Abstract
High mobility group box (HMGB) proteins belong to the high mobility group (HMG) superfamily of non-histone nuclear proteins that are involved in chromatin remodeling, regulation of gene expression, and DNA repair. When extracellular, HMGBs serve as alarmins inducing inflammation, and this is attributed to the proinflammatory activity of box B. Here, we show that Plasmodium HMGB1 has key amino acid changes in box B resulting in the loss of TNF-α stimulatory activity. Site-directed mutagenesis of the critical amino acids in box B with respect to mouse HMGB1 renders recombinant Plasmodium berghei (Pb) HMGB1 capable of inducing TNF-α release. Targeted deletion of PbHMGB1 and a detailed in vivo phenotyping show that PbHMGB1 knockout (KO) parasites can undergo asexual stage development. Interestingly, Balb/c mice-infected with PbHMGB1KO parasites display a protective phenotype with subsequent clearance of blood parasitemia and develop long-lasting protective immunity against the challenges performed with Pb wildtype parasites. The characterization of splenic responses shows prominent germinal centers leading to effective humoral responses and enhanced T follicular helper cells. There is also complete protection from experimental cerebral malaria in CBA/CaJ mice susceptible to cerebral pathogenesis with subsequent parasite clearance. Transcriptomic studies suggest the involvement of PbHMGB1 in pir expression. Our findings highlight the gene regulatory function of parasite HMGB1 and its in vivo significance in modulating the host immune responses. Further, clearance of asexual stages in PbHMGB1KO-infected mice underscores the important role of parasite HMGB1 in host immune evasion. These findings have implications in developing attenuated blood-stage vaccines for malaria.
Keywords: malaria, parasite, HMGB1, recombinant protein expression, site-directed mutagenesis, gene knockout, inflammation, pathogenesis, host immune evasion, parasite clearance
Malaria caused by Plasmodium spp. is a serious health concern with tremendous economic impact responsible for 249 million infections and 608,000 deaths in 2022. With almost half of the world’s population at risk, children under 5 years of age and pregnant women are the most vulnerable (https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022). Human malaria is caused by five Plasmodium species - falciparum, vivax, malariae, ovale, and knowlesi, and more than 90% of malaria cases and deaths are due to Plasmodium falciparum (Pf) infections. Plasmodium has a complex life cycle that occurs in vertebrate (human) and invertebrate (mosquito) hosts (1). Transmitted by the female Anopheline mosquito, sporozoites introduced into the blood stream reach human liver and undergo exo-erythrocytic stage development in the hepatocytes. The exo-erythrocytic merozoites released from the hepatocytes invade red blood cells (RBCs) and undergo periodic asexual cycle giving rise to new merozoites that invade fresh RBCs until they are cleared by therapeutic interventions. A small fraction of merozoites gives rise to gametocyte formation in RBCs that are ingested by mosquitoes during the blood meal. These gametocytes form gametes, undergo fertilization and complete their sexual development to form sporozoites that invade salivary glands of the mosquitoes to initiate the transmission.
The periodic asexual cycle occurring in RBCs is responsible for the entire malaria pathogenesis and the asexual stage parasites deploy various strategies to evade host immune responses and splenic clearance. Besides the advantage of intracellular residing and the lack of MHC class I molecule expression in host RBCs, the malaria parasite - P. falciparum expresses multigene families like var, rifin, and stevor encoding variant surface antigens that are expressed mainly on the surface of infected RBCs (iRBCs) promoting cytoadherence and sequestration (2, 3, 4). By switching the expression of these highly polymorphic proteins through epigenetic mechanisms, the parasite camouflages them from the host immune responses (5, 6). It is also known that iRBCs are resistant to complement-mediated cell lysis and the knob-like features of Pf erythrocyte membrane protein 1 (EMP-1) encoded by var genes can prevent the adequate deposition of IgGs (7, 8). Further, the recognition of key pathogen-associated molecular patterns (PAMPs) of malaria parasite such as hemozoin, glycosylphosphatidylinositols (GPIs) and nucleic acids by pattern-recognition receptors and cytosolic sensors, often lead to pro-/anti-inflammatory and Th1/Th2 imbalances with aberrant effector and memory T cell responses (9, 10, 11). This is further augmented by the ability of phagocytosed iRBCs and hemozoin to cause premature apoptosis and thereby, preventing the innate immune responses of macrophages, monocytes and dendritic cells (11, 12). Also, there occurs an atypical memory B cell response impeding the antibody production and long-lived immunity (13). All these culminate in preventing the development of protective immunity against asexual stage parasites.
High mobility group box (HMGB) proteins are non-histone chromosome-binding nuclear proteins that contain at least one HMG-box domain and are expressed in almost all eukaryotes. Many eukaryotes contain a large number of HMGB proteins and most of them possess one or two HMG-boxes, although transcription factors like UBF1 contain up to six HMG-boxes (14). In addition to nuclear functions such as chromatin organization, transcriptional regulation, and DNA repair (15, 16, 17), HMGB proteins have evolved to perform cytosolic and extracellular functions. A typical example for the cytosolic function is the regulation of autophagy and mitophagy by binding of human/mouse HMGB1 to key autophagy proteins like Beclin1 and Atg5 through its active shuttling between nucleus and cytosol (18, 19). When present extracellular either through active secretion or passive release, HMGB1 serves as a cytokine or danger-associated molecular pattern (DAMP) by mediating the inflammation. Depending on the overall redox state and interaction with various receptors such as TLR4, RAGE, and so on, HMGB1 can induce proinflammatory responses (20, 21, 22).
The genome of the malaria parasite encodes four HMGB proteins—HMGB1, HMGB2, HMGB3, and HMGB4 that are conserved across the Plasmodium species (23). While HMGB1 and HMGB2 are of around 100 amino acids in length with one HMG-box, HMGB3 consists of over 2000 amino acids with two HMG-boxes and HMGB4 has around 250 amino acids with one HMG-box. Of the four HMGBs, HMGB1 and HMGB2 have been studied so far. In vivo studies carried out in mice infected with Plasmodium berghei (Pb; rodent parasite) HMGB2 knockout (KO) parasites have shown significant protection from experimental cerebral malaria (ECM), and pre-immunization with iRBCs could confer long-lasting sterile protection against homologous and heterologous Pb strains (24, 25). Another study carried out in the rodent parasite, Plasmodium yoelii (Py), has shown that the deletion of HMGB2 leads to a prominent reduction of oocyst formation in mosquitoes (26). While the former study suggests the proinflammatory function of HMGB2 being responsible for ECM, the latter highlights the gene regulatory function wherein, ∼30 genes with most of them expressed in the gametocyte stages are downregulated in HMGB2KO parasites. Similarly, deletion of HMGB2 in Pf has led to a significant reduction in oocyst formation with no prominent changes in asexual stage development (27). In case of HMGB1, a ChIP-seq study carried out with PfHMGB1 wildtype (WT) and KO lines has shown that HMGB1 deletion disrupts centromere-/telomere-dependent nuclear architecture leading to a complete silencing of var expression (28). Given this background, we have sought to examine the proinflammatory nature of parasite HMGB1 and the in vivo phenotype of HMGB1 deletion using a lethal Pb ANKA strain. Our results suggest that the parasite HMGB1 lacks TNF-α stimulatory activity, and PbHMGB1KO parasites can lead to a protective phenotype in the asexual stage infections with long-lasting immunity. The parasite HMGB1 can regulate the expression of Plasmodium interspersed repeat (pir) multigene families that are associated with host-immune evasion and malaria pathogenesis. These results indicate the important role played by HMGB1 in the asexual stages of malaria parasite.
Results
PbHMGB1 lacks TNF-α stimulatory activity
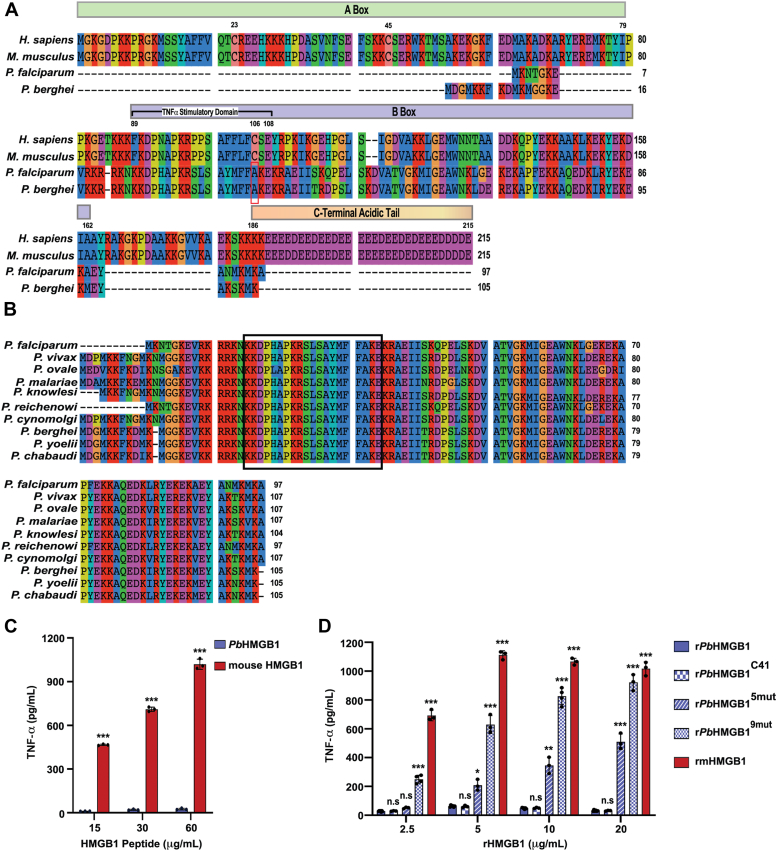
Multiple sequence alignment of Plasmodia (Pf and Pb) HMGB1 with mammalian (human and mouse) HMGB1 suggested ∼28% similarity and ∼20% identity. More importantly, Plasmodia HMGB1 lacks the A box and C-terminal acidic tail that are present in mammalian HMGB1 (Fig. 1A). The A box contains two critical cysteine residues (Cys23 and Cys45) whose redox status determines the chemoattractant and proinflammatory functions of mammalian HMGB1 (29). Further, the third critical cysteine residue (Cys106) present in the TNF-α stimulatory domain of B box in mammalian HMGB1 is also absent in Plasmodia HMGB1 (Fig. 1A). It has been shown that the mutation of Cys106 to Ala prevents the binding of mammalian HMGB1 to Toll-like receptor 4 (TLR4)/myeloid differentiation factor-2 (MD-2) complex and the subsequent release of TNF-α from macrophages (30). Interestingly, the sequence in Plasmodia HMGB1 corresponding to the TNF-α stimulatory domain of mammalian HMGB1 B box has an indigenous Ala in the place of Cys106 (Fig. 1A). Further, the characteristic features of parasite HMGB1 lacking A box, C-terminal acidic tail and Cys106 are conserved across the Plasmodium species infecting humans, primates, and rodents (Fig. 1B). All these prompted us to examine the TNF-α stimulatory activity of Plasmodia HMGB1.
Figure 1.
Sequence comparison of parasite HMGB1 and the lack of TNF-α stimulatory activity.A, multiple protein sequence alignment of mouse, human, Pf and Pb HMGB1. The alignment shows the absence of A box and C-terminal acidic tail in Plasmodia HMGB1. The cysteine residues Cys23 and Cys45 present in the A box, and Cys106 present in the B box of mammalian (mouse and human) HMGB1 are highlighted with their respective numbers. The TNF-α stimulatory domain of mammalian HMGB1 (89–108 amino acids) and the corresponding sequence in Plasmodia HMGB1 are shown. The presence of indigenous Ala in the Plasmodia HMGB1 sequence corresponding to the TNF-α stimulatory domain of mammalian HMGB1 is highlighted in red box. B, multiple protein sequence alignment of Plasmodia HMGB1 infecting humans, rodents and primates. The 20 amino acid sequence in Plasmodia HMGB1 that corresponds to TNF-α stimulatory domain of mammalian HMGB1 is highlighted in a box. The respective sequence is conserved across the represented Plasmodium species with almost 100% identity. Multiple protein sequence alignments were carried out with SeaView Version 5.0.5 (https://doua.prabi.fr/software/seaview). C, treatment of murine macrophage-like RAW 264.7 cell line with the synthetic peptide of mouse HMGB1 representing TNF-α stimulatory domain of 20 amino acid length and the corresponding synthetic peptide of PbHMGB1. After 12 h of treatment, ELISA was performed with the culture supernatants to estimate the levels of TNF-α secretion. The data (mean ± SD) represent three independent experiments (∗∗∗p < 0.001, unpaired t test; two-tailed). D, treatment of murine macrophage-like RAW 264.7 cell line with rPbHMGB1, rPbHMGB1C41, rPbHMGB15mut, rPbHMGB19mut and rmHMGB1. After 12 h of treatment, ELISA was performed with the culture supernatants to estimate the levels of TNF-α secretion. The data (mean ± SD) represent at least three independent experiments. (n.s.- not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; unpaired t test; two-tailed).
It has already been shown that the synthetic peptide of 20 amino acids representing the TNF-α stimulatory domain of mammalian HMGB1 can lead to TLR-4-dependent activation of macrophages and TNF-α release (30, 31). Therefore, to begin with, we examined the TNF-α stimulatory activity of the PbHMGB1 synthetic peptide of length 20 amino acids that corresponds to the TNF-α stimulatory domain of mammalian HMGB1 using a murine macrophage-like RAW 264.7 cell line. The respective 20 amino acid sequence of parasite HMGB1 has retained almost 100% identity within the Plasmodia species (Fig. 1B). While the synthetic peptide of mouse HMGB1 TNF-α stimulatory domain could lead to TNF-α release at a concentration of 15 μg/ml, the corresponding synthetic peptide of PbHMGB1 did not cause prominent TNF-α release even at a concentration of 60 μg/ml (Fig. 1C). To rule out the possibility of additional sequence requirement for TNF-α stimulation, the full-length recombinant PbHMGB1 (rPbHMGB1) expressed in E. coli was used. The rPbHMGB1 was purified using Ni2+-NTA resin, followed by S-Sepharose chromatography to remove additional protein impurities, and DNase I treatment and endotoxin removal to deplete DNA and lipopolysaccharide (LPS) contaminations, respectively (Fig. S1A). As observed for the synthetic peptide, rPbHMGB1 failed to induce TNF-α release even at a high concentration of 20 μg/ml. In contrast, recombinant mouse HMGB1 (rmHMGB1) could induce a strong TNF-α release at a concentration of 2.5 to 5.0 μg/ml (Fig. 1D). Since Cys106 of mammalian HMGB1 plays an important role in TLR4-dependent TNF-α release, we performed site-directed mutagenesis and replaced the indigenous Ala41 of rPbHMGB1 with Cys (rPbHMGB1C41) (Fig. S1B). The rPbHMGB1C41 was purified under identical conditions as described for rPbHMGB1 (Fig. S1A). However, the replacement of Ala with Cys could not lead to any prominent increase in TNF-α release (Fig. 1D). This in turn suggested that PbHMGB1 has additional modifications in the sequence corresponding to TNF-α stimulatory domain and a sequence comparison with mouse HMGB1 suggested another nine amino acid changes. We performed a first set of sequential site-directed mutagenesis with rPbHMGB1C41 plasmid replacing Lys42, Lys24, Leu34, and Ser33 with Ser, Phe, Pro and Pro, respectively (Fig. S1B). The purified rPbHMGB1 with five mutations (rPbHMGB15mut) including Cys41 showed ∼30% of TNF-α release with respect to mouse HMGB1 at a concentration of 5.0 μg/ml (Figs. S1, A and B and 1D). We further included four additional mutations replacing His28, Tyr37, Met38 and Phe39 with Asn, Phe, Phe, and Leu, respectively (Fig. S1, A and B). The resultant rPbHMGB1 with nine mutations (rPbHMGB19mut) matched completely with TNF-α stimulatory domain of mouse HMGB1 and showed ∼60% of TNF-α release with respect to mouse HMGB1 at a concentration of 5.0 μg/ml. At concentrations of 10 to 20 μg/ml, the levels of TNF-α release were comparable with respect to mouse HMGB1 (Fig. 1D). These findings suggested that the Plasmodia HMGB1 lacks TNF-α stimulatory activity.
