Abstract
Determining the factors influencing habitat selection and hunting success in top predators is crucial for understanding how these species may respond to environmental changes. For marine top predators, such factors have been documented in pelagic foragers, with habitat use and hunting success being linked to chlorophyll-a concentrations, sea surface temperature and light conditions. In contrast, little is known about the determinants of benthic marine predators. The Australian fur seal (Arctocephalus pusillus doriferus) is a benthic-diving forager that has a breeding and foraging distribution largely restricted to Bass Strait, the shallow (max. depth 80 m) continental shelf region between the Australian mainland and Tasmania. The species forages mostly on benthic prey and represents the greatest resident marine predator biomass in south-eastern Australia. The region is also one of the world’s fastest-warming marine areas and oceanographic changes are influencing shifts in prey distribution and abundance. In the present study, GPS-derived locations of benthic dives (n = 288,449) and dive behaviour metrics were used to determine seafloor habitat selection and factors influencing hunting success in 113 lactating adult females from Kanowna Island during the winters of 2006–2021. Individuals non-randomly selected foraging habitats comprised of deeper, steeper sloped, muddy-sandy areas with less gravel and highly disturbed regions (P < 0.01). Hunting success was greatest in shallower rocky reefs (< 30 m) and deep areas (> 40 m) characterised by moderate presence of gravel (25–50%) and substantial rock composition (50–75%) on the seabed. These findings suggest that habitat use and hunting success in adult female Australian fur seals could be impacted by predicted oceanographic changes, such as rising temperature, altered currents and waves which may modify seafloor characteristics and benthic communities.
Keywords: Habitat selection, Hunting success, Australian fur seals
Subject terms: Ecology, Animal migration, Behavioural ecology, Conservation biology, Population dynamics
Introduction
Habitat selection is the active process through which organisms select a potentially appropriate area determined by a combination of genetic and behavioural factors, guided by specific environmental cues1,2. This process is critical for understanding the complex behavioural and environmental conditions that influence the survival and fitness of individuals and is a central focus in ecology3,4. Some common features influencing the selection of a suitable habitat include the sex and age of the individuals5, the availability and abundance of food6, prey catchability7, provision of shelter, protection against predators, and other characteristics that facilitate the locomotion of individuals2. There are other less-studied factors that can also impact habitat selection, and unravelling their influence is equally important for ecologists.
Given the low probability of all necessary resources for an organism being uniformly distributed in space, the limiting resource required for each activity will be the driving force in habitat selection8,9. Consequently, habitat selection will depend on habitat features necessary to carry out the specific activity at a given moment. For instance, the short-term decision to choose a foraging habitat depends on balancing an animal’s activity budget while managing time and energy constraints, which are further shaped by factors such as physiological capacity, phenology, predation risk, competition, and the spatiotemporal distribution of food resources10. All of these factors ultimately influence foraging success11. In contrast, the long-term selection of a new breeding site depends on the essential reproductive components required for successful breeding e.g. finding a suitable partner and low interspecific competition12.
The selection of a suitable foraging habitat involves choosing an optimal region to ensure a high likelihood of foraging success. Hunting success is fundamental in predators survival and has also been correlated with reproductive success13. The determinants influencing the selection of optimal foraging areas differ between trophic levels and environment types14. For top predators, foraging areas with high hunting success have been related directly or indirectly to prey abundance, availability and reduced interspecific and intraspecific competition14. Conversely, the key determinant for prey is to occupy areas that include habitat features that comprise predator search rate and/or capture efficiency14.
Studies on habitat selection and hunting success in terrestrial predators have been facilitated by the ability to directly observe prey consumption and its relationship to environmental parameters. These direct observations have revealed that factors such as air temperature, vegetation structure, light, moon phases, and wind speed influence habitat selection and optimise hunting success in predators15. For example, direct observations have shown that long grass and dense shrubs benefit the selection of foraging areas and improve the hunting success of lion Panthera leo15. In marine environments, studies have relied on indirect observations based on tracking technologies16–18, which have shown consistent links to some environmental factors that are proxies for prey availability, and these factors can differ between pelagic and benthic predators. For pelagic predators, key factors that influence habitat selection and optimise hunting success include sea-surface chlorophyll-a concentration, sea surface temperature, and light conditions16–18. In contrast, benthic predators are affected by different factors such as seafloor sediment composition, turbidity, and bathymetry19–21. These environmental factors are expected to undergo significant changes due to climate change, with stronger wind-induced currents and wave activity likely increasing seabed sediment erosion and mobility22,23 and rising temperature affecting prey distribution24–27. Such alterations can modify species distribution which effects can scale up to top predators, potentially impacting their selection of foraging areas and success28. Therefore, identifying the environmental predictors that best explain patterns of hunting success and marine habitat use is important for understanding the role of these species within the ecosystem and predicting their response to anticipated environmental changes.
The Australian fur seal (Arctocephalus pusillus doriferus; AUFS) is a primarily benthic-diving forager comprising the greatest resident marine predator biomass in south-eastern Australia21,29–31. The species is a generalist predator, consuming a large array of prey(> 70 taxa32,33), that are predominantly benthic, with some pelagic species, and exhibits seasonal dietary variation that correlates with prey availability and its reproductive cycle32,34,35. Foraging occurs primarily on the sea floor, where benthic/demersal species such as red cod (Pseudophycis bachus), leatherjackets spp. (Family Monacanthidae), barracouta (Thyrsites atun) and octopus (e.g. pale octopus Octopus berrima/pallidus and maori octopus Octopus maoruma) are targeted during the summer. In contrast, coastal pelagic species frequently occurred in the winter diet with items like flat imperator (Beryx decadactylus), jack mackerel (Trachurus sp) and ocean jacket (Nelusetta ayraudi33,36,37). Preliminary studies on habitat selection and hunting success on AUFS have shown correlations with sea surface temperature and chlorophyll-a21,38, factors expected to be modified by climate change.
The breeding and foraging distribution of AUFS is largely restricted to Bass Strait, the shallow (maximum depth 80 m) continental shelf region between the Australian mainland and Tasmania39,40, which is located near their main colonies. Females foraging behaviour is limited to the nutrient-poor waters and low marine primary productivity of the Bass Strait region41,42, due to the need to frequently return to provision their offspring18,19. This region is also one of the fastest-warming oceanic areas in the world43,44, with anticipated oceanographic changes, such as the strengthening of wind-induced currents and wave activity22, expected to modify the distribution, diversity and abundance of species24–27. These changes are likely to impact the foraging habitat of the AUFS21, as shifting currents may alter turbidity and seafloor sediment composition22,23, affecting the benthic environment they feed on. While the effect of some environmental variables on habitat selection and hunting success has been documented, there is currently limited information on how seafloor factors influence the selection of optimal benthic foraging grounds in AUFS, despite benthic dives being the predominant foraging behaviour in this species21. This knowledge gap makes it challenging to predict the impact of anticipated oceanographic changes on their primary benthic and demersal foraging habitat.
