Abstract
Surgical site infection (SSI) following lumbar disc herniation (LDH) surgery leads to prolonged hospital stays, increased costs and reoperations. Therefore, we aim to develop and validate a nomogram to predict the risk of SSI following LDH surgery, thereby helping spine surgeons design personalized prevention strategies and promote early recovery. Data from 647 patients with SSI who underwent LDH surgery at the First Affiliated Hospital of Air Force Medical University (AFMU) from 2020 to 2023 were collected. Ultimately, 241 patients with SSI were selected based on inclusion and exclusion criteria. Patients were randomly divided into training and validation sets with a ratio of 7:3. LASSO regression, univariate, and multivariate logistic regression were utilized to identify target variables and establish the prediction model, which was subsequently validated. Six factors—Age, Body Mass Index (BMI), Postoperative Suction Drainage (PSD), Gelatin Sponge (GS), None-Preoperative Antibiotic (NPTA), and Thrombin Time (TT)—were selected to construct the nomogram model. In the training set, the area under the curve (AUC) for the nomogram was 0.818 (95% CI 0.779–0.857). In the validation set, the AUC was 0.782 (95% CI 0.717–0.846). Calibration curves for both sets showed satisfactory agreement between predicted and actual SSI probabilities. Decision curve analysis indicated that the nomogram is clinically useful with a threshold range of 1–90%. The Clinical Impact Curve (CIC) demonstrated an acceptable cost-benefit ratio. The developed nomogram model effectively predicts the risk of SSI following LDH surgery, enabling spine surgeons to formulate more professional and rational clinical prevention strategies.
Keywords: Lumbar disc herniation, Surgical site infection, Prediction model, Nomogram, Risk factor
Subject terms: Infectious diseases, Trauma, Diagnosis, Disease prevention, Public health, Risk factors, Outcomes research
Introduction
The number of surgeries for spinal diseases has been rising globally in recent years1. Lumbar disc herniation (LDH) is a prevalent issue in spinal surgery, with an incidence of 2–4%2. LDH frequently affects men aged 30 to 50, with an increasing trend in younger patients3. Non-surgical treatment is the first-line approach for LDH, while surgery is essential for patients unresponsive to conservative methods. Primary surgical procedures, including lumbar discectomy and lumbar fusion, can offer long-term symptom relief. However, these operations often carry risks. These procedures may result in various postoperative complications, such as infection, recurrence, and symptomatic epidural hematoma4.
Surgical site infection (SSI) is a serious postoperative complication, defined as an infection occurring within 30 days after surgery if no implant is present, or within one year if an implant is involved and the infection is surgery-related5. SSI not only increases patient readmission and mortality rates but also significantly escalates healthcare costs, by two to four times, imposing a substantial burden on both patients and healthcare systems6. Despite advances in infection control measures, including enhanced operating room ventilation, barrier implementation, and antibacterial prophylaxis, the incidence of SSI remains high; for instance, the reported incidence after lumbar surgery is 0.81%7. Effective management of SSI following LDH surgery is crucial for optimal postoperative outcomes and patient satisfaction.
Numerous studies have sought to identify risk factors for SSI following spinal surgery to develop preventive strategies8–10. Common risk factors include age, diabetes, obesity, smoking, alcohol, long-term steroid use, implants, prolonged surgical time, excessive blood loss, number of fusion segments, revision status, and surgical method8–12. However, the results of these studies have been inconsistent, preventing reliable conclusions about these risk factors and perpetuating debate on the optimal SSI management strategies13. Nonetheless, the importance of prevention has been consistently underscored, highlighting the critical need to identify factors linked to an elevated SSI risk14.
This study aimed to identify risk factors for SSI following LDH surgery, establish a predictive model, and provide evidence-based insights for SSI identification, assessment, and prevention. The overarching goals were to enhance patient satisfaction and treatment outcomes, reduce morbidity, and control healthcare costs.
Materials and methods
Ethics statement
The present study was approved by the Ethics Committee of the First Affiliated Hospital of the Air Force Medical University (AFMU) (Approval NO. KY20232117-C-1). Research involving human subjects complied with all relevant national regulations, institutional policies and was conducted in accordance with the tenets of the 1964 Helsinki Declaration. All participants provided written informed consent.
Patients and study design
Participants diagnosed with LDH and undergoing surgery at the First Affiliated Hospital of the AFMU between January 2020 and December 2023 were included in this population-based retrospective study. We included 241 patients with SSI following LDH surgery and screened two other patients without SSI who had undergone surgery performed by the same surgeon within a similar timeframe as a control group.
Patients meeting all the following criteria were included:
Patients who had surgery of LDH and completed the entire procedure.
Patients with complete medical records.
Patients with SSI after the initial surgery.
Patients who have signed informed consent.
Exclusion criteria encompassed:
SSI causes unrelated to the initial surgery (pressure sores, secondary trauma, etc.).
Patients with infectious spinal diseases (spinal tuberculosis, suppurative, spondylitis and brucellosis, etc.).
Patients with lumbar surgery due to other diseases (trauma, tumour, fracture, cyst, etc.).
Patients with other serious diseases (malignant tumors, heart failure, chronic kidney disease, neurological diseases, COPD, coagulation dysfunction, etc.).
Patients with incomplete data (missing > 20%).
Data collection
Data were obtained from the hospital’s electronic medical record system (EMRS). Patient information was identified using ID numbers. By combining literature review and clinical experience, we identified 21 potential risk factors for SSI. These factors include: (1) demographic indicators: gender, age, smoking status, drinking status, high blood pressure (HBP), diabetes mellitus (DM), and body mass index (BMI); (2) perioperative indicators: multi-stage surgery (MS), implant, operation time (OT), intraoperative blood loss (IBL), postoperative suction drainage (PSD), gelatin sponge (GS), postoperative anticoagulation (PTAG), and None-preoperative antibiotic (NPTA); (3) laboratory indicators: postoperative hemoglobin (PTHB), postoperative serum albumin (PTSA), liver and kidney function (LKF), K+, Ca+, and thrombin time (TT). Since the datasets used in this study contained missing values, removing all incomplete data might reduce the amount and quality of data analyzed, thereby compromising the predictive results. Therefore, we excluded data with more than 20% missing values.
