated counteracting iodide anions can serve as electron-withdrawing group (active sites) to promote electron transfer and lower H* formation energy barrier. With these dual benefits, ionized COFs materials demonstrate higher photocatalytic activities toward water splitting. The H2 evolution rate of CH3I-TpPa-1 reaches as high as 9.21 mmol g−1 h−1 without the use of Pt co-catalysts, surpassing that of most COFs photocatalysts assisted by Pt co-catalysts.
Results and discussion
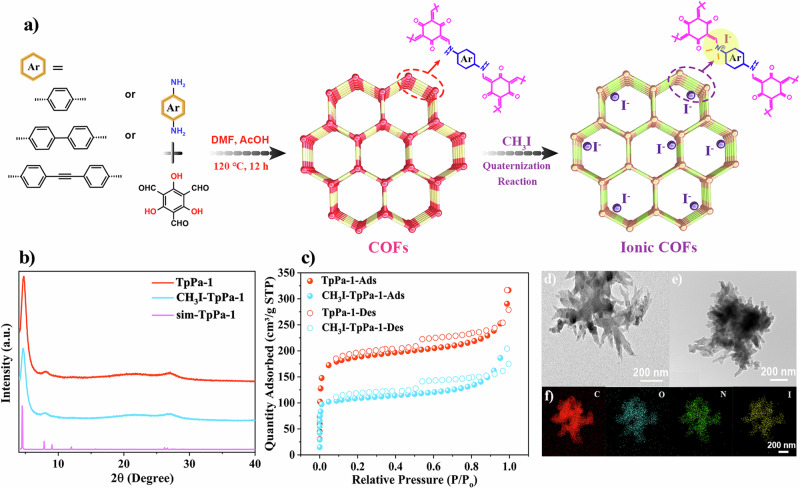
In this work, a classical β-keto-enamine-based COFs named TpPa-145, formed via polymerization of 1,3,5-triformylphloroglucinol (Tp) and p-phenylenediamine (Pa), is first selected as a representative model for examining ionic polarization effects. Subsequently, cationic COFs containing iodide counterions (CH3I-TpPa-1) are obtained through a quaternization modification. To verify the universality of the ion polarization approach, two additional β-keto-enamine-based COFs with different diamines linkers, such as 4,4’-diaminobiphenyl (BD) and 4,4’-(ethene-1,2-diyl) dianiline (EDDA), TpBD46 and TpEDDA47 have also been prepared, and they are endowed with cationic structure (CH3I-TpBD and CH3I-TpEDDA) by the same method (Fig. 1a). The high crystallinity of these samples is verified by powder X-ray diffraction (PXRD) tests. As shown in Fig. 1b, the diffraction peaks observed at 4.7° and 27° are indexed to the (100) and (001) reflection planes of TpPa-1, respectively. However, the (100) diffraction peaks of TpBD and TpEDDA shift toward smaller angles as the length of their diamine linker increases (Supplementary Figs. S2, S4, and S7). This phenomenon is related to the increased pore size, which can be measured according to the simulated COFs structure associated with ligand extension. Based on the comparison of peak location and relative intensities, the experimental PXRD profiles of all the samples are in good agreement with the simulated patterns, and their diffraction peaks consistently adhere to the AA stacking mode (Supplementary Figs. S1, S3, and S6). All of the above results demonstrate the successful preparation of the pristine COFs samples. After quaternization modification, the crystallinity of the CH3I-TpPa-1 remains well maintained, as evidenced by the sharp (100) diffraction peaks at 4.7°, which is in accordance with those observed in the pristine TpPa-1 (Fig. 1b). Similar results can also be observed in other two modified COFs (CH3I-TpBD and CH3I-TpEDDA), illustrating that the post-modification process does not destroy or alter the original structure of the pristine COFs (Supplementary Figs. S5 and S8). To assess the permanent porosity of the samples, nitrogen sorption isotherm at 77 K is conducted. As expected, all the samples display microporous adsorption behavior, and the incorporation of iodide anion decreases the Brunauer-Emmett-Teller (BET) surface area from 715 m2 g−1 of the TpPa-1 to 407 m2 g−1 for CH3I-TpPa-1 (Fig. 1c). The same trends are observed in the other two COFs (Supplementary Figs. S10 and S12). The pore size distribution results also demonstrate that the post-synthesis treatments have a negligible effect on the pore structure of the COFs (Supplementary Figs. S9, S11, and S13). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images show that the TpPa-1 nanorod crystals stack together to exhibit a flower-like morphology (Fig. 1d and Supplementary Fig. S14a). After treatment with CH3I, the CH3I-TpPa-1 maintains its original morphology and exhibits a flower-like appearance (Fig. 1e and Supplementary Fig. S14b). TpBD displays a coral-like morphology, while TpEDDA shows a stacked structure composed of small rod-like components. Similarly, both TpBD and TpEDDA maintain their original morphology after quaternization modification (Supplementary Figs. S15, S17). Furthermore, the energy dispersive spectrometer (EDS) mapping images of CH3I-TpPa-1, CH3I-TpBD, and CH3I-TpEDDA clearly reveal that apart from the C, N, O elements, I element is also uniformly distributed within the framework, providing additional evidence for the successful quaternization modification (Fig. 1f and Supplementary Figs. S16, S18).
Fig. 1. Synthesis, structure, and morphological characterization of samples.
a Schematic illustration for post-quaternization modification of series β-keto-enamine-based COFs to prepare ionic COFs. b PXRD patterns of TpPa-1, CH3I-TpPa-1 and simulated TpPa-1. c N2 adsorption–desorption isotherms of TpPa-1, CH3I-TpPa-1. d TEM images of TpPa-1. e, f TEM image of CH3I-TpPa-1 and the corresponding elemental distributions of C, N, O, and I.