Previous studies have suggested that seafloor features are crucial in shaping foraging behaviour in top predators19,45 and are, therefore, expected to have a significant influence on AUFS. Given the potential impact of climate change on these factors, the present study aimed to determine the benthic environmental factors influencing: (i) habitat selection; and (ii) hunting success in adult female Australian fur seals provisioning pups. This information was subsequently used to predict optimal foraging areas and assess how future environmental conditions might influence hunting success in AUFS.
Results
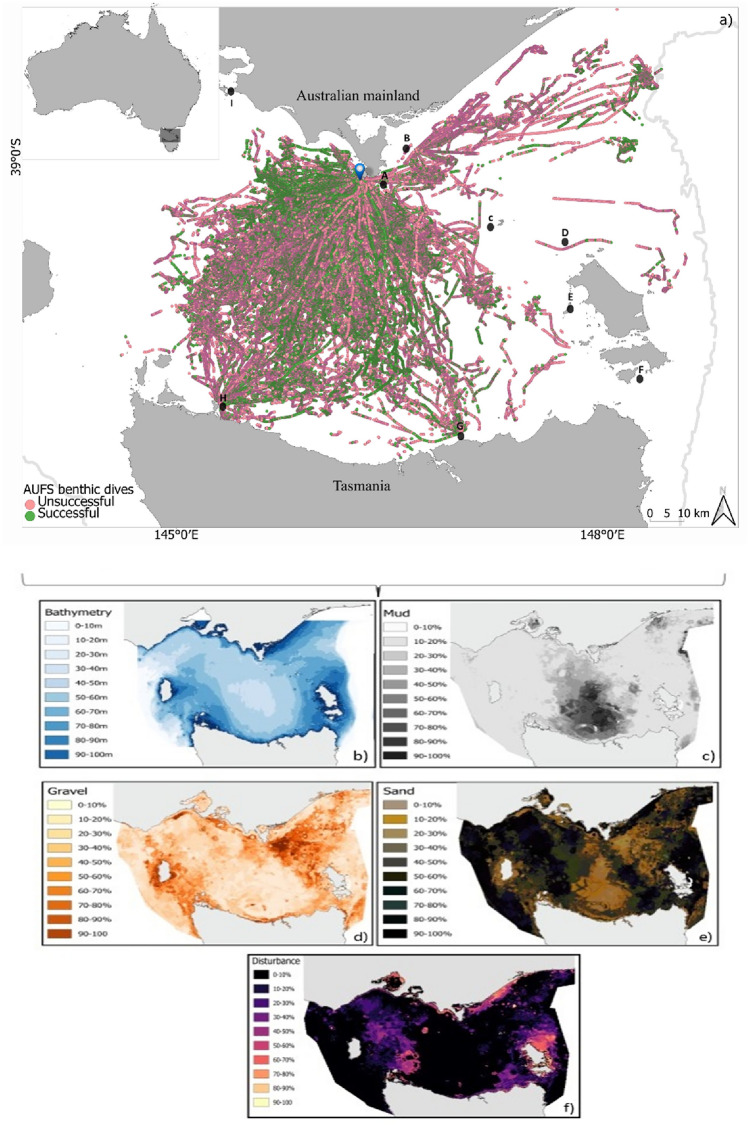
At-sea movements and diving behaviour data were collected from 113 adult females at the breeding colony of Kanowna Island. The number of deployments each year varied from 2 to 16 individuals between 2006 and 2021, resulting in a total of 288,449 dives recorded during multiple foraging trips per individual (Table 1; A2). Females predominantly travelled throughout the central Bass Strait, where approximately 90% of all dives occurred (Fig. 1). However, some individuals foraged near the Tasmania coast and several others travelled northeast of Kanowna Island (Fig. 1).
Table 1.
Summary of the deployments of Australian fur seal females at Kanowna Island from 2006 to 2021.
| Year | Seals (n) | Benthic dives (n) | Depth range (m) | Successful dives (%) |
|---|---|---|---|---|
| 2006 | 2 | 4941 | 40.5–83.5 | 44.3 |
| 2007 | 2 | 3291 | 40.5–86 | 16.5 |
| 2008 | 9 | 8868 | 40.5–89 | 31.4 |
| 2009 | 14 | 24,414 | 4.5–124.5 | 38.0 |
| 2010 | 4 | 3138 | 20.5–83 | 32.1 |
| 2011 | 8 | 12,490 | 4.5–95 | 36.7 |
| 2012 | 14 | 11,961 | 5.5–86.5 | 43.0 |
| 2013 | 7 | 24,172 | 5.5–89.5 | 56.6 |
| 2014 | 4 | 14,029 | 20.5–87.5 | 50.4 |
| 2015 | 3 | 4269 | 30.5–83.5 | 34.4 |
| 2016 | 3 | 4285 | 40.5–87 | 17.93 |
| 2017 | 8 | 12,872 | 40.5–86.5 | 37.8 |
| 2018 | 6 | 12,206 | 20.5–86.5 | 25.5 |
| 2019 | 7 | 56,413 | 40.5–93.5 | 59.3 |
| 2020 | 16 | 61,641 | 4.5–89.5 | 47.88 |
| 2021 | 6 | 29,459 | 40.5–87.5 | 51.5 |
Fig. 1.
The foraging areas of adult female Australian fur seals from Kanowna Island (blue location marker) in Bass Strait, southeastern Australia, with the locations of successful (green) and unsuccessful (pink) benthic dives (a) and the most significant environmental variables in their foraging habitat selection (b-f). Australian fur seal colonies are indicated by black location markers: A: West Moncoeur; B: Rag Island; C: Judgments Rocks; D: Wright Rocks; E: Double Island; F: Moriarty Rocks; G: Tenth Rock; H: Bull Rock; I: Seal Rock. The grey line represents the continental shelf, isobath 200 m.