Conduct test
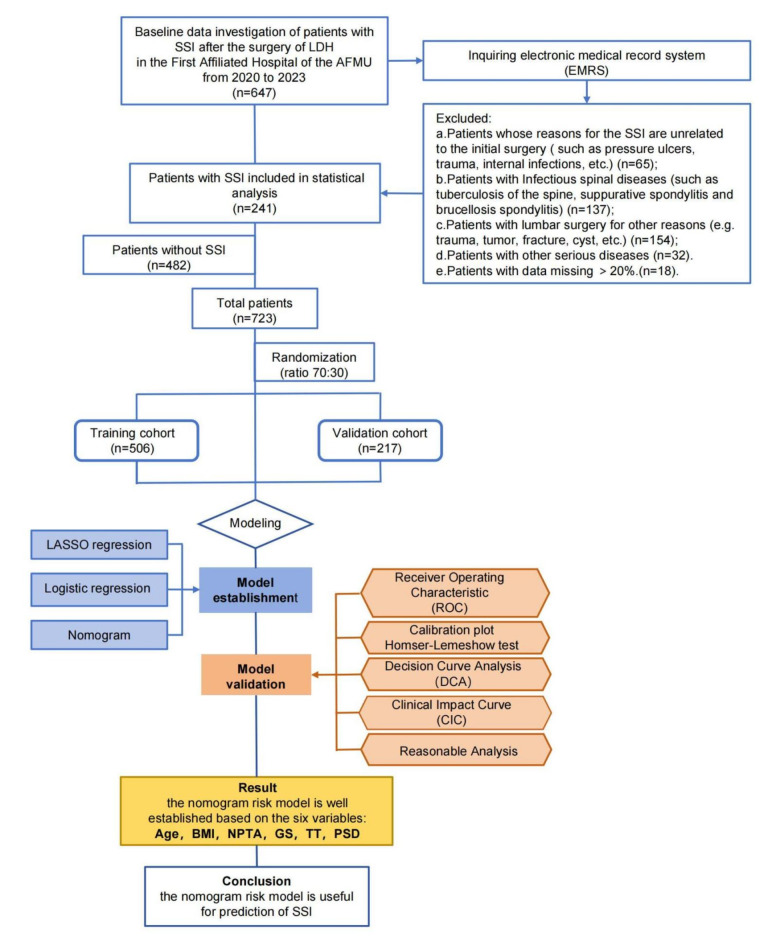
Research subjects were randomly divided into training and validation sets at a ratio of 7:3 using the R package. The training set comprised 506 participants, with 165 patients diagnosed with SSI and 341 without. The validation set, meanwhile, consisted of 217 patients, of which 76 had SSI and 141 did not. The data from the training set were utilized to discern the characteristics and patterns inherent in the sample, enabling the development of a predictive model. Conversely, the validation set served to assess the model’s performance, specifically through validation and tuning processes, whilst also evaluating its generalization capability to novel samples. A comparative analysis of indices was conducted between the SSI and non-SSI groups within both the training and validation sets. The study flow chart is shown in Fig. 1.
Fig. 1.
The workflow of the study. LDH Lumbar disc herniation, AFMU Air Force Medical University, SSI surgical site infection, LASSO least absolute shrinkage and selection operator, BMI body mass index, PSD postoperative suction drainagen, GS gelatin sponge, NPTA none-preoperative antibiotic, TT thrombin time.
Statistical analysis
Statistical analysis was primarily conducted using R software (version 4.2.1) and SPSS software (IBM, version 26.0). GraphPad Prism V9.2.0.332 was used to analyze correlation between variables. Categorical variables were shown as number and percentages, which were compared using the Chi-square (χ2) test or Fishers’ exact test. The least absolute shrinkage and selection operator (LASSO), along with univariate and multivariate logistic regression analyses, were conducted to investigate risk factors for SSI following LDH surgery. The rms package was used to construct the nomogram. The clinical prediction model underwent internal validation in validation set using the Bootstrap resampling method with B = 1000 repetitions. The ROC curve, calibration curve, Decision Curve Analysis (DCA), clinical impact curve (CIC) and reasonable analysis were used to evaluate the discrimination and predictive capability of the nomogram model. The model’s performance was graded as follows: (1) 0.5 < AUC ≤ 0.7, indicating low predictive value; (2) 0.7 < AUC ≤ 0.9, indicating moderate predictive value; and (3) 0.9 < AUC < 1, indicating high predictive value. In this study, statistical significance was defined as p < 0.05.
Results
Patient characteristics
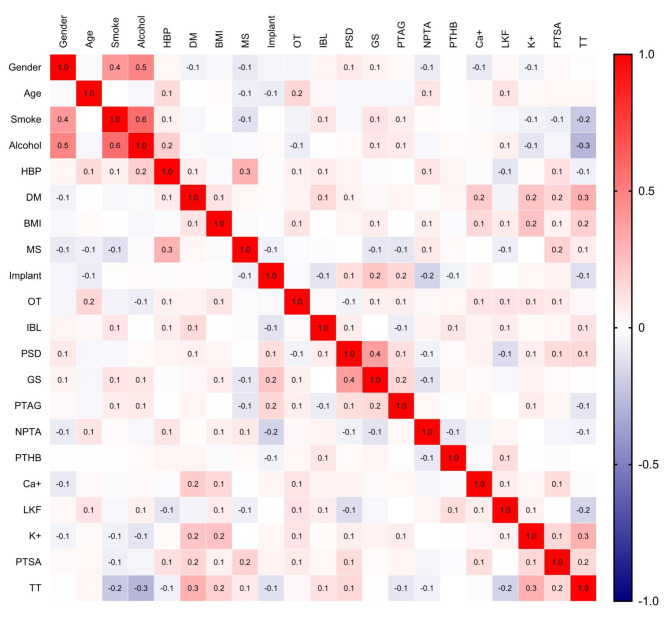
Table 1 shows variations in demographic, perioperative, and laboratory indicators between SSI and Non-SSI patients in both the training and validation sets. In the training set, there were no significant differences between SSI and Non-SSI patients in terms of gender, smoking and drinking status, HBP, and other demographic indicators. For perioperative indexes, SSI patients exhibited significantly higher ratios of OT, PSD, and GS compared to Non-SSI patients perioperative indexes such as MS, Implant, IBL, and PTAG showed no significant differences. Additionally, laboratory indicators indicated no significant differences in THB, LKF, and Ca+. The heat map shows no significant strong correlations between the variables (Fig. 2) .
Table 1.
Comparisons of demographic, perioperative and laboratory indicators between SSI and Non-SSI patients in the training and validation set.
