ABSTRACT
Background
Non‐invasive brain stimulation (NIBS) has attracted significant attention as it has been proven to be effective in facilitating upper limb motor recovery in patients with stroke. This meta‐analysis evaluates the efficacy of dual‐site non‐invasive brain stimulation (DS‐NIBS) in improving upper extremity motor function after stroke.
Methods
A PRISMA systematic search was conducted for randomized controlled trials. Two authors independently extracted data, and the quality of included studies was assessed.
Results
Ten studies were included in the current review. DS‐NIBS demonstrated a significant effect on upper extremity motor function impairment. However, only two studies showed no clear effects of DS‐tDCS on upper extremity motor function after stroke. Due to the limited number of studies, the effects of DS‐NIBS remain inconclusive.
Finding
This review found evidence for the relatively higher efficacy of DS‐NIBS on post‐stroke upper extremity motor function impairment, compared to the sham and SS‐NIBS. Additionally, DS‐TMS was found to generate better improvement than DS‐tDCS.
Keywords: meta‐analysis, stroke, transcranial direct current stimulation, transcranial magnetic stimulation, upper extremity
This meta‐analysis evaluates the efficacy of dual‐site non‐invasive brain stimulation (DS‐NIBS) in improving upper extremity motor function after stroke. The analysis included 10 trials with 426 participants, indicating that DS‐NIBS showed higher efficacy on post‐stroke upper extremity motor function impairment compared to the sham and single‐NIBS. Additionally, dual‐site repetitive transcranial magnetic stimulation was found to generate better improvement than dual‐site transcranial direct current stimulation.
1. Introduction
Stroke is one of the most important challenges faced by the world medical community, being a leading cause of death and disability (Collaborators 2021; Owolabi et al. 2022; Stinear et al. 2020). Hemiplegia is the most common functional disability after stroke, and upper limb hemiplegia (including hand dysfunction) has always been an important and difficult problem in stroke rehabilitation, affecting daily living and increasing the burden on these patients and their families (Lawrence et al. 2001; Min and Min 2015; Vlisides and Mashour 2016). Conventional rehabilitation training techniques for upper limb dysfunction are limited, including occupational therapy, neurostimulation techniques, strength training, motor relearning, restriction‐induced motor training, and so on (Gittler and Davis 2018; Langhorne, Bernhardt, and Kwakkel 2011), and often show variable and limited effectiveness (Barreca et al. 2003).
Various patterns of neural network reorganization occur in both the lesioned and unaffected hemispheres after stroke (Cramer 2008), and functional recovery is associated with neural plastic changes in the brain (Volz et al. 2016), including neurogenesis, gliogenesis, axonal sprouting, changes in excitation/inhibition balance, and many more. The relationship between stroke motor recovery and cortical reorganization has been explored by many scientists (Sinke et al. 2018; Vidal et al. 2018; Xerri et al. 2014), revealing the importance of the inter‐hemispheric activation balance in motor‐related cortices for motor recovery of stroke patients (Tang et al. 2015). Therefore, cortical excitability regulation has become a therapeutic strategy for stroke.
Non‐invasive brain stimulation (NIBS) entails the modulation of brain excitability and activity (Caglayan et al. 2019; Cheng et al. 2023). At the cellular level, NIBS is capable of enhancing cellular neuromodulation. This modulation includes the reduction of the inflammatory response, autophagy suppression, antiapoptotic effects, angiogenesis enhancement, alterations in the blood‐brain barrier permeability, attenuation of oxidative stress, influence on neurotransmitter metabolism, neurogenesis, and enhanced structural neuroplasticity (Badoiu et al. 2023; Ferreira et al. 2024). Transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) were considered the two most common methods used for NIBS (Li et al. 2023). Existing evidence suggests that tDCS can elicit either an excitatory or inhibitory effect. More specifically, anodal tDCS can increase the function of the targeted cortical areas, whereas cathodal tDCS elicits a suppressive effect (Fertonani and Miniussi 2017). These effects were commonly modulated through Ca2+ signaling. In synapses, Ca2+ influxes into the postsynaptic cell through activating AMPA‐subtype glutamate receptors to trigger synapse plasticity, such as long‐term potentiation and long‐term depression, to improve neuroplasticity (Shepherd and Huganir 2007). Similarly, repetitive transcranial magnetic stimulation (rTMS) has dual effects of facilitation (high frequency, > 1 Hz, commonly used > 5 Hz) or inhibition (low frequency, ≤ 1 Hz, commonly used 1 Hz) on the excitability of the cerebral cortex and has been widely used in the movement disorders caused by stroke (Bai, Zhang, and Fong 2022; Edwards et al. 2021).
However, although there are many reports and related guidelines on the application of NIBS for stroke hemiplegia rehabilitation, there is still no consensus on the target, mode, or strategy for stimulation (Khedr et al. 2013; Kubis 2016; Peruzzotti‐Jametti et al. 2013; Wong et al. 2022). This may be related to the degree of brain damage, the course of the disease, the patient's response to interventions, and so on (Boddington and Reynolds 2017).
The concept of interhemispheric competition is the mainstream theory behind neural regulation. Stroke causes damage to one hemisphere, thereby reducing the ipsilateral hemisphere's ability to inhibit the contralateral hemisphere. As a result, the contralateral hemisphere increases inhibition on the affected hemisphere, resulting in an imbalance between the two hemispheres and affecting functional recovery. The Vicariation model complements interhemispheric competition, but some strategies are diametrically opposed. It believes that the lesion of the stroke causes dysfunction, and the over‐activation of both the peri‐lesional brain area and the contralateral hemisphere may be not a maladaptive but rather a vicarious mechanism. Interhemispheric competition and vicariation model have two diametrically opposite directions on whether the contralateral hemisphere cortex is inhibited or excited, causing difficulty for clinicians in choosing neuromodulation strategies for stroke hemiplegia rehabilitation. The bimodal balance‐recovery model moderates some differences between the two models above and introduces the concept of “structural reserve” (Pino et al. 2014). However, it remains controversial what constitutes high or low structural retention.