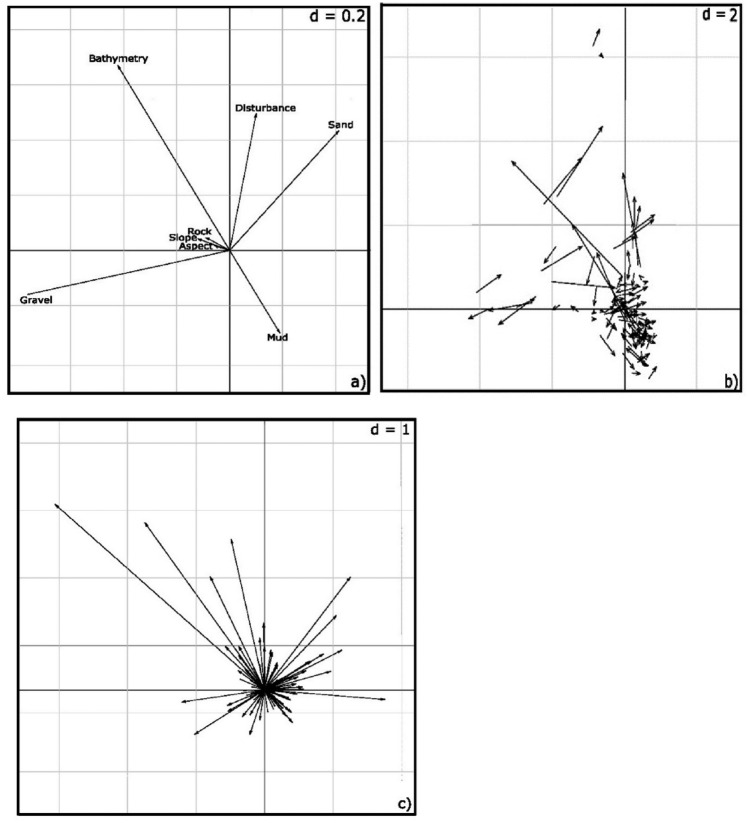
The K-select analysis resulted in the first two axes accounting for 73% of the marginality of individuals. The randomization test conducted on the marginality vectors revealed habitat selection was significantly non-random for all the animals (P < 0.05, Fig. 2a). Individuals selected foraging habitats comprised of deeper, steeper sloped, muddy-sandy areas with less gravel and highly disturbed seafloor (P < 0.001; Fig. 2b). Some females exhibited similar preferences for habitat characteristics by selecting deeper areas or regions with greater mud content or choosing locations with increased bottom floor disturbance and sandy substrates. However, the females had no distinct preference between rocky or sandy seabed structure (Fig. 2c).
Fig. 2.
Habitat selection by 113 adult female Australian fur seals from Kanowna Island. (a) variables loadings on the two first factorial axes (axis 1: x axis; axis 2: y axis) , (b) the marginality vectors of individuals on the first factorial plane, the end of the arrows correspond to the mean characteristics of the habitat on the relocation of individuals, and (c) the marginality vectors of individuals after re-centering each home range composition which is axis 1 and axis 2.
The dive classification indicated that 47% (n = 136,080) were successful. Out of the 63 models fitted in the FSSgam test, the most parsimonious model identified bathymetry, gravel, and rock as the most influential variables in determining hunting success. These factors collectively explained the highest variation in the model subset, suggesting AUFS exhibit greater success in benthic dives when encountering these habitat features. The GAMM outcomes revealed that hunting success in AUFS was greatest in shallower waters (< 30 m; Fig. 3a) and deep areas (> 40 m), characterised by the moderate presence of gravel (25–50%; Fig. 3b) and substantial rock composition (50–75%) on the seafloor (Fig. 3c; P < 0.001). The spatial prediction derived from the GAMM predictors indicated that the central and northeast regions of Bass Strait have the most conducive habitat conditions for hunting success for AUFS (Fig. 4).
Fig. 3.
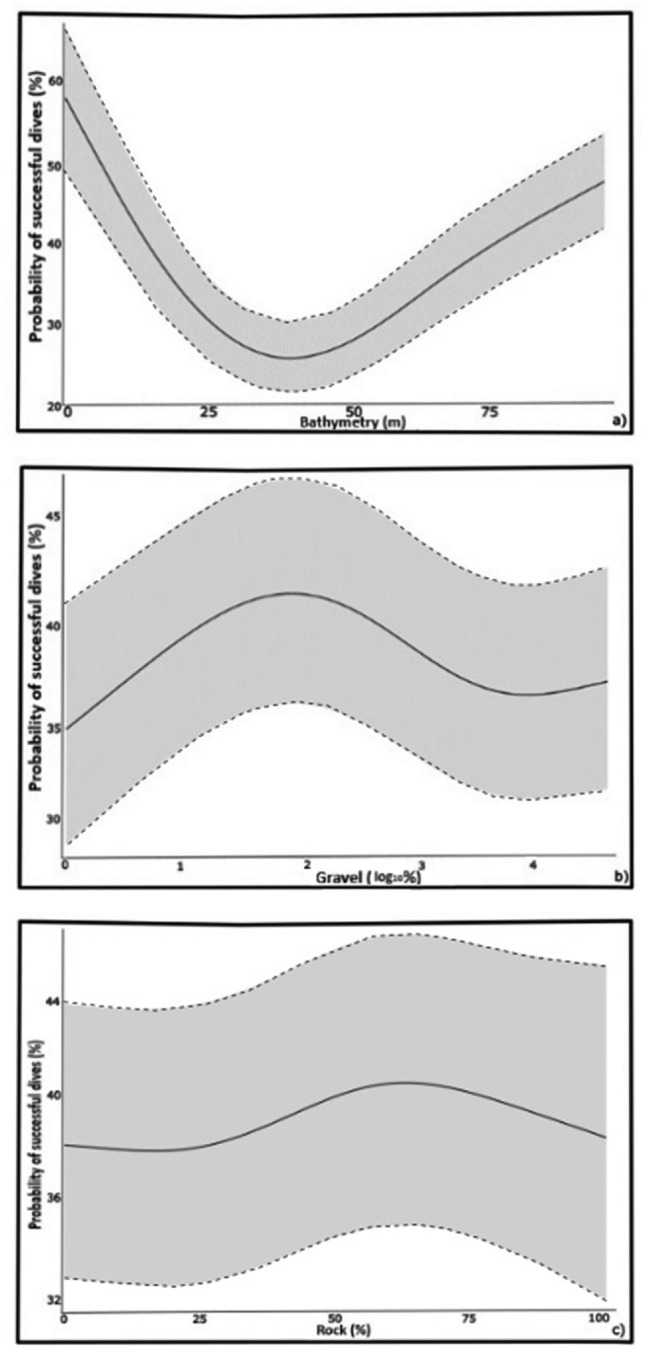
Relationship between the benthic environmental variables of bathymetry (a), gravel cover (log10%; b) and rock (c) and hunting success in 113 adult female Australian fur seals.
Fig. 4.