| Variables | Training set | Validation set | ||||
|---|---|---|---|---|---|---|
| SSI | Non-SSI | P-value | SSI | Non-SSI | P-value | |
| (N = 165) | (N = 341) | (N = 76) | (N = 141) | |||
| Demographic Indicators | ||||||
| Gender | 0.20 | 1.00 | ||||
| Female | 79 (47.88%) | 141 (41.35%) | 34 (44.74%) | 64 (45.39%) | ||
| Male | 86 (52.12%) | 200 (58.65%) | 42 (55.26%) | 77 (54.61%) | ||
| Age | 0.001 | 0.21 | ||||
| <60 years | 98 (59.39%) | 253 (74.19%) | 52 (68.42%) | 109 (77.30%) | ||
| ≥ 60 years | 67 (40.61%) | 88 (25.81%) | 24 (31.58%) | 32 (22.70%) | ||
| Smoke status | 0.72 | 0.03 | ||||
| Never | 107 (64.85%) | 214 (62.76%) | 41 (53.95%) | 99 (70.21%) | ||
| Ever/current | 58 (35.15%) | 127 (37.24%) | 35 (46.05%) | 42 (29.79%) | ||
| Drink status | 0.35 | 0.72 | ||||
| Never | 134 (81.21%) | 263 (77.13%) | 58 (76.32%) | 103 (73.05%) | ||
| Ever/current | 31 (18.79%) | 78 (22.87%) | 18 (23.68%) | 38 (26.95%) | ||
| HBP | 0.63 | 0.03 | ||||
| No | 109 (66.06%) | 234 (68.62%) | 47 (61.84%) | 108 (76.60%) | ||
| Yes | 56 (33.94%) | 107 (31.38%) | 29 (38.16%) | 33 (23.40%) | ||
| DM | 0.001 | 0.001 | ||||
| No | 97 (58.79%) | 251 (73.61%) | 40 (52.63%) | 106 (75.18%) | ||
| Yes | 68 (41.21%) | 90 (26.39%) | 36 (47.37%) | 35 (24.82%) | ||
| BMI | <0.001 | 0.004 | ||||
| <28 kg/m2 | 51 (30.91%) | 185 (54.25%) | 25 (32.89%) | 77 (54.61%) | ||
| ≥ 28 kg/m2 | 114 (69.09%) | 156 (45.75%) | 51 (67.11%) | 64 (45.39%) | ||
| Perioperative indicators | ||||||
| MS: | 0.96 | 0.10 | ||||
| No | 115 (69.70%) | 240 (70.38%) | 47 (61.84%) | 104 (73.76%) | ||
| Yes | 50 (30.30%) | 101 (29.62%) | 29 (38.16%) | 37 (26.24%) | ||
| Implant | 0.15 | 0.10 | ||||
| No | 18 (10.91%) | 55 (16.13%) | 9 (11.84%) | 31 (21.99%) | ||
| Yes | 147 (89.09%) | 286 (83.87%) | 67 (88.16%) | 110 (78.01%) | ||
| OT: | 0.03 | 0.56 | ||||
| < 3 h | 78 (47.27%) | 197 (57.77%) | 47 (61.84%) | 80 (56.74%) | ||
| ≥ 3 h | 87 (52.73%) | 144 (42.23%) | 29 (38.16%) | 61 (43.26%) | ||
| IBL | 0.09 | 1.00 | ||||
| < 200 ml | 144 (87.27%) | 315 (92.38%) | 68 (89.47%) | 127 (90.07%) | ||
| ≥ 200 ml | 21 (12.73%) | 26 (7.62%) | 8 (10.53%) | 14 (9.93%) | ||
| PSD | <0.001 | <0.001 | ||||
| < 300 ml | 21 (12.73%) | 165 (48.39%) | 12 (15.79%) | 70 (49.65%) | ||
| ≥ 300 ml | 144 (87.27%) | 176 (51.61%) | 64 (84.21%) | 71 (50.35%) | ||
| GS | <0.001 | 0.005 | ||||
| No | 12 (7.27%) | 84 (24.63%) | 4 (5.26%) | 29 (20.57%) | ||
| Yes | 153 (92.73%) | 257 (75.37%) | 72 (94.74%) | 112 (79.43%) | ||
| PTAG | 0.60 | 0.37 | ||||
| No | 43 (26.06%) | 98 (28.74%) | 18 (23.68%) | 43 (30.50%) | ||
| Yes | 122 (73.94%) | 243 (71.26%) | 58 (76.32%) | 98 (69.50%) | ||
| NPTA | 0.009 | 0.02 | ||||
| No | 110 (66.67%) | 266 (78.01%) | 45 (59.21%) | 106 (75.18%) | ||
| Yes | 55 (33.33%) | 75 (21.99%) | 31 (40.79%) | 35 (24.82%) | ||
| Laboratory indicators | ||||||
| PTHB | 0.32 | 0.09 | ||||
| Normal | 57 (34.55%) | 135 (39.59%) | 32 (42.11%) | 42 (29.79%) | ||
| Abnormal | 108 (65.45%) | 206 (60.41%) | 44 (57.89%) | 99 (70.21%) | ||
| PTSA | 0.004 | 0.002 | ||||
| Normal | 91 (55.15%) | 234 (68.62%) | 36 (47.37%) | 98 (69.50%) | ||
| Abnormal | 74 (44.85%) | 107 (31.38%) | 40 (52.63%) | 43 (30.50%) | ||
| LKF | 0.71 | 0.09 | ||||
| Normal | 67 (40.61%) | 146 (42.82%) | 40 (52.63%) | 56 (39.72%) | ||
| Abnormal | 98 (59.39%) | 195 (57.18%) | 36 (47.37%) | 85 (60.28%) | ||
| K+ | 0.001 | 0.65 | ||||
| Normal | 43 (26.06%) | 141 (41.35%) | 27 (35.53%) | 56 (39.72%) | ||
| Abnormal | 122 (73.94%) | 200 (58.65%) | 49 (64.47%) | 85 (60.28%) | ||
| Ca+ | 0.18 | 0.37 | ||||
| Normal | 96 (58.18%) | 221 (64.81%) | 43 (56.58%) | 90 (63.83%) | ||
| Abnormal | 69 (41.82%) | 120 (35.19%) | 33 (43.42%) | 51 (36.17%) | ||
| TT | <0.001 | <0.001 | ||||
| Normal | 56 (33.94%) | 251 (73.61%) | 39 (51.32%) | 108 (76.60%) | ||
| Abnormal | 109 (66.06%) | 90 (26.39%) | 37 (48.68%) | 33 (23.40%) | ||
SSI surgical site infection, HBP high blood pressure, DM diabetes mellitus, BMI body mass index, MS multi-stage surgery, OT operation time, IBL intraoperative blood loss, PSD postoperative suction drainagen, GS gelatin sponge, PTAG postoperative anticoagulation, NPTA none-preoperative antibiotic, PTHB postoperative hemoglobin, PTSA postoperative serum albumin, LKF liver and kidney function, TT thrombin time. Categorical variables were presented as number and percentage (n, %). The bold text means that the P value was < 0.05.
Fig. 2.
Heat map of the correlations between all the variables. The illustration reveals that there are no strong correlations among the variables (correlation coefficient < 0.7). HBP high blood pressure, DM diabetes mellitus, BMI body mass index, MS multi-stage surgery, OT operation time, IBL intraoperative blood loss, PSD postoperative suction drainagen, GS gelatin sponge, PTAG postoperative anticoagulation, NPTA none-preoperative antibiotic, PTHB postoperative hemoglobin, PTSA postoperative serum albumin, LKF liver and kidney function, TT thrombin time.
Univariate logistic regression of surgical site infection following lumbar disc herniation surgery
Univariate logistic regression analysis was conducted on 21 independent variables to assess the impact of each variable on the occurrence of SSI. The results indicated that the following variables were statistically significant: Age, DM, BMI, OT, IBL, PSD, GS, NPTA, K+, PTSA, TT, as shown in Table 2. A p-value of less than 0.1 was considered statistically significant.
Table 2.