To achieve better clinical outcomes, some studies also have used dual‐site NIBS for upper extremity motor dysfunction after stroke, focusing on brain circuits (Achacheluee et al. 2018; Cho et al. 2017). Cortico‐cortical paired associative stimulation (ccPAS), which applies repeated pairing of TMS pulses over two distinct cortical sites at precise interpulse intervals, can more effectively alter the excitability of the motor cortex, resulting in better recovery of the upper limb function (Duan et al. 2022). Moreover, cerebello‐motor PAS has also been found effective compared to sham in improving hand dexterity but not grip strength (Rosso et al. 2022). Some researchers also explore the effect of dual‐site tDCS. In one study (Lei et al. 2023), the investigators set up a new method of tDCS in which the cathodal electrode is placed on the ischemic hemisphere and the anodal electrode on the contralateral hemisphere of rats subjected to ischemia‐reperfusion injury. This new method protected against neuronal death and improved the functional recovery of stroke animals, suggesting a potential endogenous therapy. A meta‐analysis on 657 stroke patients demonstrated that bilateral transcranial electric stimulation and cathodal tDCS over the contralesional hemisphere were superior to other stimulation montages/patterns/protocols on gait, balance and/or lower limb motor recovery in stroke patients (Veldema and Gharabaghi 2022).
The present systematic review and meta‐analysis of randomized controlled trials aimed to explore the effects of dual‐site NIBS on the upper limb (UL) motor impairments and functional performance post‐stroke. The secondary goals included investigating whether the NIBS types enhance motor recovery, identifying factors that may contribute to better motor outcomes, and exploring potential adverse effects of using dual‐site NIBS in patients with stroke.
2. Methods
2.1. Search Strategy
This systematic review and meta‐analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement and was registered at the International Prospective Register of Systematic Reviews (number CRD42022370564). The literature research was conducted in MEDLINE (via PubMed), Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), WeiPu, WanFang, and the Chinese Biomedical Literature Database (CBM) up to September 30, 2022, without language restriction.
The following keywords were used: stroke, upper extremity, transcranial direct current stimulation, and transcranial magnetic current stimulation. The full search strategy can be found in Supplementary Material (SM), Table SM1.
2.2. Study Selection
The inclusion criteria to identify for qualifying articles were:
randomized control trials (RCTs) that included post‐stroke adult participants (≥ 18 years);
the focus was on the effects on the upper limb in post stroke patients;
the types of intervention were dual‐site NIBS;
the control group included single‐site or sham NIBS;
reported at least one standardized outcome measure that evaluated the upper‐limb performance, ICF body structure/body function domain (e.g., Fugl‐Meyer Assessment Scale (FMA), Ashworth or modified Ashworth Scale, force and range of motion) or activity level (e.g., Wolf Motor Function Test (WMFT), Action Research Arm Test (ARAT), Box and Blocks Test (BBT), Jebsen‐Taylor Hand Function Test (JTT), Nine Hole Peg Test, and Motor Assessment Scale).
Exclusion criteria were as follows:
failure to provide relevant data on the outcome measures;
the dual‐site NIBS was used not only in one group;
NIBS was applied in combination with other techniques (i.e., use of virtual reality or electrostimulation in adjunction to single‐site NIBS intervention);
central‐peripheral paired associative stimulation;
poor quality RCTs (PEDro < 5) were also excluded (This is discussed in detail in Quality Assessment section).
After searching, duplicate records were excluded. Two independent investigators reviewed study titles and abstracts, and studies that satisfied the inclusion criteria were retrieved for full‐text assessment. Two reviewers independently identified the eligible studies according to the pre‐formulated inclusion and exclusion criteria. If any discrepancies arose, a third investigator was consulted and made the final decision.
2.3. Data Extraction
Two authors independently extracted data from the included studies. If any discrepancies arose, a third investigator was consulted and made the final decision. If information was missing or unclear in articles, their authors were contacted. For crossover studies, only data from the first intervention were extracted for meta‐analyses.
When multiple outcomes were assessed to measure body function or structure, the Fugl‐Meyer assessment was considered the priority of analysis according to the recommendations of the measurement working group of the Stroke Recovery and Rehabilitation Round table. To measure activity limitation, the ARAT was considered the priority of analysis according to recommendations of the same panel. If these scales were not present in the study, the most frequent outcomes across the selected studies were chosen.
An electronic data extraction form was used to collect the following information: study design, number of subjects, sample characteristics (i.e., mean age, gender, stroke duration, lesion side, and phase), treatment protocol (i.e., frequency, intensity, number of pulses, the time of intervention, the number of sessions, and the target), and outcome measures. In instances where results were only presented in figures and the authors did not report further information despite attempts to contact them, a Plot Digitizer program was used to extract values. This program digitizes uploaded figures by calibrating the image's axes, allowing data points to be extracted by clicking on any data point on the figure. If a study did not report sufficient quantifiable results and the authors did not respond to requests, then the study was excluded.
2.4. Quality Assessment
The Physiotherapy Evidence Database (PEDro) scale was used to assess the methodological quality of each included RCT by two independent reviewers. The PEDro scale is an 11‐item scale that has been widely used for rating the methodological quality of RCTs (Baniqued et al. 2021; Brunt, Albines, and Hopkins‐Rosseel 2019). Each satisfied item pertained to internal validity received one point, except the first item, which is rated as YES or NO (maximum score = 10 points). Studies scoring four or higher on the PEDro scale were considered of sufficient quality (Foley et al. 2003). Studies scoring 6 or higher in which the critical criteria 2 or 3 (randomization and concealment of allocation, respectively) were absent were downgraded to fair quality. Poor‐quality studies (scores lower than 4) were excluded in the present study.
2.5. Statistical Analysis
For studies using the same scale to evaluate the outcome (i.e., Fugl‐Meyer for body structure/function), the number of participants in each group, mean scores, and SDs after interventions in the active and control groups were analyzed in RevMan 5.3. (Review Manager 5.3). For the Fugl‐Meyer, a higher score was regarded as positive.
For types of outcomes assessed with different scales (i.e., activity limitation), measures of postintervention and preintervention of each subject were assessed after contacting the authors and requesting individual data. The mean and SD of the change scores relative to the baseline or the posttreatment mean and SD were recorded for each outcome measure in the experimental and control groups. For scales in which a lower score was regarded as positive compared with a higher score, the mean was multiplied by −1. The means and SDs of relative differences in active and control groups were analyzed in RevMan 5.3. Regarding the continuous outcomes, if the unit of measurement was consistent across trials, the results were presented as the weighted mean difference (MD) with 95% confidence intervals (95% CIs). Standardized mean differences (SMDs), instead of the mean difference (MD), with 95% confidence intervals (95% CIs), were used if the outcome measurement scale was not identical between studies.
Funnel plots were used to detect publication bias if more than 10 articles were involved in the meta‐analysis.