Spatial prediction from a general additive mixed effect model of successful foraging areas for adult female Australian fur seals from Kanowna Island. Darker shades of orange indicate areas of predicted greater hunting success based on the important variables derived from the model.
Discussion
Habitat selection by predators is a crucial process in shaping ecological communities and maintaining ecosystem function46. The selection of a foraging habitat influences hunting success and, ultimately, individual survival47,48. Understanding the factors affecting both parameters in top predators is essential for predicting their responses to environmental changes. The present study investigated how benthic habitat features impact habitat selection and hunting success in adult female AUFS provisioning pups. The results reveal that female AUFS exhibited a non-random selection of foraging areas, with preferences for deeper locations, where they were also more successful. Sediment composition played a key role in both habitat selection and hunting success, while seafloor disturbance only impacted the selection of foraging grounds. Given the expected changes in the hydrodynamic seabed morphology and sediment characteristics22,23 due to the strengthening of wind-induced currents and waves22,23 in southeastern Australia, shifts in the benthic habitat preferred by female AUFS are anticipated. These alterations could affect the availability and quality of their foraging habitats, potentially impacting their hunting success and leading to population-level consequences. To assess the population-level impact, further dedicated research across different sex and age classes is needed.
Habitat selection
The selection of foraging habitat by predators involves identifying areas that increase the likelihood of successful prey capture. However, individuals face different challenges when selecting a proper foraging area, balancing the trade-offs between food availability, competition, and predation risk while dealing with environmental constraints11,49. In the present study, habitat selection by adult female AUFS was significantly influenced by bathymetry, sediment composition and disturbance of the seafloor. These results highlight the importance of environmental variables in shaping the foraging behaviour of female AUFS, emphasising the need to understand how changes in these parameters could influence their habitat selection.
Consistent with previous studies on other top predators (e.g. penguins, seals19,45), seafloor sediment composition influenced the habitat selection of AUFS. Sediment type and structure (e.g., reefs, gravel outcrops, flat seabed) are known to influence the distribution and abundance of benthic fish50 and play a key role in shaping the benthic habitat of key prey species for AUFS21,29,33,51–53. Female AUFS preferred benthic habitats with similar sediment composition and hydrodynamics to those selected by other predators, such as the yellow-eyed penguin, (Megadyptes antipodes)19,54. For example, yellow-eyed penguins selected sand ripple areas54, likely shaped by strong seafloor currents55, where their primary benthic prey, opal fish (Hemerocoetes monopterygius) and blue cod (Parapercis colias) are present. The selection of muddy-sandy areas and a higher seafloor disturbance index by female AUFS may be linked to the high-energy environment of the Bass Strait, which supports rich benthic communities56 and hosts a largely endemic invertebrate and benthic fish fauna57. Although little is known about the relationship between the distribution of the benthic fish and cephalopods that AUFS consume32,33,58,59 and the sandy-muddy areas, these sediment could likely reflect the habitat preferences of their prey or indicate favourable conditions that facilitate prey capture by AUFS. Understanding how AUFS’ prey species relate to hydrodynamics and sediment composition would help elucidate the influence of these factors on habitat selection by female AUFS.
Bathymetry has previously been reported as an influential factor in the habitat selection of female AUFS38, as well as for other demersal predators (e.g., gray seals, Halichoerus grypus45, yellow-eyed penguins60), consistent with the findings in the present study. Although individuals preferred greater depths in the present study, the most successful hunting occurred in moderately deep areas near the breeding colony in the central Bass Strait, where depths range from 60 to 80 m. This behaviour is likely driven by their central-place foraging strategy, which constraints individuals to forage within a limited area as they must return regularly to provision their offspring61. In addition, the foraging behaviour of the individuals from Kanowna Island may also be restricted by the high intraspecific competition, especially given the proximity of other AUFS breeding colonies62,63, which increases pressure from conspecifics. For example, females from the Seal Rock breeding colony have been recorded foraging in the northeast and central Bass Strait, potentially competing for resources with AUFS from Kanowna Island, which could lead to exclusion or avoidance behaviour by Kanowna seals38. Alternatively, this could reflect the distribution of the preferred prey of AUFS64, which has been reported to increase in diversity with depth in Bass Strait56.
Factors influencing foraging habitat selection have often been linked to acquiring high-quality resources 12, while the role of these areas in minimising predation risk remains poorly understood10,61. Female AUFS may be selecting these deeper foraging grounds to avoid predators, restricting their activity to safer areas with fewer predators as detected in other species65. For example, Cape fur seals (A. p. pusillus) reduce their activity in deep open water in favour of areas with abundant refugia (e.g. kelp forest66) to avoid white sharks (Carcharodon carcharias). In addition, pinnipeds are known to be major prey items for white sharks, which aggregate near fur seal breeding colonies in the shallow waters of South Australia67,68 ). Although the distribution of this predator and the predation risk faced by AUFS in the Bass Strait remain unknown, the presence of white sharks may drive the seals to select deeper areas farther from their breeding colonies. Gaining insights into the distribution of AUFS predators and the role of habitat features in mitigating predation risk would enhance our understanding of AUFS habitat selection.
Hunting success
Seafloor sediment composition influenced hunting success in female AUFS. Individuals achieved higher hunting success rates in areas with greater rock composition and lower gravel concentrations. Both substrates could serve as orientation or navigational aids for seals, helping them to forage in areas with predictable prey, consistent with findings from other benthic-foraging top predators19,69,70. For instance, seafloor visual cues in yellow-eyed penguins reduce the randomness of their movement paths, guiding the individuals towards prey-rich areas and enhancing foraging efficiency70. Similarly, solid substrates may guide the benthic diving behaviour of AUFS, enabling the creation of memorised landscape maps that improve their hunting success. In addition, rocky substrates provide suitable habitats for AUFS’ key prey species, such as gurnards, leatherjackets71 and Octopus spp.51, facilitating foraging opportunities for the females that target these areas. While rocky regions enhance hunting success, gravel-rich areas can hinder AUFS’ ability to efficiently target and capture prey by reducing visibility and accessibility, making these habitats less ideal for seals for successful foraging successfully, despite their high species richness72. Understanding the importance of these habitats for the prey consumed by AUFS would be beneficial for comprehending the high foraging success associated with this type of seafloor sediment.