To further identify the successful quaternization modification of TpPa-1, a combination of X-ray photoelectron spectroscopy (XPS, Fig. 2a–d) and solid-state 13C cross-polarization magic angle spinning nuclear magnetic resonance (13C CP/MAS NMR, Fig. 2e) tests are performed. The XPS survey spectra demonstrate that C, N, O, and I elements existed in the modified samples with no other impurity peak observed. In the high-resolution C 1 s XPS spectrum of TpPa-1, three peaks located at 284.4, 285.5, and 288.5 eV can be observed, corresponding to C = C, C-N, and C = O bonds, respectively (Fig. 2b). The high-resolution N 1 s spectrum reveals a single peak located at 399.5 eV, matching well with the C-N-H bond, revealing the structural transition from Enol to Keto and the successful construction of TpPa-1 (Fig. 2c). As for the modified sample, CH3I-TpPa-1 displays additional peeks at 286.8 and 402.5 eV in C 1 s and N 1 s spectra, respectively, ascribed to the formation of C-N+ bonds (Fig. 2a–c)48. Moreover, the I 3 d spectrum displays two peaks at 629.4 and 617.9 eV, corresponding to I 3d3/2 and I 3d5/2, respectively, indicating that I element is present in COFs as a monovalent anion (Fig. 2d). Similar results can also be obtained in other two pairs of COFs (Supplementary Figs. S19–S26). Interestingly, in comparison with TpPa-1, a significant peak appears at 57 ppm in the 13C CP/MAS NMR spectrum of CH3I-TpPa-1, representing the presence of N+-CH3 in the modified framework (Fig. 2e)49. Moreover, the NMR spectrum of the product obtained by treating the monomer synthesized from Tp and aniline with CH3I further confirms the feasibility of the methylation reaction (Supplementary Fig. S27)16. Combining the above results, the successful functionalization of COFs can be fully validated. In addition, the binding energy between iodine anions and the cationic COF framework is calculated as high as − 3.23 eV (see atomic coordinates of optimized computational models in Supplementary Data 1), demonstrating that the structure of CH3I-TpPa-1 is not only rational but also highly stable (Fig. 2f).
Fig. 2. The bonding structure of materials.
a The survey XPS spectra of TpPa-1 and CH3I-TpPa-1. b C 1 s High-resolution XPS spectra of TpPa-1 and CH3I-TpPa-1. c N 1 s High-resolution XPS spectra of TpPa-1 and CH3I-TpPa-1. d I 3 d High-resolution XPS spectra of CH3I-TpPa-1. e 13C CP/MAS NMR spectra of TpPa-1 and CH3I-TpPa-1. f The simulated structure of a fragment of CH3I-TpPa-1.
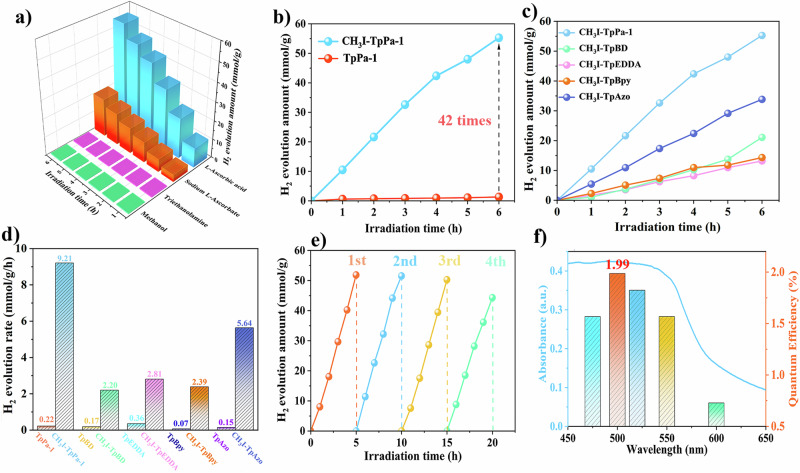
The photocatalytic tests are performed to validate our hypothesis that ion polarization can make great contributions to promoting the photocatalytic process, with initial experiments focusing on photocatalytic H2 production under different sacrificial reagents. When methanol or triethanolamine are used as the sacrificial reagent, only trace amounts of H2 are detected. However, when employing L-ascorbic acid sodium salt or L-ascorbic acid as the sacrificial reagent, the H2 production rate is significantly increased, with L-ascorbic acid demonstrating the highest efficiency on H2 production (Fig. 3a). Following these findings, subsequent photocatalytic tests are conducted under visible light irradiation with L-ascorbic acid as a sacrificial reagent. It is worth mentioning that the entire photocatalytic system is operated without any other metal or Pt co-catalysts. Due to the well-recognized issues such as high exciton dissociation energy and sluggish charge transfer, the original TpPa-1 does not exhibit high photocatalytic activity, only with an H2 evolution rate of 0.22 mmol g−1 h−1. However, after quaternization modification, the photocatalytic performance of CH3I-TpPa-1 is significantly enhanced, resulting in an H2 evolution rate as high as 9.21 mmol g−1 h−1. This represents an approximately 42-fold increase compared to the original TpPa-1, even surpassing most of those reported COFs photocatalysts with Pt as co-catalysts (Fig. 3b and Supplementary Table S2)50–52. The experiment with varying amounts of catalyst is also conducted, confirming that the catalyst exhibits high photocatalytic activity at different dosages (Supplementary Fig. S28). At the same time, photocatalytic hydrogen production experiments are conducted under seawater conditions, and the comparably photocatalytic activity suggests the potential for significant future applications in hydrogen production from seawater (Supplementary Fig. S29). In order to confirm the universality of ion polarization, we also investigated the photocatalytic activities of the other two pairs of COFs (TpBD vs CH3I-TpBD and TpEDDA vs CH3I-TpEDDA). Similar activity trends can be observed that after the quaternization modification, the activity of β-keto-enamine-based COFs series can be significantly enhanced (Fig. 3c, d and Supplementary Figs. S30, S32). In addition to catalytic activity, recyclability is also a crucial factor for evaluating the practical value of photocatalysts. As shown in Fig. 3e and Supplementary Figs. S31, S33, after undergoing 4 times (20 h) cycling tests, the cationic COFs can still maintain their high photocatalytic performance without any observable decline. The crystallinity and morphology of the three COFs can also be maintained after the reaction (Supplementary Figs. S34–S39). Moreover, inductively coupled plasma spectrometry (ICP) is used to quantitatively analyze the leakage of I- after the cycling tests. The results show only a minimal increase in iodine content in the solution, proving that there is almost no leakage of iodine during the reaction (Supplementary Table S1). The above findings have all substantiated the recyclability of the material. In addition, the apparent quantum efficiency (AQY) of CH3I-TpPa−1, CH3I-TpBD, and CH3I-TpEDDA at a wavelength of 500 nm is calculated to be 1.99, 0.85 and 0.7 %, respectively (Fig. 3f and Supplementary Figs. S40, 41). Furthermore, the other β-keto-enamine-based COFs, such as TpBpy53 and TpAzo54, with higher nitrogen content, are also synthesized and subjected to modification and photocatalytic tests under the same conditions (Supplementary Figs. S42, S43). The results are equally gratifying, as their activities are greatly enhanced, further validating the universality of this method (Fig. 3c, d).