Univariate logistic analysis based on training groups.
| Variable | B | SE | OR | 95% CI | P |
|---|---|---|---|---|---|
| Gender | − 0.265 | 0.19073 | 0.767 | 0.767 (0.528–1.116) | 0.17 |
| Age | 0.676 | 0.20111 | 1.966 | 1.966 (1.325–2.916) | 0.001 |
| Smoke status | − 0.091 | 0.19782 | 0.913 | 0.913 (0.618–1.343) | 0.65 |
| Drink status | − 0.248 | 0.23737 | 0.78 | 0.78 (0.485–1.232) | 0.30 |
| HBP | 0.116 | 0.20162 | 1.124 | 1.124 (0.754–1.665) | 0.56 |
| DM | 0.67 | 0.20028 | 1.955 | 1.955 (1.32–2.896) | 0.001 |
| BMI | 0.975 | 0.20049 | 2.651 | 2.651 (1.798–3.949) | < 0.001 |
| MS | 0.033 | 0.20679 | 1.033 | 1.033 (0.686–1.545) | 0.88 |
| Implant | 0.451 | 0.28989 | 1.571 | 1.571 (0.906–2.839) | 0.12 |
| OT | 0.423 | 0.19062 | 1.526 | 1.526 (1.051–2.22) | 0.03 |
| IBL | 0.569 | 0.31016 | 1.767 | 1.767 (0.954–3.24) | 0.07 |
| PSD | 1.861 | 0.2575 | 6.429 | 6.429 (3.957–10.90) | < 0.001 |
| GS | 1.427 | 0.32506 | 4.167 | 4.167 (2.285–8.259) | < 0.001 |
| PTAG | 0.135 | 0.21394 | 1.144 | 1.144 (0.756–1.751) | 0.53 |
| NPTA | 0.573 | 0.21063 | 1.773 | 1.773 (1.171–2.678) | 0.007 |
| PTHB | 0.216 | 0.19765 | 1.242 | 1.242 (0.845–1.836) | 0.27 |
| Ca+ | 0.28 | 0.19434 | 1.324 | 1.324 (0.903–1.936) | 0.15 |
| LKF | 0.091 | 0.19263 | 1.095 | 1.095 (0.752–1.601) | 0.64 |
| K+ | 0.693 | 0.20867 | 2 | 2 (1.337–3.033) | 0.001 |
| PTSA | 0.576 | 0.19525 | 1.778 | 1.778 (1.213–2.609) | 0.003 |
| TT | 1.692 | 0.20525 | 5.428 | 5.428 (3.648–8.163) | < 0.001 |
HBP high blood pressure, DM diabetes mellitus, BMI body mass index, MS multi-stage surgery, OT operation time, IBL intraoperative blood loss, PSD postoperative suction drainagen, GS gelatin sponge, PTAG postoperative anticoagulation, NPTA none-preoperative antibiotic, PTHB postoperative hemoglobin, PTSA postoperative serum albumin, LKF liver and kidney function, TT thrombin time. The bold text means that the P value was<0.05.
LASSO regression
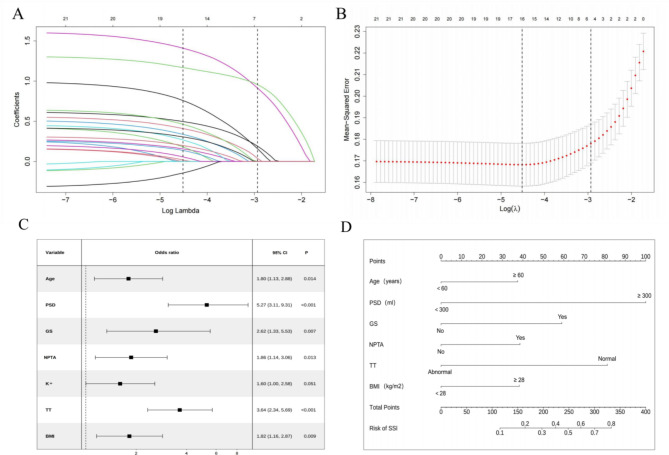
LASSO regression was employed to reduce the number of independent variables, and ten-fold cross-validation was conducted to select variables with the highest correlation based on lambda.1-SE. From the 21 variables, we identified seven with nonzero coefficients (Fig. 3A,B).
Fig. 3.
Establishment of nomogram prediction model. (A) LASSO coefficient profiles of the factors were analyzed. The outcomes of cross-validation for the LASSO regression models are presented. A vertical line is drawn at the point of optimum with the minimum criterion, as well as at 1 standard error (1-SE) of the minimum criterion. At 1-SE, seven variables were selected for logistic regression analysis. (B) Employ cross-validation to determine the optimal penalty parameter lambda. The coefficient profile plot of predictors illustrates the seven factors that displayed significant differences between patients with SSI and those without SSI. (C) The forest plot of multivariate logistic regression. The results showed that the selection of six independent variables as significant risk factors for SSI following LDH surgery. (D) The six factors are used to construct a nomogram for predicting SSI. Each independent predictor is assigned a score on the upper scale, while the total score of the six factors for each case is represented on the lower scale. The total score at the bottom of the chart corresponds to the likelihood of postoperative SSI diagnosis, providing an assessment of the risk for patients with LDH. SSI surgical site infection, LASSO least absolute shrinkage and selection operator, BMI body mass index, PSD postoperative suction drainagen, GS gelatin sponge, NPTA none-preoperative antibiotic, TT thrombin time.
Subsequently, seven significant variables were identified, combining meaningful indicators from univariate logistic regression and those screened by LASSO regression, including age, BMI, PSD, GS, NPTA, K+, and TT.
Multivariate logistic regression analysis
The seven identified variables were subsequently included in a multivariate logistic regression analysis. The results indicated that the following variables were statistically significant: age (OR 1.80, 95% CI 1.13–2.88, p = 0.014), PSD (OR 5.27, 95% CI 3.11–9.31, p< 0.001), GS (OR 2.62, 95% CI 1.33–5.53, p = 0.007), NPTA (OR 1.86, 95% CI 1.14–3.06, p = 0.013), TT (OR 3.64, 95% CI 2.34–5.69, p< 0.001), BMI (OR 1.82, 95% CI 1.16–2.87, p = 0.009) (Fig. 3C).
Creation of a nomogram for predicting the risk of SSI following LDH surgery
Based on the multivariate logistic regression results, we developed a nomogram model utilizing six indicators to predict the probability of postoperative SSI in LDH patients. In personalized medicine, nomogram points can be determined based on specific values for age, BMI, PSD, GS, NPTA and TT. (Fig. 3D).