PbHMGB1 is undetectable in plasma
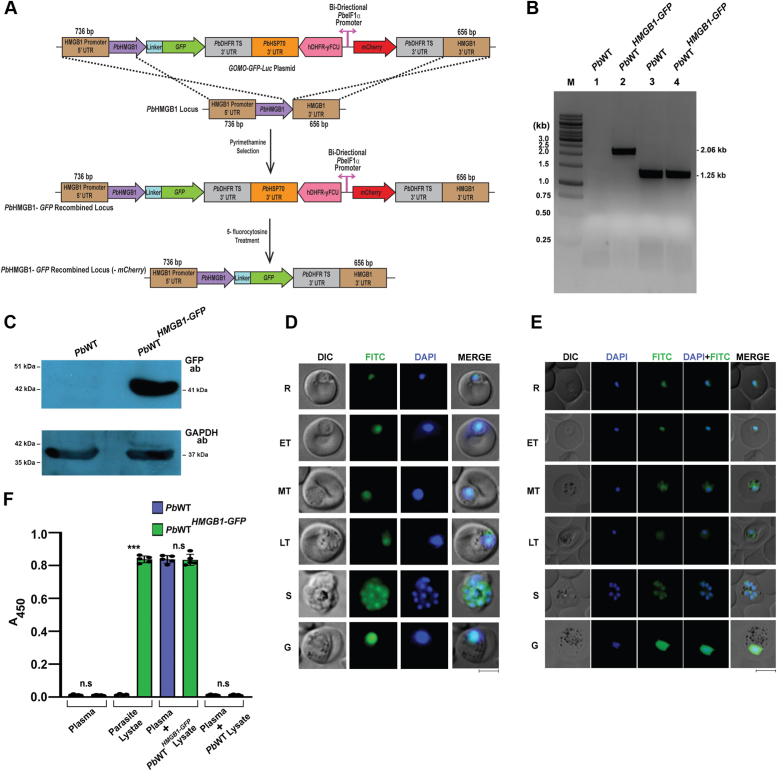
Our next interest was to examine whether PbHMGB1 undergoes active secretion or passive release. For this, we generated PbWTHMGB1-GFP transgenic parasites through double-crossover recombination wherein, the C-terminus of endogenous PbHMGB1 was tagged in-frame with a linker followed by green fluorescence protein (GFP) (Fig. 2A). The modification of PbHMGB1 locus in PbWTHMGB1-GFP parasites was confirmed by genomic DNA PCR analysis (Fig. 2B). Western blot analysis carried out with the lysates of PbWTHMGB1-GFP parasites confirmed the expression of a 41 kDa PbHMGB1-GFP fusion protein (Fig. 2C). Live imaging studies performed with PbWTHMGB1-GFP transgenic parasites showed the co-localization of GFP fluorescence with the DNA staining of 4′,6-diamidino-2-phenylindole (DAPI) suggesting the nuclear localization of PbHMGB1 (Fig. 2D). These results were further confirmed by indirect immunofluorescence analysis performed with GFP antibodies (Fig. 2E). To examine the extracellular presence of PbHMGB1, plasma samples were collected from mice infected with PbWTHMGB1-GFP parasites showing ∼10% blood parasitemia (∼109 parasites/ml of blood) and ELISA was carried out with GFP antibodies. The GFP antibodies could detect PbHMGB1-GFP at as low as 104 PbWTHMGB1-GFP parasites (Fig. S2A). For control, plasma samples of PbWT-infected mice were used. The results obtained suggested that PbHMGB1-GFP could not be detected in the plasma samples of PbWTHMGB1-GFP-infected mice (Fig. 2F). There was only a background signal as observed for PbWT-infected mice plasma. However, GFP antibodies could react with the endogenous PbHMGB1-GFP present in the PbWTHMGB1-GFP parasite lysates. To further ensure these findings, the respective plasma samples were independently spiked with the lysates of PbWTHMGB1-GFP and PbWT parasites. As expected, GFP antibodies could readily detect the plasma samples spiked with PbHMGB1-GFP lysates, but not with PbWT lysates (Fig. 2F). All these results obtained with PbWTHMGB1-GFP parasites suggested that PbHMGB1 remains undetectable in the plasma samples of infected mice and it is neither actively secreted nor passively released.
Figure 2.
Generation of PbWTHMGB1-GFPparasites and examination of the extracellular presence of parasite HMGB1.A, schematic representation of double-crossover recombination strategy followed to generate PbWTHMGB1-GFP parasites. B, genomic DNA PCR confirmation for site-specific integration of HMGB1-GFP in PbWTHMGB1-GFP parasites. Lane M: 1 kb ladder. Lane 1 and 2: Integration-specific product of 2.06 kb amplified using forward primer upstream to the promoter sequence of PbHMGB1 and GFP-specific reverse primer. Lane 3 and 4: PbGAPDH control (1.25 kb). C, Western blot analysis of PbHMGB1-GFP protein expression in PbWTHMGB1-GFP parasites. 100 μg of total protein was used. D, live imaging of PbWTHMGB1-GFP parasites showing the localization of endogenous PbHMGB1-GFP. DAPI treatment was carried out to stain the nuclear DNA. Images were captured using 100× objective lens. Scale bar = 5 μm. E, indirect immunofluorescence analysis of PbHMGB1-GFP localization in PbWTHMGB1-GFP parasites using GFP antibodies. DAPI treatment was carried out to stain the nuclear DNA. Images were captured using 100× objective lens. Scale bar = 5 μm. F, ELISA of plasma samples collected from PbWT- and PbWTHMGB1-GFP-infected mice using GFP antibodies. 100 μl plasma samples were used to coat the wells. For parasite lysate control, 50 μg of PbWT and PbWTHMGB1-GFP lysates were used. For spiking, the respective plasma samples were spiked with 50 μg of PbWTHMGB1-GFP or PbWT lysates. The data (mean ± SD) represent five different samples (n.s.- not significant, ∗∗∗p < 0.001; unpaired t test; two-tailed). ET, early trophozoite; G, gametocyte; LT, late trophozoite; MT, mid trophozoite; R, ring; S, schizont.
PbHMGB1 deletion leads to the clearance of asexual stages
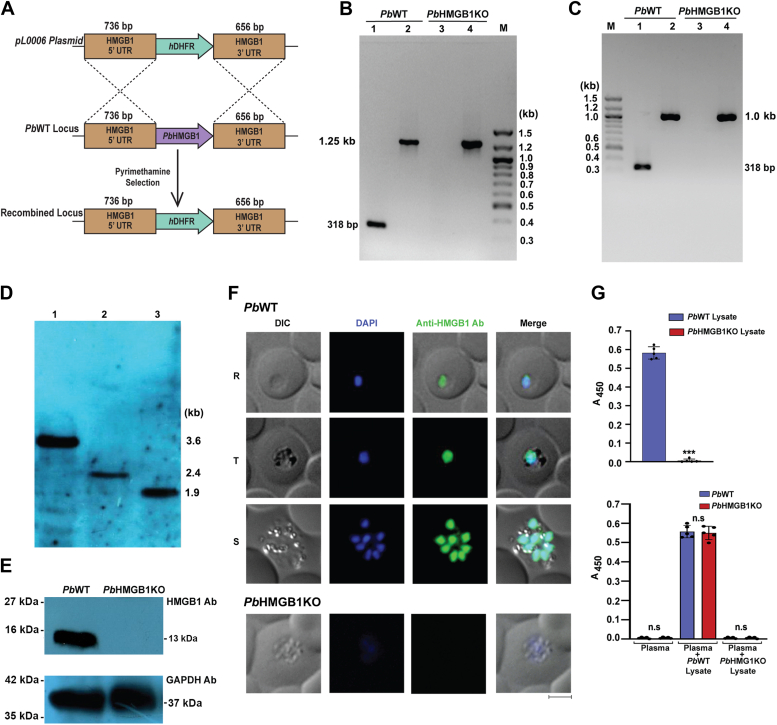
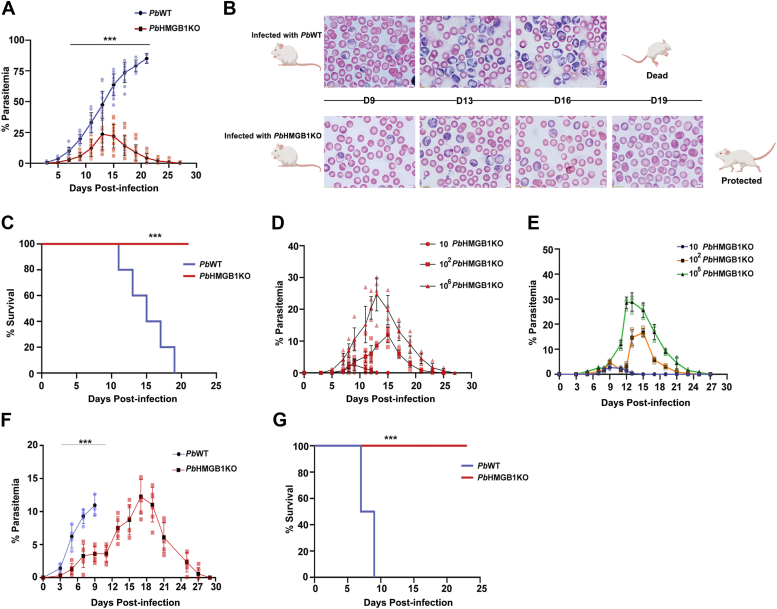
To understand the significance of HMGB1 in the life cycle of Pb, targeted deletion was carried out through double-crossover recombination (Fig. 3A). The successful deletion of PbHMGB1 was verified by genomic DNA PCR, RT-PCR and Southern analyses (Fig. 3, B–D). We raised polyclonal antibodies against rPbHMGB1 and Western analysis carried out with the lysates of PbWT and PbHMGB1KO parasites showed the presence of a 13 kDa PbHMGB1 in PbWT, but not in PbHMGB1KO parasites (Fig. 3E). This was also true for immunofluorescence analysis wherein, nuclear localization of PbHMGB1 could only be detected in PbWT parasites, but not in the PbHMGB1KO parasites (Fig. 3F). Having confirmed the specificity, we further utilized PbHMGB1 polyclonal antibodies to verify the ELISA results obtained for the absence of extracellular HMGB1 in PbWTHMGB1-GFP-infected mice with GFP antibodies. The PbHMGB1 polyclonal antibodies could detect at least 0.1 to 1.0 ng of rPbHMGB1, and also the native PbHMGB1 at as low as 104 PbWT parasites (Fig. S2, B and C). Once again, PbHMGB1 antibodies could only detect PbHMGB1 in PbWT parasite lysates, but not in the plasma samples of the PbWT-infected mice. As expected, no reactivity could be observed for PbHMGB1KO parasite lysates and plasma samples of PbHMGB1KO-infected mice (Fig. 3G). Our next interest was to examine the asexual phenotype of PbHMGB1KO parasites. Growth analysis performed for the asexual stages in Balb/c mice infected with 105 PbHMGB1KO parasitized RBCs through intraperitoneal route suggested a delay of ∼3 days in comparison with PbWT parasite growth. Importantly, mice infected with PbHMGB1KO parasites displayed a subsequent clearance when the blood parasitemia reached around 15 to 30% (Fig. 4, A and B). While all the mice infected with PbWT parasites succumbed to anemia within day 19 post-infection, PbHMGB1KO-infected mice were completely protected (Fig. 4C). To rule out any reappearance of asexual stage parasites in the blood due to recrudescence, the protected mice were monitored over a period of 6 months by examining the presence of parasites in tail vein blood smears prepared at an interval of 5 days. However, no parasites could be detected suggesting the complete clearance of blood parasitemia and the absence of recrudescence.
Figure 3.
Generation of PbHMGB1KO parasites.A, double cross-over recombination strategy followed to generate PbHMGB1KO parasites. B, genomic DNA PCR confirmation of HMGB1 deletion in PbHMGB1KO parasites. Lane 1 and 3: PCR amplification of PbHMGB1 (318 bp). Lane 2 and 4: PbGAPDH control (1.25 kb). Lane M: 100 bp ladder. C, RT-PCR confirmation of HMGB1 deletion in PbHMGB1KO parasites. Lane 1 and 3: PCR amplification of HMGB1 (318 bp). Lane 2 and 4: PbGAPDH control (1.0 kb). Lane M: 100 bp ladder. D, southern blot confirmation of site-specific integration in PbHMGB1KO parasites. Lane 1: Recombinant plasmid used for transfection as a control to rule out the presence of episomes. Lane 2 and 3: Genomic DNA isolated from PbWT and PbHMGB1KO parasites, respectively. Genomic DNA and plasmid samples were digested with SphI and HindIII and hybridized with 3′UTR-specific probe of PbHMGB1. PbWT and PbHMGB1KO genomic DNA showed the hybridized fragments of size 2.4 kb and 1.9 kb, respectively. For recombinant plasmid, the fragment size was 3.6 kb. E, Western blot confirmation of HMGB1 deletion in PbHMGB1KO parasites using PbHMGB1 polyclonal antibodies. 150 μg of total protein was used for SDS-PAGE. F, immunofluorescence analysis of HMGB1 localization in PbWT parasites and HMGB1 deletion in PbHMGB1KO parasites. Scale bar = 5 μM. G, ELISA analysis of plasma samples collected from PbWT- and PbHMGB1KO-infected mice using polyclonal PbHMGB1 antibodies. 100 μl plasma samples were used to coat the wells. For parasite lysates, 50 μg of PbWT and PbHMGB1KO lysates were used. The data (mean ± SD) represent five different samples (n.s.- not significant, ∗∗∗p < 0.001; unpaired t test; two-tailed).
Figure 4.
Characterization of a sexual phenotype of PbHMGB1KO parasites.A, growth analysis of PbWT (n = 13) and PbHMGB1KO (n = 16) in Balb/c mice. 105 parasites were used for infection. The data (mean ± SD) represent three different batches (∗∗∗p < 0.001; Two-way ANOVA; Tukey test). B, Geimsa-stained images of blood smears prepared from tail vein blood of PbWT and PbHMGB1KO parasite-infected mice. Scale bar = 10 μM. C, Survival analysis of Balb/c mice infected with PbWT (n = 5) and PbHMGB1KO (n = 11) parasites (∗∗∗p < 0.001; log-rank (Mantel-Cox) test). D, clearance of blood parasitemia in Balb/c mice (n = 6) infected with 10, 102 or 106 parasitized RBCs through intravenous route. The data (mean ± SD) represent three different batches. (∗∗∗p < 0.001; Two-way ANOVA; Tukey test). E, growth analysis of PbHMGB1KO parasites collected during protective phase. The naïve Balb/c mice (n = 3) were infected with 10, 102 or 105 parasitized RBCs through intravenous route. F, growth analysis of PbWT (n = 4) and PbHMGB1KO (n = 6) in CBA/CaJ mice. 105 parasites were used for infection. The data (mean ± SD) represent three different batches (∗∗∗p < 0.001; Two-way ANOVA; Tukey test). G, survival analysis of CBA/CaJ mice (n = 12) infected with PbWT and PbHMGB1KO parasites (∗∗∗p < 0.001 log-rank (Mantel-Cox) test).