Fig. 3. Evaluation of H2 production performance.
a Photocatalytic hydrogen evolution performance of CH3I-TpPa-1 under different sacrificial agents. b–d Photocatalytic hydrogen evolution activity of the synthesized products. e Recycling performance of CH3I-TpPa-1. f Wavelength-dependent apparent quantum efficiency (AQY) of CH3I-TpPa-1.
To explore the factors contributing to the improvement in the photocatalytic performance of β-keto-enamine-based COFs after CH3I treatment, the optical absorption characteristics of samples are first studied using UV-vis diffuse reflectance spectroscopy (UV-vis DRS). These three COFs exhibit significant absorption in the visible light region, indicating great potential for visible light photocatalysis. The absorption edge of the samples is only slightly changed after quaternization modification with CH3I, suggesting a minor variation in their band gaps. According to the Kubelka-Munk (KM) equation and Tauc plots, the band gap energies of TpPa-1 and CH3I-TpPa−1 are determined to be 2.10 and 2.09 eV, respectively. For TpBD and CH3I-TpBD, the band gaps are found to be 2.24 and 2.19 eV, and it is calculated to be 2.13 and 2.17 eV for TpEDDA and CH3I-TpEDDA, respectively (Fig. 4a–c and Supplementary Figs. S44–S49). Subsequently, Mott-Schottky measurements are performed to further elucidate their energy band structures. All samples exhibit n-type semiconductor behavior, as evidenced by the positive slopes observed in their curves. According to the value of intersection at the x-axis, the flat-band potentials (Efb vs. Ag/AgCl) of TpPa-1, TpBD, and TpEDDA are determined to be − 0.73, − 0.57, and − 0.84 eV, respectively (insert images of Fig. 4b, c and Supplementary Figs. S50, S52). According to the equation E(NHE) = E(Ag/AgCl) + 0.2 eV, the normal hydrogen electrode (NHE) potentials of TpPa-1, TpBD, and TpEDDA are estimated to be − 0.53, − 0.37 and − 0.64 V, respectively. It is widely accepted that for n-type semiconductors, the lowest unoccupied molecular orbital (LUMO) is typically around 0.2 eV above the Efb55. Consequently, the LUMO energies of TpPa-1, TpBD, and TpEDDA are estimated to be − 0.73, − 0.57, and − 0.84 eV, respectively. In comparison with pristine COFs, intriguing patterns in experimentation can be observed that after CH3I treatment, all the modified samples exhibit a more negative LUMO energy to be − 0.92, − 0.63, and − 0.90 eV, indicating an enhancement in their reduction potential, which would be more favorable for H+ reduction (Fig. 4c, d and Supplementary Figs. S51 and S53). This phenomenon may be attributed to the electron-withdrawing nature of the iodide anion in their framework, which can stabilize the LUMO and lower its energy. Moreover, in conjugation with the band gaps results, their highest occupied molecular orbitals (HOMO) can also be easily obtained (Fig. 4d). It is well acknowledged that a built-in electric field refers to a generalized electric field existing within a material. It is primarily formed due to charge redistribution caused by spontaneous electron flow at material interfaces or by charge movement within the material under the influence of a weak internal electric field. The built-in electric field (BIEF) extends from regions with low charge density to regions with high charge density within the material. In this study, the enhancement of the BIEF is mainly attributed to the iodide-induced ionic polarization effect. This effect strengthens the non-uniform charge distribution within the COF framework, thereby significantly enhancing the BIEF. To illustrate the effectiveness of quaternization modification in polarizing the COFs and establishing a stronger BIEF in their framework, the charge density distribution of the TpPa-1 and CH3I-TpPa-1 are first studied through density functional theory (DFT) method47,56–59. According to the results of electrostatic potential distribution (ESP), CH3I-TpPa-1 (1.35 Debye) exhibits a larger dipole moment compared to TpPa-1 (0.46 Debye), along with a more significant potential difference (Fig. 4e and Supplementary Fig. S54, S55). The electrostatic potential of the iodide ion unit is significantly higher than that of the Tp unit, highlighting an uneven electron distribution. This imbalance arises from the induced polarization effect, which is caused by electron transfer between the iodide ion unit and the Tp unit. Consequently, the BIEF is established between these two units, oriented from the Tp unit toward the iodide ion unit37. Moreover, the BIEF of both materials is quantitatively measured through the kelvin probe force microscopy (KPFM) tests60,61. As shown in Fig. 4f, g, CH3I-TpPa-1 has a significantly higher surface potential (ΔE = 281 mV) than that of TpPa-1 (ΔE = 51 mV). Utilizing the method reported by Kanata et al.62, it is confirmed that CH3I-TpPa-1 exhibits a 6.4-fold enhancement in the intensity of its BIEF compared with TpPa-1 (Fig. 4h), as calculated by combining the surface charge density and the surface voltage results. All these results consistently indicate that ionic polarization has effectively enhanced the BIEF of the COFs.