2.6. Additional Analysis
To identify potential influencing factors on motor recovery, subgroup analyses were also performed based on NIBS types (tDCS versus rTMS), post‐stroke duration (acute [⩽ 1 week] versus subacute [1 week to 6 months] versus chronic [> 6 months]), targeted region, theoretical model, lesion location (subcortical versus nonspecified), and treatment sessions. If two or fewer studies were identified for a single analysis objective, only qualitative description instead of meta‐analysis would be performed.
Heterogeneity between studies was assessed using the Cochran Q test and the Higgins’ I2 statistic. An I2 value below 20% was considered to indicate low levels of heterogeneity, while an I2 value above 50% indicated high levels of heterogeneity. A fixed effects model was applied if I2 < 50%; otherwise, a random‐effects model would be used.
Publication bias was evaluated using Egger's linear regression test and visual inspection of the funnel plot. Sensitivity analysis was conducted to explore the impact on the effect size when low‐quality studies and studies with cross‐over design were excluded. The level of significance was set at p < 0.05 for all statistical analyses. Stata (version 16.0) was used for Egger's linear regression test. Statistical significance was set at two‐tailed p < 0.05. Finally, effect sizes were classified as small (0.2), medium (0.2–0.8), and large (0.8).
3. Results
3.1. Study Characteristics
The initial database search yielded a total of 2,741 relevant studies. Only 10 studies were identified (N = 426) by two independent reviewers based on the inclusion criteria. The flow diagram of the selection process is shown in Figure 1.
FIGURE 1.
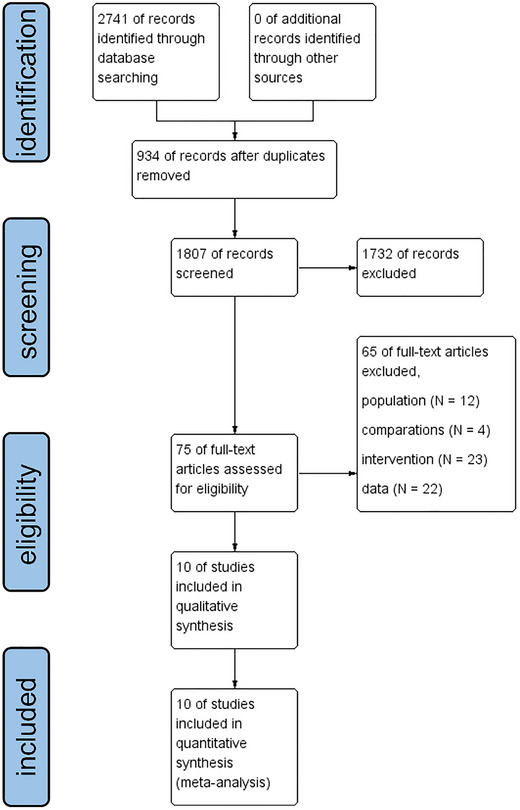
PRISMA Flow Chart diagram.
The characteristics of recruited participants included in this meta‐analysis are detailed in Table 1. All relevant information regarding the studies that meet the inclusion criteria is presented in Table 2. The study protocols contained NIBS with various conditions. In four studies (Hsu et al. 2021; Ji 2014; Kim 2021; Lindenberg et al. 2010), the control group received sham NIBS. In another four studies (Cai 2020; Cao et al. 2022; Fleming et al. 2017; Ren et al. 2018), the control group received single‐site NIBS. The remaining two studies (Long et al. 2018; Taud et al. 2021) contain both sham and single‐site NIBS groups.
TABLE 1.
Characteristics of recruited participants included in this meta‐analysis.
| Reference |
Total N |
Age (year) [Mean (SD)] |
Gender [Male (%)] |
Time post stroke [Mean (SD)] |
Lesion side [right (%)] | phase |
|---|---|---|---|---|---|---|
| Fleming et al. (2017) | 25 |
DS: 59.8 (13.1) SS: 59.8 (13.1) |
NR |
DS: 19.7 (27.4) m SS: 19.7 (27.4) m |
DS: 12(48%) SS: 12(48%) |
chronic |
| Long et al. (2018) | 62 |
DS: 55.90 (8.89) SS: 57 (11.78) Sham:56.85(5.48) |
DS: 16 (76%) SS: 16 (76%) Sham: 15(75%) |
DS: 19.81 (2.98) d SS: 19.57 (2.34) d Sham: 19.05(2.74) |
DS: 11(52%) SS: 11(52%) Sham: 11(55%) |
subacute |
| Taud et al. (2021) | 30 |
DS: 58.3 (12.8) SS: 60.3 (10.3) |
DS: 11 (73%) SS: 12 (80%) |
DS: 21.9 (17.2) m SS: 28.8 (35.3) m |
DS: 8(53%) SS: 8(53%) |
chronic |
| Cai (2020) | 103 |
DS: 55.37 (8.16) SS: 55.00 (7.55) |
DS: 29 (56%) SS: 27 (53%) |
DS: 60.31 (16.94) d SS: 61.39 (15.49) d |
DS: 26(50%) SS: 27(53%) |
subacute |
| Cao et al. (2022) | 40 |
DS: 51.80 (15.45) SS: 48.75 (13.24) |
DS: 12 (60%) SS: 11 (55%) |
DS: 45.85 (20.56) d SS: 41.90 (24.86) d |
DS: 12(60%) SS: 7(35%) |
subacute |
| Ren et al. (2018) | 60 |
DS: 51.2(3.6) SS:49.6 (4.3) |
DS: 19 (63%) SS: 17 (57%) |
NR | NR | NR |
| Hsu et al. (2021) | 27 |
DS: 59.1 (11.4) Sham: 59.2 (11.8) |
DS: 9 (69%) Sham: 6 (43%) |
DS: 20.7 (3.5) d Sham: 21.1 (5.3)d |
DS: 9 (69%) Sham: 7 (50%) |
subacute |
| Kim (2021) | 30 |
DS: 60.2(5.3) Sham: 60.33(6.33) |
DS: 7 (47%) Sham: 8 (53%) |
DS: 12.13(1.84)m Sham: 10.93(1.94)m |
DS: 7 (47%) Sham: 8 (53%) |
chronic |
| Lindenberg et al. (2010) | 20 |
DS: 61.7 (14.7) Sham: 55.8(12.9) |
DS: 8 (80%) Sham: 7 (70%) |
DS: 30.5(21.4)m Sham: 40.3(23.4)m |
DS: 4 (40%) Sham: 3 (30%) |
chronic |
| Ji (2014) | 29 |
DS: 64.2(11.9) Sham: 62.3(12.2) |
DS: 11 (73%) Sham: 10(71%) |
DS: 8.1 (1.5) m Sham: 8.2 (1.6)m |
NR | chronic |
Abbreviations: DS: dual‐site NIBS; NR: not reported; SS: single‐site NIBS.