The clearance of PbHMGB1KO parasites and the protective phenotype of PbHMGB1KO-infected mice were also reproducible with the inoculum of 10, 102, or 106 parasitized RBCs through the intravenous route (Fig. 4D). To rule out the subsequent clearance of PbHMGB1KO asexual stage infections occurring due to any growth defect in the parasite per se, PbHMGB1KO parasites were collected during the protective phase when the blood parasitemia fell to 0.1%. When such parasites were injected into naive Balb/c mice, they could successfully establish the blood stage infections leading to an increase in the blood parasitemia followed by subsequent clearance (Fig. 4E). We next examined the protective phenotype of PbHMGB1KO parasites in CBA/CaJ mice - an in vivo mouse model for ECM, by injecting 105 PbHMGB1KO-parasitized RBCs through the intraperitoneal route. As observed for Balb/c mice, PbHMGB1KO parasites displayed a growth delay in CBA/CaJ mice (Fig. 4F). While all the PbWT-infected CBA/CaJ mice died within 9 days post-infection with ∼80% of them showing the typical symptoms of ECM, none of the PbHMGB1KO-infected mice showed ECM. There was also a complete protection from mortality due to ECM and/or anemia with subsequent clearance of blood parasitemia (Fig. 4G). All these findings suggested the important role played by parasite HMGB1 in sustaining the in vivo asexual stage infections.
Splenic clearance of PbHMGB1KO parasites
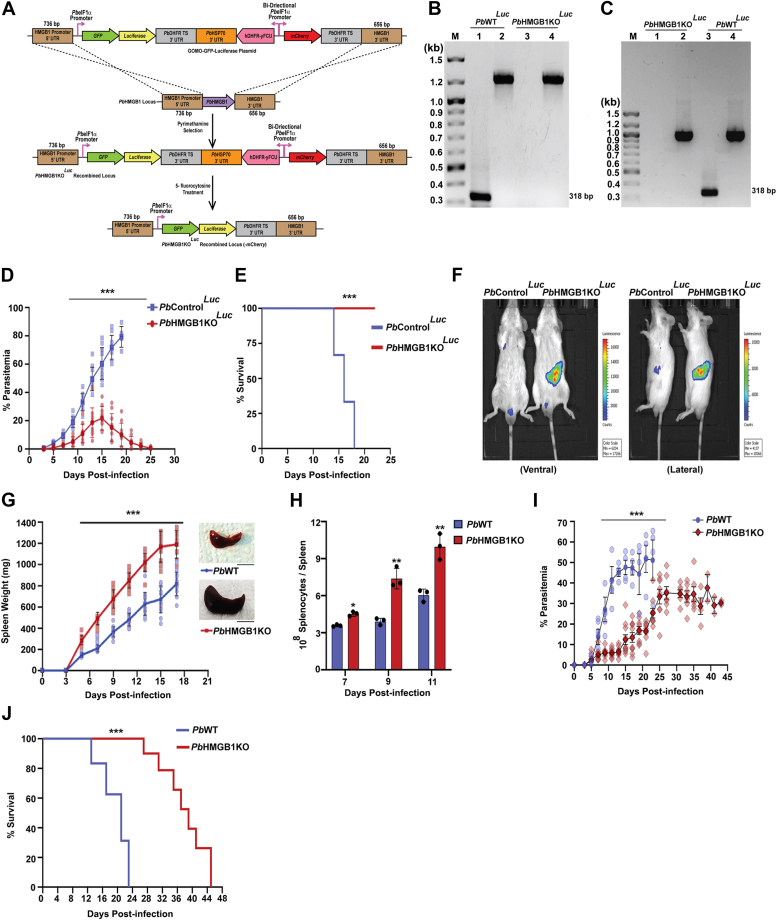
We sought to verify the protective phenotype of PbHMGB1KO parasites with an independent transgenic deletion line wherein, PbHMGB1 was replaced with GFP-luciferase (PbHMGB1KOLuc) through double-crossover recombination (Fig. 5A). The targeted deletion was confirmed by genomic DNA (Fig. 5B) and RT-PCR (Fig. 5C) analyses. As observed for PbHMGB1KO parasites, mice infected with PbHMGB1KOLuc parasites showed protective phenotype in asexual stage infections. The blood parasitemia reached to a maximum of ∼25%, followed by the clearance with complete protection from mortality due to anemia (Fig. 5, D and E). Since PbHMGB1KOLuc parasites expressed luciferase, they offered the advantage of performing bioluminescence studies to track the parasites in vivo. Bioluminescence imaging carried out on day 7 post-infection showed prominent signal in the spleen of mice infected with PbHMGB1KOLuc parasites (Fig. 5F). This was observed despite the blood parasitemia of PbHMGB1KOLuc-infected mice being less when compared with PbWT-infected mice, suggesting an important role played by spleen in the clearance of PbHMGB1KOLuc parasites. In agreement, mice infected with PbHMGB1KO parasites displayed splenomegaly with ∼2 times increase in the spleen weight with respect to PbWT-infected mice (Fig. 5G). The increase in spleen weight of PbHMGB1KO-infected mice was reflected in the total number of splenocytes (Fig. 5H). These findings prompted us to examine the asexual growth of PbHMGB1KO parasites in splenectomized mice. Interestingly, the splenectomized mice could not clear the asexual stages of PbHMGB1KO parasites and they died due to anemia (Fig. 5, I and J). However, there was a significant delay in mortality in comparison with PbWT-infected mice and this could be due to the relatively slow growth of PbHMGB1KO parasites (Fig. 5J). These results suggested that splenic clearance is responsible for the protective phenotype of mice infected with PbHMGB1KO parasites.
Figure 5.
In vivo bioluminescence studies with PbHMGB1KOLucparasites and the effect of splenectomy on the clearance of PbHMGB1KO parasites.A, double cross-over recombination strategy followed to generate PbHMGB1KOLuc parasites. B, genomic DNA PCR confirmation of HMGB1 deletion in PbHMGB1KOLuc parasites. Lane M: 100 bp ladder. Lane 1 and 3: PCR amplification of HMGB1 (318 bp). Lane 2 and 4: GAPDH control (1.25 kb). C, RT-PCR confirmation of HMGB1 deletion in PbHMGB1KOLuc parasites. Lane M: 100 bp ladder. Lane 1 and 3: PCR amplification of HMGB1 (318 bp). Lane 2 and 4: PbGAPDH control (1.0 kb). Lane M: 100 bp ladder. D, growth analysis of PbControlLuc (n = 10) and PbHMGB1KOLuc (n = 10) in Balb/c mice. 105 parasites were used for infection. The data (mean ± SD) represent three different batches (∗∗∗p < 0.001; Two-way ANOVA; Tukey test). E, survival analysis of Balb/c mice infected with PbControlLuc (n = 6) and PbHMGB1KOLuc (n = 6) parasites (∗∗∗p < 0.001, log rank (Mantel-Cox) test). F, in vivo bioluminescence imaging of Balb/c mice infected with PbControlLuc and PbHMGB1KOLuc parasites. 105 parasites were used for infection. G, Spleen weight of Balb/c mice infected with PbWT (n = 14) and PbHMGB1KO (n = 16). The data (mean ± SD) represent three different batches (∗∗∗p < 0.001; Two way ANOVA; Tukey test). Scale bar = 1 cm. H, total number of splenocytes from PbWT- and PbHMGB1KO-infceted mouse spleen. The data (mean ± SD) represent three mice (∗p < 0.05, ∗∗p < 0.01; unpaired t test; two-tailed). I, growth analysis of PbWT (n = 5) and PbHMGB1KO (n = 10) parasites in splenectomized Balb/c mice. 105 parasites were used for infection. The data represent mean ± SD; ∗∗∗p < 0.001; Two-way ANOVA; Tukey test). J, survival analysis of splenectomized Balb/c mice infected with PbWT (n = 4) and PbHMGB1KO (n = 7) parasites; (∗∗∗p < 0.001; log-rank (Mantel-Cox) test].
Genetic complementation in PbHMGB1KO parasites restores the lethal phenotype
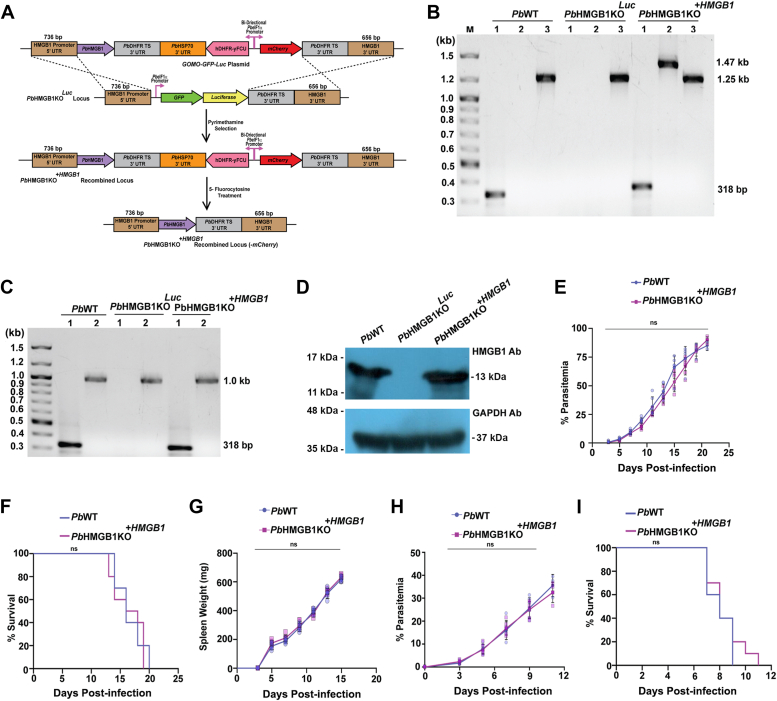
To further ensure the findings on the protective phenotype of PbHMGB1KO parasites, PbHMGB1KOLuc-infected mice were treated with 5-fluorocytosine to remove the hDHFR-yFCU selection cassette. The transgenic parasites lacking pyrimethamine selection marker were then subjected to transfection for reintroducing PbHMGB1 into its endogenous locus (Fig. 6A). The genetic complementation of PbHMGB1 in the KO parasites (PbHMGB1KO+HMGB1) and its site-specific integration were confirmed by genomic DNA and RT-PCR analyses (Fig. 6, B and C). Western analysis with the lysates of PbHMGB1KO+HMGB1parasites suggested the restoration of PbHMGB1 protein expression to the levels comparable with the PbWT parasites (Fig. 6D). As expected, PbHMGB1KO+HMGB1parasites could regain the lethal phenotype of PbWT parasites and all the Balb/c mice infected with PbHMGB1KO+HMGB1parasites died of anemia within day 17 post-infection (Fig. 6, E and F). The spleen weight of mice infected with PbHMGB1KO+HMGB1parasites was similar to that of PbWT-infected mice (Fig. 6G). There was also restoration of ECM phenotype and mortality in CBA/CaJ mice infected with PbHMGB1KO+HMGB1parasites (Fig. 6, H and I). The findings from genetic complementation studies further confirmed that the protective phenotype observed in PbHMGB1KO-infected mice is indeed due to the deletion of PbHMGB1.
Figure 6.
Genetic complementation of PbHMGB1 in PbHMGB1KOLucparasites.A, double cross-over recombination strategy followed to generate PbHMGB1KO+HMGB1 parasites. The arrows denote the promoters present in the plasmid. The expression of PbHMGB1 was restored at its endogenous locus with its own promoter. B, genomic DNA PCR confirmation for the genetic complementation of PbHMGB1 and its site-specific integration in PbHMGB1KOLuc parasites. C, RT-PCR confirmation showing the expression of PbHMGB1 RNA in PbHMGB1KO+HMGB1 parasites. D, Western analysis showing the expression of PbHMGB1 in PbHMGB1KO+HMGB1 parasites. E, Growth analysis of PbHMGB1KO+HMGB1 parasites in Balb/c mice (n = 6). 105 parasites were used for infection. For control, PbWT-infected mice (n = 6) were used. The data (mean ± SD) represent two different batches (n.s., not significant; Two-way ANOVA; Tukey test). F, survival analysis of Balb/c mice infected with PbHMGB1KO+HMGB1 parasites. The data represent ten mice (n.s., not significant; log-rank (Mantel-Cox) test). G, Spleen weight of Balb/c mice infected with PbWT (n = 6) and PbHMGB1KO+HMGB1 parasites (n = 6). H, growth analysis of PbHMGB1KO+HMGB1 parasites (n = 6) in CBA/CaJ mice. 105 parasites were used for infection. For control, PbWT-infected mice (n = 6) were used. The data (mean ± SD) represent two different batches (n.s., not significant; Two-way ANOVA; Tukey test). I, survival analysis of CBA/CaJ mice infected with PbWT and PbHMGB1KO parasites. The data represent ten mice (n.s., not significant; log-rank (Mantel-Cox) test).
Splenic architecture and germinal center responses in PbHMGB1KO-infected mice
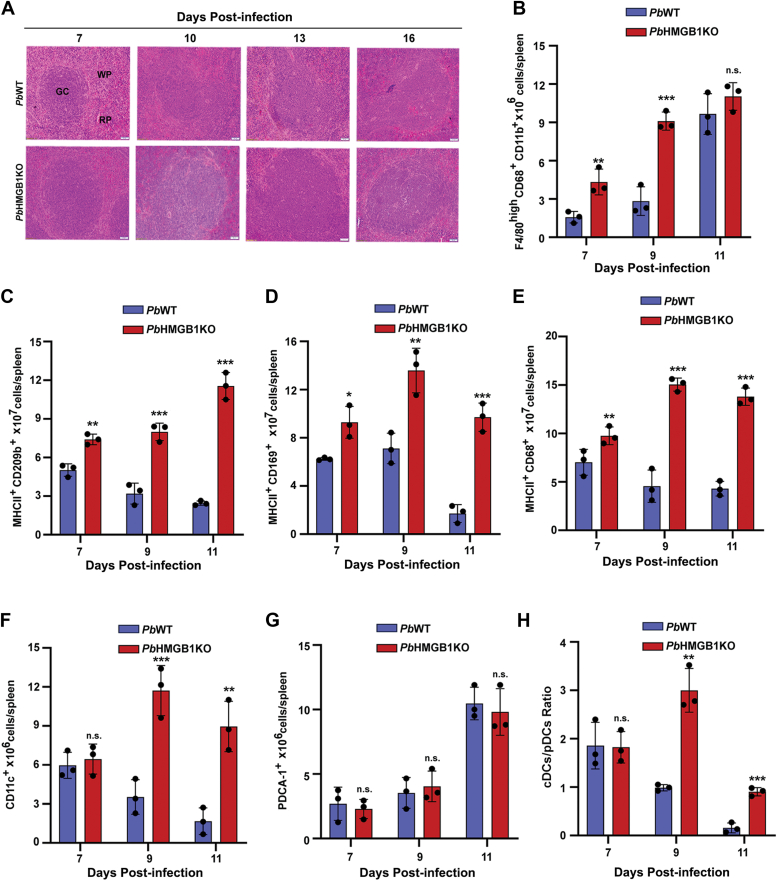
The dissolution of splenic architecture occurs in malaria infections (32, 33). Hematoxylin and eosin (H&E)-staining of the spleen sections suggested that the splenic architecture of PbHMGB1KO-infected mice differed significantly from PbWT-infected mice. While the demarcations of red pulp and white pulp compartments were comparable between PbWT- and PbHMGB1KO-infected mice on day 7 post-infection, there was a progressive disruption in the splenic architecture of PbWT-infected mice on subsequent days. In contrast, PbHMGB1KO-infected mice displayed a well-preserved splenic architecture throughout the infection. The presence of intact and well-demarcated red and white pulp regions in the spleen sections of PbHMGB1KO-infected mice could be observed even on day 16 post-infection corresponding to the clearance phase (Fig. 7A). Flow cytometry analysis showed a significant increase in the red pulp macrophages (F4/80high CD68+ CD11b+) of PbHMGB1KO-infected mouse spleen on Days 7 and 9 post-infection (Figs. 7B and S3). There was also a prominent increase in the marginal zone and white pulp macrophages such as marginal zone macrophages (MHCII+ CD209b+), marginal metallophilic macrophages (MHCII+ CD169+), and tingible body macrophages (MHCII+ CD68+) (Figs. 7, C–E and S4) between day 7 and 11 post-infection. Similarly, there was a significant increase in the CD11c+ conventional dendritic cells (cDCs) of PbHMGB1KO-infected mouse spleen on Days 9 and 11 (Figs. 7F and S5). However, the levels of PDCA-1+ plasmacytoid dendritic cells (pDCs) in the spleen remained comparable between PbWT- and PbHMGB1KO-infected mice (Figs. 7G and S5). This was also reflected in the ratio of cDCs to pDCs in the spleen of PbHMGB1KO-infected mouse (Fig. 7H).