Fig. 4. Electronic characterizations of COFs samples.
a UV–vis diffuse reflectance spectra of TpPa-1 and CH3I-TpPa-1. b, c Tauc plot and Mott–Schottky plots of TpPa-1 and CH3I-TpPa-1. d the band structure of series β-keto-enamine-based COFs and modified samples. e The calculated electrostatic potential maps and the corresponding diagrams for TpPa-1 and CH3I-TpPa-1. f, g Surface potentials of TpPa-1 and CH3I-TpPa-1 tested by the Kelvin probe force microscopy (KPFM). h A comparison of BIEF intensity for TpPa-1 and CH3I-TpPa-1.
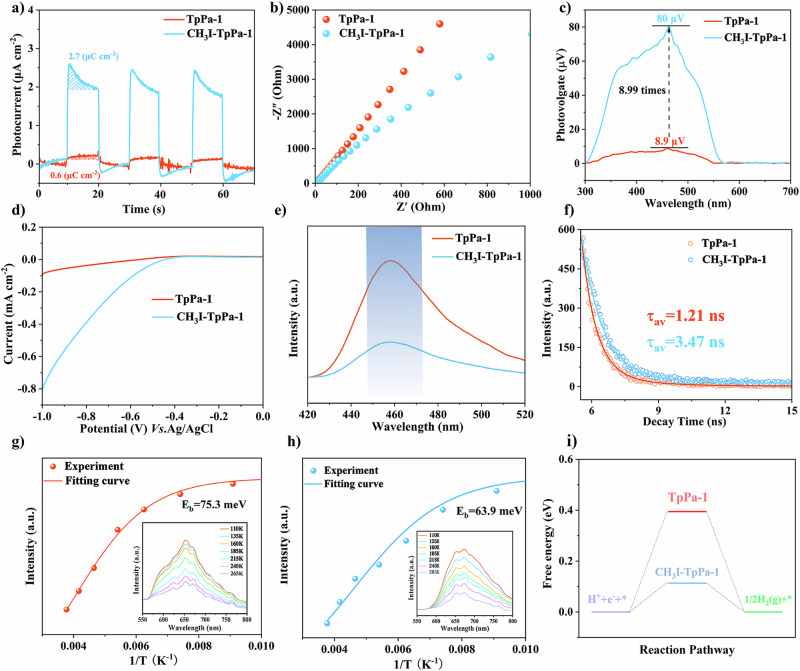
To identify the important role of ionic polarization in promoting the electron-hole pairs migration/separation efficiency, several photoelectrochemical experiments are additionally conducted. The photocurrent response of the CH3I-TpPa-1 is much stronger than that of the TpPa-1, indicating a more charge transfer/separation efficiency (Fig. 5a). The Nyquist curve of CH3I-TpPa-1 exhibits a significantly smaller semicircle diameter compared to TpPa-1, demonstrating a significant improvement in electron transfer efficiency (Fig. 5b). Similar results can also be found in other two pairs of COFs (Supplementary Figs. S56–S59), suggesting the universality of the ionic polarization method. Surface photogenerated voltage (SPV) spectra can also be employed to identify the charge transfer property. As shown in Fig. 5c, the signal of CH3I-TpPa-1 reaches 80 μV, far surpassing that of TpPa-1, which is only 8.9 μV. This significant disparity implies that CH3I-TpPa-1 with a stronger SPV signal has a higher charge separation efficiency. All these findings strongly suggest that the enhanced BIEF associated with ionic polarization can make great contributions to promoting charge migration and separation. The linear sweep voltammetry (LSV) curves clearly show that the electrocatalytic H2 evolution potential of CH3I-TpPa-1 is dramatically lower than that of TpPa-1, implying an enhanced reduction capability for facilitating the H2 evolution reaction after ionic polarization (Fig. 5d). The photoluminescence (PL) and time-resolved photoluminescence (TRPL) spectra are then used to investigate the separation and dynamic behavior of photogenerated charges. Under excitation at 362 nm, the fluorescence intensity of the pristine TpPa-1 is higher than that of the CH3I-TpPa-1, which can be attributed to the role of BIEF in enhancing carrier separation (Fig. 5e). Similar trends are observed in other two pairs COFs (Supplementary Figs. S60–S63). Based on the TRPL results, the average lifetime of CH3I-TpPa-1 is 3.47 ns, while that of TpPa-1 is 1.21 ns, indicating an extended lifetime for photo-induced charges in CH3I-TpPa-1 (Fig. 5f). Excitons dissociation efficiency is another decisive factor for COFs photocatalysts63,64. Thus, temperature-dependent PL measurements are also conducted at different temperatures ranging from 110 to 265 K. The PL intensity of both COFs decreases as the temperature increases, primarily due to the thermally activated nonradiative recombination process. The exciton binding energies Eb of TpPa-1 and CH3I-TpPa-1 are estimated to be 75.3 and 63.9 meV, respectively (Fig. 5g, h). This suggests that the excitons generated in CH3I-TpPa-1 are more likely to undergo dissociation65,66, highlighting the beneficial effect of ionic polarization in lowering the exciton dissociation energy for COFs. Moreover, contact angle tests are also performed to detect possible changes in the hydrophilicity of materials, and the results indicate that the three modified COFs have superior hydrophilicity, which will improve the H+ transfer efficiency and promote the H+ reduction process (Supplementary Figs. S64–S66). The formation of H* is typically recognized as the rate-determining step for H+ reduction67, while Pt is well recognized as an active site for this process due to favorable adsorption and desorption. Here, we verify our experimental results by computation. The results show that the Gibbs free energy (ΔGH*) for the iodine anion as an active site in CH3I-TpPa-1 is only 0.12 eV, much lower than the oxygen site in TpPa-1, which is 0.40 eV, implying that the iodine anion is more likely to serve as the active site for hydrogen production in the modified samples can more effectively balance the hydrogen ions adsorption and desorption processes (Supplementary Figs. S67 and S68), playing a similar function of Pt. In contrast, the higher ΔGH* for TpPa-1 suggests limited release of hydrogen ions, thereby inhibiting photocatalytic activity (Fig. 5i).