TABLE 2.
Characteristics of the included studies in this meta‐analysis.
| Reference | Type of NIBS | DS intervention group | Comparation group | Outcome measure |
|---|---|---|---|---|
| Fleming et al. (2017) | tDCS | The anode was placed over ipsilesional M1 and the cathode over contralesional M1. |
Anodal tDCS: anodal to ipsilesional M1 and the cathode over the contralateral supraorbital ridge, 1 mA 0.04 mA/cm2 Cathodal tDCS: cathodal to contralesional M1 the anode was placed over ipsilesional M1 and the cathode over contralesional M1. Sham |
JTT |
| Long et al. (2018) | TMS |
1 Hz rTMS to the cM1 followed by 10 Hz rTMS to the iM1 With an interstimulus interval of 50 seconds. 90% RMT, 1000 pulses, 15 sessions |
LF‐rTMS: cM1, 1 Hz, 90% RMT, 1000 pulses, 15 sessions Sham: The coil was held at an angle of 90° to the scalp |
FMA‐UL WMFT |
| Taud et al. (2021) | tDCS | Active anode placed over the iM1 and a smaller active cathode was placed over the cM1. 0.03 mA/cm2,23 min, 5 sessions |
Anodal tDCS: active anode placed over the iM1 and the cathode was placed over the contralesional supraorbital ridge. Sham: the electrode set‐up was pseudo‐randomly assigned to participants (either anodal or dual) and balanced across the group. |
FMA‐UL WMFT |
| Cai (2020) | TMS | cM1, 1 Hz, 90% RMT, 1000 pulse, 24 sessions and iM1, 10 Hz, 90% RMT, 1000 pulses, 24 sessions |
LF‐rTMS: cM1, 1Hz, 90% RMT, 1000 pulses, 24 sessions HF‐rTMS: iM1, 10 Hz,90% RMT, 1000 pulses, 24 sessions |
FMA‐UL |
| Cao et al. (2022) | TMS |
iTBS applied to contralateral cerebellar cortex and iM1 separately, an interval of 5s was set between these two stimulations. iTBS: 80%∼100% RMT, repetitive bursts of 3 stimuli at a frequency of 50 Hz repeated at 5 Hz,600 pulses, 24 sessions |
iTBS: iM1, 80%∼100% RMT, 600 pulses, 24 sessions | FMA‐UL ARAT |
| Ren et al. (2018) | TMS | cM1,80%RMT, 1 Hz, 3000 pulses, 14 sessions and iM1,80%RMT, 5 Hz, 3000 pulses, 14 sessions | iM1, 80%RMT, 5 Hz, 3000 pulses, 14 sessions | FMA‐UL |
| Hsu et al. (2021) | tDCS | iM1 anode and cM1 cathode, 0.08 mA/cm2, 20 min, 20 sessions | Sham: tDCS settings were similar except that the direct current ceased after 2 min. | FMA‐UL ARAT |
| Kim (2021) | tDCS | iM1 anodal electrode, and the cM1 cathodal electrode, 1 mA, 20 min, 20 sessions | Sham: no current flows | FMA‐UL |
| Lindenberg et al. (2010) | tDCS | Anodal tDCS to iM1 and cathodal tDCS to cM1, 0.09 mA/cm2, 30 min, 5 sessions | Sham: The current was ramped up to 1.5 mA and slowly decreased over 30 seconds | FMA‐UL WMFT |
| Ji (2014) | TMS | cM1, 1 Hz, 80–100%RMT, 900 pulses and iM1, iTBS, 80–120%RMT, 15 min, 30 sessions | Sham: The coil was held at an angle of 90° to the scalp | FMA‐UL |
Abbreviations: ARAT: Action Research Arm Test; FMA‐UL: Fugl‐Meyer Assessment upper limb; JTT: Jebsen‐Taylor Hand Function Test; LF‐rTMS: low‐frequency repetitive transcranial magnetic stimulation; tDCS: transcranial direct current stimulation; WMFT: Wolf Motor Function Test.
The study intervention contained various NIBS. Five studies (Fleming et al. 2017; Hsu et al. 2021; Klomjai et al. 2022; Lindenberg et al. 2010; Taud et al. 2021) applied tDCS, while five studies (Cai 2020; Cao et al. 2022; Ji 2014; Long et al. 2018; Ren et al. 2018) applied rTMS. According to the outcome measures of upper limb function, nine studies reported FMA‐UL, while only one study (Fleming et al. 2017) reported JTT.
3.2. Adverse Effects
All participants tolerated DS‐NIBS without significant adverse events. No adverse effects were observed by the investigators or reported by the DS‐NIBS patients.
3.3. Quality Assessment
Table 3 presents the methodological quality assessment of the included studies, as evaluated using the PEDro scale. All of the included studies scored more than 4 points on the PEDro scale, indicating sufficient quality. The mean score of PEDro was 6.7 points (SD = 0.82), ranging from 5 to 8 points.
TABLE 3.
Methodological quality of included studies.
| Reference | Eligibility criteria specified (Yes/No) | Random allocation (0/1) | Concealed allocation (0/1) | Comparable at baseline (0/1) | Blinded subjects (0/1) | Blinded therapists (0/1) | Blinded assessors (0/1) | Adequate follow‐up (0/1) | Intention‐totreat analysis (0/1) | Between group comparisons (0/1) | Point estimates and variability (0/1) | Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fleming et al. (2017) | Yes | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Long et al. (2018) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Taud et al. (2021) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Cai (2020) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Cao et al. (2022) | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Ren et al. (2018) | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Hsu et al. (2021) | Yes | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Kim (2021) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
| Lindenberg et al. (2010) | Yes | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Ji (2014) | Yes | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 7 |
3.4. Meta‐Analysis Results
The effect of dual‐site NIBS on upper limb function after stroke compared with sham and single‐site NIBS was evaluated by pooling post‐intervention data from 10 studies involving 426 participants. The pooled meta‐analysis showed a significant improvement on Fugl‐Meyer Assessment upper limb (FMA‐UL) scores after dual‐site NIBS in the clinical population (p = 0.008), compared with SS‐NIBS (Figure 2).
FIGURE 2.