Validation of the nomogram
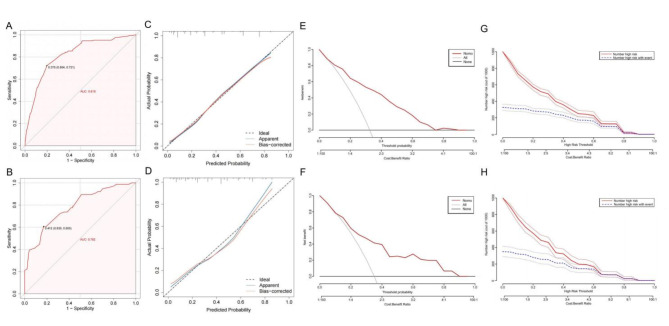
ROC curves were generated to evaluate the model’s predictive efficacy in both the training and validation sets (Fig. 4A,B). Figure 4A shows the strong predictive ability of the nomogram model in the training sets, with an AUC of 0.818 (95% CI 0.779–0.857, cutoff value 0.376, sensitivity 0.721, specificity 0.804). The validation set also exhibited high predictive performance, with an AUC of 0.782 (95% CI 0.717–0.846, cutoff 0.412, sensitivity 0.605, specificity 0.823) (Fig. 4B). These findings indicate that the clinical prediction model developed in this study demonstrated moderate predictive value (AUC > 0.7).
Fig. 4.
Validation of nomogram prediction model. (A) Receiver operating characteristic (ROC) curves for distinguishing surgical site infection (SSI) from Non-SSI in the training set. (B) ROC curves for distinguishing SSI from Non-SSI in the validation set. The horizontal axis represents 1-specificity, and the vertical axis represents sensitivity, both with maximum values of 1. The dotted line is the reference line (AUC = 0.5), and the red curve is the ROC curve. The further the ROC curve is from the reference line, the greater the AUC value, indicating better model predictive performance. The results showed that the model had good predictive performance in both the training and validation sets (AUC > 0.75). (C) Calibration curve of the nomogram in the training set. (D) Calibration curve of the nomogram in the validation set. Calibration curves depict the calibration of each model in terms of agreement between predicted SSI risks and observed SSI outcomes. The Y-axis represents the actual incidence of SSI. The X-axis represents the predicted SSI risk. The diagonal dotted line represents a perfect prediction by an ideal model. The solid pink line represents the performance of the nomogram; a closer fit to the diagonal dotted line indicates better predictive accuracy. The Hosmer-Lemeshow goodness-of-fit test shows favorable results in both the training set and validation set. (E) Decision curve analysis (DCA) for the nomogram model in the training set. (F) DCA for the nomogram model in the validation set. The results indicated that both the training set and the validation set exhibit substantial net benefit across a specified range of SSI threshold probabilities (0–0.8). (G) Clinical impact curve (CIC) of the nomogram based on data from the training set. (H) CIC of the nomogram based on data from the validation set. The horizontal axis represents the probability threshold, while the vertical axis indicates the number of individuals. The solid red lines represent the number of individuals deemed by the model to be at high risk for SSI at different probability thresholds. The dashed blue line represents the number of individuals predicted by the model to be at high risk for SSI who actually experienced the outcome at different probability thresholds. The bottom axis indicates the benefit ratio, representing the ratio of loss to gain at different probability thresholds.
Figure 4C.D presents the calibration curves for the training and validation sets respectively, created using the Bootstrap recalibration method (repeated 1000 times). The apparent and deviation-corrected lines indicate that in the training set, the Brier score is 0.156, the slope is 1.000, and P = 0.839 (Fig. 4C). In the validation sets, the Brier score was 0.174, the slope was 0.956, and P = 0.280 (Fig. 4D). These results demonstrate strong concordance between the predicted and actual probabilities. The P-value of the Homser-Lemeshow test is 0.318 (validation set is 0.583), indicating that the clinical prediction model for SSI is well-calibrated. DCA was performed to assess the clinical utility of the model. The results show that in the training set, when the threshold of the model is set between 1% and 90%, the decision curve is above the NONE and ALL lines, indicating clinical practicability within this range (Fig. 4E). Similarly, the validation set demonstrated reasonable clinical practicability with the model threshold set between 1% and 90% (Fig. 4F).
Additionally, the clinical impact curve (CIC) demonstrated that within the most favorable threshold probability range, the number of expected high-risk patients consistently exceeded the number of actual patients, accompanied by an acceptable cost-benefit ratio (Fig. 4G,H).
Reasonable analysis
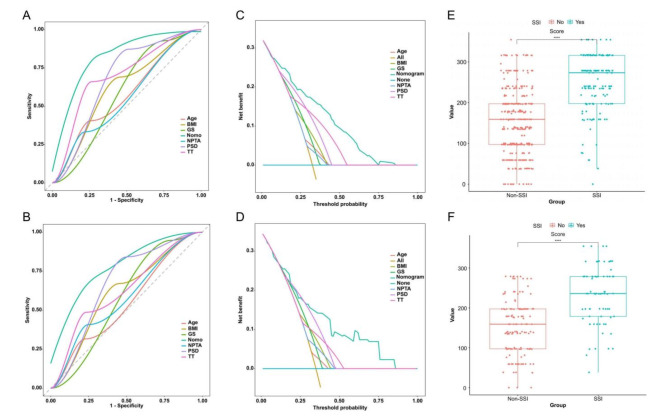
ROC curves of reasonable analysis of the training and validation sets showed that the nomogram model had superior predictive ability compared to individual variables (Fig. 5A,B). Furthermore, Decision Curve Analysis (DCA) showed that the nomogram had greater clinical practicality than any single variables (Fig. 5C,D). Analysis of nomogram scores revealed statistically significant differences between SSI and Non-SSI groups in both the training and validation sets (Fig. 5E,F).
Fig. 5.
The reasonable analysis of the established clinical prediction model. (A) Receiver Operating Characteristic (ROC) diagram of reasonable analysis in the training set. (B) ROC diagram of reasonable analysis in the validation set. The results showed that the Area Under the Curve (AUC) of the nomogram model is higher than that of single-variable models in both the training set and the validation set. This indicates that the predictive performance of the six-variable model is superior to that of any single-variable model. (C) Decision curve analysis (DCA) diagram of reasonable analysis in the training set. (D) DCA diagram of reasonable analysis in the validation set. The results of the DCA diagram of reasonable analysis showed that the net benefit rate of the nomogram model is higher than that of a simple model with thresholds ranging from 0.1 to 0.8 in the training set and from 0.3 to 0.8 in the validation set. This indicates that the net benefit of the six-variable model is superior to that of single-variable models in both the training and validation sets. (E) The results of the model score comparison the Significant differences in nomogram scores between the SSI and Non-SSI groups in the training set. (F) The results of the model score comparison the Significant differences in nomogram scores between the SSI and Non-SSI groups in the validation sets. The results of the model score comparison indicating the model’s effectiveness. P values were calculated via two independent samples t-tests. SSI surgical site infection, BMI body mass index, PSD postoperative suction drainagen, GS gelatin sponge, NPTA none-preoperative antibiotic, TT thrombin time.
