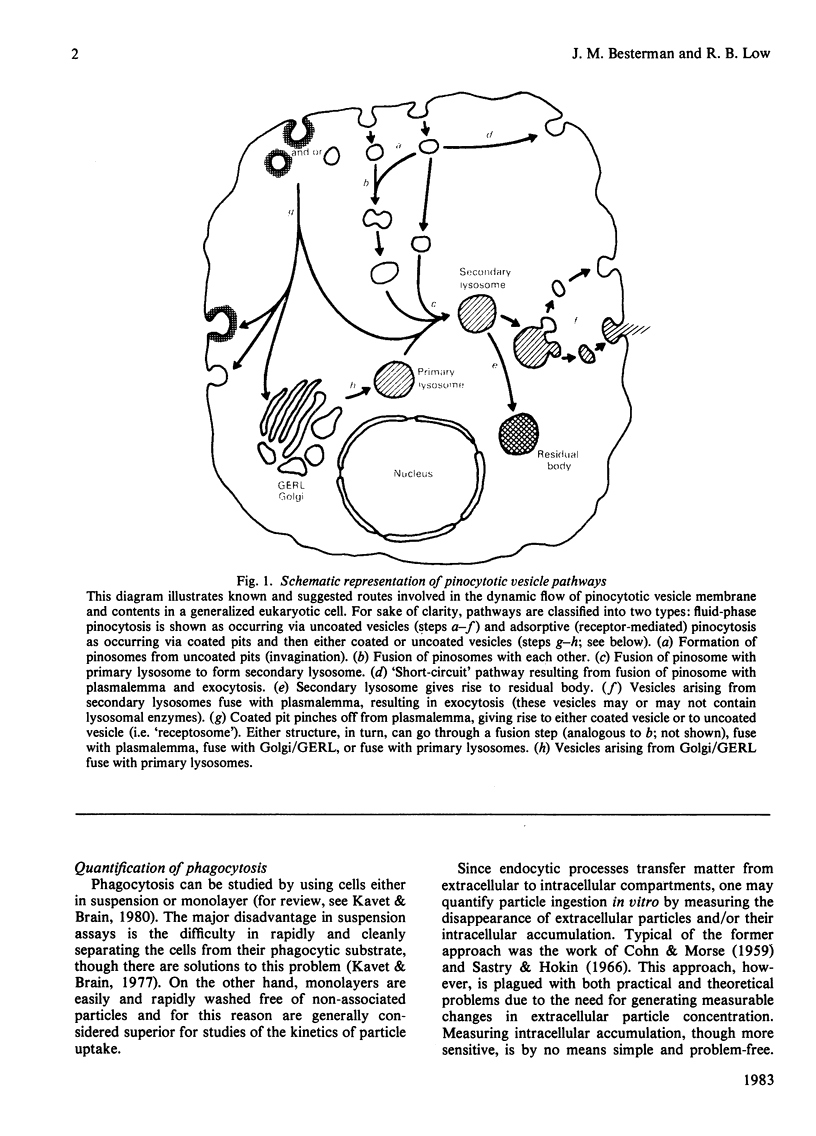
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ibrahim M. S., Chandra R., Kishore R., Valentine F. T., Lawrence H. S. A micromethod for evaluating the phagocytic activity of human macrophages by ingestion of radio-labelled polystyrene particles. J Immunol Methods. 1976 Mar;10(2-3):207–218. doi: 10.1016/0022-1759(76)90172-1. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Brown M. S., Goldstein J. L. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977 Mar;10(3):351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Goldstein J. L., Brown M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977 Dec 22;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G., Ashwood-Smith M. J. Endocytosis in Chinese hamster fibroblasts. Inhibition by glucose. Exp Cell Res. 1973 Dec;82(2):310–314. doi: 10.1016/0014-4827(73)90346-7. [DOI] [PubMed] [Google Scholar]
- Berger R. R., Karnovsky M. L. Biochemical basis of phagocytosis. V. Effect of phagocytosis on cellular uptake of extracellular fluid, and on the intracellular pool of L-alpha-glycerophosphate. Fed Proc. 1966 May-Jun;25(3):840–845. [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Surface functions during mitosis. II. Quantitation of pinocytosis and kinetic characterization of the mitotic cycle with a new fluorescence technique. J Cell Biol. 1980 Jun;85(3):660–671. doi: 10.1083/jcb.85.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M., Walter R. J. Surface functions during Mitosis I: phagocytosis, pinocytosis and mobility of surface-bound Con A. Cell. 1978 Oct;15(2):327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Airhart J. A., Low R. B. Macrophage phagocytosis: analysis of particle binding and internalization. Am J Physiol. 1982 May;242(5):C339–C346. doi: 10.1152/ajpcell.1982.242.5.C339. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Airhart J. A., Woodworth R. C., Low R. B. Exocytosis of pinocytosed fluid in cultured cells: kinetic evidence for rapid turnover and compartmentation. J Cell Biol. 1981 Dec;91(3 Pt 1):716–727. doi: 10.1083/jcb.91.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Bowers B. Comparison of pinocytosis and phagocytosis in Acanthamoeba castellanii. Exp Cell Res. 1977 Dec;110(2):409–417. doi: 10.1016/0014-4827(77)90307-x. [DOI] [PubMed] [Google Scholar]
- Bowers B., Olszewski T. E. Pinocytosis in Acanthamoeba castellanii. Kinetics and morphology. J Cell Biol. 1972 Jun;53(3):681–694. doi: 10.1083/jcb.53.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Freeman A. R. Plasma membrane: substructural changes correlated with electrical resistance and pinocytosis. Science. 1967 Feb 3;155(3762):582–585. doi: 10.1126/science.155.3762.582. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Directed lipid flow in cell membranes. Nature. 1976 Mar 4;260(5546):21–23. doi: 10.1038/260021a0. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Thomson J. N., Pearse B. M. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzuchowska W. Use of radioactive isotopes in studies on phagocytosis in vitro. Nature. 1966 Oct 8;212(5058):210–211. doi: 10.1038/212210b0. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. I. Observations on the requirements and consequences of particle ingestion. J Exp Med. 1960 May 1;111:667–687. doi: 10.1084/jem.111.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo C., Bongrand P., Benoliel A. M., Depieds R. Non-specific recognition in phagocytosis: ingestion of aldehyde-treated erythrocytes by rat peritoneal macrophages. Immunology. 1979 Mar;36(3):501–508. [PMC free article] [PubMed] [Google Scholar]
- Carlson S. S., Kelly R. B. An antiserum specific for cholinergic synaptic vesicles from electric organ. J Cell Biol. 1980 Oct;87(1):98–103. doi: 10.1083/jcb.87.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter R. R., Barsales P. B. Uptake by mononuclear phagocytes of protein-coated bentonite particles stabilized with a carbodiimide. J Immunol. 1967 Apr;98(4):844–853. [PubMed] [Google Scholar]