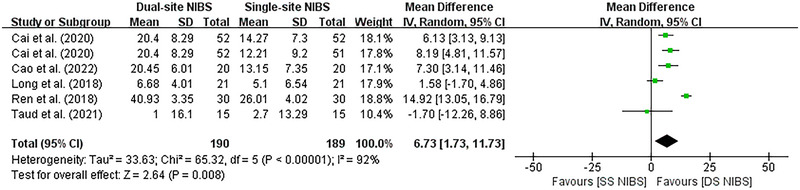
Forest plot of DS‐NIBS versus SS‐NIBS. Results of meta‐analysis comparing the FMA‐UL of DS‐NIBS versus SS‐NIBS in treating upper extremity motor function after stroke. FMA‐UL: Fugl‐Meyer Assessment upper limb; DS‐NIBS: dual‐site non‐invasive brain stimulation; SS‐NIBS: single‐site non‐invasive brain stimulation.
Dual‐site NIBS was significantly more effective than sham simulation (MD, 3.18; 95% CI, 1.73 to 4.63) and single‐site NIBS (MD, 9.65; 95% CI, 8.4 to 10.89) with respect to motor function, respectively. However, significant evidence of inter‐study heterogeneity was observed for the meta‐analysis in sham simulation and single‐site NIBS (I2 = 44%, p < 0.0001 and I2 = 92%, p = 0.008, respectively).
A further subgroup meta‐analysis was conducted according to the different NIBS types. Because there are only two studies using tDCS for DS‐NIBS versus SS‐NIBS, the subgroup meta‐analysis was only performed for DS‐NIBS versus Sham‐NIBS. When considering the three DS‐tDCS trials alone, there was a moderate but non‐significant pooled effect size (0.52, p = 0.15) favoring the stimulation intervention. The three trials investigating DS‐rTMS on post‐stroke upper limb function impairment demonstrated a similar, but significant, pooled effect size (0.56, p < 0.001), indicating that TMS was associated with significantly better improvement in upper limb function than tDCS (Figure 3). Due to the limited number of studies, other additional analysis was not possible.
FIGURE 3.
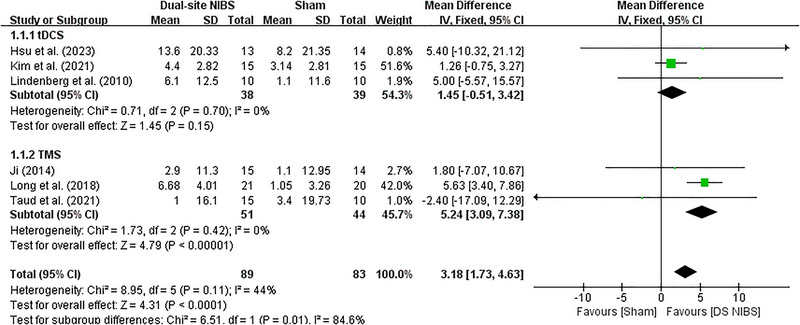
Forest plot of DS‐NIBS versus Sham‐NIBS. Results of subgroup analysis comparing the FMA‐UL of DS‐NIBS versus Sham‐NIBS in treating upper extremity motor function after stroke. FMA‐UL: Fugl‐Meyer Assessment upper limb; DS‐NIBS: dual‐site non‐invasive brain stimulation; SS‐NIBS: single‐site non‐invasive brain stimulation.
3.5. Publication Bias
Figure 4 describes the total risk of bias in the 10 studies.
FIGURE 4.
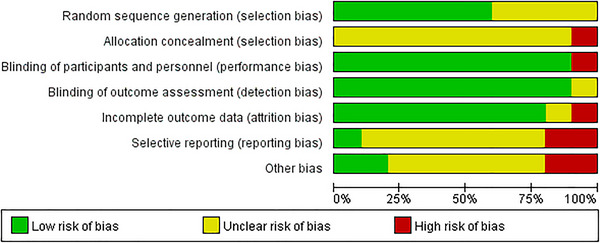
Results of the risk of bias analysis.
4. Discussion
The main purpose of this meta‐analysis was to analyze and summarize the current scientific literature in order to assess the efficacy of dual‐site NIBS on the upper limb motor impairments in post‐stroke patients. Our results show that dual‐site NIBS can yield improved upper limb function compared with sham and single‐site NIBS. This is the first meta‐analysis to report improvements in limb motor with DS‐rTMS compared to placebo stimulation, while no significant improvement was found with DS‐tDCS, suggesting DS‐rTMS may be superior to DS‐tDCS in improving upper limb motor after stroke.
Upper extremity motor impairment is a significant challenge in rehabilitation treatment after stroke. NIBS techniques are widely used to improve deficits following neuronal damage and have been reported to be successful in a proportion of treated patients (Davis and Koningsbruggen 2013). However, the choice of stimulation parameters and targets determines the effect of acupuncture therapy to a large extent. Some studies demonstrated high efficacy of dual‐site NIBS compared to single‐site NIBS (Achacheluee et al. 2018; Cho et al. 2017). As shown in Figures 2 and 3, our trial has yielded robust and consistent findings that support the benefits of dual‐site NIBS despite differences in NIBS types, post‐stroke duration, and treatment sessions.
One study (Taud et al. 2021) found no significant difference between DS‐tDCS and SS‐tDCS in facilitating recovery of upper extremity function. This result should be interpreted cautiously due to the small sample size and high inter‐individual variance in baseline motor function, lesion site, location and extent, time since stroke, age, and so on. Another study (Fleming et al. 2017) showed significant improvements in JTT performance after anodal or cathodal tDCS but not after bihemispheric stimulation. The reason why bihemispheric tDCS was ineffective is unknown. It might be due to differences in the structures that be stimulated and the changes in connectivity between brain regions relative to the unilateral arrangements. A meta‐analysis found that the linear response did not necessarily exist between session and tDCS effect; the effect of tDCS ≤ 10 sessions on upper limb function recovery in stroke patients was significantly higher than that of other sessions both with anode and cathode stimulation (Bai et al. 2019).