Hunting success was influenced by bathymetry, with success increasing in depths both at deeper regions (> 40 m) and at shallower depths (< 30 m). The increased success at greater depth may be attributed to the spatial and temporal predictability of their key prey items, supported by previous studies that have documented higher benthic species diversity with depth in Bass Strait28,43,56. Most foraging activity by individuals from Kanowna Island occurred within the central Bass Strait, where local bathymetry determined a maximum depth of around 60–80 m, suggesting bathymetry is more of a threshold predictor for foraging behaviour. A high probability of hunting success also occurred at shallower depths, such as near the coast of Tasmania and Flinders Island (40 S 148 03′ E), where rocky-reef habitats are prevalent73 and host a large abundance of demersal fish species71,74 that are known prey of AUFS37,59,75. In addition, these shallow waters near other breeding colonies (e.g. Wright Rocks, Double Island; Moriarty Rocks), offer suitable haul-out sites on land and may allow individuals from Kanowna Island to exploit profitable foraging grounds for longer periods61,76. However, despite the high success associated with these shallow benthic dives, they were observed in only 10 individuals and accounted for less than 1% of all dives. Such limited occurrence may reflect individual foraging specialisations in shallow rocky reef habitats, which is expected in benthic foragers77,78 and has been previously reported in AUFS79. Such specialised behaviour has been shown to enhance hunting success in generalist populations by reducing intraspecific competition, which may offer a buffer against environmental changes80, potentially benefiting female AUFS.
Potential impacts of oceanographic changes
Numerous studies have established associations between climatic variations and both habitat use and hunting success in marine top predators (e.g. 81,82). Marine communities are undergoing negative impacts of anthropogenic climate change globally, including shifts in community composition and structure83–86. For example, fish assemblages in south-eastern Australia have shown significant changes in their diversity, abundance and distribution in response to rising sea temperatures26,43. Such changes may lead to the selection of different habitats and prey types, driven by the significant dietary plasticity that AUFS have shown in response to the rising sea temperature32,75. Their adaptability could be advantageous for female AUFS to maintain hunting success and resilience under the expected changes in the marine environment.
Projected climate change scenarios predict a strengthening of wind-induced currents and wave activity in the Bass Strait22. These changes are expected to disturb the seafloor and impact seabed mobility and erosion22,23, which could affect the smaller sediments preferred by female AUFS and influence their habitat selection. Such alterations in the benthic environment may affect species assemblages and result in shifts in the distribution of benthic prey consumed by AUFS, potentially influencing their habitat selection and hunting success. The magnitude and direction of these environmental changes, along with the specific benthic variables affected, will determine the extent of the AUFS’s response. Wind-induced currents can bring nutrient-rich waters closer to central Bass Strait87, potentially leading to shifts in prey preference that may impact hunting success in female AUFS. During periods of stronger winds32, certain prey species such as redbait occur more frequently in their diet when winds are stronger, which may influence hunting success and shift preferred foraging areas.
In summary, the present study revealed that habitat selection and hunting success in female AUFS are influenced by different environmental factors, with bathymetry and sediment composition playing critical roles. Individuals non-randomly selected foraging areas where they are not necessarily more successful. In addition, anticipated oceanographic changes that are expected to alter the sea floor characteristics and benthic communities could impact habitat selection and hunting success in adult female AUFS. To better understand how these potential changes may influence the AUFS population, further investigation is needed to explore how benthic environments may respond to the predicted climate-induced alterations and how these shifts may impact different age classes and sexes.
Methods
Ethics statement
All animal handling procedures for the present study were conducted in accordance with the regulations, guidelines and approval of the Deakin University Animal Ethics Committee (Approval A33/2004, A16/2008, A14/2011, B16/2014, B04/2017, B05/2020), and the Department of Sustainability and Environment (Victoria, Australia) Wildlife Research Permits (10,000,187, 10,000,706, 10,001,143, 10,001,672, 10,002,269, 10,005,362, 10,007,153, 10,008,286 and 10,005,848).
Study site and animal handling
The study was conducted at Kanowna Island (39°10’S, 146°18’E; Fig. 1), northern Bass Strait (south-eastern Australia). The island hosts the third-largest breeding colony for the species31. During the autumn/winter months (May–August) of 2006–2021, adult female nursing pups were selected at random and captured with a modified hoop net (Fuhrman Diversified, Seabrook, Texas, USA). This sampling period corresponds to peak lactation for the species88 and, thus, the period of greatest nutritional demand for adult females provisioning young89,90. Individuals were then anesthetized for safe handling with isofluorane delivered via a portable gas vaporizer (Stinger, Advanced Anaesthesia Specialists, Gladesville, NSW, Australia) before being placed on a flat board for processing. For identification, uniquely numbered plastic tags (Super Tags, Dalton, Woolgoogla. Australia) were inserted in the trailing edge of each fore-flipper.
Individuals were then instrumented with a dive behaviour data logger (Mk06, Mk07, Mk08, Mk09, or Mk10, Wildlife Computers Ltd., Redmond, WA, USA) and a Fastloc GPS data logger (F1G Sirtrack Ltd, Havelock North, New Zealand), or a combined dive behaviour/Fastloc GPS data logger (MK10AF, Wildlife Computers Ltd). The devices were glued in series to the fur of the dorsal midline just posterior to the scapula using quick-setting epoxy resin (RS Components, Corby, UK). The dive behaviour and GPS data loggers were programmed to sample depth and location at 1 s or 5 s and 10 min, respectively. To assist with relocation for recapture, individuals were also instrumented with a VHF transmitter (Sirtrack Ltd., Havelock North, New Zealand) posterior to the other devices on the dorsal midline. The combined mass and cross-sectional area of the devices used were less than < 1% and, therefore, are likely to have had a negligible effect on the hunting success of individuals91. Following recovering from anaesthesia, individuals were released to resume normal behaviours. After one or more complete foraging trips, animals were recaptured using the previously described method and the data loggers were removed by cutting the fur beneath them.
Data processing and analyses
The GPS data were filtered to remove erroneous locations and linearly interpolated along each foraging trip using the trip package version 1.8.792,93 in the R statistical environment94. The dive behaviour records were first corrected for any potential drift in depth and the metrics of the dives (dive time, dive duration, maximum depth, bottom time, descent and ascent rate) were summarised using the diveMove package version 1.6.195. The GPS tracks were then merged with the dive behaviour data to geolocate all dives. As fur seals may enter the water around colonies to thermoregulate61, only immersions > 8 h were considered foraging trips. In addition, to exclude dives in the proximity of the colony that may be conducted for predator avoidance61, all dives occurring within a 1 km buffer of the colony and haul-out sites were excluded.