- Cheng S. Y., Maxfield F. R., Robbins J., Willingham M. C., Pastan I. H. Receptor-mediated uptake of 3,3',5-triiodo-L-thyronine by cultured fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3425–3429. doi: 10.1073/pnas.77.6.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Benson B. The in vitro differentiation of mononuclear phagocytes. 3. The reversibility of granule and hydrolytic enzyme formation and the turnover of granule constituents. J Exp Med. 1965 Sep 1;122(3):455–466. doi: 10.1084/jem.122.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. 3. The induction of vesicle formation by nucleosides and nucleotides. J Exp Med. 1967 Mar 1;125(3):457–466. doi: 10.1084/jem.125.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn Z. A., Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967 Feb 1;125(2):213–232. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Membrane sialic acid on target particles modulates their phagocytosis by a trypsin-sensitive mechanism on human monocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3831–3835. doi: 10.1073/pnas.75.8.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Opsonin-independent phagocytosis of activators of the alternative complement pathway by human monocytes. J Immunol. 1978 Apr;120(4):1132–1138. [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Ross R. Mediation of pinocytosis in cultured arterial smooth muscle and endothelial cells by platelet-derived growth factor. J Cell Biol. 1978 Dec;79(3):663–671. doi: 10.1083/jcb.79.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Poole A. R., Lazarus G. S., Barrett A. J. Immunoinhibition of intracellular protein digestion in macrophages. J Exp Med. 1973 May 1;137(5):1124–1141. doi: 10.1084/jem.137.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. The mechanism of release of catecholamines from the adrenal medulla. Pharmacol Rev. 1966 Mar;18(1):471–480. [PubMed] [Google Scholar]
- EAGLE H., PIEZ K. A. The utilization of proteins by cultured human cells. J Biol Chem. 1960 Apr;235:1095–1097. [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phago-lysosome formation. J Exp Med. 1974 Nov 1;140(5):1364–1386. doi: 10.1084/jem.140.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Zwiebel R., Cohn Z. A. The pinocytic rate of activated macrophages. J Exp Med. 1975 Nov 1;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich B. A., Cohn Z. A. The uptake and digestion of iodinated human serum albumin by macrophages in vitro. J Exp Med. 1967 Nov 1;126(5):941–958. doi: 10.1084/jem.126.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimer N. B., Ogmundsdóttir H. M., Blackwell C. C., Sutherland I. W., Graham L., Weir D. M. The role of cell wall carbohydrates in binding of microorganisms to mouse peritoneal exudate macrophages. Acta Pathol Microbiol Scand B. 1978 Apr;86(2):53–57. doi: 10.1111/j.1699-0463.1978.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Farquhar M. G. Functions of coated vesicles during protein absorption in the rat vas deferens. J Cell Biol. 1967 Nov;35(2):357–376. doi: 10.1083/jcb.35.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze J. J., Kramer M. F. Function of coated membranes and multivesicular bodies during membrane regulation in stimulated exocrine pancreas cells. Cell Tissue Res. 1974;156(1):1–20. doi: 10.1007/BF00220098. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Faust J. R., Beaudet A. L., Brown M. S. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975 Nov 10;250(21):8487–8495. [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A. Augmentation of macrophage complement receptor function in vitro. II. Characterization of the effects of a unique lymphokine upon the phagocytic capabilities of macrophages. J Immunol. 1980 Aug;125(2):844–849. [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Silverstein S. C. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med. 1976 Sep 1;144(3):788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Mullinax P. J. Augmentation of macrophage complement receptor function in vitro. III. C3b receptors that promote phagocytosis migrate within the plane of the macrophage plasma membrane. J Exp Med. 1981 Aug 1;154(2):291–305. doi: 10.1084/jem.154.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Silverstein S. C. Segmental response of the macrophage plasma membrane to a phagocytic stimulus. J Exp Med. 1974 Feb 1;139(2):323–336. doi: 10.1084/jem.139.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. A., Griffin F. M., Jr Augmentation of macrophage complement receptor function in vitro. I. Characterization of the cellular interactions required for the generation of a T-lymphocyte product that enhances macrophage complement receptor function. J Exp Med. 1979 Sep 19;150(3):653–675. doi: 10.1084/jem.150.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudewicz P. W., Molnar J., Lai M. Z., Beezhold D. W., Siefring G. E., Jr, Credo R. B., Lorand L. Fibronectin-mediated uptake of gelatin-coated latex particles by peritoneal macrophages. J Cell Biol. 1980 Nov;87(2 Pt 1):427–433. doi: 10.1083/jcb.87.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. J Cell Biol. 1979 Oct;83(1):82–90. doi: 10.1083/jcb.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J. A., 3rd, Rannels D. E. Protein turnover in pulmonary macrophages. Utilization of amino acids derived from protein degradation. Biochem J. 1981 Jul 15;198(1):53–65. doi: 10.1042/bj1980053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. II. Metabolic fate of iodinated polypeptides of mouse L cells. J Cell Biol. 1975 Feb;64(2):461–479. doi: 10.1083/jcb.64.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hällgren R., Sjöström P., Bill A. The uptake of IgG and IgM coated sheep erythrocytes in perfused rabbit liver. Immunology. 1978 Feb;34(2):347–351. [PMC free article] [PubMed] [Google Scholar]
- Ito T., Ueda M. J., Okada T. S., Ohnishi S. Phagocytosis by macrophages. II. The dissociation of the attachment and ingestion steps. J Cell Sci. 1981 Oct;51:189–201. doi: 10.1242/jcs.51.1.189. [DOI] [PubMed] [Google Scholar]
- Kanaseki T., Kadota K. The "vesicle in a basket". A morphological study of the coated vesicle isolated from the nerve endings of the guinea pig brain, with special reference to the mechanism of membrane movements. J Cell Biol. 1969 Jul;42(1):202–220. doi: 10.1083/jcb.42.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. Pinocytic activity of rabbit alveolar macrophages in vitro. J Reticuloendothel Soc. 1978 Dec;24(6):673–685. [PubMed] [Google Scholar]
- Kavet R. I., Brain J. D. Methods to quantify endocytosis: a review. J Reticuloendothel Soc. 1980 Feb;27(2):201–221. [PubMed] [Google Scholar]
- Kavet R. I., Brain J. D. Phagocytosis: quantification of rates and intercellular heterogeneity. J Appl Physiol Respir Environ Exerc Physiol. 1977 Mar;42(3):432–437. doi: 10.1152/jappl.1977.42.3.432. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Klein H. P., Stockem W. Pinocytosis and locomotion of amoebae: XII. Dynamics and motive force generation during induced pinocytosis in A. proteus. Cell Tissue Res. 1979 Mar 19;197(2):263–279. doi: 10.1007/BF00233919. [DOI] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. A lectin-like receptor on mammalian macrophages. Biochem Biophys Res Commun. 1978 Nov 29;85(2):678–683. doi: 10.1016/0006-291x(78)91215-9. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Reiss E., Cherniak R. Concomitant but not causal association between surface charge and inhibition of phagocytosis by cryptococcal polysaccharide. Infect Immun. 1980 Aug;29(2):295–300. doi: 10.1128/iai.29.2.295-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Dubbeldeman-Rempt I., van Furth R. Kinetics of phagocytosis of Staphylococcus aureus and Escherichia coli by human granulocytes. Immunology. 1979 Jun;37(2):453–465. [PMC free article] [PubMed] [Google Scholar]