It is worth considering that all included studies in this meta‐analysis employ neuromodulation based on the inter‐hemispheric inhibitory competition model. The interhemispheric competition mechanism shows a reciprocal inhibition of the neural activity between bilateral hemispheres in a healthy brain, which is realized by the transcallosal fibers. After a unilateral stroke, the balance between the bilateral hemispheres of patients is broken, resulting in excessive excitation of the unaffected hemisphere and increased inhibition of the affected hemisphere (Bertolucci, Chisari, and Fregni 2018). The subsequent recovery is related to the connection of the brain network between the two hemispheres (Swayne et al. 2008). Therefore, rebalance of the brain is the key for the recovery of function (Tang et al. 2015). Bilateral NIBS is more conducive to achieving this balance. Nine of the ten included studies activate the affected hemisphere's M1 and suppress the unaffected hemisphere's M1, thereby correcting imbalanced interhemispheric competition. Only one study (Cao et al. 2022) considers the possible role of cerebella and activates the contralateral cerebellar cortex and iM1 (nonsimultaneous stimulation of contralateral cerebellar cortex before iM1). Some researchers found the interhemispheric imbalance resulting from stroke is time‐dependent, increasing in the early weeks after stroke. Sasaki et al. (Sasaki, Kakuda, and Abo 2014) compared a combined protocol of 10‐Hz ipsilesional rTMS and 1‐Hz contralesional rTMS with a single 10‐Hz ipsilesional rTMS in 58 patients (< 15 days poststroke). They found that the bilateral rTMS group showed significantly greater improvement in the Bruunstrom Recovery Stage than the 10‐Hz rTMS group. A meta‐analysis showed that both theta burst stimulation (TBS) and rTMS were found to be significantly more effective in the acute phase of stroke, but TBS was more effective than rTMS. However, rTMS was found to be more effective than TBS stimulation in patients in the subacute and chronic phases of stroke (Chen et al. 2022).
This meta‐analysis could not perform sub‐analyses to investigate effects of different post‐stroke duration and different stimulation parameters due to the limited number of studies and variability in stimulation parameters that have been reported, thus limiting our understanding of the positive changes in upper limb motor function promoted by DS‐NIBS.
In addition to the competition mechanism, a vicariation model and a bimodal balance‐recovery model have been proposed. The vicariation model suggests that the over‐activation of the contralesional hemisphere may be not a maladaptive but rather a vicarious mechanism through which the non‐lesioned hemisphere compensates for the affected one's functional and structural damage. The bimodal balance‐recovery model suggests that the mechanisms at the basis of an improvement after the lesion might change according to the amount of damage (Chen et al. 2023). The question as to whether the two hemispheres are in an inhibitory or facilitatory relationship and to what extent one mechanism takes over the other is still an open matter. However, there has been no research on DS‐NIBS for upper limb function impairment after stroke based on the other two models.
4.1. Limitations
Despite great efforts to minimize methodology differences across the selected studies, heterogeneity is yet unavoidable due to the large variability in the characteristics of patients across studies (chronic or acute, ischemic or hemorrhagic, cortical or subcortical lesion) and the lack of a standardized intervention protocol, making it difficult to reach a definite conclusion. Further, limited research on DS‐NIBS in the treatment of post‐stroke upper limb function impairment restricted further analysis.
5. Conclusion
DS‐NIBS showed a relatively higher effect than the sham and SS‐NIBS. In addition, DS‐rTMS demonstrated better therapeutic effects compared to DS‐tDCS on upper limb function impairment after stroke.
Author Contributions
Meng Ren: writing–original draft, writing–review and editing, conceptualization. Jingjing Xu: writing–original draft, conceptualization. Wenjing Wang: software, visualization. Lexian Shen: writing–review & editing. Chaojie Wang: methodology, visualization. Haoyang Liu: data curation. Lu Chen: data curation. Chanjing Liu: data curation. Yongheng Tang: validation. Tiantian Liu: resources. Jiening Wang: resources.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.70145.
Supporting information
Supporting Information
Funding: This work was supported by the Construction of the National Integrated Traditional Chinese Medicine Development Reform Experimental Zone in Pudong New Area, Shanghai ‐ Li Diangui National Master of Traditional Chinese Medicine Studio [grant numbers PDZY‐2023‐0701]; Shanghai University of Traditional Chinese Medicine 2023 Annual “Outstanding PhD Students Cultivation Project” [grant number GJ2023020]; The funders of the study played no role in study design, data collection, data analysis, or writing of the manuscript.
Meng Ren and Jingjing Xu made equal contributions to this study.
Contributor Information
Jiening Wang, Email: drwjn0606@sina.com.
Tiantian Liu, Email: tiantianliu66@163.com.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Achacheluee, S. T. , Rahnama L., Karimi N., Abdollahi I., Arslan S. A., and Jaberzadeh S.. 2018. “The Effect of Unihemispheric Concurrent Dual‐Site Transcranial Direct Current Stimulation of Primary Motor and Dorsolateral Prefrontal Cortices on Motor Function in Patients With Sub‐acute Stroke.” Frontiers in Human Neuroscience 12: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoiu, A. , Mitran S. I., Catalin B., et al. 2023. “From Molecule to Patient Rehabilitation: The Impact of Transcranial Direct Current Stimulation and Magnetic Stimulation on Stroke—A Narrative Review.” Neural Plasticity 2023: 5044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, X. , Guo Z., He L., Ren L., McClure M. A., and Mu Q.. 2019. “Different Therapeutic Effects of Transcranial Direct Current Stimulation on Upper and Lower Limb Recovery of Stroke Patients With Motor Dysfunction: A Meta‐Analysis.” Neural Plasticity 2019: 1372138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Z. , Zhang J., and Fong K. N. K.. 2022. “Effects of Transcranial Magnetic Stimulation in Modulating Cortical Excitability in Patients With Stroke: A Systematic Review and Meta‐Analysis.” Journal of NeuroEngineering and Rehabilitation 19: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniqued, P. D. E. , Stanyer E. C., Awais M., et al. 2021. “Brain‐Computer Interface Robotics for Hand Rehabilitation After Stroke: A Systematic Review.” Journal of Neuroengineering and Rehabilitation 18: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca, S. , Wolf S. L., Fasoli S., and Bohannon R.. 2003. “Treatment Interventions for the Paretic Upper Limb of Stroke Survivors: A Critical Review.” Neurorehabilitation and Neural Repair 17: 220–226. [DOI] [PubMed] [Google Scholar]