Figure 7.
Assessment of splenic architecture, macrophages and dendritic cells in PbHMGB1KO-infected mice.A, H&E stained spleen sections of mice infected with PbWT and PbHMGB1KO parasites. The spleen samples were collected on the respective days post-infection. Images were captured using 10× objective. Scale bar = 50 μm. n = 3 independent experiments. B–G, Flow cytometry analyses of F4/80high CD68+ CD11b+ red pulp macrophages (B), MHCII+ CD209b+ marginal zone macrophages (C), MHCII+ CD169+ marginal metallophilic macrophages (D), MHCII+ CD68+ tingible body macrophages (E), CD11c+ cDCs (F) and PDCA-1+ pDCs (G). The spleen samples of PbWT- and PbHMGB1KO-infected mice were collected and the total splenocytes were prepared to carry out the isolation and staining of macrophages and dendritic cells. The flow cytometry data (mean ± SD) represents three different mice for the respective days (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, n.s., not significant; unpaired t test; two-tailed). H, cDCs/pDCs ratio in the spleen samples of PbWT- and PbHMGB1KO-infected mice. The data represent the mice used for flow cytometry analysis of cDCs and pDCs (n.s., not significant, ∗∗p < 0.01, ∗∗∗p < 0.001; unpaired t test; two-tailed). GC, germinal centers; RP, red pulp; WP, white pulp.
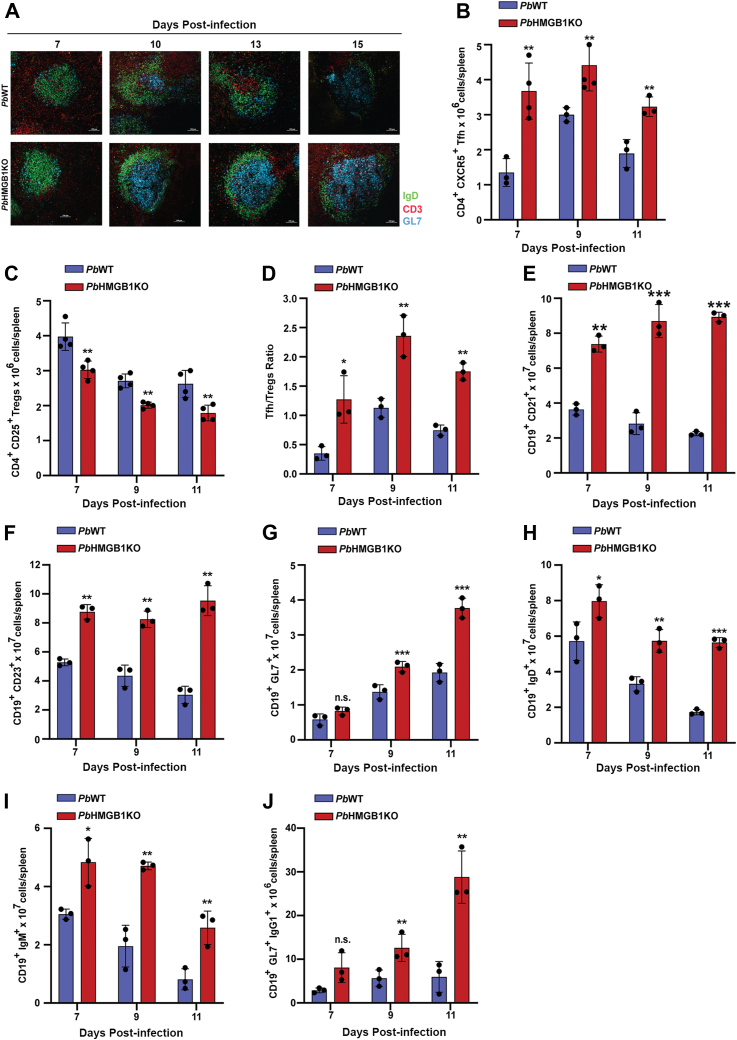
Germinal centers (GCs) offer the conducive niche for the interaction of T follicular helper cells with B cells leading to the proliferation and differentiation of B cells (34, 35). It has been shown that malaria infections can abrogate the germinal center responses leading to impaired protective immunity (36, 37, 38). Immunofluorescence analysis of the GCs in the spleen sections using GL7, IgD and CD3 antibodies that are specific for GCs, B cell follicles and T cell zones, respectively, suggested the restoration of GCs in the spleen of PbHMGB1KO-infected mouse. While GCs were hardly detectable in the spleen of PbWT-infected mice on day 7 to 15 post-infection, they were prominent in PbHMGB1KO-infected mice with organized T cell zone and B cell follicles (Fig. 8A). Since T-follicular helper (Tfh) cells play an important role in determining GC responses and protective immunity, and regulatory T cells (Tregs) can impede the function of Tfh (37, 38), we examined the levels of CD4+ CXCR5+ Tfh and CD4+ CD25+ Tregs in PbHMGB1KO-infected mice and determined the ratio of Tfh:Tregs. There was a significant increase in the number of Tfh (Figs. 8B and S6) with a concomitant decrease in Tregs (Figs. 8C and S6), and as a result, Tfh:Tregs ratio of PbHMGB1KO-infected mouse spleen was ∼3 times higher when compared with PbWT-infected mouse (Fig. 8D). There was also an increase in the marginal zone B cells (CD19+ CD21+), follicular B cells (CD19+ CD23+), IgM/IgD secreting B cells (CD19+ IgM+/IgD+), GC B cells (CD19+ GL+), and GC B cells expressing IgG1 (CD19+ GL+ IgG1) (Figs. 8, E–J and S7). These data suggested the robust GC responses of PbHMGB1KO-infected mouse spleen.
Figure 8.
Characterization of GC responses in PbHMGB1KO-infected mice.A, immunofluorescence analyses of GCs in the spleen sections of PbWT- and PbHMGB1KO-infected mice. Images were captured using 20× objective. Scale bar = 50 μm. n = 3 independent experiments. B, Tfh in the spleen samples of PbWT- and Pb-HMGB1KO-infected mice. C, tregs in the spleen samples. D, tregs/Tfh ratio in the spleen samples. The flow cytometry data (mean ± SD) represent at least three different mice for the respective days (∗p < 0.05, ∗∗p < 0.01; unpaired t test; two-tailed). E–J, B cell subtypes in the spleen samples of PbWT- and Pb-HMGB1KO-infected mice. CD19+ CD21+ marginal zone B cells (E), CD19+ CD23+ follicular B cells (F), CD19+ GL+ GC B cells (G), IgD secreting CD19+ B cells (H), IgM secreting CD19+ B cells (I) and CD19+ GL+ GC B cells expressing IgG1 (J). The flow cytometry data (mean ± SD) represent three different mice (n.s.- not significant,∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; unpaired t test; two-tailed).
Long-term protection of PbHMGB1KO-infected mice against PbWT challenges
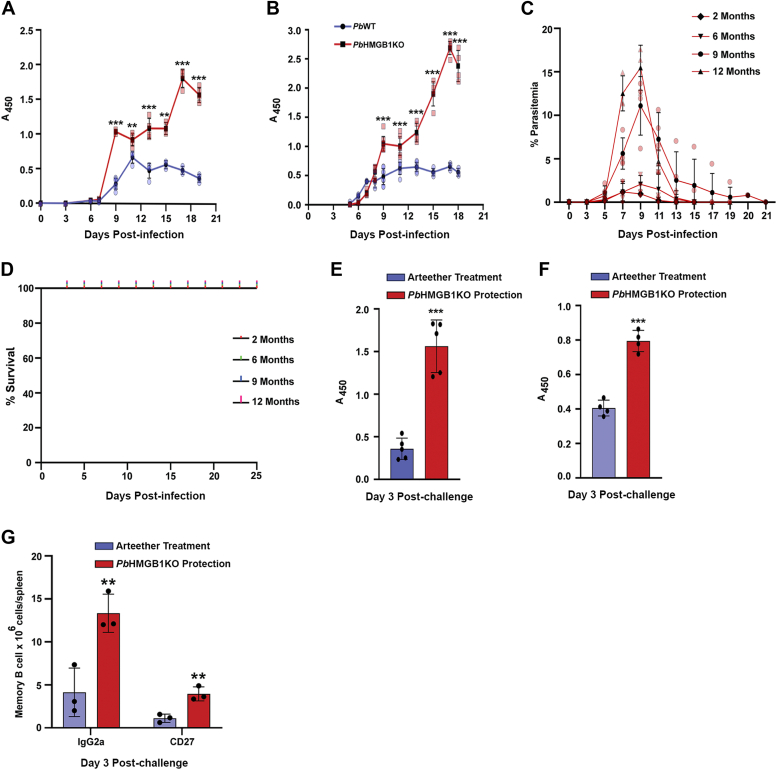
Since PbHMGB1KO-infected mice could clear the blood parasitemia and exhibit better GC responses, we examined the levels of parasite antigen-specific IgG during the course of infection in PbWT- and PbHMGB1KO-infected mice. ELISA analyses were carried out by coating the wells individually with PbWT parasite lysates (Fig. 9A) and infected RBC (iRBC) lysates (Fig. 9B). The iRBC lysates were included to represent the parasite antigens that are recruited on RBC membrane. In both the cases, parasite antigen-specific IgG levels were significantly higher in the sera collected from PbHMGB1KO-infected mice in comparison with PbWT-infected mice. In particular, the parasite antigen-specific IgG levels were at least 4 times higher in the sera collected during the clearance phase of PbHMGB1KO-infected mice (Fig. 9, A and B). We then assessed the long-term protection of PbHMGB1KO-infected mice against PbWT parasite challenges at 2, 6, 9, and 12 months post-protection by challenging them intravenously with 105 PbWT parasites. Interestingly, all the mice were protected and the protection could last long even at 12 months (Fig. 9, C and D). Our next interest was to assess the memory response for which, we challenged PbHMGB1KO-infected mice with intravenous administration of 106 PbWT parasites after 2 months of parasite clearance. For control, we used PbWT-infected mice cleared with a single dose of arteether (2 mg/mouse) when the blood parasitemia was around ∼20%. It is known that the clearance of parasites in PbWT-infected mice with antimalarials can lead to increased levels of parasite antigen-specific IgG and memory responses against the subsequent challenge with PbWT parasites. On day 3 post-challenge, the sera were collected to assess the levels of the parasite antigen-specific IgG, and spleens were collected to examine the memory responses. Interestingly, the parasite antigen-specific IgG levels of PbHMGB1KO-infected mice were at least three times higher in the case of wells coated with parasite lysates (Fig. 9E) and two times higher in the case of wells coated with iRBC lysates than the antimalarial-treated PbWT-infected mice (Fig. 9F). This was also reflected in the increased levels of CD27+ and IgG2a+ memory B cells isolated from the spleen (Figs. 9G and S8). These results suggested that the clearance of blood parasitemia in PbHMGB1KO-infected mice could lead to an efficient long-lasting memory response.
Figure 9.
Long-term protection of PbHMGB1KO-infected mice.A and B, ELISA analyses of the sera collected from PbHMGB1KO-infected mice against PbWT parasite lysates (A) and iRBC lysates (B) to assess the parasite antigen-specific IgG. For control, sera collected from PbWT-infected mice were used. The wells were coated with 100 μg of the total protein from parasite lysates or iRBC lysates. The data (mean ± SD) represent at least three different mice (∗∗p < 0.01, ∗∗∗p < 0.001; unpaired t test; two-tailed). C, blood parasitemia of PbHMGB1KO-infected mice challenged with PbWT parasites at 2, 6, 9 and 12 months post-protection. D, survival curves of the respective mice used for PbWT challenge. The data (mean ± SD) represent at least three different mice for each time interval. E and F, assessment of parasite antigen-specific IgG in the sera of the protected mice infected earlier with PbHMGB1KO parasites and challenged with PbWT parasites. 100 μg of the total protein from PbWT parasite lysates (E) and iRBC lysates (F) were used to coat the wells. Sera collected from day 3 post-challenge were used. For control, sera from PbWT-infected mice cleared with a single dose of arteether (2 mg/mouse) and challenged with PbWT parasites were used. The data (mean ± SD) represent at least three different mice (∗∗∗p < 0.001; unpaired t test; two-tailed). G, levels of CD27+ and IgG2a+ memory B cells isolated from the spleen of PbHMGB1KO-infection protected mice on day 3 post-challenge. The flow cytometry data (mean ± SD) represent three different mice (∗∗p < 0.01; unpaired t test; two-tailed).