- Magnusson K. E., Stendahl O., Stjernström I., Edebo L. Reduction of phagocytosis, surface hydrophobicity and charge of Salmonella typhimurium 395 MR10 by reaction with secretory IgA (SIgA). Immunology. 1979 Mar;36(3):439–447. [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. S., Steinman R. M., Unkeless J. C., Cohn Z. A. Selective iodination and polypeptide composition of pinocytic vesicles. J Cell Biol. 1980 Sep;86(3):712–722. doi: 10.1083/jcb.86.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Pancake S. J., Noseworthy J., Karnovsky M. L. Measurement of rates of phagocytosis: the use of cellular monolayers. J Cell Biol. 1969 Jan;40(1):216–224. doi: 10.1083/jcb.40.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages II. Dissociation of the inhibitory effects of 2-deoxyglucose on phagocytosis and ATP generation. J Exp Med. 1976 Dec 1;144(6):1484–1493. doi: 10.1084/jem.144.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages. I. Description of the inhibitory effect. J Exp Med. 1976 Dec 1;144(6):1465–1483. doi: 10.1084/jem.144.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Silverstein S. C. Role of macrophage receptors in the ingestion phase of phagocytosis. Birth Defects Orig Artic Ser. 1978;14(2):99–117. [PubMed] [Google Scholar]
- Mudd S., Mudd E. B. CERTAIN INTERFACIAL TENSION RELATIONS AND THE BEHAVIOR OF BACTERIA IN FILMS. J Exp Med. 1924 Oct 31;40(5):647–660. doi: 10.1084/jem.40.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system I. Analysis of a novel method of intralysosomal iodination. J Cell Biol. 1980 Jul;86(1):292–303. doi: 10.1083/jcb.86.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system. II. Bidirectional flow between secondary lysosomes and plasma membrane. J Cell Biol. 1980 Jul;86(1):304–314. doi: 10.1083/jcb.86.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel R. J., Rosen N., Bloom B. R. Isolation of variants in phagocytosis of a macrophage-like continuous cell line. J Exp Med. 1977 Jan 1;145(1):175–186. doi: 10.1084/jem.145.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel R. J., Rosen N., Rosen O. M., Bloom B. R. Modulation of Fc-mediated phagocytosis by cyclic AMP and insulin in a macrophage-like cell line. J Immunol. 1977 Nov;119(5):1813–1820. [PubMed] [Google Scholar]
- Neufeld E. F., Sando G. N., Garvin A. J., Rome L. H. The transport of lysosomal enzymes. J Supramol Struct. 1977;6(1):95–101. doi: 10.1002/jss.400060108. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Ukena T. E., Berlin R. D. Effects of phagocytosis and colchicine on the distribution of lectin-binding sites on cell surfaces. Proc Natl Acad Sci U S A. 1974 Feb;71(2):394–398. doi: 10.1073/pnas.71.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Malaisse-Lagae F., Ravazzola M., Amherdt M., Renold A. E. Exocytosis-endocytosis coupling in the pancreatic beta cell. Science. 1973 Aug 10;181(4099):561–562. doi: 10.1126/science.181.4099.561. [DOI] [PubMed] [Google Scholar]
- Painter R. G., McIntosh A. T. The regional association of actin and myosin with sites of particle phagocytosis. J Supramol Struct. 1979;12(3):369–384. doi: 10.1002/jss.400120308. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–250. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. On the structural and functional components of coated vesicles. J Mol Biol. 1978 Dec 25;126(4):803–812. doi: 10.1016/0022-2836(78)90021-9. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L. Role of surface wettability in phagocytosis of Neurospora crassa spores. J Reticuloendothel Soc. 1979 Nov;26(5):549–552. [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Quie P. G. Influence of temperature on opsonization and phagocytosis of staphylococci. Infect Immun. 1977 Jan;15(1):175–179. doi: 10.1128/iai.15.1.175-179.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty H. R., Hafeman D. G., McConnell H. M. Disappearance of macrophage surface folds after antibody-dependent phagocytosis. J Cell Biol. 1981 May;89(2):223–229. doi: 10.1083/jcb.89.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty H. R., Hafeman D. G., McConnell H. M. Specific antibody-dependent phagocytosis of lipid vesicles by RAW264 macrophages results in the loss of cell surface Fc but not C3b receptor activity. J Immunol. 1980 Dec;125(6):2391–2396. [PubMed] [Google Scholar]