- Bertolucci, F. , Chisari C., and Fregni F.. 2018. “The Potential Dual Role of Transcallosal Inhibition in Post‐Stroke Motor Recovery.” Restorative Neurology and Neuroscience 36: 83–97. [DOI] [PubMed] [Google Scholar]
- Boddington, L. J. , and Reynolds J. N. J.. 2017. “Targeting Interhemispheric Inhibition With Neuromodulation to Enhance Stroke Rehabilitation.” Brain Stimulation 10: 214–222. [DOI] [PubMed] [Google Scholar]
- Brunt, A. , Albines D., and Hopkins‐Rosseel D.. 2019. “The Effectiveness of Exercise on Cognitive Performance in Individuals With Known Vascular Disease: A Systematic Review.” Journal of Clinical Medicine 8: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglayan, A. B. , Beker M. C., Caglayan B., et al. 2019. “Acute and Post‐Acute Neuromodulation Induces Stroke Recovery by Promoting Survival Signaling, Neurogenesis, and Pyramidal Tract Plasticity.” Frontiers in Cellular Neuroscience 13: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Q. 2020. Effect of Repetitive Transcranial Magnetic Stimulation on Upper Limb Motor Function in Patients With Cerebral Infarction. Southeast University. [Google Scholar]
- Cao, Z. , Feng H., Li Y., et al. 2022. “Effects of Multi‐Target iTBS Between Cerebral Hemispheres on Upper Limb Function in Stroke Patients.” Chinese Journal of Rehabilitation Theory and Practice 28: 502–507. [Google Scholar]
- Chen, G. , Lin T., Wu M., et al. 2022. “Effects of Repetitive Transcranial Magnetic Stimulation on Upper‐Limb and Finger Function in Stroke Patients: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials.” Frontiers in Neurology 13: 940467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Zhang X., Chen X., et al. 2023. “The Assessment of Interhemispheric Imbalance Using Functional Near‐Infrared Spectroscopic and Transcranial Magnetic Stimulation for Predicting Motor Outcome After Stroke.” Frontiers in Neuroscience 17: 1231693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, S. , Xin R., Zhao Y., Wang P., Feng W., and Liu P.. 2023. “Evaluation of fMRI Activation in Post‐Stroke Patients With Movement Disorders After Repetitive Transcranial Magnetic Stimulation: A Scoping Review.” Frontiers in Neurology 14: 1192545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, J. Y. , Lee A., Kim M. S., et al. 2017. “Dual‐Mode Noninvasive Brain Stimulation Over the Bilateral Primary Motor Cortices in Stroke Patients.” Restorative Neurology and Neuroscience 35: 105–114. [DOI] [PubMed] [Google Scholar]
- Collaborators, G. S. 2021. “Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019.” The Lancet Neurology 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer, S. C. 2008. “Various Patterns of Neural Reorganization Occur After Stroke.” Annals of Neurology 63: 272–287. [DOI] [PubMed] [Google Scholar]
- Davis, N. J. , and Koningsbruggen M. G.v. 2013. ““Non‐Invasive” Brain Stimulation Is Not Non‐Invasive.” Frontiers in Systems Neuroscience 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, Y.‐J. , Hua X.‐Y., Zheng M.‐X., et al. 2022. “Corticocortical Paired Associative Stimulation for Treating Motor Dysfunction After Stroke: Study Protocol for a Randomised Sham‐Controlled Double‐Blind Clinical Trial.” Bmj Open 12: e053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, J. D. , Black S. E., Boe S., et al. 2021. “Canadian Platform for Trials in Noninvasive Brain Stimulation (CanStim) Consensus Recommendations for Repetitive Transcranial Magnetic Stimulation in Upper Extremity Motor Stroke Rehabilitation Trials.” Neurorehabilitation and Neural Repair 35: 103–116. [DOI] [PubMed] [Google Scholar]
- Ferreira, S. A. , Pinto N., Serrenho I., Pato M. V., and Baltazar G.. 2024. “Contribution of Glial Cells to the Neuroprotective Effects Triggered by Repetitive Magnetic Stimulation: A Systematic Review.” Neural Regeneration Research 19: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertonani, A. , and Miniussi C.. 2017. “Transcranial Electrical Stimulation: What We Know and Do Not Know About Mechanisms.” The Neuroscientist 23: 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming, M. K. , Rothwell J. C., Sztriha L., Teo J. T., and Newham D. J.. 2017. “The Effect of Transcranial Direct Current Stimulation on Motor Sequence Learning and Upper Limb Function After Stroke.” Clinical Neurophysiology 128, no. 7: 1389–1398. [DOI] [PubMed] [Google Scholar]
- Foley, N. C. , Teasell R. W., Bhogal S. K., and Speechley M. R.. 2003. “Stroke Rehabilitation Evidence‐Based Review: Methodology.” Topics in Stroke Rehabilitation 10: 1–7. [PubMed] [Google Scholar]
- Gittler, M. , and Davis A. M.. 2018. “Guidelines for Adult Stroke Rehabilitation and Recovery.” Jama 319: 820–821. [DOI] [PubMed] [Google Scholar]
- Hsu, S. P. , Lu C. F., Tang C. W., et al. 2021. “Dual Transcranial Direct Current Stimulation for Subacute Stroke Patients With Compromised Corticospinal Integrity: A Randomized, Double‐Blind, Sham‐Controlled Study.” Brain Stimulation 14: 1702. [Google Scholar]
- Ji, A. 2014. Effect of Repeated Transcranial Magnetic Stimulation on Motor Function and MEP in Stroke Patients. Jinan, China: Shandong University. [Google Scholar]
- Khedr, E. M. , Shawky O. A., El‐Hammady D. H., et al. 2013. “Effect of Anodal versus Cathodal Transcranial Direct Current Stimulation on Stroke Rehabilitation: A Pilot Randomized Controlled Trial.” Neurorehabilitation and Neural Repair 27: 592–601. [DOI] [PubMed] [Google Scholar]
- Kim, S. H. 2021. “Effects of Dual Transcranial Direct Current Stimulation and Modified Constraint‐Induced Movement Therapy to Improve Upper‐Limb Function After Stroke: A Double‐Blinded, Pilot Randomized Controlled Trial.” Journal of Stroke and Cerebrovascular Diseases 30: 105928. [DOI] [PubMed] [Google Scholar]