Discussion
Postoperative SSI following spinal surgery presents significant challenges, often requiring repeated irrigation and debridement procedures, while prolonged antibiotic use elevates the risk of resistance. Severe cases might necessitate the removal of internal fixation devices, potentially resulting in postoperative nonunion, which has serious implications for both patients and surgeons15. Furthermore, SSI imposes a substantial medical burden, encompassing prolonged care, additional surgeries, unplanned readmissions, and delayed return to work. Our study found an overall SSI incidence of 4.46% following LDH surgery, consistent with previous research6. Identifying associated factors is crucial for SSI prevention. Previous studies have reported numerous potential risk factors without reaching a consensus16,17.
Integrating a nomogram into clinical practice enhances the conversation with patients. This visual tool, derived from a multivariate logistic regression model, assigns scores to each risk factor based on its impact on outcomes, enabling bedside calculations during routine clinical care. Previous researchers have successfully utilized this tool across diverse medical fields. Our study focused on LDH patients, thereby making our model more specific to this population18,19.
Our study demonstrates that logistic regression modeling effectively evaluates factors influencing SSI risk in LDH patients by using demographic, perioperative, and laboratory indicators. After rigorous statistical analysis, including univariate, multivariate logistic regression, and LASSO regression, age, BMI, PSD, GS, NPTA and TT emerged as significant predictors of SSI. Utilizing these variables, we constructed a nomogram to predict SSI risk, which exhibited robust predictive performance, with AUCs of 0.818 (95% CI 0.779–0.857) and 0.782 (95% CI 0.718–0.846) in the training and validation sets, respectively. Furthermore, our model effectively discriminated between regular and SSI patients, as evidenced by significant score differences in both sets.
Elderly individuals are often predisposed to postoperative spinal infections due to diminished physiological resilience, increased comorbidities, and impaired tissue healing capacity20,21. Studies by Fei et al. and Dubory et al. have highlighted ages > 60 and > 65 years, respectively, as significant risk factors for postoperative SSI in spinal surgery22,23. Given that China categorizes individuals aged over 60 as elderly, our study compared this age group with non-elderly individuals, offering valuable insights for SSI management. Our findings revealed a 1.8-fold increased SSI risk in individuals aged ≥ 60 years, underscoring the need for enhanced perioperative care and infection prevention strategies for elderly patients.
Global obesity rates are rising, with numerous studies linking obesity (or elevated BMI) to increased SSI risk post-spinal surgery9,10. Higher BMI has consistently correlated with increased SSI incidence in postoperative patients, as demonstrated by studies conducted by Ming, Piper, and others24–27. Obese individuals often present with comorbidities such as diabetes, metabolic disorders, and immune dysfunction, making them more susceptible to infections12. Consistent with prior research, our study defined obesity as a BMI ≥ 28 kg/m², reaffirming the heightened SSI risk in obese individuals compared to non-obese counterparts.
Negative pressure wound therapy (NPWT) and closed suction irrigation systems (CSIS) have become widely accepted in spinal surgery for postoperative wound management. NPWT facilitates continuous antibiotic irrigation and pollutant absorption, providing anti-inflammatory effects. It also reduces hematoma and edema through negative pressure, enhances local blood circulation, and promotes neovascularization and granulation tissue formation for accelerated wound healing28–31. Although closed drainage systems have been implicated in retrograde infections, studies by Liu et al. found no significant difference in wound infection rates compared to open drainage32,33. Notably, postoperative drainage volume, indicative of tissue edema and wound healing, emerged as a significant risk factor for postoperative SSI, emphasizing the importance of managing drainage flow rather than just using negative pressure devices. Therefore, we believe that rather than focusing solely on the use of negative pressure drainage devices, it is more important to explore the impact of drainage volume on SSI. Our results showed that a total postoperative drainage volume > 300 ml was a significant risk factor for postoperative SSI after LDH (OR: 5.27, 95% CI 3.11–9.31). Therefore, perioperative strategies such as minimizing tissue exposure, optimizing surgical timing, and ensuring prompt hemostasis are crucial for controlling postoperative drainage.
Gelatin sponge, known for its biocompatibility, biodegradability, and cost-effectiveness, is widely used in biomedical and tissue engineering. Its porous structure facilitates cell migration and provides structural support for tissue regeneration34–36. Despite its liquid absorption and hemostatic properties, the gelatin sponge lacks intrinsic antibacterial effects37. However, its use in postoperative hemostasis may inadvertently disrupt local blood flow, impede wound healing and foster a microbial-friendly environment, thus increasing the risk of SSI. Our study found a 2.62-fold higher SSI risk associated with gelatin sponge usage, emphasizing the need for alternative hemostatic techniques such as electrotome and ligation to mitigate infection risk.
Guidelines for antibiotic use in spinal surgery remain debated among practitioners. While Trampu et al. advocate for antibiotic prophylaxis in both clean and contaminated incisions, concerns over antibiotic resistance have prompted some to question its preoperative use without comprehensive research on resistance patterns38. Despite evidence supporting preoperative antibiotic prophylaxis to reduce postoperative infection rates, there is no consensus on its optimal duration. Lai et al. observed a correlation between the omission of preoperative antibiotics and postoperative SSI in lumbar spine surgeries39. While prophylactic antibiotics reduce postoperative infection rates by 3.4–6%, no study unequivocally favors prolonged postoperative antibiotic use over preoperative administration for SSI prevention38,40. Therefore, our study specifically investigated the impact of preoperative antibiotic use on SSI development, revealing a 1.86-fold increase SSI risk in patients without preoperative antibiotic coverage. Given the unique anatomical challenges of spinal surgery, short-term preoperative antibiotic prophylaxis is recommended.
TT is a crucial indicator of both intrinsic and extrinsic coagulation pathways, sensitively reflecting patients’ coagulation status41. Management of antiplatelet and anticoagulant medications preoperatively and postoperatively adhered to guidelines, with no preoperative history of such drugs among patients42. Given TT’s ability to reflect intrinsic coagulation dynamics, it is a superior risk indicator compared to other clotting parameters for assessing postoperative SSI risk. Therefore, we use TT as a risk indicator rather than other clotting parameters. Abnormal TT values conferred a 3.64-fold increased SSI risk, underscoring the importance of preoperative coagulation assessments to minimize bleeding-related complications.
Previous studies have suggested that internal implants may incite soft tissue inflammation, hematoma formation, and microbial proliferation43. Additionally, metal debris from implant fretting can foster granuloma formation, providing a conducive environment for bacterial colonization44. Contrary to expectations, our study did not identify internal implants as a significant risk factor for SSI. Notably, while DM is commonly implicated as a key SSI risk factor due to impaired immune responses and delayed wound healing, our findings did not confirm its independent association with SSI22,45. Hyperglycemia impairs neutrophil activity and chemotactic function, leading to prolonged wound healing and increased susceptibility to infection46. Although a statistical difference in DM prevalence existed between SSI and Non-SSI patients, the sample size of this study may have influenced the outcome. Future research should explore the impact of postoperative hyperglycemia on SSI beyond the status of DM.