PbHMGB1 regulates pir expression
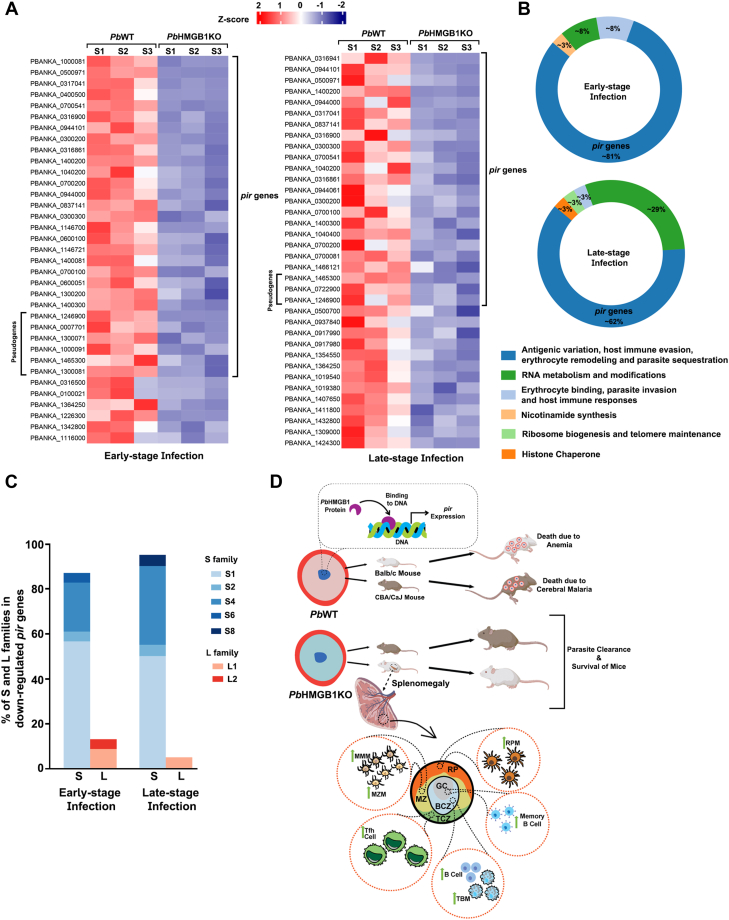
Transcriptomic studies were carried out to examine the reasons behind the self-clearance phenotype of PbHMGB1KO parasites, and the lack of cerebral pathogenesis and long-term protection observed for PbHMGB1KO-infected mice. For this, RNA-Seq analyses were performed for PbWT and PbHMGB1KO parasites isolated from early-stage (day seven-eighths post-infection) and late-stage (day 12–15 post-infection) infections. Despite having severe growth limitations and self-clearance phenotype, PbHMGB1KO parasites showed a significant down-regulation for only a small repertoire of genes. Interestingly, of the total number of 36 genes that were down-regulated in the early-stage infections, ∼81% of them represented pir gene family that is known to be associated with the antigenic variation, host immune evasion, erythrocyte remodeling, and parasite sequestration (Fig. 10, A and B and Dataset S1). A similar pattern was also observed for the late-stage infections wherein, ∼62% of the total 37 genes down-regulated belonged to pir gene family (Fig. 10, A and B and Dataset S1). Within the down-regulated pir genes, ∼41% and ∼26% of them were unique for early- and late-stage infections, respectively, suggesting the dynamic changes in pir gene expression during the course of blood-stage infections. In particular, ∼87% of the early-stage and ∼95% of the late-stage down-regulated pir genes represented the short (S) family of S1, S2, S4 S6, and S8 clades, and almost all of them are expressed in the blood stages (39). The contribution of the long (L) family to the downregulated pir genes was strikingly low (Fig. 10C and Dataset S1). The rest of the downregulated genes included fam proteins, small nucleolar RNAs, and other proteins that are associated with RNA modifications and metabolism, and ribosomal functions. The down-regulation of small nucleolar RNA genes appeared to be more pronounced in late-stage infections (Fig. 10B and Dataset S1).
Figure 10.
Transcriptomics of PbHMGB1KO parasites and schematic representation of the parasite clearance and long-lasting protective immunity in PbHMGB1KO-infected mice.A, list of downregulated genes in PbHMGB1KO parasites during early- and late-stage infections. The genes that showed significant down-regulation with greater than 1.5-fold change, FDR < 0.05 and adjusted p-value < 0.05 were considered (Benjamini-Hochberg procedure; multiple hypothesis testing). RNA-Seq analyses were carried out for three independent parasite pellets of PbWT and PbHMGB1KO parasites for early- and late-stage infections, respectively. B, donut chart representing the gene ontologies of significantly down-regulated genes based on the functional annotations available in PlasmoDB and published literature. C, percentage of S and L families in the down-regulated pir genes and the proportion of various clades. The entire details of RNA-Seq analyses are provided in Dataset S1. D, model depicting the role of PbHMGB1 in regulating the expression of pir genes and the splenic events leading to parasite clearance and long-lasting protective immunity in PbHMGB1KO-infected mice. The protection from anemia and cerebral malaria mortality due to the clearance of PbHMGB1KO parasites in Balb/c and CBA/CaJ mice is shown. The preservation of splenic architecture with effective germinal center responses in PbHMGB1KO-infected mice is represented. BCZ, B cell zone; GC, germinal center; MZ, marginal zone; MMM, marginal metallophilic macrophages; MZM, marginal zone macrophages; RP, red pulp; RPM, red pup macrophages; TBM, tingible body macrophages; TCZ, T cell zone.
The analysis of up-regulated genes in PbHMGB1KO parasites suggested significant changes in the transcript levels of 65 genes for early-stage and 54 genes for late-stage infections. There was also an upregulation of a few pir genes that represented ∼30% of the total genes. Further, ∼50% of the early- and late-stage up-regulated pir genes were pseudogenes in contrast to ∼20% that was observed for the down-regulated pir genes (Fig. S9, A and B and Dataset S1). Of the 9 pir genes that were up-regulated in early- and late-stage infections, two-third belonged to the S family (Fig. S9C and Dataset S1). Interestingly, there was a prominent increase in the percentage of fam family genes and other genes that encode reticulocyte binding proteins, erythrocyte membrane antigen 1, and exported proteins. These proteins play important role in erythrocyte binding, parasite invasion, and host immune responses, and they represented ∼50% of the up-regulated genes in the early- and late-stage infections of PbHMGB1KO parasites (Fig. S9B and Dataset S1). It is also worthwhile to mention that there were no significant changes in the transcript levels of the other three HMGBs - HMGB2, HMGB3, and HMGB4 in PbHMGB1KO parasites (Dataset S1). All these data suggested that pir gene expression is altered in PbHMGB1KO parasites displaying a prominent down-regulation with a concomitant up-regulation of genes that are involved in erythrocyte binding and parasite invasion.
Discussion
HMGB proteins are ubiquitous non-histone proteins playing important nuclear functions such as regulation of gene expression, DNA replication and repair, telomere homeostasis, and chromosome stability (14, 15, 16, 17, 40, 41). While human and mouse HMGB1 have been extensively studied (22, 29), there are also quite a few studies on HMGB2 and other HMGB proteins (42, 43, 44, 45). In addition to the nuclear functions, HMGB1 regulates autophagy and apoptosis by shuttling between the nucleus and cytosol (18, 19, 46). It is also actively secreted into the extracellular space by the activated immune cells or passively released during necrosis to function as a DAMP/cytokine. The interaction of extracellular HMGB1 with receptors such as RAGE, TLR4, and other surface molecules can trigger various downstream signaling events that activate MyD88, nuclear factor κB, MAPK, phosphatidylinositol3-kinase (PI3K), and IFN regulatory factor pathways (20, 22, 47). All these eventually render HMGB1 performing a plethora of functions that include cell differentiation, proliferation, migration and senescence, metastasis, angiogenesis, inflammation, and immune responses (22, 48). The present study highlights that Plasmodium HMGB1 lacks the characteristic features of mammalian HMGB1. It has been shown that the disulfide bond formation between Cys23 and Cys45 present in box A is required for the translocation of mouse and human HMGB1 from the nucleus to the cytosol regulating autophagy, and for the extracellular release. Interestingly, the entire box A is absent in the parasite HMGB1. Similarly, Cys106 in box B is essential for the nucleocytoplasmic translocation, and proinflammatory and immunomodulatory functions of mouse and human HMGB1 (20, 22, 29, 30, 49). Although the parasite HMGB1 has retained box B, it lacks the corresponding Cys residue and contains Ala instead of Cys.
We show that the parasite HMGB1 does not exhibit TNF-α stimulatory activity and the TNF-α stimulatory domain of box B present in the parasite HMGB1 has undergone extensive modifications. The synthetic peptide of PbHMGB1 corresponding to the TNF-α stimulatory domain of mammalian HMGB1 and the rPbHMGB1 do not induce TNF-α release in the murine macrophage cell line. Further, the mutation of Ala to Cys in box B is inadequate to restore the TNF-α stimulatory activity of rPbHMGB1. Our results from site-directed mutagenesis studies suggest that eight independent mutations are additionally required in box-B of the parasite HMGB1 to show similar levels of TNF-α stimulatory activity as mouse HMGB1. An earlier study alluded to the proinflammatory activity of parasite HMGB1 by performing in vitro TNF-α release assays with rPfHMGB1 and showing the substantial release of TNF-α at higher concentrations (100–300 μg/ml) of rPfHMGB1 in contrast to mouse HMGB1 that could exhibit TNF-α release at a concentration of 0.4 to 2.5 μg/ml. Further, rPfHMGB1 was not subjected to DNase treatment, and polymyxin B was added along with rPfHMGB1 while performing the assays (50). In the present study, rPbHMGB1 is subjected to DNase treatment and high-capacity endotoxin removal before performing the in vitro assays. The results are also verified with the synthetic peptides corresponding to TNF-α stimulatory domain and mutagenesis studies. Our findings lay the platform to examine the TNF-α stimulatory activity of HMGBs from other apicomplexan parasites. A comparison of HMGBs from the closely related non-parasitic (Chromera and Vitrella) and parasitic alveolates suggests sequence homology with the TNF-α stimulatory domain of mammalian HMGBs, although there are considerable changes as observed for Plasmodium HMGB1 (Fig. S10). Interestingly, Cys106 of mammalian HMGB1 is conserved in HMGBs of Vitrella, Haemoproteus, and Cryptosporidium, but not in Chromera and other apicomplexans. There are also additional HMG box-containing proteins present and further studies are required to address whether the loss of TNF-α stimulatory activity is a common feature of apicomplexan HMGBs.
In agreement with the lack of TNF-α stimulatory activity and the three Cys residues that are essential for extracellular functions, PbHMGB1 is undetectable in the plasma of Pb-infected mice. Extracellular mouse/human HMGB1 serves as a potent proinflammatory cytokine and it is associated with systemic inflammation like sepsis and various other inflammatory diseases (21, 51, 52). The parasitized-RBCs encounter numerous host immune cells and are phagocytosed extensively by monocytes, macrophages, and dendritic cells present in the circulation and organs like spleen, liver, and lungs (2, 53, 54). This, in turn, may lead to the exposure of immune cells to the parasite HMGB1 and if the parasite HMGB1 is proinflammatory, such exposure may trigger abrupt immune signaling events resulting in an uncontrolled inflammation that will be deleterious to the host. This is also true for the release of parasite HMGB1 which may happen during the clearance of parasitized-RBCs in the spleen. Hence, the loss of extracellular and proinflammatory functions of parasite HMGB1 might be advantageous in the context of prolonged host survival that is required for the successful transmission to the definitive hosts - Anopheline mosquitoes.
Another interesting finding is the deletion of HMGB1 in a lethal rodent parasite strain, Pb ANKA, leading to complete protection from mortality due to anemia and cerebral malaria (Fig. 10D). PbHMGB1KO parasites are completely cleared when the blood parasitemia reaches around 20%. The ability of PbHMB1KO parasites collected from the clearance phase to induce fresh infections in naïve mice suggests the absence of growth defects in the parasite, and the protection is mediated by host immune responses. Splenic macrophages play an important role in eliminating the parasitized RBCs and the control of blood parasitemia (55, 56, 57). PbHMGB1KO-infected mice display a significant increase in the red pulp, marginal zone, and white pulp macrophages. This is also true for the cDCs that are involved in T cell priming. However, the levels of pDCs do not seem to differ between the spleen samples of PbWT- and PbHMGB1KO-infected mice. More importantly, the splenic architecture and GCs are well-preserved in PbHMB1KO-infected mice. This is in stark contrast to the PbWT-infected mice with disease severity wherein, a profound disorganization of splenic architecture and germinal centers is observed. In the context of GC reactions being crucial for adaptive immune responses and generation of parasite-specific antibodies (36, 37, 58), PbHMGB1KO-infected mice show a robust parasite-specific antibody response. There is also an increase in the marginal zone, follicular, and GC B cells (Fig. 10). In particular, Tfh cells essential for GC formation, affinity maturation, and generation of high-affinity antibodies and memory B cells are significantly higher in the PbHMB1KO-infected mouse spleen. A number of studies with rodent and human infections have highlighted the importance of Tfh cells in the development of functional antibodies and long-lasting immunity against malaria (37, 38, 59, 60, 61). In agreement with these results, mice recovered from PbHMGB1KO infections display long-lasting immunity and protection when re-challenged with PbWT parasites. Altogether, our in vivo findings emphasize the important role of PbHMGB1 in modulating the host splenic responses. It is worthwhile to mention that the phenotype of PbHMGB1KO differs significantly from HMGB2 deletion. While HMGB2 deletion in PbNK65 strain could lead to self-clearance and protection in infected mice, its deletion in highly lethal PbANKA or PyYM strains leads to 100% mortality in infected mice with a slight attenuation of asexual stage growth (24, 25, 26). Further, microarray studies carried out for blood-stage parasites have suggested the prominent role of PyHMGB2 in regulating the genes expressed in gametocytes that remain translationally repressed until the onset of mosquito stages (26). PyHMGB2KO parasites display a significant reduction in oocyst and sporozoite formation, and this has also been shown for HMGB2 deletion in Pf parasites (27). It is possible that the function of HMGB2 is more confined to the parasite development in the mosquitoes.
Intrigued by the absence of TNF-α stimulatory activity and extracellular release, we have examined the gene regulatory function of PbHMGB1 for the reasons behind robust splenic responses, self-clearance, and protection phenotype in PbHMGB1KO-infected mice. Chromosome conformation capture and transcriptome analyses of in vitro cultured Pf parasites lacking HMGB1 have suggested the loss of centromere/telomere organization resulting in the silencing of var genes that are associated with parasite sequestration, disease pathogenesis, and host immune evasion (28). However, var genes are present only in the Plasmodia species of Laverania subgenus such as Pf and Plasmodium reichenowi (62). Other non-Laverania species infecting humans (vivax, malariae, ovale and knowlesi), non-human primates (cynomolgi, coatneyi etc.) and rodents (berghei, yoelii etc.) have Plasmodium interspersed repeat (pir) multigene families with suggested similar roles as var. It is also known that unlike the mutually exclusive expression of one var gene at a time, multiple pir genes are expressed together in various stages of the parasite life cycle (63, 64, 65, 66). Our findings suggest that the deletion of PbHMGB1 leads to a prominent down-regulation of pir genes that are expressed in the blood stages (Fig. 10D). Interestingly, a larger proportion of these down-regulated pir genes belong to S family and further ChIP-Seq studies are required to gain insights on the promoter occupancy of PbHMGB1 and other co-regulators. The down-regulation of small nucleolar RNAs in the late-stage infections of PbHMGB1KO parasites is also noteworthy and it could be a reflection of the decreased pir expression. With increasing evidence on the functions of small nucleolar RNAs in RNA modifications, mRNA abundance, and translational regulation, it would be of interest to examine their role in regulating the pir expression. There is also a small subset of pir genes that are upregulated in PbHMGB1KO parasites and it is not clear at this stage whether their expressions are controlled by other HMGBs or transcription factors. Another prominent aspect is the upregulation of fam, erythrocyte binding, and exported proteins involved in host receptor interactions, parasite invasion, and immune responses. Such processes might help PbHMGB1KO parasites to deploy compensatory mechanisms for sustaining the blood stage growth and overcoming splenic clearance. There is also a scope for understanding the significance of HMGB1 in the other stages of the Plasmodium life cycle. In summary, the present study highlights the gene regulatory function of parasite HMGB1 and its significance in modulating disease pathogenesis, splenic responses, and host immune evasion in blood-stage infections. Such insights would help us to understand the virulence and immune evasion mechanisms of malaria parasites, and explore the possibility of developing a genetically attenuated blood-stage vaccine for malaria if HMGB1 deletion can lead to self-clearance and protection for human parasites.
Experimental Procedures
Multiple sequence alignment of HMGB1
The HMGB1 sequences of mouse (NP_034569.1) and human (CAG33144.1) were retrieved from NCBI (https://www.ncbi.nlm.nih.gov/), and the sequences of Pb (PBANKA_0601900), Pf (PF3D7_1202900), Plasmodium vivax (PVP01_1302200), Plasmodium ovale (PocGH01_13013100), P malariae (PmUG01_13013200), Plasmodium knowlesi (PKA1H_130007700), P. reichenowi (PRCDC_1202300), Plasmodium cynomolgi (PcyM_1302700), P. yoelii (PY17X_0604400) and Plasmodium chabaudi (PCHAS_0603700) were retrieved from PlasmoDB (https://plasmodb.org/plasmo/app). Multiple sequence alignment was carried out with SeaView Version 5.0.5 (https://doua.prabi.fr/software/seaview) using MUSCLE (Multiple Sequence Comparison by Log-Expectation) algorithm.