- Klomjai, W. , Aneksan B., Chotik‐Anuchit S., et al. 2022. “Effects of Different Montages of Transcranial Direct Current Stimulation on Haemodynamic Responses and Motor Performance in Acute Stroke: A Randomized Controlled Trial.” Journal of Rehabilitation Medicine 54: jrm00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubis, N. 2016. “Non‐Invasive Brain Stimulation to Enhance Post‐Stroke Recovery.” Frontiers in Neural Circuits 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne, P. , Bernhardt J., and Kwakkel G.. 2011. “Stroke Rehabilitation.” Lancet 377: 1693–1702. [DOI] [PubMed] [Google Scholar]
- Lawrence, E. S. , Coshall C., Dundas R., et al. 2001. “Estimates of the Prevalence of Acute Stroke Impairments and Disability in a Multiethnic Population.” Stroke; A Journal of Cerebral Circulation 32: 1279–1284. [DOI] [PubMed] [Google Scholar]
- Lei, R. , Wang S., Liu A., et al. 2023. “Bilateral Transcranial Direct‐Current Stimulation Promotes Migration of Subventricular Zone‐Derived Neuroblasts Toward Ischemic Brain.” FASEB BioAdvances 5: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K.‐P. , Wu J.‐J., Zhou Z.‐L., et al. 2023. “Noninvasive Brain Stimulation for Neurorehabilitation in Post‐Stroke Patients.” Brain Sciences 13: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg, R. , Renga V., Zhu L. L., Nair D., and Schlaug G.. 2010. “Bihemispheric Brain Stimulation Facilitates Motor Recovery in Chronic Stroke Patients.” Neurology 75: 2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, H. , Wang H., Zhao C., et al. 2018. “Effects of Combining High‐ and Low‐frequency Repetitive Transcranial Magnetic Stimulation on Upper Limb Hemiparesis in the Early Phase of Stroke.” Restorative Neurology and Neuroscience 36: 21–30. [DOI] [PubMed] [Google Scholar]
- Min, K.‐B. , and Min J.‐Y.. 2015. “Health‐Related Quality of Life Is Associated With Stroke Deficits in Older Adults.” Age and Ageing 44: 700–704. [DOI] [PubMed] [Google Scholar]
- Owolabi, M. O. , Thrift A. G., Mahal A., et al. 2022. “Primary Stroke Prevention Worldwide: Translating Evidence Into Action.” The Lancet Public Health 7: e74–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzotti‐Jametti, L. , Cambiaghi M., Bacigaluppi M., et al. 2013. “Safety and Efficacy of Transcranial Direct Current Stimulation in Acute Experimental Ischemic.” Stroke 44: 3166–3174. [DOI] [PubMed] [Google Scholar]
- Pino, G. D. , Pellegrino G., Assenza G., et al. 2014. “Modulation of Brain Plasticity in Stroke: A Novel Model for Neurorehabilitation.” Nature Reviews Neurology 10: 597–608. [DOI] [PubMed] [Google Scholar]
- Ren, P. , Wang X., Fan Y., and Tan J.. 2018. “Efficacy of Low Frequency Combined With High Frequency Transcranial Magnetic Therapy for Upper Limb Motor Dysfunction After Stroke.” ACTA MEDICINAE SINICA 31: 114–116. [Google Scholar]
- Rosso, C. , Moulton E. J., Kemlin C., et al. 2022. “Cerebello‐Motor Paired Associative Stimulation and Motor Recovery in Stroke: A Randomized, Sham‐Controlled.” Double‐Blind Pilot Trial Neurotherapeutics 19: 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, N. , Kakuda W., and Abo M.. 2014. “Bilateral High‐ and Low‐Frequency rTMS in Acute Stroke Patients With Hemiparesis: A Comparative Study With Unilateral High‐Frequency rTMS.” Brain Injury 28: 1682–1686. [DOI] [PubMed] [Google Scholar]
- Shepherd, J. D. , and Huganir R. L.. 2007. “The Cell Biology of Synaptic Plasticity: AMPA Receptor Trafficking.” Annual Review of Cell and Developmental Biology 23: 613–643. [DOI] [PubMed] [Google Scholar]
- Sinke, M. R. , Otte W. M., Meer M. P.v., Toorn A.v.d., and Dijkhuizen R. M.. 2018. “Modified Structural Network Backbone in the Contralesional Hemisphere Chronically After Stroke in Rat Brain.” Journal of Cerebral Blood Flow and Metabolism 38: 1642–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear, C. M. , Lang C. E., Zeiler S., and Byblow W. D.. 2020. “Advances and Challenges in Stroke Rehabilitation.” Lancet Neurology 19: 348–360. [DOI] [PubMed] [Google Scholar]
- Swayne, O. B. C. , Rothwell J. C., Ward N. S., and Greenwood R. J.. 2008. “Stages of Motor Output Reorganization After Hemispheric Stroke Suggested by Longitudinal Studies of Cortical Physiology.” Cerebral Cortex 18: 1909–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Q. , Li G., Liu T., et al. 2015. “Modulation of Interhemispheric Activation Balance in Motor‐Related Areas of Stroke Patients With Motor Recovery: Systematic Review and Meta‐Analysis of fMRI Studies.” Neuroscience and Biobehavioral Reviews 57: 392–400. [DOI] [PubMed] [Google Scholar]
- Taud, B. , Lindenberg R., Darkow R., et al. 2021. “Limited Add‐On Effects of Unilateral and Bilateral Transcranial Direct Current Stimulation on Visuo‐Motor Grip Force Tracking Task Training Outcome in Chronic Stroke. A Randomized Controlled Trial.” Frontiers in Neurology 12: 736075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldema, J. , and Gharabaghi A.. 2022. “Non‐Invasive Brain Stimulation for Improving Gait, Balance, and Lower Limbs Motor Function in Stroke.” Journal of NeuroEngineering and Rehabilitation 19: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal, A. C. , Banca P., Pascoal A. G., et al. 2018. “Bilateral Versus Ipsilesional Cortico‐Subcortical Activity Patterns in Stroke Show Hemispheric Dependence.” International Journal of Stroke 12, no. 1: 1747493018767164. [DOI] [PubMed] [Google Scholar]
- Vlisides, P. , and Mashour G. A.. 2016. “Perioperative Stroke.” Canadian Journal of Anaesthesia 63: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz, L. J. , Rehme A. K., Michely J., et al. 2016. “Shaping Early Reorganization of Neural Networks Promotes Motor Function After Stroke.” Cerebral Cortex 26: 2882–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, P.‐L. , Yang Y.‐R., Tang S.‐C., Huang S.‐F., and Wang R.‐Y.. 2022. “Comparing Different Montages of Transcranial Direct Current Stimulation on Dual‐Task Walking and Cortical Activity in Chronic Stroke: Double‐Blinded Randomized Controlled Trial.” BMC Neurology 22: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri, C. , Zennou‐Azogui Y., Sadlaoud K., and Sauvajon D.. 2014. “Interplay Between Intra‐ and Interhemispheric Remodeling of Neural Networks as a Substrate of Functional Recovery After Stroke: Adaptive Versus Maladaptive Reorganization.” Neuroscience 283: 178–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