Dives were classified as benthic or pelagic using the Intra-depth Zone (IDZ) method96 which considers sequential dives with maximum depths of ± 10% to be benthic. Subsequently, all pelagic dives, which represented a smaller proportion of the total dives, were excluded from further analyses. The geolocated benthic dives were then used as temporally independent locations for a habitat-selection analysis. The potential available habitat was determined as the at-sea area within a 300 km radius of the colony, restricted to the continental shelf (depth < 200 m), corresponding to the maximum observed distance from the colony attained by lactating female AUFS during foraging trips38. A 0.01° × 0.01° grid was then overlaid over this area and the number of dives within grid cells was compared to environmental variables.
An initial exploratory analysis was conducted to identify oceanographic variables influencing habitat selection (Table 2), applying an exclusion criterion to the ones with strong collinearity through the VIF method (car package version 3.1–2). The degree of habitat selection of AUFS was explored within the home ranges using K-select analysis adehabitatHS package version 0.3.1738,97–99. This analysis employs the marginality concept, evaluating the strength of habitat selection based on the mean difference between the environmental conditions of the home ranges (habitat uses) and habitat availability, which is the same for all animals design II97. The K-select provides a linear combination of environmental variables maximising mean marginality97,100. The orientation of the marginality vectors reveals the habitat selection of individuals, indicating whether they share habitat preferences97.
Table 2.
Environmental variables of the habitat availability K-select model of Australian fur seals at 0.01(°) of resolution.
| Environmental variables | Description | Source | Categories |
|---|---|---|---|
| Bathymetry | Digital sea floor elevation data (m) | GEBCO | 10 m |
| Slope | Derived from the digital elevation data (°) | GEBCO | 10° |
| Aspect | Derived from the digital elevation data (°) | GEBCO | 10° |
| Mud | Interpolation of available online data (%) | 101 | 10% |
| Sand | Interpolation of available online data (%) | 101 | 10% |
| Gravel | Interpolation of available online data (%) | 101 | 10% |
| Disturbance index | Sediment mobility number (% of time) | 101 | 10% |
Previous studies have demonstrated a correlation between dive profile metrics and prey capture, enabling the probability of whether an individual captured prey during a dive to be determined using dive behaviour data only102. In the present study, benthic dives were classified as successful (1, with at least one prey capture event) or unsuccessful (0, with no prey capture events) based on a previously derived relationship between dive metrics such as descent rate, ascent rate, bottom time, and the duration of previous dives and prey capture events measured using accelerometry (see Supplementary Material A1). The classified dives were then used in a habitat suitability model, to investigate the seafloor factors that impact hunting success, with successful dives as presence (1) and unsuccessful dives as absence (0).
To determine which environmental variables influenced hunting success, a habitat distribution model using a full subset generalised additive mixed model FSSgam103 was constructed with the Fssgam package version 1.11103. The same environmental covariates as used in the habitat selection analyses were included in the model, with the addition of depth and current velocity at the same resolution Bio ORACLE104. The function ‘generate.model.set’ was used to evaluate the importance of the variables and select the optimal model according to the Akaike’s Information Criterion AIC105. The FSSgam evaluated all viable candidate models, explored the relative importance of each potential indicator without overfitting, and identified the best-fitting model (s) influencing the successful dives103. To assess the viability of potential predictors, models with ΔAICc < 4 were considered supported106. All possible combinations of potential predictors were tested, with a limited maximum number of 3 explanatory variables and the number of knots for each parameter within a given model was restricted to 4. Environmental variables were transformed where necessary to meet the normality assumption required by the model. To investigate the relationship between the probability of dives being successful and the most representative covariates from the FSSgam, a generalized additive mixed effect model GAMM107 was fitted with individuals included as a random effect. Penalised thin plate regression splines were used to fit the smooth terms to all the predictor variables and the ‘gam. check’ function was used to determine the adequate number of knots in each smooth term108. A spatial prediction was generated to identify the optimal areas for successful dives using the GAMM predictors. The model was fitted by using mgcv package version 1.8–36108. All the statistical analyses were conducted in the R Statistical environment version 4.3.194 and the maps were made with Quantum Geographic Information System (Qgis) version 3.30.1109.
Supplementary Information
Acknowledgements
The assistance of the many volunteer field assistants and students who have been involved in data collection for this study over the years is gratefully acknowledged. Logistical support was provided by Parks Victoria, in particular the rangers from the Foster and Tidal River offices, Prom Adventurer Boat Charters (Geoff Boyd) and Best Helicopters (Sean Best and Cameron Lang) was crucial for the success of the study. Financial support was provided by research grants from the Australian Research Council, Winifred Violet Scott Charitable Trust, Holsworth Wildlife Research Endowment, Office of Naval Research and Deakin University internal funds.
Author contributions
S.N.B. and J.P.Y.A. conceived the ideas and designed the methodology; S.N.B., J.P.Y.A., and C.J. collected the data; S.N.B. and J.M. analysed the data; S.N.B. and J.P.Y.A. M.AH & D.P.C: provided resources and assisted in the interpretation of the results. led the writing of the manuscript. All authors contributed critically to the manuscript preparation and gave final approval for publication.
Funding
This work was funded by Deakin University, University of Tasmania, University of Colorado Boulder, University of Santa Cruz, Australian Research Council, Winifred Violet Scott Charitable Trust, Holsworth Wildlife Research Endowment, Office of Naval Research.
Data availability
The datasets generated and/or analysed during the current study are available in the Zenodo repository.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78643-5.
References
- 1.Smith, R. L., Smith, T. M., Hickman, G. C. & Hickman, S. M. Elements of ecology. (1998).
- 2.Hilden, O. Habitat selection in birds: a review. In Annales Zoologici Fennici.2(1), 53–75 (1965). [Google Scholar]
- 3.Hutto, R. L. Habitat selection by nonbreeding, migratory land. Habitat selection in birds 455. (1985).
- 4.Block, W. M. & Brennan, L. A. The habitat concept in ornithology: theory and applications. Curr. Ornithol.11, 35–91 (1993). [Google Scholar]
- 5.Di Stefano, J., York, A., Swan, M., Greenfield, A. & Coulson, G. Habitat selection by the swamp wallaby (Wallabia bicolor) in relation to diel period, food and shelter. Austral Ecol.34, 143–155 (2009). [Google Scholar]
- 6.Bjørneraas, K. et al. Habitat quality influences population distribution, individual space use and functional responses in habitat selection by a large herbivore. Oecologia168, 231–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, Z. et al. Environmental determinants of habitat and kill site selection in a large carnivore: scale matters. J. Mammal.93, 677–685 (2012). [Google Scholar]
- 8.Lacher, T. E. Jr. & Mares, M. A. Availability of resources and use of space in eastern chipmunks, Tamias striatus. J. Mammal.77, 833–849 (1996). [Google Scholar]
- 9.Stevens, M. A. Influences of social and habitat features on selection and use of breeding habitat and pup survival in South American fur seals. University of New Hampshire. (2002).