Expression and purification of rPbHMGB1, rPbHMGB1C41, rPbHMGB15mut, rPbHMGB19mut and rmHMGB1
The cDNA sequences of PbHMGB1 and mouse HMGB1 (BC110667.1) were retrieved from PlasmoDB (https://plasmodb.org/plasmo/app) and NCBI GenBank (https://www.ncbi.nlm.nih.gov/), respectively. RNeasy Mini Kit (Qiagen, 74104) was utilized for the isolation of total RNA from Pb parasites and mouse liver according to the manufacturer’s protocol. RevertAid Reverse Transcriptase (Thermo Fisher Scientific, EP0442) was used for the synthesis of cDNA from 1 μg of total RNA followed by PCR amplification with Phusion High-Fidelity DNA Polymerase (New England Biolabs, M0530). The following are the forward and reverse primers used for PbHMGB1: 5′-GCCAGGAGCTCATGGATGGCATGAAAAAATTTAAAGATATGAAAATGGGTG-3′ and 5′-GCCAGGAATTCTTATTTCATTTTACTTTTGGCATATTCCATTTTTTCTTTT-3′. SacI and EcoRI restriction sites used for cloning are underlined. The following are the forward and reverse primers used for mouse HMGB1: 5′-GCCAGGATCCATGGGCAAAGGAGATCCTAAGAAGCCGAG-3′ and 5′-GCCCGGTACCTTATTCATCATCATCATCTTCTTCTTCATC-3’. BamHI and KpnI restriction sites are underlined. The PCR products were subjected to digestion with the respective restriction enzymes and the digested products were cloned in-frame into pRSETA plasmid (Thermo Fisher Scientific) having 6xHis tag at the N-terminus region. Protein expression was carried out in E. coli Rosetta2DE3pLysS strain (Novagen). In brief, E. coli Rosetta2DE3PLysS strain transformed with recombinant plasmid was grown to an A600 of 0.8 at 30 °C. The protein induction was carried out using 1 mM isopropyl-β-D-thiogalactoside (IPTG) (MP Biomedicals, 11IPTG0001) at 18 °C for 12 h. The overexpressed recombinant (r) PbHMGB1 or mouse HMGB1 (mHMGB1) with N-terminal histidine tag was purified under identical conditions using Ni2+-NTA agarose resin (Qiagen, 30210). For this, the respective bacterial cell pellet expressing the recombinant protein was resuspended in lysis buffer containing 50 mM Tris pH 8.0, 500 mM NaCl, 2% glycerol, 0.5% Triton X-100 and protease inhibitors, sonicated and centrifuged at 43,000g for 60 min. The supernatant was collected and loaded onto a column packed with Ni2+-NTA resin, and the column was washed consecutively with lysis buffer containing 5 and 50 mM imidazole. The recombinant protein was eluted with lysis buffer containing 100 mM imidazole. The purified protein was then dialyzed against 25 mM Tris pH 8.0 containing 100 mM NaCl and 2% glycerol, followed by further purification by S-Sepharose cation exchange chromatography (Macro-Prep High S Media, Bio-Rad, 1560030). The dialyzed recombinant protein was loaded onto a column packed with S-Sepharose resin and the column was washed consecutively with 25 mM Tris buffer pH 8.0 containing 125 mM and 250 mM, respectively, followed by elution in the same buffer containing 500 mM NaCl. The purified recombinant protein was subjected to enterokinase (New England Biolabs, P8070S) cleavage to remove the 6xHis tag and Xpress epitope, followed by DNase I (New England Biolabs, M0303S) treatment (20 Units for 1 mg of recombinant protein) to remove any bacterial genomic DNA contaminants. The resultant protein was then loaded on Pierce High Capacity Endotoxin Removal Resin (88,270) (1 ml of resin for 1 mg of recombinant protein) to remove LPS contamination as per the manufacturer’s protocol. The primers used to perform the site-directed mutagenesis for generating - rPbHMGB1C41, rPbHMGB15mut, and rPbHMGB19mut plasmids are provided in Table S1. PCR amplification was carried out with the respective primers and the amplified products were subjected to DpnI (New England Biolabs, R0176S) digestion to remove the recombinant plasmid used as a template, followed by transformation into E. coli NovaBlue (Novagen) bacterial strain. The plasmid isolation was carried out for the individual recombinant colonies and the presence of respective mutation was confirmed by DNA sequencing using Sanger’s method. For the generation of rPbHMGB1C41, rPbHMGB1 plasmid was used as a template. For the subsequent mutations, the mutant plasmid generated for the previous mutation was used as a template for the next mutation. The mutant proteins - rPbHMGB1C41, rPbHMGB15mut, and rPbHMGB19mut were also purified and processed following identical conditions.
TNF-α stimulatory activity of synthetic peptides and recombinant proteins
Murine macrophage-like Raw 264.7 cell line was cultured in DMEM medium (Gibco 12,100,038, Thermo fisher) containing 10% FBS and 1% penicillin-streptomycin (v/v) (15,140–122 Gibco Thermo fisher) under 5% CO2 at 37 °C and 95% humidity (30). 2 x 104 cells were seeded and treated for 12 h with different concentrations of synthetic peptides and recombinant proteins. The 20-mer peptide of mouse HMGB1 (FKDPNAPKRPPSAFFLFCSE) and PbHMGB1 (KKDPHAPKRSLSAYMFFAKE) was purchased from GL Biochem (Shangai) Ltd, with greater than 90% purity as confirmed by HPLC. The respective peptides were dissolved in DMSO and diluted further with sterile PBS to achieve the working stocks as per the manufacturer’s instruction. The recombinant protein concentrations were estimated using the Pierce BCA Protein Assay Kit (23,225). The culture supernatants collected after 12 h were centrifuged at 5000g for 5 min at 4 °C to remove the cell debris, if any. The resultant supernatants were used to perform ELISA for estimating the levels of TNF-α released using TNF alpha Mouse ELISA Kit (ab46105) as per the manufacturer’s protocol. The culture supernatants of the cells treated with PBS containing DMSO or buffer containing 25 mM Tris pH 8.0 and 500 mM NaCl were used as negative controls for the background subtraction. TNF-α release induced by LPS (10 μg/ml) treatment was used as a positive control in all the experiments.
Generation of PbWTHMGB1-GFP transgenic parasites
To generate PbWTHMGB1-GFP transgenic parasites, a 736 bp upstream promoter sequence along with the entire coding sequence of PbHMGB1 was amplified using Phusion DNA polymerase with the following forward and reverse primers: 5′- GCCACCGCGGCGGTTTATTTTGGCAAAATTAAAAGGG-3′ and 5′-GCAAGGATCCTCCAGCACCAGCAGCAGCACCTTTCATTTTACTTTTGGCATATTCCAT-3′. SacII and BamHI restriction sites are underlined and the 21 bp linker sequence included in the reverse primer is highlighted in bold. The amplified fragment of 1076 bp was cloned in-frame upstream to the GFP sequence of pL0031 plasmid (67). The cloned fragment with in-frame GFP was then amplified with the forward primer and a GFP-specific reverse primer: 5′-GCAAGAATTCTTATTTGTATAGTTCATCCATGCCATG-3′. EcoRI restriction site in the GFP-specific reverse primer is underlined. The resultant fragment of 1795 bp was cloned into the GOMO-GFP-luciferase plasmid (68, 69) by replacing the GFP-luciferase sequence. To perform double-crossover recombination, a 3′UTR sequence of PbHMGB1 (656 bp) was amplified using the forward and reverse primers: 5′-GCCACTCGAGGGAAAGTATATATAATAAAATATTATATGAATGTG-3′ and 5′-GCCCGGTACCGAACGTGCTAAAATAACACCA-3′, and cloned into the afore-mentioned plasmid. XhoI and KpnI restriction sites are underlined. The recombinant plasmid was then digested with SacII and KpnI restriction sites and the released fragment was transfected into PbWT schizonts using 4D-Nucleofector (Lonza). The transfected parasites were then injected intravenously into naïve Balb/c mice and selected using pyrimethamine (70 μg/ml) in drinking water. The clonal selection was performed by limiting dilution and the transgenic PbWTHMGB1-GFP parasites obtained were then subjected to 5-fluorocytosine treatment in drinking water (1 mg/ml) to remove the mCherry reporter cassette (70) The site-specific integration in PbWTHMGB1-GFP transgenic parasites was confirmed using a forward primer designed upstream to the promoter sequence of PbHMGB1 used for transfection (5′-ACGGATATATATATAAGAAAATCGCATTTAAATATG-3′) and GFP-specific reverse primer.
Localization of PbHMGB1
Live imaging of GFP fluorescence in PbWTHMGB1-GFP parasites expressing PbHMGB1-GFP fusion protein was carried out as described (71, 72). Balb/c mice were infected intraperitoneally with 105 PbWTHMGB1-GFP-parasitized RBCs and when the blood parasitemia reached around 5% on day 6, 10 μl of tail vein blood was collected in heparinized PBS. The blood was then centrifuged to remove the plasma and the cells were washed twice with PBS. The cells were then resuspended in PBS containing DAPI (1 μg/ml) and incubated at room temperature for 20 min, followed by three washes with PBS. The cells containing iRBCs were then resuspended in PBS and transferred to glass-bottom Petri dishes for live imaging. Indirect immunofluorescence analysis of PbHMGB1-GFP localization in PbWTHMGB1-GFP parasites was carried out as described earlier (71). In brief, the tail vein blood collected from the infected mice was washed thrice with PBS and overlaid on poly-L-lysine coated coverslip for 1 h, followed by fixing with 4% paraformaldehyde in PBS (w/v) containing 0.0075% glutaraldehyde (v/v) for 30 min at room temperature. The cells were permeabilized with 0.1% Triton X-100 (v/v) in PBS for 10 min, treated with 0.1 M glycine for 10 min and subsequently blocked with 2% BSA (w/v) for 3 h at room temperature. The cells were then incubated with rabbit polyclonal anti-GFP antibody (Abcam; ab290; 1:500 dilution) for 4 h at room temperature, washed with PBS, and then treated with FITC-conjugated donkey anti-rabbit IgG (SantaCruz, sc-2090; 1:200 dilution) for 2 h at room temperature. After staining with DAPI (1 μg/ml in PBS) for 20 min and subsequent washing with PBS, the coverslip was dried and mounted on a glass slide with ProLong TM Gold antifade (Invitrogen). The localization of PbHMGB1 in PbWT parasites using polyclonal anti-rPbHMGB1 antibodies (1:1000 dilution) was also carried out in a similar fashion. All the images were captured with 100X objective using an Olympus IX83 inverted microscope having a high-performance camera (DP73) and processed using Olympus CellSens Dimension software.
ELISA for the extracellular presence of HMGB1
To examine the extracellular presence of PbHMGB1-GFP, whole blood samples were collected form the mice infected with PbWTHMGB1-GFP parasites through cardiac puncture using heparin as an anticoagulant. The blood samples were then centrifuged at 2000g at 4 °C and the separated plasma samples were used to coat the wells of ELISA plate in 50 mM carbonate buffer pH 9.6 for overnight at 4 °C. After blocking with 3% BSA (w/v) in PBS containing 0.05% (v/v) Tween 20 (PBST) at room temperature for 2 h, the wells were treated for 3 h with rabbit polyclonal anti-GFP antibody (Abcam; ab290; 1:3000 dilution) and washed thrice with PBST, followed by 2 h treatment with peroxidase-conjugated goat anti-rabbit IgG antibody (ab97051; 1:25,000 dilution). After washing thrice with PBST, 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate was added, followed by stopping with 1 N H2SO4 and measuring the absorbance at 450 nm. For control and spiking the plasma samples, PbWT and PbWTHMGB1-GFP parasite lysates were prepared by resuspending the respective parasite pellets in 50 mM Tris pH 7.5 containing 100 mM NaCl, 0.05% Tween 20 (Sigma-Aldrich) and 1× Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific), followed by sonication in ice. The lysates were then centrifuged at 13,000g for 15 min at 4 °C and the supernatants collected were used to coat the wells of ELISA plate or for spiking the plasma samples after estimating the total protein content. For examining the presence of PbHMGB1 in the plasma samples of PbWT-infected mice and PbWT parasite lysates using polyclonal anti-rPbHMGB1 antibodies, a high-sensitivity method was followed (73) wherein, perchloric acid addition to the samples was carried out to the final concentration of 3% (v/v), followed by centrifugation at 15,000g for 5 min at 4 °C. The supernatants collected were then neutralized with 1.5 N NaOH and used to coat the wells of ELISA plate. Polyclonal anti-rPbHMGB1 antibodies were used at 1:3000 dilution.
Generation of PbHMGB1KO and PbHMGB1KOLuc parasites
To generate PbHMGB1KO parasites, 5′UTR region of PbHMGB1 was amplified from PbWT genomic DNA using the following forward and reverse primers: 5′-GCCAGGGCCCCGGTTTATTTTGGCAAAATAAAAGGG-3′ and 5′-GCCAGATCTAGAAAACCTTTTTTTTTAAAATATAAAGGACAGG-3’. ApaI and XbaI restriction sites are underlined. Similarly, 3′UTR region of PbHMGB1 was amplified using the following forward and reverse primers: 5′-GCCAGGTACCGGAAAGTATATATAATAAAATATTATATGAATGTG-3′ and 5′-GCCCGCGGCCGCGAACGTGCTAAAATAACACCA-3′. KpnI and NotI restriction sites are underlined. The resultant 5′UTR and 3′UTR fragments were digested with respective restriction enzymes and cloned into pL0006 plasmid on either side flanking the human DHFR selection cassette (68). The recombinant plasmid was then digested with ApaI and NotI, and the released fragment was transfected into PbWT schizonts using 4D-Nucleofector (Lonza). The transfected parasites were then injected intravenously into naïve mice and selected using pyrimethamine. The pyrimethamine-resistant parasites were subjected to clonal selection by limiting dilution. The protective phenotype of PbHMGB1KO parasites was confirmed with two independent clones. To generate PbHMGB1KOLuc parasites, a similar set of primers were used except for the changes in the restriction sites. For 5′UTR forward and reverse primers, SacII and NotI restriction sites were used. For 3′UTR forward and reverse primers, XhoI and KpnI restriction sites were used. The digested 5′UTR and 3′UTR fragments were cloned into GOMO-GFP-Luc plasmid on either side flanking GFP-Luc expressing cassette and a drug selection cassette expressing hDHFR fused with yFCU (yeast cytosine deaminase-uridyl phosphoribosyl transferase) and m-Cherry (69). After digesting the recombinant plasmid with SacII and KpnI, transfection was carried out as described for PbHMGB1KO parasites. For control in bioluminescence studies, PbControlLuc parasites reported in our earlier studies were used (70).