Fig. 5. Photoelectrochemical characterization and Gibbs free energy calculations.
a The transient photocurrent response of TpPa-1 and CH3I-TpPa-1. b EIS Nyquist images of TpPa-1 and CH3I-TpPa-1. c SPV spectra of TpPa-1 and CH3I-TpPa-1. d LSV plots of TpPa-1 and CH3I-TpPa-1. e, f The photoluminescence spectra, and time-resolved fluorescence emission decay spectra of TpPa-1 and CH3I-TpPa-1. g, h Temperature-dependent PL spectra with excitation wavelength at 362 nm and extracted exciton binding energies of TpPa-1 and CH3I-TpPa-1. i Calculated free energy diagrams of hydrogen evolution for TpPa-1 and CH3I-TpPa-1.
After validating through the aforementioned experimental and theoretical calculations tests, the boosted photocatalytic mechanism can be demonstrated. Through a unique post-synthetic quaternization reaction, ionic polarization can be achieved in a series of β-keto-enamine-based COFs, thus endowing them with separate charge centers comprising cationic skeleton and iodide counter-anions. Due to the different electrostatic potential values between the iodide anions unit and the cationic framework unit, a local electric field can be formed between the iodide anions unit and the cationic framework unit, with the direction from the cationic framework unit to the iodide anions unit, thus establishing robust BIEF within their framework. Benefitting from the enhanced BIEF, the charge separation and the exciton dissociation efficiency of the modified COFs can be significantly enhanced, which can be proved by a series of optical and spectroscopy tests. In addition, in pristine β-keto-enamine-based COFs, the oxygen atom can act as the active site to promote the H2 evolution. After being modified through quaternization reaction, due to the electron-withdrawing effect, the introduced iodide anion not only serves as an additional active site to reduce the energy barrier of H* formation but also acts as an electron extractant to promote the electron-hole pairs separation/migration, confirmed by series of photoelectric and theoretical calculation tests. Therefore, under the synergistic effect, the photocatalytic activity of series modified β-keto-enamine-based COFs materials for hydrogen production is significantly enhanced.
In conclusion, we have successfully constructed a series of cationic covalent organic frameworks (COFs) incorporating iodine anions via a post-synthetic method. The ionic framework of these COFs induces ionic polarization, resulting in a stronger built-in electric field, as validated through KPFM tests and DFT calculations. Moreover, the presence of iodine ions can provide more electron-rich reduction centers, thereby improving the efficiency of electron-hole pair separation and promoting photocatalytic reactions. It is worth mentioning that the addition of Pt co-catalysts is typically required in most reported COFs photocatalysts. However, with the help of ionic polarization, the photocatalytic H2 evolution performance of the modified samples is significantly improved and can even display commendable H2 evolution performance without the addition of Pt co-catalysts. This is particularly evident in CH3I-TpPa-1, where the H2 production rate reaches as high as 9.21 mmol g−1 h−1. Overall, this work paves the way for addressing the issues of high exciton dissociation energy and sluggish charge transfer via rational molecule-level structure design of ion-polarized COFs. Moreover, the great potential of ion-polarized COFs as highly efficient photocatalysts and is significant for clean energy generation.
Methods
Preparation of COFs
TpPa-1 was synthesized based on previously reported methods with slight adjustments. Initially, 0.1 mmol (purity > 8%) of 1,3,5-triformylphloroglucinol (Tp) and 0.15 mmol (purity > 99.3%) of p-phenylenediamine (Pa-1) ligands were effectively dispersed in 3 mL of N, N-dimethylformamide (DMF, purity > 99.5%) utilizing ultrasound. Next, 0.5 mL of 3 M acetic acid was introduced into the tube. After achieving uniform ultrasonication of the solution, the tube was transferred to liquid nitrogen (77 K) for rapid freezing and subjected to three rounds of vacuum treatment. Then, the tube was hermetically sealed and placed inside an oven, where it was subjected to heating at 120 °C for a period of 3 days. The resulting product was obtained through centrifugation, followed by multiple washes with anhydrous tetrahydrofuran (THF, purity > 99.5%) and anhydrous acetone (purity > 99.8%). Finally, the product was dried under vacuum at 60 °C for a duration of 24 h to get TpPa-1. Using a similar approach, the replacement of p-phenylenediamine (Pa-1) with 0.15 mmol (purity > 99.5%) of 4,4’-Diaminobiphenyl (BD) or 0.15 mmol (purity > 99.7%) of 4,4’-(ethyne-1,2-diyl)dianiline (EDDA) was carried out to synthesize TPBD or TPEDDA.
TpBpy was prepared using a solvothermal synthesis method. In this process, 0.3 mmol of Tp and 0.45 mmol of (2,2’-bipyridine)-5,5’-diamine (Bpy) (purity > 98.8%) were dissolved in a solvent mixture containing 4.5 mL of N,N-dimethylacetamide (DMAc) (purity > 99.0%) and 1.5 mL of ortho-dichlorobenzene (ODCB). In addition, 0.6 mL of 6 M acetic acid was added. The resulting solution was ultrasonicated for 15 min to ensure complete dispersion and then subjected to three cycles of freeze-pump-thaw to remove dissolved gases. After degassing, the sealed reaction mixture was heated at 120 °C for 72 h. Once the reaction was complete, the product was collected by filtration and washed thoroughly with deionized water and DMAc to remove unreacted precursors and oligomeric byproducts. The final purification step involved rinsing the material with acetone, followed by vacuum drying at 60 °C for 24 h68.
TpAzo was synthesized via a solvothermal method. Initially, 0.3 mmol of Tp, along with the corresponding diamine 4,4′-azodianiline (Azo) (purity > 99.2%), 3 mL of DMAc, and 3 mL of ODCB, were combined and sonicated for 10 min to form a uniform dispersion. The mixture was then quickly frozen in a liquid nitrogen bath (77 K) and degassed by performing three cycles of freeze-pump-thaw. After sealing the tube, it was heated at 120 °C for 3 days. The final sample was washed with acetone and then dried under a vacuum at 60 °C for 24 h.