- Phaire-Washington L., Wang E., Silverstein S. C. Phorbol myristate acetate stimulates pinocytosis and membrane spreading in mouse peritoneal macrophages. J Cell Biol. 1980 Aug;86(2):634–640. doi: 10.1083/jcb.86.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratten M. K., Williams K. E., Lloyd J. B. A quantitative study of pinocytosis and intracellular proteolysis in rat peritoneal macrophages. Biochem J. 1977 Dec 15;168(3):365–372. doi: 10.1042/bj1680365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusch R. D. Bulk solute extrusion as a mechanism conferring solute uptake specificity by pinocytosis in Amoeba proteus. Science. 1981 Aug 7;213(4508):668–670. doi: 10.1126/science.7256268. [DOI] [PubMed] [Google Scholar]
- Prusch R. D. Endocytotic sucrose uptake in Amoeba proteus induced with the calcium ionophore A23187. Science. 1980 Aug 8;209(4457):691–692. doi: 10.1126/science.6771873. [DOI] [PubMed] [Google Scholar]
- Prusch R. D., Hannafin J. A. Sucrose uptake by pinocytosis in Amoeba proteus and the influence of external calcium. J Gen Physiol. 1979 Oct;74(4):523–535. doi: 10.1085/jgp.74.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintart J., Leroy-Houyet M. A., Trouet A., Baudhuin P. Endocytosis and chloroquine accumulation during the cell cycle of hepatoma cells in culture. J Cell Biol. 1979 Sep;82(3):644–653. doi: 10.1083/jcb.82.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. PARTICLE UPTAKE BY POLYMORPHONUCLEAR LEUCOCYTES AND EHRLICH ASCITES-CARCINOMA CELLS. Biochem J. 1963 Oct;89:150–156. doi: 10.1042/bj0890150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYSER H., AUB J. C., CAULFIELD J. B. Studies on protein uptake by isolated tumor cells. II. Quantitative data on the adsorption and uptake of I-131-serum albumin by Ehrlich ascites tumor cells. J Cell Biol. 1962 Dec;15:437–449. doi: 10.1083/jcb.15.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967 Apr;46(1):19–28. doi: 10.1016/0014-4827(67)90405-3. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Axline S. G. Subplasmalemmal microfilaments and microtubules in resting and phagocytizing cultivated macrophages. J Cell Biol. 1973 Oct;59(1):12–27. doi: 10.1083/jcb.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Roberts A. V., Williams K. E., Lloyd J. B. The pinocytosis of 125I-labelled poly(vinylpyrrolidone), [14C]sucrose and colloidal [198Au]gold by rat yolk sac cultured in vitro. Biochem J. 1977 Nov 15;168(2):239–244. doi: 10.1042/bj1680239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen N., Piscitello J., Schneck J., Muschel R. J., Bloom B. R., Rosen O. M. Properties of protein kinase and adenylate cyclase-deficient variants of a macrophage-like cell line. J Cell Physiol. 1979 Jan;98(1):125–136. doi: 10.1002/jcp.1040980114. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. Uptake of protein by mammalian cells: an underdeveloped area. The penetration of foreign proteins into mammalian cells can be measured and their functions explored. Science. 1968 Jan 26;159(3813):390–396. doi: 10.1126/science.159.3813.390. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Sastry P. S., Hokin L. E. Studies on the role of phospholipids in phagocytosis. J Biol Chem. 1966 Jul 25;241(14):3354–3361. [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D. Disappearance and recovery of human monocyte IgG receptor activity after phagocytosis. J Immunol. 1972 Oct;109(4):914–917. [PubMed] [Google Scholar]