- 10.Patenaude-Monette, M., Belisle, M. & Giroux, J.-F. Balancing energy budget in a central-place forager: which habitat to select in a heterogeneous environment?. PLoS ONE9, e102162 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decaestecker, E., De Meester, L. & Ebert, D. In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc. Natl. Acad. Sci.99, 5481–5485 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron, E. Z. Behavioural Ecology: An Evolutionary Perspective on Behaviour, É. Danchin, L.A. Giraldeau, F. Cézilly (Eds.), Oxford University Press (2008), 874 pp, Elsevier. (2009).
- 13.Morse, D. H. & Fritz, R. S. in Foraging behavior 443–455. Springer. (1987).
- 14.Hugie, D. M. & Dill, L. M. Fish and game: a game theoretic approach to habitat selection by predators and prey. J. Fish Biol.45, 151–169 (1994). [Google Scholar]
- 15.Funston, P., Mills, M. & Biggs, H. Factors affecting the hunting success of male and female lions in the Kruger National Park. Journal of Zoology253, 419–431 (2001). [Google Scholar]
- 16.Guinet, C. et al. Spatial distribution of foraging in female Antarctic fur seals Arctocephalus gazella in relation to oceanographic variables: a scale-dependent approach using geographic information systems. Marine Ecology Progress Series219, 251–264 (2001). [Google Scholar]
- 17.Bradshaw, C. J., Higgins, J., Michael, K. J., Wotherspoon, S. J. & Hindell, M. A. At-sea distribution of female southern elephant seals relative to variation in ocean surface properties. ICES Journal of Marine Science61, 1014–1027 (2004). [Google Scholar]
- 18.Guinet, C. et al. Southern elephant seal foraging success in relation to temperature and light conditions: insight into prey distribution. Marine Ecology Progress Series499, 285–301 (2014). [Google Scholar]
- 19.Hickcox, R. P. et al. Staying close to home: Marine habitat selection by foraging yellow-eyed penguins using spatial distribution models. Frontiers in Marine Science9, 967741 (2022). [Google Scholar]
- 20.Wynn-Simmonds, S., Planque, Y., Huon, M., Lovell, P. & Vincent, C. Foraging behavior and habitat selection of harbor seals (Phoca vitulina vitulina) in the archipelago of Saint-Pierre-and-Miquelon. Northwest Atlantic. Marine Mammal Science10.1111/mms.13134 (2024). [Google Scholar]
- 21.Speakman, C. N. et al. Environmental influences on foraging effort, success and efficiency in female Australian fur seals. Sci Rep10, 17710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, J. et al. The wave climate of Bass Strait and south-east Australia. Ocean Modelling172, 101980 (2022). [Google Scholar]
- 23.Li, F., Dyt, C., Griffiths, C. & McInnes, K. L. Predicting seabed change as a function of climate change over the next 50 yr in the Australian southeast. (2007).
- 24.Johnson, C. R. et al. Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology400, 17–32 (2011). [Google Scholar]
- 25.Ling, S., Barrett, N. & Edgar, G. Facilitation of Australia’s southernmost reef-building coral by sea urchin herbivory. Coral Reefs37, 1053–1073 (2018). [Google Scholar]
- 26.Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nature Climate Change3, 919–925 (2013). [Google Scholar]
- 27.Gervais, C. R., Champion, C. & Pecl, G. T. Species on the move around the Australian coastline: A continental-scale review of climate-driven species redistribution in marine systems. Global change biology27, 3200–3217 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henkel, S. K., Suryan, R. M. & Lagerquist, B. A. Marine renewable energy and environmental interactions: baseline assessments of seabirds, marine mammals, sea turtles and benthic communities on the Oregon Shelf. Marine renewable energy technology and environmental interactions, 93–110. (2014).
- 29.Arnould, J. P. Y. & Warneke, R. Growth and condition in Australian fur seals (Arctocephalus pusillus doriferus)(Carnivora: Pinnipedia). Australian Journal of Zoology50, 53–66 (2002). [Google Scholar]
- 30.Warneke, R. & Shaughnessy, P. Arctocephalus pusillus, the South African and Australian fur seal: taxonomy, evolution, biogeography, and life history. Studies of sea mammals in south latitudes, 53–77. (1985).
- 31.McIntosh, R. R. et al. Understanding meta-population trends of the Australian fur seal, with insights for adaptive monitoring. PLoS One13, e0200253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kliska, K. et al. Environmental correlates of temporal variation in the prey species of Australian fur seals inferred from scat analysis. Royal Society Open Science9, 211723 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy, N. et al. Assessing the trophic ecology of top predators across a recolonisation frontier using DNA metabarcoding of diets. Marine Ecology Progress Series573, 237–254 (2017). [Google Scholar]
- 34.Page, B., McKenzie, J. & Goldsworthy, S. D. Dietary resource partitioning among sympatric New Zealand and Australian fur seals. Marine Ecology Progress Series293, 283–302 (2005). [Google Scholar]
- 35.Arnould, J. P. Y., Cherel, Y., Gibbens, J., White, J. & Littnan, C. Stable isotopes reveal inter-annual and inter-individual variation in the diet of female Australian fur seals. Marine Ecology Progress Series422, 291–302 (2011). [Google Scholar]
- 36.Littnan, C., Arnould, J. P. Y. & Harcourt, R. Effect of proximity to the shelf edge on the diet of female Australian fur seals. Marine Ecology Progress Series338, 257–267 (2007). [Google Scholar]
- 37.Deagle, B. E., Kirkwood, R. & Jarman, S. N. Analysis of Australian fur seal diet by pyrosequencing prey DNA in faeces. Molecular ecology18, 2022–2038 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Arnould, J. P. Y. & Kirkwood, R. Habitat selection by female Australian fur seals (Arctocephalus pusillus doriferus). Aquatic Conservation: Marine and Freshwater Ecosystems17, S53–S67 (2007). [Google Scholar]
- 39.Kirkwood, R. et al. Continued population recovery by Australian fur seals. Marine and freshwater research61, 695–701 (2010). [Google Scholar]
- 40.Kirkwood, R. & Arnould, J. P. Y. Foraging trip strategies and habitat use during late pup rearing by lactating Australian fur seals. Australian Journal of Zoology59, 216–226 (2012). [Google Scholar]
- 41.Newell, B. Hydrology of south-east Australian waters: Bass Strait and New South Wales tuna fishing areas. (1961).