Preparation of Iodine ion modified COFs
20 mg of the above product was added to 3 mL of DMF, followed by the addition of 1 mL of CH3I. The mixture was stirred in the dark at room temperature for 24 hours. The resulting product was then washed twice with acetone, collected by centrifugation, and finally dried under vacuum at 60 oC for 4 h. The product was named CH3I-TpPa-1. The same method was applied to obtain CH3I-TpBD and CH3I-TpEDDA. The product was named CH3I-TpPa-1. The same method was applied to obtain CH3I-TpBD, CH3I-TpEDDA, CH3I-TpBpy, and CH3I-TpAzo.
Photocatalytic H2 production rests
Photocatalytic H2 production experiments were conducted using a full glass automatic online trace gas analysis system (Labsolar-6A, Beijing Perfectlight Technology Co., Ltd.). The light source was a 300 W Xenon lamp with a 420 nm filter (PLS-SXE300/300UV, light intensity: 100 mW·cm−², Beijing Perfectlight Technology Co., Ltd.). Hydrogen detection and quantitative analysis were performed using a GC7900 gas chromatography system (Shanghai Tianmei Scientific Instrument Co.) with nitrogen as the carrier gas at 30-minute intervals. The catalyst was placed in a custom quartz glass reactor, and the reaction temperature was maintained at 5 °C using a water condenser (Supplementary Fig. S69). 10 mg of catalyst was added to 100 mL of deionized water containing 100 mg of L-Ascorbic acid as a hole scavenger. The suspension was then stirred in a 200 mL custom quartz reactor. The suspension was then transferred to the custom quartz reactor for the photocatalytic reaction. Prior to each photocatalytic reaction, the system was vacuum-treated several times to remove dissolved air. The entire reaction system was irradiated with Xe light using a UV cut-off filter (λ ≥ 420 nm). The vertical distance between the Xe lamp and the reactor was approximately 3 cm. In addition, the apparent quantum efficiency (AQE) was measured using a xenon lamp equipped with bandpass filters. Photocatalytic experiments were performed with 10 mg of catalysts, using filters with wavelengths of 475, 500, 520, 550, and 600 nm, respectively.
Photoelectrochemical characterization
The electrochemical measurements were conducted using an electrochemical workstation, model CHI 760. The experiment employed a three-electrode system, where a Pt sheet served as the counter electrode and an Ag/AgCl electrode as the reference electrode (The reference electrode calibration method: First, clean the reference electrode using deionized water to remove any contaminants. Then, adjust the potential of the reference electrode via an external circuit until it reaches a stable state. Finally, allow the potential to stabilize and check again). 0.2 M Na2SO4 solution (pH = 7) was used as the electrolyte and prepared fresh before each use (Supplementary Fig. S70). A xenon lamp with a filter (λ ≥ 420 nm) provided the visible light source. To prepare the working electrode, 5 mg of the catalyst sample and 20 μL of Nafion solution were dissolved in 1 mL of anhydrous ethanol, sonicated for 30 min to ensure thorough mixing, and then applied onto FTO glass (1 cm × 1 cm). (The above electrochemical measurement results have not been corrected for iR compensation.)
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 52071171, 52202248, 22101105), Liaoning Province Centrally Guided Local Science and Technology Development Fund Program (2024JH6/100700010, 2024JH6/100700011). Open Project of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry (2024-35), Open Research Fund of Guangdong Advanced Carbon Materials Co., Ltd (Kargen-2024B1001). T.M. acknowledged the Australian Research Council (ARC) through Future Fellowship (FT210100298), Discovery Project (DP220100603), Linkage Project (LP210200504, LP220100088, LP230200897) and Industrial Transformation Research Hub (IH240100009) schemes, the Australian Government through the Cooperative Research Centers Projects (CRCPXIII000077), the Australian Renewable Energy Agency (ARENA) as part of ARENA’s Transformative Research Accelerating Commercialization Program (TM021), and European Commission’s Australia-Spain Network for Innovation and Research Excellence (AuSpire). X.L. acknowledged the support of the ARC DECRA fellowship(DE250100232)
Author contributions
M.T. and S.X. proposed the project and designed experiments. Z.J. and Hu.H. synthesized and characterized the samples and performed experiments and data analysis. L.H. and Hu.H. contributed to the analyses of KPFM results. S.X. wrote the paper. S.X., M.T. and L.X. polished the writing of the paper. Z.J. and L.X. contributed equally to this work. All authors discussed the results and commented on the paper.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data are included in the main text or the supplementary information. Source data are provided in this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiahe Zhang, Xiaoning Li.
Contributor Information
Xiaodong Sun, Email: sunxiaodong@lnu.edu.cn.
Tianyi Ma, Email: tianyi.ma@rmit.edu.au.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53834-w.
References
- 1.Wang, Z., Li, C. & Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev.48, 2109–2125 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Lei, Y. et al. Designing atomic active centers for hydrogen evolution electrocatalysts. Angew. Chem. Int. Ed.59, 20794–20812 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Ran, J., Zhang, J., Yu, J., Jaroniec, M. & Qiao, S. Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev.43, 7787–7812 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Feng, X., Ding, X. & Jiang, D. Covalent organic frameworks. Chem. Soc. Rev.41, 6010–6022 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Ding, S.-Y. & Wang, W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev.42, 548–568 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Freund, R. et al. The current status of MOF and COF applications. Angew. Chem. Int. Ed.60, 23975–24001 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Yong, Z. & Ma, T. Solar‐to‐H2O2 catalyzed by covalent organic frameworks. Angew. Chem. Int. Ed.135, e202308980 (2023). [DOI] [PubMed] [Google Scholar]
- 8.He, T. & Zhao, Y. Covalent organic frameworks for energy conversion in photocatalysis. Angew. Chem. Int. Ed.62, e202303086 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Wang, H. et al. Covalent organic framework photocatalysts: structures and applications. Chem. Soc. Rev.49, 4135–4165 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Zhang, F. M. et al. Rational design of MOF/COF hybrid materials for photocatalytic H2 evolution in the presence of sacrificial electron donors. Angew. Chem. Int. Ed.57, 12106–12110 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Wang, H. et al. Integrating suitable linkage of covalent organic frameworks into covalently bridged inorganic/organic hybrids toward efficient photocatalysis. J. Am. Chem. Soc.142, 4862–4871 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Shen, R., Liang, G., Hao, L., Zhang, P. & Li, X. In situ synthesis of chemically bonded 2D/2D covalent organic frameworks/O‐vacancy WO3 Z‐scheme heterostructure for photocatalytic overall water splitting. Adv. Mater.35, 2303649 (2023). [DOI] [PubMed] [Google Scholar]
- 13.Hu, H. et al. Construction of a 2D/2D crystalline porous materials-based S‐scheme heterojunction for efficient photocatalytic H2 production. Adv. Energy Mater. 14, 2303638 (2024).