- Schneider Y. J., Tulkens P., de Duve C., Trouet A. Fate of plasma membrane during endocytosis. II. Evidence for recycling (shuttle) of plasma membrane constituents. J Cell Biol. 1979 Aug;82(2):466–474. doi: 10.1083/jcb.82.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Shaw D. R., Griffin F. M., Jr Phagocytosis requires repeated triggering of macrophage phagocytic receptors during particle ingestion. Nature. 1981 Jan 29;289(5796):409–411. doi: 10.1038/289409a0. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O. I., Hartwig J. H., Brotschi E. A., Stossel T. P. Distribution of actin-binding protein and myosin in macrophages during spreading and phagocytosis. J Cell Biol. 1980 Feb;84(2):215–224. doi: 10.1083/jcb.84.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Edebo L., Magnusson K. E., Tagesson C., Hjertén S. Surface-charge characteristics of smooth and rough Salmonella typhimurium bacteria determined by aqueous two-phase partitioning and free zones electrophoresis. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):334–340. doi: 10.1111/j.1699-0463.1977.tb01984.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Edebo L. Phagocytosis of mutants of Salmonella typhimurium by rabbit polymorphonuclear cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):481–488. doi: 10.1111/j.1699-0463.1972.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Tagesson C., Edebo L. Influence of hyperimmune immunoglobulin G on the physicochemical properties of the surface of Salmonella typhimurium 395 MS in relation to interaction with phagocytic cells. Infect Immun. 1974 Aug;10(2):316–319. doi: 10.1128/iai.10.2.316-319.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Tagesson C., Edebo M. Partition of Salmonella typhimurium in a two-polymer acqueous phase system in relation to liability to phagocytosis. Infect Immun. 1973 Jul;8(1):36–41. doi: 10.1128/iai.8.1.36-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Tagesson C., Magnusson K. E., Edebo L. Physiochemical consequences of opsonization of Salmonella typhimurium with hyperimmune IgG and complement. Immunology. 1977 Jan;32(1):11–18. [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H. Interactions of actin, myosin, and a new actin-binding protein of rabbit pulmonary macrophages. II. Role in cytoplasmic movement and phagocytosis. J Cell Biol. 1976 Mar;68(3):602–619. doi: 10.1083/jcb.68.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Quantitative studies of phagocytosis. Kinetic effects of cations and heat-labile opsonin. J Cell Biol. 1973 Aug;58(2):346–356. doi: 10.1083/jcb.58.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. The mechanism of phagocytosis. J Reticuloendothel Soc. 1976 Apr;19(4):237–245. [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Blinks J. R., Reynolds G. Contractile basis of ameboid movement. VII. Aequorin luminescence during ameboid movement, endocytosis, and capping. J Cell Biol. 1980 Aug;86(2):599–607. doi: 10.1083/jcb.86.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Wang Y. L., Heiple J. M. Contractile basis of ameboid movement. VII. The distribution of fluorescently labeled actin in living amebas. J Cell Biol. 1980 Aug;86(2):590–598. doi: 10.1083/jcb.86.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher S. G., Yoshida T., Van Oss C. J., Cohen S., Rose N. R. Alteration of macrophage interfacial tension by supernatants of antigen-activated lymphocyte cultures. J Immunol. 1973 Feb;110(2):321–326. [PubMed] [Google Scholar]
- Tsan M. F., Berlin R. D. Effect of phagocytosis on membrane transport of nonelectrolytes. J Exp Med. 1971 Oct 1;134(4):1016–1035. doi: 10.1084/jem.134.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher F., Dobronyi I., Gergel J. IgM-Fc receptor-mediated phagocytosis of rat macrophages. Immunology. 1981 Mar;42(3):419–425. [PMC free article] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F., Neumann A. W. Phagocytosis as a surface phenomenon. IV. The minimum size and composition of antigen-antibody complexes that can become phagocytized. Immunol Commun. 1974;3(1):77–84. doi: 10.3109/08820137409055746. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- Vogel G., Thilo L., Schwarz H., Steinhart R. Mechanism of phagocytosis in Dictyostelium discoideum: phagocytosis is mediated by different recognition sites as disclosed by mutants with altered phagocytotic properties. J Cell Biol. 1980 Aug;86(2):456–465. doi: 10.1083/jcb.86.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Rosenstreich D. L. Defective Fc receptor-mediated phagocytosis in C3H/HeJ macrophages. I. Correction by lymphokine-induced stimulation. J Immunol. 1979 Dec;123(6):2842–2850. [PubMed] [Google Scholar]