This study reveals that age, BMI, PSD, GS, NPTA, and TT constitute risk factors for postoperative infection in patients with LDH. Examination of the nomogram underscores PSD as the primary influencing factor, with TT and GS emerging as two additional significant contributors. This underscores the clinical importance of preoperative TT assessment; for patients with severe abnormalities, surgical intervention should be deferred or carefully considered. During surgery, minimizing the use of gelatin sponges is advisable, with electrocautery and ligation serving as viable alternatives to mitigate the risk of postoperative infection. Although negative pressure drainage is a common postsurgical practice, our findings indicate that a drainage volume exceeding 300 ml represents a crucial factor in the development of infection. Consequently, meticulous monitoring of patients’ drainage volumes within the initial three postoperative days is imperative. For patients whose drainage volume surpasses 300 ml, prompt symptomatic treatment is essential. This includes cleaning the drainage site and timely removal of the drainage tube when the daily drainage volume drops below 50 ml. Furthermore, utilizing B-ultrasound to detect wound fluid accumulation is a pivotal preventive measure against infection.
The study has several limitations. Firstly, the study was conducted at a single center, and despite internal validation, no external validation was conducted. Secondly, the retrospective design may introduce subjective and selection biases. Lastly, although the nomogram achieved good calibration and optimal discrimination through internal validation, it requires external validation with additional datasets.
Conclusion
The nomogram model developed in this study enables early identification of SSI risk post-surgery for LDH, provides valuable references for clinical decision-making, and optimizes medical resource allocation. Furthermore, we constructed and validated a nomogram to predict SSI probability in patients following LDH surgery.
Acknowledgements
We acknowledged Dr. Ji Yu fei and Wang Long chao from the First Affiliated Hospital of Air Force Medical University for their writing assistance in this work.
Abbreviations
- SSI
Surgical site infection
- HBP
High blood pressure
- DM
Diabetes mellitus
- BMI
Body mass index
- MS
Multistage surgery
- OT
Operation time
- IBL
Intraoperative blood loss
- PSD
Postoperative suction drainagen
- GS
Gelatin sponge
- PTAG
Postoperative anticoagulation
- NPTA
None preoperative antibiotic
- PTHB
Postoperative hemoglobin
- PTSA
Postoperative serum albumin
- LKF
Liver and kidney function
- TT
Thrombin time
Author contributions
H.Q.: Conceptualization, methodology, software, data curation, visualization, investigation, and writing—original draft. Y.Z.: Conceptualization, formal analysis, methodology, writing - review & editing. D.L.: Formal analysis, methodology. F.S.: Data curation, writing—review and editing. C.L.: Methodology, supervision, writing—review and editing. J.D.: Data curation, writing—review and editing. Y.Y.: Data curation, formal analysis, methodology. X.H.: Data curation, methodology. W.L.: Conceptualization, Formal analysis, methodology, writing—review and editing. All authors gave their consent for publication.
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Lei, Email: leiwei@fmmu.edu.cn.
Yang Zhang, Email: zhangyang@fmmu.edu.cn.
References
- 1.Sivasubramaniam, V., Patel, H. C., Ozdemir, B. A. & Papadopoulos, M. C. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England: a 15-year time-series study. BMJ Open.5, e009011. 10.1136/bmjopen-2015-009011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartvigsen, J. et al. What low back pain is and why we need to pay attention. Lancet (London England). 391, 2356–2367. 10.1016/s0140-6736(18)30480-x (2018). [DOI] [PubMed] [Google Scholar]
- 3.Jordan, J. & Konstantinou, K. & O’Dowd, J. Herniated lumbar disc. BMJ Clin. Evid. (2009). [PMC free article] [PubMed]
- 4.Lurie, J. D. et al. Surgical versus nonoperative treatment for lumbar disc herniation: eight-year results for the spine patient outcomes research trial. Spine. 39, 3–16. 10.1097/brs.0000000000000088 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangram, A. J., Horan, T. C., Pearson, M. L., Silver, L. C. & Jarvis, W. R. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 27, 97–132 (1999) (quiz 133–134; discussion 196). [PubMed] [Google Scholar]
- 6.Blumberg, T. J., Woelber, E., Bellabarba, C., Bransford, R. & Spina, N. Predictors of increased cost and length of stay in the treatment of postoperative spine surgical site infection. Spine J. Offi. J. N. Am. Spine Soc.18, 300–306. 10.1016/j.spinee.2017.07.173 (2018). [DOI] [PubMed] [Google Scholar]
- 7.De la Garza-Ramos, R. et al. Deep-wound and organ-space infection after surgery for degenerative spine disease: an analysis from 2006 to 2012. Neurol. Res.38, 117–123. 10.1080/01616412.2016.1138669 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Xing, D. et al. A methodological, systematic review of evidence-based independent risk factors for surgical site infections after spinal surgery. Eur. Spine J.22, 605–615. 10.1007/s00586-012-2514-6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebastian, A. et al. Risk factors for surgical site infection after posterior cervical spine surgery: an analysis of 5,441 patients from the ACS NSQIP 2005–2012. Spine J.16, 504–509. 10.1016/j.spinee.2015.12.009 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Lee, M. J., Cizik, A. M., Hamilton, D. & Chapman, J. R. Predicting surgical site infection after spine surgery: a validated model using a prospective surgical registry. Spine J.14, 2112–2117. 10.1016/j.spinee.2013.12.026 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Manoso, M. W. et al. Medicaid status is associated with higher surgical site infection rates after spine surgery. Spine. 39, 1707–1713. 10.1097/brs.0000000000000496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen, C. J., Murphy, K. E. & Fernandez, M. L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. (Bethesda Md). 7, 66–75. 10.3945/an.115.010207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin, D., Liu, B., Chang, Y., Gu, H. & Zheng, X. Management of late-onset deep surgical site infection after instrumented spinal surgery. BMC Surg.18, 121. 10.1186/s12893-018-0458-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alamanda, V. K. & Springer, B. D. The prevention of infection: 12 modifiable risk factors. Bone Jt J. 101–b. 10.1302/0301-620x.101b1.Bjj-2018-0233.R1 (2019). [DOI] [PubMed]
- 15.Abdul-Jabbar, A. et al. Surgical site infection in spinal surgery: description of surgical and patient-based risk factors for postoperative infection using administrative claims data. Spine. 37, 1340–1345. 10.1097/BRS.0b013e318246a53a (2012). [DOI] [PubMed] [Google Scholar]