- 14.Yang, H. et al. Local electron donor defects induce dipole polarization boosting on covalent organic frameworks to promote photocatalysis. ACS Mat. Lett.5, 2877–2886 (2023). [Google Scholar]
- 15.Zheng, Q. et al. A covalent organic framework onion structure. Mater. Today60, 98–105 (2022). [Google Scholar]
- 16.Weng, W. & Guo, J. The effect of enantioselective chiral covalent organic frameworks and cysteine sacrificial donors on photocatalytic hydrogen evolution. Nat. Commun.13, 5768 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou, W. et al. Heterogenization of salen metal molecular catalysts in covalent organic frameworks for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed.62, e202214143 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Li, Y. et al. In situ photodeposition of platinum clusters on a covalent organic framework for photocatalytic hydrogen production. Nat. Commun.13, 1355 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong, P. et al. Platinum single atoms anchored on a covalent organic framework: boosting active sites for photocatalytic hydrogen evolution. ACS Catal.11, 13266–13279 (2021). [Google Scholar]
- 20.Qian, Y. et al. Computation-based regulation of excitonic effects in donor-acceptor covalent organic frameworks for enhanced photocatalysis. Nat. Commun.14, 3083 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, W. et al. Thiazolo [5, 4‐d] thiazole‐based donor-acceptor covalent organic framework for sunlight‐driven hydrogen evolution. Angew. Chem. Int. Ed.60, 1869–1874 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Yang, J. et al. Protonated imine‐linked covalent organic frameworks for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed.60, 19797–19803 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang, J. et al. Constitutional isomerism of the linkages in donor–acceptor covalent organic frameworks and its impact on photocatalysis. Nat. Commun.13, 6317 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang, Q., Luo, M., Liu, K., Cao, H. & Yan, H. Covalent organic frameworks for photocatalytic applications. Appl. Catal. B Environ.276, 119174 (2020). [Google Scholar]
- 25.Rajak, S., Vu, N.-N., Kaur, P., Duong, A. & Nguyen-Tri, P. Recent progress on the design and development of diaminotriazine based molecular catalysts for light-driven hydrogen production. Coord. Chem. Rev.456, 214375 (2022). [Google Scholar]
- 26.Chen, F., Huang, H., Guo, L., Zhang, Y. & Ma, T. The role of polarization in photocatalysis. Angew. Chem. Int. Ed.58, 10061–10073 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Pan, L. et al. Manipulating spin polarization of titanium dioxide for efficient photocatalysis. Nat. Commun.11, 418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao, Y. et al. The polarization effect in surface‐plasmon‐induced photocatalysis on Au/TiO2 nanoparticles. Angew. Chem. Int. Ed.132, 18375–18380 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Ben, H. et al. Local spatial polarization induced efficient charge separation of squaraine‐linked COF for enhanced photocatalytic performance. Adv. Funct. Mater.32, 2104519 (2022). [Google Scholar]
- 30.Huang, J. et al. Gradient tungsten-doped Bi3TiNbO9 ferroelectric photocatalysts with additional built-in electric field for efficient overall water splitting. Nat. Commun.14, 7948 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao, X. et al. Rational regulation of the exciton effect of acrylonitrile-linked covalent organic framework toward boosting visible-light-driven hydrogen evolution. ACS Catal.14, 533–546 (2023). [Google Scholar]
- 32.Xu, H. et al. Programming tetrathiafulvalene‐based covalent organic frameworks for promoted photoinduced molecular oxygen activation. Angew. Chem. Int. Ed.63, e202405476 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Wang, Y. et al. Linkages make a difference in the photoluminescence of covalent organic frameworks. Angew. Chem. Int. Ed.135, e202310794 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Qian, Y., Li, D., Han, Y. & Jiang, H.-L. Photocatalytic molecular oxygen activation by regulating excitonic effects in covalent organic frameworks. J. Am. Chem. Soc.142, 20763–20771 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Fu, Y. et al. Zwitterionic covalent organic frameworks: attractive porous host for gas separation and anhydrous proton conduction. ACS Nano15, 19743–19755 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Chen, S. et al. Tuning proton dissociation energy in proton carrier doped 2D covalent organic frameworks for anhydrous proton conduction at elevated temperature. J. Mater. Chem. A8, 13702–13709 (2020). [Google Scholar]
- 37.Li, J. et al. Symmetrical localized built-in electric field by Induced polarization effect in ionic covalent organic frameworks for selective imaging and killing bacteria. ACS Nano18, 4539–4550 (2024). [DOI] [PubMed] [Google Scholar]
- 38.Zhang, Z. & Xu, Y. Hydrothermal synthesis of highly crystalline zwitterionic vinylene-linked covalent organic frameworks with exceptional photocatalytic properties. J. Am. Chem. Soc.145, 25222–25232 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Zhang, P., Wang, Z., Cheng, P., Chen, Y. & Zhang, Z. Design and application of ionic covalent organic frameworks. Coord. Chem. Rev.438, 213873 (2021). [Google Scholar]
- 40.Xie, Y. et al. Ionic functionalization of multivariate covalent organic frameworks to achieve an exceptionally high iodine‐capture capacity. Angew. Chem. Int. Ed.60, 22432–22440 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Bera, S. et al. Odd-even alternation in tautomeric porous organic cages with exceptional chemical stability. Angew. Chem. Int. Ed.56, 2123–2126 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Majumder, P. et al. Proximity-enabled photochemical C-H functionalization using a covalent organic framework-confined Fe2IV-μ-oxo species in water. J. Am. Chem. Soc.145, 18855–18864 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Yin, L. et al. Structure-property relationship in β-keto-enamine-based covalent organic frameworks for highly efficient photocatalytic hydrogen production. Chem. Eng. J.419, 129984 (2021). [Google Scholar]