Genetic complementation of PbHMGB1 in PbHMGB1KOLuc parasites
For genetic complementation, PbHMGB1KOLuc-infected mice were treated with 5-fluorocytosine (1 mg/ml) to remove hDHFR-yFCU selection gene cassette (68, 69) and the resultant marker-free PbHMGB1KOLuc parasites were confirmed by the loss of m-cherry fluorescence. The parasites were then transfected with GOMO-GFP-Luc plasmid, wherein the PbeIF1α promoter and the downstream luciferase were replaced with the PbHMGB1 gene under its endogenous promoter. The corresponding sequence of −736 to +318 bp was PCR amplified with the following forward and reverse primers: 5′-GCCACCGCGGCGGTTTATTTTGGCAAAATTAAAAGGG-3′ and 5′-GCAAGAATTCTTATTTCATTTTACTTTTGGCATATTCCATTTTTTCTTTT-3′. SacII and EcoRI restriction sites used for cloning are underlined. The 3′UTR fragment of PbHMGB1 was cloned using XhoI and KpnI restriction sites. The recombinant plasmid was then digested with SacII and KpnI and transfection was carried out as described earlier. The site-specific integration in the PbHMGB1KO+HMGB1 parasites was confirmed using a forward primer 5′-ACGGATATATATATAAGAAAATCGCATTTAAATATG-3′ designed upstream to the promoter sequence of PbHMGB1 used for transfection and a PbDHFR specific reverse primer 5′- TAGCTAAAATTATGAACATTTTATTTTTTGTTCAGAAAAAA-3′.
P. berghei infection studies
All the mice studies were carried out with the approval (ILS/IAEC-114-AH/APR-18) of the Institutional Animal Ethics Committee (IAEC) of the Institute of Life Sciences, Bhubaneswar, as per the national guidelines of “The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA)”. The mice were bred at the animal house facility of the Institute of Life Sciences and maintained under the standard conditions of 25 °C and 40 to 50% relative humidity with a periodic 12 h light/dark cycle and ad libitum feed and water. P. berghei (Pb) ANKA strain was propagated in 7 to 8 weeks old male/female Balb/c mice and the asexual stage infections were initiated by injecting parasitized RBCs into the naïve mice through the intraperitoneal route. The deletion of PbHMGB1, and the generation of PbHMGB1KO, PbHMGB1KOLuc, and PbWTHMGB1-GFP parasites were performed in the PbANKA strain. For growth analyses of PbWT and transgenic parasites, assessment of protective phenotype in PbHMGB1KO-infected mice, and challenging experiments, the required number of parasitized RBCs for the respective parasites were injected through intraperitoneal/intravenous route in Balb/c mice. Giemsa-stained smears were routinely prepared from the tail vein blood of the infected mice to assess the blood parasitemia (70). For the collection of spleen samples to perform immunohistochemical analyses and splenocyte isolations, infected mice were subjected to transcardial perfusion with PBS on the respective days. For ECM experiments, 7 to 8 weeks old male/female CBA/CaJ mice were used for the propagation of the respective parasites and for the subsequent ECM analyses.
Histological analyses
To perform H&E staining of the spleen sections, PbWT- and PbHMGB1KO-infected mice were subjected to transcardial perfusion with PBS. The spleens were then dissected out and formalin-fixed for 72 h, followed by serial dehydrations with ethanol and clearing with xylene for 1 h. The paraffin blocks were then prepared and tissue sections of 5 μm thickness were generated using Leica RM2125RT rotary microtome. The sections were deparaffinized and serially hydrated before staining with hematoxylin and eosin (70). For immunohistochemistry, the spleen samples fixed with 4% (w/v) paraformaldehyde in PBS were cryoprotected in 30% (w/v) sucrose in PBS for 24 h at 4 °C followed by embedding in tissue freezing medium (Leica Biosystems) (71). The spleen sections of 7 μm thickness were then prepared using Leica CM1850 cryostat microtome and mounted on poly-L-lysine-coated slides. The sections were then blocked using 2% BSA for 2 h followed by incubation with Alexa Flour 488 anti-mouse IgD (Biolegend, 11-26c.2a) or biotin-conjugated anti-mouse GL7 (eBioscience Thermo Fisher, 13-5902-82) or Alexa Fluor 647 anti-mouse CD3 (Biolegend, 17A2) antibodies for 3 h at room temperature. The concentration of antibodies used was 2 μg/ml. For GL-7 detection, streptavidin-Alexa Flour 594 (Biolegend) was used at a concentration of 2.5 μg/ml. The sections were then washed, dried, and mounted with ProLong TM Gold antifade (Invitrogen). The fluorescence images were captured with a DP73 high-performance camera using an Olympus IX83 inverted microscope.
Flow cytometry analyses
The spleen samples for the respective days were collected from the infected mice perfused with PBS. To isolate the splenocytes, the spleen samples were minced and digested with 2 mg/ml (Collagenase D > 0.15 U/mg, from Roche Diagnostics) in Collagenase D buffer containing 10 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2. The cells were then passed through a 70 μm nylon cell strainer followed by a 40 μm strainer to obtain the single-cell suspensions. The single-cell suspensions were then centrifuged at 300g for 10 min at 4 °C, and the pellets obtained were resuspended in 2 ml of RBC lysis buffer pH 7.4 containing 155 mM NH4Cl and 2 mM EDTA followed by 4 min incubation at room temperature. The cells were then washed and resuspended in PBS containing 0.5% BSA and 2 mM EDTA (70). For the flow cytometry analyses of cDCs and pDCs, DCs were enriched by subjecting the splenocytes to MACS cell separation using Pan Dendritic Cell Isolation Kit, mouse (Miltenyi Biotech, 130-100-875). The enriched DCs were then stained with anti-mouse CD11c-APC (Miltenyi Biotech, 130-102-800) and anti-mouse PDCA-1-FITC antibodies (Miltenyi Biotech, 130-102-827). For red pulp macrophage analysis, F4/80+ macrophages were first isolated using Anti-F4/80 Microbeads Ultrapure, mouse (Miltenyi Biotech, 130-110-443), followed by staining with anti-mouse CD11b-APC (Miltenyi Biotech; 130-113-802) and anti-mouse CD68-FITC (Biolegend; 137005). For the analyses of marginal zone and white pulp macrophages, splenocytes were stained with anti-mouse MHCII-PE (Miltenyi Biotech, 130-102-896), anti-mouse CD209b-Alexa Fluor 488 (eBioscience Thermo Fisher, eBIO22D1), anti-mouse CD169-APC (Biolegend, 142417) and anti-mouse CD68-FITC (Biolegend, 137005) antibodies. To analyse the levels of Tregs, CD4+CD25+ Tregs were isolated from the splenocytes using CD4+CD25+ Regulatory T Cell Isolation Kit, mouse (Miltenyi Biotech, 130-091-041). For Tfh cells, the flow-through fraction containing CD4+CD25- cells obtained after the positive selection of CD4+CD25+ Tregs was stained with anti-mouse CXCR5-APC (Miltenyi Biotech, 130-119-129) and used for flow cytometry. For B cell analyses, B cells from the mouse spleen were isolated using B cell isolation kit (Miltenyi Biotech, 130-090-862) and stained with anti-mouse CD19-PE (Miltenyi Biotech, 130-111-883), anti-mouse CD21/CD35-PerCP-VIO700 (Miltenyi Biotech, 130-111-734), anti-mouse CD23-APC (Miltenyi Biotech,130-118-761), anti-mouse GL7-Biotin (eBioscience Thermo Fisher, 13-5902-82), anti-mouse IgD-VioBlue (Miltenyi Biotech, 130-102-268), anti-mouse IgM-PerCP-VIO700 (Miltenyi Biotech, 130-106-061) and anti-mouse IgG1-APC (Miltenyi Biotech, 130-102-308) antibodies. For the detection of markers stained with biotin-conjugated antibodies, Streptavidin-FITC (Miltenyi Biotech, 130-106-932) was used. All the antibodies were used at concentrations or dilutions recommended by the manufacturers. After incubating with the respective antibodies, cells were washed with PBS containing 0.5% BSA and 2 mM EDTA, and acquired using BD LSR Fortessa and CytoFLEX S. To compensate for the spectral overlap, single fluorochrome staining was performed for each experiment. The data were analyzed using FlowJo v10.6.1 software.
Transcriptomic studies
Pb infections were carried out in Balb/c mice and the total RNA was extracted from PbWT and PbHMGB1KO parasites using RNeasy Mini Kit (Qiagen, 74104). The quality of RNA samples was assessed using Bioanalyzer (Agilent Technologies, Inc) and their concentrations were determined using Qubit (Thermo Fischer Scientific). For RNA-Seq library preparations, NEBNext Ultra II RNA Library Prep Kit for Illumina (NEB#E7770S) was utilized. Briefly, mRNAs isolated using magnetic oligo-d(T) beads were used for the first- and second-strand cDNA synthesis followed by the end preparation of the cDNA libraries and adapter ligations using the manufacturer’s protocols. The cDNA libraries were quantified using Qubit HS Assay (Invitrogen, Cat# Q32854) and the pooled libraries of 160 pM concentrations were then loaded on to 25B flow cells and sequenced using Illumina NovaSeq X Plus to generate 150 bp paired-end reads. The raw BCL files were converted to FASTQ files and the sequenced reads were demultiplexed. The quality checks were performed using the FastQC v.0.11.5 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). PbANKA genome (v68) and its corresponding genomic feature files were downloaded from PlasmoDB. Genome index was generated in STAR (v2.5.3a) and the reads were mapped using the STAR RNA-Seq alignment tool. The count matrix for the mapped genes was generated using featureCounts v.1.6.4 from Subread package (74) and was used with Q = 10 for mapping quality, and these count files were used as input for downstream differential gene expression analysis with DESeq2 version 1.14.1. Normalization and differential expression were carried out using the DESeq2 Bioconductor package with the R statistical programming environment. The false discovery rate (FDR < 0.05) was calculated using Benjamini-Hochberg procedure to control multiple hypothesis testing. The genes exhibiting a substantial magnitude of fold-change (≥1.5 fold) with a stringent statistical threshold (adjusted p-value < 0.05) were classified as significantly differentially expressed. To generate the heatmaps, differential gene lists from each comparison were used along with their normalized values using complex heatmap Bioconductor package.
Other procedures
Parasite isolations were carried out by lysing the infected RBC pellets with an equal volume of 0.15% saponin (Sigma-Aldrich, S4521) in PBS (w/v). The samples were then centrifuged at 10,000 g for 10 min at 4 °C to obtain the parasites and the supernatants collected after saponin lysis were used as iRBC lysates. The parasite lysates were prepared by sonicating the parasite pellets resuspended in lysis buffer containing 50 mM Tris pH 7.5, 100 mM NaCl, 0.05% Tween 20 (Sigma-Aldrich) and 1× Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific). The lysates were then centrifuged at 30,000g for 15 min at 4 °C, and the supernatants were collected and used for further analyses. Protein estimations were carried out using a Micro BCA protein assay kit (ThermoFisher Scientific). To perform ELISA, wells were coated with the parasite or iRBC lysates or rPbHMGB1 in 0.05 M carbonate buffer pH 9.6 and incubated overnight at 4 °C. Blocking was carried out with 3% BSA in PBS containing 0.1% Tween-20. Rabbit polyclonal GFP antibody (ab290, Abcam) and rabbit polyclonal serum raised against rPbHMGB1 were used at 1:3000 dilution. HRP-conjugated goat anti-rabbit IgG secondary antibody (ab97051) was used 1:25,000 dilution. The HRP activity was detected using a tetramethylbenzidine liquid substrate and after stopping the reaction with 2 M H2SO4, the absorbance was measured at 450 nm. For the sensitivity assays, the percentage of blood parasitemia was calculated using Giemsa smears prepared from the tail vein blood, and the total number of RBCs present in the mouse blood collected for parasite preparations was estimated using a hemocytometer. Based on these, the total number of parasites present in the pellets obtained after saponin lysis was determined. The parasite lysates were prepared by sonication and centrifuged to remove membrane debris, and the lysates corresponding to the defined number of parasites were used to coat the wells of ELISA plates. In vivo bioluminescence studies were carried out as described (70, 71) using D-luciferin substrate (Promega VivoGlo). Immunization of rabbits to generate polyclonal sera against recombinant PbHMGB1 was carried out using Freund’s complete and incomplete adjuvant (Sigma-Aldrich, F5881, and F5506). The specificity of HMGB1 antibodies was confirmed with HMGB1KO parasites. The splenectomy for mice was carried out as described earlier (75). SDS-PAGE and Western blot analyses were carried out following the standard protocols and the blots were developed using Pierce ECL Western Blotting Substrate (ThermoFisher Scientific, 32106). Genomic DNA isolation was carried out using a DNeasy Blood & Tissue Kit (Qiagen, 69506). Southern analysis was carried out using the standard protocol. DIG DNA Labeling Kit (Roche, 11093657910) was used to generate PbHMGB1 3′UTR-specific probe labeled with Digoxigenin (DIG). The hybridization of the DIG-labelled probe was detected using DIG Luminescent Detection Kit (Roche, 11363514910).
Statistical analyses
GraphPad Prism 8.0 (GraphPad Software) and Microsoft Excel were used to plot the graphs and analyze the data. Statistical analyses were carried out using two-way ANOVA, Tukey test, and two-tailed, unpaired t test. The log-rank (Mantel-Cox) test was used to determine the significance in survival curve analysis. Normalization and differential expression analysis of RNA-Seq were carried out using the DESeq2 Bioconductor package with the R statistical programming. The false discovery rate was calculated using Benjamini-Hochberg procedure to control multiple hypothesis testing.
Data availability
All data are included within the article or supporting information. The raw files of RNA-Seq data are available at biostudies@ebi.ac.uk under accession number E-MTAB-14378.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We acknowledge the support rendered by flow cytometry and animal house facilities of Institute of Life Sciences, Bhubaneswar. We thank Mr Paritosh Nath for the flow cytometry experiments. We extend our thanks to Dr Sarita Jena and the technical assistant Biswajit Patro for their support in conducting animal studies. We thank MedGenome Labs Ltd, Bangalore, for performing the RNA-Seq runs. We thank Dr Santosh Chauhan for providing the RAW 264.7 murine macrophage cell line. The authenticity of the cell line was verified by morphology and TNF-α stimulatory assays, and confirmed for the absence of mycoplasma. We extend our gratitude to Malaria Research and Reference Reagent Resource Center (MR4), ATCC Manassas, Virginia for providing Pb ANKA strain (MRA-311) deposited by Thomas F. McCutchan; plasmid pL0006 (MRA-775), pL0031 (MRA-800) contributed by Andrew P. Waters. We are thankful to Addgene for providing us with the GOMO-GFP-LUC plasmid (#60976) deposited by Olivier Silvie.
Author contributions
R. D., P. M. V., B. R., G. P., and V. A. N. writing–review & editing; R. D., P. M. V., and V. A. N. writing–original draft; R. D., P. M. V., H. S. C., V. A. N., M. C., S. G., and A. A. visualization; R. D., P. M. V., H. S. C., V. A. N., M. C., S. G., and A. A. validation; R. D., P. M. V., H. S. C., V. A. N., M. C., S. G., and A. A. methodology; R. D., P. M. V., H. S. C., V. A. N., M. C., S. G., and A. A. investigation; R. D., P. M. V., H. S. C., B. R., G. P., V. A. N., S. G., K. C. M., and A. A. formal analysis; R. D., P. M. V., H. S. C., V. A. N., M. C., and K. C. M. data curation; P. M. V. and V. A. N. project administration; V. A. N. supervision; V. A. N. funding acquisition; V. A. N. conceptualization.
Funding and additional information
This study was supported by intramural support from Institute of Life Sciences (ILS/18–22, V. A. N.), and Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India (EMR/2016/005664, V. A. N.).