- 16.Lee, N. J. et al. Incidence, impact, and risk factors for 30-Day wound complications following elective adult spinal deformity surgery. Glob. Spine J.7, 417–424. 10.1177/2192568217699378 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaichana, K. L. et al. Risk of infection following posterior instrumented lumbar fusion for degenerative spine disease in 817 consecutive cases. J. Neurosurg. Spine. 20, 45–52. 10.3171/2013.10.Spine1364 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Wang, H. et al. A nomogram to predict the risk of prolonged length of stay following primary total hip arthroplasty with an enhanced recovery after surgery program. J. Orthop. Surg, Res.10.1186/s13018-021-02877-6 (2021). [DOI] [PMC free article] [PubMed]
- 19.Lu, C. X., Huang, Z. B., Chen, X. M. & Wu, X. D. Predicting prolonged postoperative length of stay risk in patients undergoing lumbar fusion surgery: development and assessment of a novel predictive nomogram. Front. Surg.9, 925354. 10.3389/fsurg.2022.925354 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogihara, S. et al. Risk factors for surgical site infection after lumbar laminectomy and/or discectomy for degenerative diseases in adults: a prospective multicenter surveillance study with registry of 4027 cases. PLoS One. 13, e0205539. 10.1371/journal.pone.0205539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habiba, S. et al. Risk factors for surgical site infections among 1,772 patients operated on for lumbar disc herniation: a multicentre observational registry-based study. Acta Neurochir.159, 1113–1118. 10.1007/s00701-017-3184-2 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Fei, Q. et al. Risk factors for surgical site infection after spinal surgery: a meta-analysis. World Neurosurg.95, 507–515. 10.1016/j.wneu.2015.05.059 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Dubory, A. et al. Surgical-site infection in spinal injury: incidence and risk factors in a prospective cohort of 518 patients. Eur. Spine J.24, 543–554. 10.1007/s00586-014-3523-4 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Schoenfeld, A. J., Carey, P. A., Cleveland, A. W. 3, Bader, J. O., Bono, C. M. & rd, & Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5,887 patients. Spine J.13, 1171–1179. 10.1016/j.spinee.2013.02.071 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Rao, S. B. et al. Risk factors for surgical site infections following spinal fusion procedures: a case-control study. Clin. Infect. Dis.53, 686–692. 10.1093/cid/cir506 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Piper, K. F. et al. Risk factors for wound complications following spine surgery. Surg. Neurol. Int.8, 269. 10.4103/sni.sni_306_17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng, F., Cao, J. & Meng, X. Risk factors for surgical site infections following spinal surgery. J. Clin. Neurosci.22, 1862–1866. 10.1016/j.jocn.2015.03.065 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Webb, L. X. The impact of negative pressure wound therapy on orthopaedic infection. Qld. Gov. Min. J.48, 167–179. 10.1016/j.ocl.2016.12.004 (2017). [DOI] [PubMed] [Google Scholar]
- 29.van den Bulck, R. et al. Initial clinical experiences with a new, portable, single-use negative pressure wound therapy device. Int. Wound J.10, 145–151. 10.1111/j.1742-481X.2012.00954.x (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery, S., Leaper, D., Armstrong, D. & Lantis, J. Using negative pressure wound therapy to prevent surgical site infection. J. Wound Care. 27, S5–s13. 10.12968/jowc.2018.27.Sup3.S5 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Glass, G. E., Murphy, G. F., Esmaeili, A., Lai, L. M. & Nanchahal, J. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br. J. Surg.101, 1627–1636. 10.1002/bjs.9636 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Liu, Y., Li, Y. & Miao, J. Wound drains in posterior spinal surgery: a meta-analysis. J. Orthop. Surg. Res.1110.1186/s13018-016-0351-8 (2016). [DOI] [PMC free article] [PubMed]
- 33.Chen, Z. Y., Gao, Y., Chen, W., Li, X. & Zhang, Y. Z. Is wound drainage necessary in hip arthroplasty? A meta-analysis of randomized controlled trials. Eur. J. Orthop. Surg. Traumatol. Orthopedie Traumatologie. 24, 939–946. 10.1007/s00590-013-1284-0 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Rath, G., Hussain, T., Chauhan, G., Garg, T. & Goyal, A. K. Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mater. Sci. Eng. C Mater. Biol. Appl.58, 242–253. 10.1016/j.msec.2015.08.050 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Pham-Nguyen, O. V., Shin, J. U., Kim, H. & Yoo, H. S. Self-assembled cell sheets composed of mesenchymal stem cells and gelatin nanofibers for the treatment of full-thickness wounds. Biomater. Sci.8, 4535–4544. 10.1039/d0bm00910e (2020). [DOI] [PubMed] [Google Scholar]
- 36.Kang, M. G. et al. Nanogels derived from fish gelatin: application to drug delivery system. Mar. Drugs. 1710.3390/md17040246 (2019). [DOI] [PMC free article] [PubMed]
- 37.Alinezhad Sardareh, E. et al. Antimicrobial activity of blow Spun PLA/Gelatin nanofibers containing green synthesized silver nanoparticles against Wound infection-causing Bacteria. Bioeng. (Basel Switzerland). 910.3390/bioengineering9100518 (2022). [DOI] [PMC free article] [PubMed]
- 38.Takahashi, H. et al. Antimicrobial prophylaxis for spinal surgery. J. Orthop. Sci.. 14, 40–44. 10.1007/s00776-008-1296-5 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Lai, Q. et al. Risk factors for acute surgical site infections after lumbar surgery: a retrospective study. J. Orthop. Surg. Res.1210.1186/s13018-017-0612-1 (2017). [DOI] [PMC free article] [PubMed]
- 40.Christodoulou, A. G., Givissis, P., Symeonidis, P. D., Karataglis, D. & Pournaras, J. Reduction of postoperative spinal infections based on an etiologic protocol. Clin. Orthop. Relat. Res.444, 107–113. 10.1097/01.blo.0000201174.10506.cc (2006). [DOI] [PubMed] [Google Scholar]
- 41.Modi, H. N., Lee, D. Y. & Lee, S. H. Postoperative spinal epidural hematoma after microscopic lumbar decompression: a prospective magnetic resonance imaging study in 89 patients. J. Spin. Disord. Tech.24, 146–150. 10.1097/BSD.0b013e3181e1958e (2011). [DOI] [PubMed] [Google Scholar]
- 42.Narouze, S. et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (Second Edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine. Reg. Anesth. Pain Med.43, 225–262. 10.1097/aap.0000000000000700 (2018). the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. [DOI] [PubMed] [Google Scholar]
- 43.Pull ter Gunne, A. F., van Laarhoven, C. J. & Cohen, D. B. Surgical site infection after osteotomy of the adult spine: does type of osteotomy matter? Spine J.10, 410–416. 10.1016/j.spinee.2009.11.017 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Grajek, A., Białecki, J., Marczyński, W., Walczak, P. & Macias, J. A. Retrospective analysis of bacteriological studies of surgical site infections in a monoprofile, Multidepartmental Orthopedic Hospital. Ortop. Traumatol. Rehabil.17, 275–288. 10.5604/15093492.1162427 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Pull ter Gunne, A. F. et al. A methodological systematic review on surgical site infections following spinal surgery: part 1: risk factors. Spine. 37, 2017–2033. 10.1097/BRS.0b013e31825bfca8 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Ogihara, S. et al. Prospective multicenter surveillance and risk factor analysis of deep surgical site infection after posterior thoracic and/or lumbar spinal surgery in adults. J. Orthop. Sci.. 20, 71–77. 10.1007/s00776-014-0669-1 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.