- 44.Basak, A., Karak, S. & Banerjee, R. Covalent organic frameworks as porous pigments for photocatalytic metal-free C-H borylation. J. Am. Chem. Soc.145, 7592–7599 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Kandambeth, S. et al. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc.134, 19524–19527 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Biswal, B. P. et al. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc.135, 5328–5331 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Pachfule, P. et al. Diacetylene functionalized covalent organic framework (COF) for photocatalytic hydrogen generation. J. Am. Chem. Soc.140, 1423–1427 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Liu, M. et al. Post-synthetic modification of covalent organic frameworks for CO2 electroreduction. Nat. Commun.14, 3800 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou, L.-L., Guan, Q., Zhou, W., Kan, J.-L. & Dong, Y.-B. An iodide-containing covalent organic framework for enhanced radiotherapy. Chem. Sci.14, 3642–3651 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, J., Jing, J., Li, W. & Zhu, Y. Electron donor–acceptor interface of TPPS/PDI boosting charge transfer for efficient photocatalytic hydrogen evolution. Adv. Sci.9, 2201134 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, Z., Zhu, Y., Chen, X., Zhang, H. & Wang, J. A full‐spectrum metal‐free porphyrin supramolecular photocatalyst for dual functions of highly efficient hydrogen and oxygen evolution. Adv. Mater.31, 1806626 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Pu, Z., Amiinu, I. S., Kou, Z., Li, W. & Mu, S. RuP2‐based catalysts with platinum‐like activity and higher durability for the hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed.56, 11559–11564 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Paul, S. et al. Covalent organic frameworks for the purification of recombinant enzymes and heterogeneous biocatalysis. J. Am. Chem. Soc.146, 858–867 (2024). [DOI] [PubMed] [Google Scholar]
- 54.Kumar Mahato, A. et al. Covalent organic framework cladding on peptide-amphiphile-based biomimetic catalysts. J. Am. Chem. Soc.145, 12793–12801 (2023). [DOI] [PubMed] [Google Scholar]
- 55.Xu, H.-Q., Yang, S., Ma, X., Huang, J. & Jiang, H.-L. Unveiling charge-separation dynamics in CdS/metal-organic framework composites for enhanced photocatalysis. ACS Catal.8, 11615–11621 (2018). [Google Scholar]
- 56.Banerjee, T. et al. Single-site photocatalytic H2 evolution from covalent organic frameworks with molecular cobaloxime co-catalysts. J. Am. Chem. Soc.139, 16228–16234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen, W. et al. Modulating benzothiadiazole‐based covalent organic frameworks via halogenation for enhanced photocatalytic water splitting. Angew. Chem. Int. Ed.132, 17050–17057 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Liu, Y. et al. One-dimensional covalent organic frameworks with atmospheric water harvesting for photocatalytic hydrogen evolution from water vapor. Appl. Catal. B Environ.338, 123074 (2023). [Google Scholar]
- 59.Bi, S. et al. Two-dimensional semiconducting covalent organic frameworks via condensation at arylmethyl carbon atoms. Nat. Commun.10, 2467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo, Y., Shi, W. & Zhu, Y. Internal electric field engineering for steering photogenerated charge separation and enhancing photoactivity. EcoMat1, e12007 (2019). [Google Scholar]
- 61.Yue, J. Y. et al. Regulating the topology of covalent organic frameworks for boosting overall H2O2 photogeneration. Angew. Chem. Int. Ed.63, e202405763 (2024). [DOI] [PubMed] [Google Scholar]
- 62.Kanata-Kito, T., Matsunaga, M., Takakura, H., Hamakawa, Y. & Nishino, T. Photoreflectance characterization of built-in potential in MBE-produced As-grown GaAs surface; proceedings of the Modulation Spectroscopy. SPIE News.1286, 56–65 (1990). [Google Scholar]
- 63.Hao, L. et al. Fluorenone-based covalent organic frameworks with efficient exciton dissociation and well-defined active center for remarkable photocatalytic hydrogen evolution. Appl. Catal. B Environ.330, 122581 (2023). [Google Scholar]
- 64.Li, G. et al. Boosting exciton dissociation by regulating dielectric constant in covalent organic framework for photocatalysis. Chem Catal.2, 1734–1747 (2022). [Google Scholar]
- 65.Chen, Q., Wang, Y. & Luo, G. Photoenzymatic CO2 reduction dominated by collaborative matching of linkage and linker in covalent organic frameworks. J. Am. Chem. Soc.146, 586–598 (2023). [DOI] [PubMed] [Google Scholar]
- 66.Hou, Y., Liu, F., Nie, C., Li, Z. & Tong, M. Boosting exciton dissociation and charge transfer in triazole-based covalent organic frameworks by increasing the donor unit from one to two for the efficient photocatalytic elimination of emerging contaminants. Environ. Sci. Technol.57, 11675–11686 (2023). [DOI] [PubMed] [Google Scholar]
- 67.Yan, G. et al. Integrating covalent organic framework with transition metal phosphide for noble‐metal‐free visible‐light‐driven photocatalytic H2 evolution. Small18, 2201340 (2022). [DOI] [PubMed] [Google Scholar]
- 68.Xu, H., Gao, J. & Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem.7, 905–912 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data are included in the main text or the supplementary information. Source data are provided in this paper.