- Vogel S. N., Weedon L. L., Oppenheim J. J., Rosenstreich D. L. Defective Fc-mediated phagocytosis in C3H/HeJ macrophages. II. Correction by cAMP agonists. J Immunol. 1981 Feb;126(2):441–445. [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Walter H., Krob E. J., Garza R. Factors in the partition of red blood cells in aqueous dextran-polyethylene glycol two-phase systems. Biochim Biophys Acta. 1968 Oct 15;165(3):507–514. doi: 10.1016/0304-4165(68)90231-6. [DOI] [PubMed] [Google Scholar]
- Walter R. J., Berlin R. D., Pfeiffer J. R., Oliver J. M. Polarization of endocytosis and receptor topography on cultured macrophages. J Cell Biol. 1980 Jul;86(1):199–211. doi: 10.1083/jcb.86.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M. N., Papadimitriou J. M. Phagocytosis: a review. CRC Crit Rev Toxicol. 1978 Sep;5(4):377–421. doi: 10.3109/10408447809081012. [DOI] [PubMed] [Google Scholar]
- Warr G. A. A macrophage receptor for (mannose/glucosamine)-glycoproteins of potential importance in phagocytic activity. Biochem Biophys Res Commun. 1980 Apr 14;93(3):737–745. doi: 10.1016/0006-291x(80)91139-0. [DOI] [PubMed] [Google Scholar]
- Warren L., Glick M. C. Membranes of animal cells. II. The metabolism and turnover of the surface membrane. J Cell Biol. 1968 Jun;37(3):729–746. doi: 10.1083/jcb.37.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Dickson R., Pastan I. Microinjection of anticlathrin antibodies into fibroblasts does not interfere with the receptor-mediated endocytosis of alpha2-macroglobulin. Cell. 1981 Jul;25(1):105–119. doi: 10.1016/0092-8674(81)90235-x. [DOI] [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Plasma membrane synthesis in the macrophage following phagocytosis of polystyrene latex particles. J Biol Chem. 1972 Apr 25;247(8):2439–2446. [PubMed] [Google Scholar]
- Wilkinson P. C. Recognition and response in mononuclear and granular phagocytes. Clin Exp Immunol. 1976 Sep;25(3):355–366. [PMC free article] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. I. Kinetics of uptake of (125I)polyvinylpyrrolidone by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):113–122. doi: 10.1083/jcb.64.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Keen J. H., Pastan I. H. Ultrastructural immunocytochemical localization of clathrin in cultured fibroblasts. Exp Cell Res. 1981 Apr;132(2):329–338. doi: 10.1016/0014-4827(81)90108-7. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Maxfield F. R., Pastan I. H. alpha 2 Macroglobulin binding to the plasma membrane of cultured fibroblasts. Diffuse binding followed by clustering in coated regions. J Cell Biol. 1979 Sep;82(3):614–625. doi: 10.1083/jcb.82.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The receptosome: an intermediate organelle of receptor mediated endocytosis in cultured fibroblasts. Cell. 1980 Aug;21(1):67–77. doi: 10.1016/0092-8674(80)90115-4. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Roth T. F. Coated vesicles: characterization, selective dissociation, and reassembly. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4394–4398. doi: 10.1073/pnas.75.9.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Zaner K. S., Stossel T. P. Ca2+ control of actin gelation. Interaction of gelsolin with actin filaments and regulation of actin gelation. J Biol Chem. 1980 Oct 10;255(19):9494–9500. [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]