Reviewed by members of the JBC Editorial Board. Edited by Ronald Wek
Supporting information
References
- 1.Cox F.E. History of the discovery of the malaria parasites and their vectors. Parasit. Vectors. 2010;3:5. doi: 10.1186/1756-3305-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deroost K., Pham T.T., Opdenakker G., Van den Steen P.E. The immunological balance between host and parasite in malaria. FEMS Microbiol. Rev. 2016;40:208–257. doi: 10.1093/femsre/fuv046. [DOI] [PubMed] [Google Scholar]
- 3.Templeton T.J. The varieties of gene amplification, diversification and hypervariability in the human malaria parasite, Plasmodium falciparum. Mol. Biochem. Parasitol. 2009;166:109–116. doi: 10.1016/j.molbiopara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Sherman I.W., Eda S., Winograd E. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect. 2003;5:897–909. doi: 10.1016/s1286-4579(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 5.Duraisingh M.T., Skillman K.M. Epigenetic variation and regulation in malaria parasites. Annu. Rev. Microbiol. 2018;72:355–375. doi: 10.1146/annurev-micro-090817-062722. [DOI] [PubMed] [Google Scholar]
- 6.Guizetti J., Scherf A. Silence, activate, poise and switch! Mechanisms of antigenic variation in Plasmodium falciparum. Cell Microbiol. 2013;15:718–726. doi: 10.1111/cmi.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiyuka P.K., Meri S., Khattab A. Complement in malaria: immune evasion strategies and role in protective immunity. FEBS Lett. 2020;594:2502–2517. doi: 10.1002/1873-3468.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J.O., Howell K.B., Reiling L., Ataide R., Mackintosh C.L., Fowkes F.J.I. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J. Clin. Invest. 2012;129:3227–3238. doi: 10.1172/JCI62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunst J., Kamena F., Matuschewski K. Cytokines and chemokines in cerebral malaria pathogenesis. Front. Cell Infect. Microbiol. 2017;7:324. doi: 10.3389/fcimb.2017.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mbengue B., Niang B., Niang M.S., Varela M.L., Fall B., Fall M.M., et al. Inflammatory cytokine and humoral responses to Plasmodium falciparum glycosylphosphatidylinositols correlates with malaria immunity and pathogenesis. Immun. Inflamm. Dis. 2016;4:24–34. doi: 10.1002/iid3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivier M., Van Den Ham K., Shio M.T., Kassa F.A., Fougeray S. Malarial pigment hemozoin and the innate inflammatory response. Front. Immunol. 2014;5:25. doi: 10.3389/fimmu.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gowda D.C., Wu X. Parasite recognition and signaling mechanisms in innate immune responses to malaria. Front. Immunol. 2018;9:3006. doi: 10.3389/fimmu.2018.03006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez-Mazliah D., Ndungu F.M., Aye R., Langhorne J. B-cell memory in malaria: Myths and realities. Immunol. Rev. 2020;293:57–69. doi: 10.1111/imr.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malarkey C.S., Churchill M.E. The high mobility group box: the ultimate utility player of a cell. Trends Biochem. Sci. 2012;37:553–562. doi: 10.1016/j.tibs.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi M.E., Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 2005;15:496–506. doi: 10.1016/j.gde.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Lange S.S., Mitchell D.L., Vasquez K.M. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10320–10325. doi: 10.1073/pnas.0803181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlitz G., Hock R., Ueda T., Bustin M. The dynamics of HMG protein-chromatin interactions in living cells. Biochem. Cell Biol. 2009;87:127–137. doi: 10.1139/O08-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang D., Kang R., Livesey K.M., Cheh C.W., Farkas A., Loughran P., et al. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang R., Livesey K.M., Zeh H.J., Lotze M.T., Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011;7:1256–1258. doi: 10.4161/auto.7.10.16753. [DOI] [PubMed] [Google Scholar]
- 20.Yang H., Wang H., Andersson U. Targeting inflammation driven by HMGB1. Front. Immunol. 2020;11:484. doi: 10.3389/fimmu.2020.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H., Lundbäck P., Ottosson L., Erlandsson-Harris H., Venereau E., Bianchi M.E., et al. Redox modifications of cysteine residues regulate the cytokine activity of HMGB1. Mol. Med. 2021;27:58. doi: 10.1186/s10020-021-00307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R., Kang R., Tang D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022;54:91–102. doi: 10.1038/s12276-022-00736-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briquet S., Boschet C., Gissot M., Tissandié E., Sevilla E., Franetich J.F., et al. High-mobility-group box nuclear factors of Plasmodium falciparum. Eukaryot. Cell. 2006;5:672–682. doi: 10.1128/EC.5.4.672-682.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briquet S., Lawson-Hogban N., Boisson B., Soares M.P., Péronet R., Smith L., et al. Disruption of parasite hmgb2 gene attenuates Plasmodium berghei ANKA pathogenicity. Infect. Immun. 2015;83:2771–2784. doi: 10.1128/IAI.03129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briquet S., Lawson-Hogban N., Peronet R., Mécheri S., Vaquero C. A genetically hmgb2 attenuated blood stage P. berghei induces crossed-long live protection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gissot M., Ting L.M., Daly T.M., Bergman L.W., Sinnis P., Kim K. High mobility group protein HMGB2 is a critical regulator of Plasmodium oocyst development. J. Biol. Chem. 2008;283:17030–17038. doi: 10.1074/jbc.M801637200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S., Kappe S.H.I. PfHMGB2 has a role in malaria parasite mosquito infection. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.1003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu B., Liu M., Gu L., Li Y., Shen S., Guo G., et al. The architectural factor HMGB1 is involved in genome organization in the human malaria parasite Plasmodium falciparum. mBio. 2021;12 doi: 10.1128/mBio.00148-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janko C., Filipovic M., Munoz L.E., Schorn C., Schett G., Ivanovic-Burmazovic I., et al. Redox modulation of HMGB1-related signaling. Antioxid. Redox Signal. 2014;20:1075–1085. doi: 10.1089/ars.2013.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H., Hreggvidsdottir H.S., Palmblad K., Wang H., Ochani M., Li J., et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Kokkola R., Tabibzadeh S., Yang R., Ochani M., Qiang X., et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 32.Urban B.C., Hien T.T., Day N.P., Phu N.H., Roberts R., Pongponratn E., et al. Fatal Plasmodium falciparum malaria causes specific patterns of splenic architectural disorganization. Infect. Immun. 2005;73:1986–1994. doi: 10.1128/IAI.73.4.1986-1994.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh D., Stumhofer J.S. The spleen: "epicenter" in malaria infection and immunity. J. Leukoc. Biol. 2021;110:753–769. doi: 10.1002/JLB.4RI1020-713R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young C., Brink R. The unique biology of germinal center B cells. Immunity. 2021;54:1652–1664. doi: 10.1016/j.immuni.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Victora G.D., Nussenzweig M.C. Germinal centers. Annu. Rev. Immunol. 2022;40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- 36.Ryg-Cornejo V., Ioannidis L.J., Ly A., Chiu C.Y., Tellier J., Hill D.L., et al. Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation. Cell Rep. 2016;14:68–81. doi: 10.1016/j.celrep.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Kurup S.P., Obeng-Adjei N., Anthony S.M., Traore B., Doumbo O.K., Butler N.S., et al. Regulatory T cells impede acute and long-term immunity to blood-stage malaria through CTLA-4. Nat. Med. 2017;23:1220–1225. doi: 10.1038/nm.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan J.A., Loughland J.R., de Labastida Rivera F., SheelaNair A., Andrew D.W., Dooley N.L., et al. Th2-like T follicular helper cells promote functional antibody production during Plasmodium falciparum infection. Cell Rep. Med. 2020;9 doi: 10.1016/j.xcrm.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Little T.S., Cunningham D.A., Vandomme A., Lopez C.T., Amis S., Alder C., et al. Analysis of pir gene expression across the Plasmodium life cycle. Malar. J. 2021;20:445. doi: 10.1186/s12936-021-03979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida R., Fernández-Justel J.M., Santa-María C., Cadoret J.C., Cano-Aroca L., Lombrana R., et al. Chromatin conformation regulates the coordination between DNA replication and transcription. Nat. Commun. 2018;9:1590. doi: 10.1038/s41467-018-03539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amato J., Cerofolini L., Brancaccio D., Giuntini S., Iaccarino N., Zizza P., et al. Insights into telomeric G-quadruplex DNA recognition by HMGB1 protein. Nucleic Acids Res. 2019;47:9950–9966. doi: 10.1093/nar/gkz727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kucirek M., Bagherpoor A.J., Jaros J., Hampl A., Stros M. HMGB2 is a negative regulator of telomerase activity in human embryonic stem and progenitor cells. FASEB J. 2019;33:14307–14324. doi: 10.1096/fj.201901465RRR. [DOI] [PubMed] [Google Scholar]
- 43.Gong W., Guo Y., Yuan H., Hu X., Chai R., Zheng B., et al. HMGB3 is a potential therapeutic target by affecting the migration and proliferation of colorectal cancer. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.891482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen B., Wei Y.T., Zhao K. The role of high mobility group protein B3 (HMGB3) in tumor proliferation and drug resistance. Mol. Cell Biochem. 2021;476:1729–1739. doi: 10.1007/s11010-020-04015-y. [DOI] [PubMed] [Google Scholar]
- 45.Rouhiainen A., Zhao X., Vanttola P., Qian K., Kulesskiy E., Kuja-Panula J., et al. HMGB4 is expressed by neuronal cells and affects the expression of genes involved in neural differentiation. Sci. Rep. 2016;6 doi: 10.1038/srep32960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan S., Liu Z., Xu Z., Liu J., Zhang J. High mobility group box 1 (HMGB1): a pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020;13:91. doi: 10.1186/s13045-020-00920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venereau E., Ceriotti C., Bianchi M.E. DAMPs from cell death to new life. Front. Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Xiang L., Li H., Chen P., Feng Y., Zhang J., et al. The role of HMGB1 signaling pathway in the development and progression of hepatocellular carcinoma: a review. Int. J. Mol. Sci. 2015;16:22527–22540. doi: 10.3390/ijms160922527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwak M.S., Kim H.S., Lkhamsuren K., Kim Y.H., Han M.G., Shin J.M., et al. Peroxiredoxin-mediated disulfide bond formation is required for nucleocytoplasmic translocation and secretion of HMGB1 in response to inflammatory stimuli. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar K., Singal A., Rizvi M.M., Chauhan V.S. High mobility group box (HMGB) proteins of Plasmodium falciparum: DNA binding proteins with pro-inflammatory activity. Parasitol. Int. 2008;57:150–157. doi: 10.1016/j.parint.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Deng M., Tang Y., Li W., Wang X., Zhang R., Zhang X., et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. 2018;49:740–753.e7. doi: 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang W., Tang Y., Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 53.Dobbs K.R., Crabtree J.N., Dent A.E. Innate immunity to malaria-The role of monocytes. Immunol. Rev. 2020;293:8–24. doi: 10.1111/imr.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yap X.Z., Lundie R.J., Beeson J.G., O'Keeffe M. Dendritic cell responses and function in malaria. Front. Immunol. 2019;10:357. doi: 10.3389/fimmu.2019.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henry B., Volle G., Akpovi H., Gineau L., Roussel C., Ndour P.A., et al. Splenic clearance of rigid erythrocytes as an inherited mechanism for splenomegaly and natural resistance to malaria. EBioMedicine. 2022;82 doi: 10.1016/j.ebiom.2022.104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta P., Lai S.M., Sheng J., Tetlak P., Balachander A., Claser C., et al. Tissue-resident CD169(+) macrophages form a crucial front line against Plasmodium infection. Cell Rep. 2016;16:1749–1761. doi: 10.1016/j.celrep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Lai S.M., Sheng J., Gupta P., Renia L., Duan K., Zolezzi F., et al. Organ-specific fate, recruitment, and refilling dynamics of tissue-resident macrophages during blood-stage malaria. Cell Rep. 2018;25:3099–3109.e3. doi: 10.1016/j.celrep.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 58.Fontana M.F., Ollmann Saphire E., Pepper M. Plasmodium infection disrupts the T follicular helper cell response to heterologous immunization. Elife. 2023;12 doi: 10.7554/eLife.83330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Figueiredo M.M., Costa P.A.C., Diniz S.Q., Henriques P.M., Kano F.S., Tada M.S., et al. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan J.A., Loughland J.R., de la Parte L., Okano S., Ssewanyana I., Nalubega M., et al. Age-dependent changes in circulating Tfh cells influence development of functional malaria antibodies in children. Nat. Commun. 2022;13:4159. doi: 10.1038/s41467-022-31880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soon M.S.F., Nalubega M., Boyle M.J. T-follicular helper cells in malaria infection and roles in antibody induction. Oxf. Open Immunol. 2021;2 doi: 10.1093/oxfimm/iqab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larremore D.B., Sundararaman S.A., Liu W., Proto W.R., Clauset A., Loy D.E., et al. Ape parasite origins of human malaria virulence genes. Nat. Commun. 2015;6:8368. doi: 10.1038/ncomms9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hang J.W., Tukijan F., Lee E.Q., Abdeen S.R., Aniweh Y., Malleret B. Zoonotic malaria: non-Laverania Plasmodium biology and invasion mechanisms. Pathogens. 2021;10:889. doi: 10.3390/pathogens10070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Otto T.D., Böhme U., Jackson A.P., Hunt M., Franke-Fayard B., Hoeijmakers W.A., et al. A comprehensive evaluation of rodent malaria parasite genomes and gene expression. BMC Biol. 2014;12:86. doi: 10.1186/s12915-014-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison T.E., Reid A.J., Cunningham D., Langhorne J., Higgins M.K. Structure of the Plasmodium-interspersed repeat proteins of the malaria parasite. Proc. Natl. Acad. Sci. U. S. A. 2020;117:32098–32104. doi: 10.1073/pnas.2016775117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giorgalli M., Cunningham D.A., Broncel M., Sait A., Harrison T.E., Hosking C., et al. Differential trafficking and expression of PIR Proteins in acute and chronic Plasmodium infections. Front. Cell Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.877253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt-Christensen A., Sturm A., Horstmann S., Heussler V.T. Expression and processing of Plasmodium berghei SERA3 during liver stages. Cell Microbiol. 2008;10:1723–1734. doi: 10.1111/j.1462-5822.2008.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manzoni G., Briquet S., Risco-Castillo V., Gaultier C., Topçu S., Ivanescu M.L., et al. A rapid and robust selection procedure for generating drug-selectable marker-free recombinant malaria parasites. Sci. Rep. 2014;4:4760. doi: 10.1038/srep04760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orr R.Y., Philip N., Waters A.P. Improved negative selection protocol for Plasmodium beghei in the rodent malaria model. Malar. J. 2012;11:103. doi: 10.1186/1475-2875-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anand A., Chandana M., Ghosh S., Das R., Singh N., Vaishalli P.M., et al. Significance of Plasmodium berghei amino acid transporter 1 in food vacuole functionality and its association with cerebral pathogenesis. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.04943-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chandana M., Anand A., Ghosh S., Das R., Beura S., Jena S., et al. Malaria parasite heme biosynthesis promotes and griseofulvin protects against cerebral malaria in mice. Nat. Commun. 2022;13:4028. doi: 10.1038/s41467-022-31431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghosh S., Kundu R., Chandana M., Das R., Anand A., Beura S., et al. Distinct evolution of type I glutamine synthetase in Plasmodium and its species-specific requirement. Nat. Commun. 2023;14:4216. doi: 10.1038/s41467-023-39670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barnay-Verdier S., Gaillard C., Messmer M., Borde C., Gibot S., Marechal V. PCA-ELISA: a sensitive method to quantify free and masked forms of HMGB1. Cytokine. 2011;55:4–7. doi: 10.1016/j.cyto.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 75.Dotson A.L., Wang J., Saugstad J., Murphy S.J., Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J. Neuroimmunol. 2015;278:289–298. doi: 10.1016/j.jneuroim.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included within the article or supporting information. The raw files of RNA-Seq data are available at biostudies@ebi.ac.uk under accession number E-MTAB-14378.