- 42.Gibbs, C., Tomczak, M. Jr. & Longmore, A. The nutrient regime of Bass Strait. Marine and Freshwater Research37, 451–466 (1986). [Google Scholar]
- 43.Hobday, A. J. & Pecl, G. T. Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Reviews in Fish Biology and Fisheries24, 415–425 (2014). [Google Scholar]
- 44.Ridgway, K. R. Long-term trend and decadal variability of the southward penetration of the East Australian Current. Geophysical Research Letters.10.1029/2007GL030393 (2007). [Google Scholar]
- 45.Huon, M. et al. Habitat selection of gray seals (Halichoerus grypus) in a marine protected area in France. The Journal of Wildlife Management79, 1091–1100 (2015). [Google Scholar]
- 46.Morris, D. W. Toward an ecological synthesis: a case for habitat selection. Oecologia136, 1–13 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Bechard, M. J. Effect of vegetative cover on foraging site selection by Swainson’s Hawk. The Condor84, 153–159 (1982). [Google Scholar]
- 48.Janke, A. K., Gates, R. J. & Terhune, T. M. II. Habitat influences northern bobwhite survival at fine spatiotemporal scales. The Condor: Ornithological Applications117, 41–52 (2015). [Google Scholar]
- 49.Bates, E. M. & Ballard, B. M. Factors influencing behavior and success of foraging Reddish Egrets (Egretta rufescens). Waterbirds37, 191–202 (2014). [Google Scholar]
- 50.Schultz, A. L. et al. Sediment variability affects fish community structure in unconsolidated habitats of a subtropical marine park. Marine Ecology Progress Series532, 213–226 (2015). [Google Scholar]
- 51.Krueck, N., Hill, N., Hartmann, K. & Fraser, K. Tasmanian octopus fishery assessment 2019/20. Assessment, 20. (2019).
- 52.Morrison, M., Jones, E. G., Parsons, D. M. & Grant, C. Habitats and areas of particular significance for coastal finfish fisheries management in New Zealand: A review of concepts and life history knowledge, and suggestions for future research. Ministry for Primary Industries Wellington New Zealand. (2014).
- 53.Lavering, I. H. Marine environments of Southeast Australia (Gippsland Shelf and Bass Strait) and the impact of offshore petroleum exploration and production activity. Marine georesources & geotechnology12, 201–226 (1994). [Google Scholar]
- 54.Mattern, T., McPherson, M. D., Ellenberg, U., van Heezik, Y. & Seddon, P. J. High definition video loggers provide new insights into behaviour, physiology, and the oceanic habitat of a marine predator, the yellow-eyed penguin. PeerJ6, e5459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harms, J. C. Hydraulic significance of some sand ripples. Geological Society of America Bulletin80, 363–396 (1969). [Google Scholar]
- 56.Coleman, N., Gason, A. S. & Poore, G. C. High species richness in the shallow marine waters of south-east Australia. Marine Ecology Progress Series154, 17–26 (1997). [Google Scholar]
- 57.Poore, G. Marine biogeography of Australia 189–212 (Marine biology. Longman Cheshire, 1994). [Google Scholar]
- 58.Gales, R., Pemberton, D., Lu, C. & Clarke, M. Cephalopod diet of the Australian fur seal: variation due to location, season and sample type. Marine and Freshwater Research44, 657–671 (1993). [Google Scholar]
- 59.Hume, F., Hindell, M. A., Pemberton, D. & Gales, R. Spatial and temporal variation in the diet of a high trophic level predator, the Australian fur seal (Arctocephalus pusillus doriferus). Marine Biology144, 407–415 (2004). [Google Scholar]
- 60.Mattern, T. Modelling marine habitat utilisation by yellow-eyed penguins along their mainland distribution: baseline information. Ministry for Primary Industries. (2020).
- 61.Arnould, J. P. Y. & Hindell, M. A. Dive behaviour, foraging locations, and maternal-attendance patterns of Australian fur seals (Arctocephalus pusillus doriferus). Canadian Journal of Zoology79, 35–48 (2001). [Google Scholar]
- 62.Wakefield, E. D. et al. Space partitioning without territoriality in gannets. Science341, 68–70 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Adams, E. S. Approaches to the study of territory size and shape. Annual review of ecology and systematics32, 277–303 (2001). [Google Scholar]
- 64.Kailola, P. J.,Williams, M. J., Stewart, P. C., Reichelt, R. E., & McNee, A. Australian fisheries resources. (1993).
- 65.Caro, T. M. Antipredator defenses in birds and mammals. University of Chicago Press. (2005).
- 66.Jewell, O. J. et al. Core habitat use of an apex predator in a complex marine landscape. Marine Ecology Progress Series506, 231–242 (2014). [Google Scholar]
- 67.Bruce, B. D. Preliminary observations on the biology of the white shark, Carcharodon carcharias, in south Australian waters. Marine and Freshwater Research43, 1–11 (1992). [Google Scholar]
- 68.Shaughnessy, P. D. Instances of predation on fur seals by white sharks in South Australia. Australian Mammalogy28, 107–110 (2006). [Google Scholar]
- 69.van Eeden, R., Reid, T., Ryan, P. G. & Pichegru, L. Fine-scale foraging cues for African penguins in a highly variable marine environment. Marine Ecology Progress Series543, 257–271 (2016). [Google Scholar]
- 70.Mattern, T. et al. Straight line foraging in yellow-eyed penguins: new insights into cascading fisheries effects and orientation capabilities of marine predators. PLoS One8, e84381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams, J., Jordan, A., Harasti, D., Davies, P. & Ingleton, T. Taking a deeper look: Quantifying the differences in fish assemblages between shallow and mesophotic temperate rocky reefs. PloS one14, e0206778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gray, J. S. Species richness of marine soft sediments. Marine ecology progress series244, 285–297 (2002). [Google Scholar]
- 73.Ingleton, T. et al. in Seafloor Geomorphology as Benthic Habitat 487–502. Elsevier.10.1016/B978-0-12-814960-7.00028-2 (2020). [Google Scholar]
- 74.Hosack, G. R. & Dambacher, J. M. (2012). Ecological Indicators for the Exclusive Economic Zone of Australia’s South East Marine Region. A report prepared for the Australian Government Department of Sustainability, Environment, Water, Population and Communities. CSIRO Wealth.
- 75.Kirkwood, R., Hume, F. & Hindell, M. Sea temperature variations mediate annual changes in the diet of Australian fur seals in Bass Strait. Marine Ecology Progress Series369, 297–309 (2008). [Google Scholar]
- 76.Boyd, I. L. Time and energy constraints in pinniped lactation. The American Naturalist152, 717–728 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Baylis, A. M. M. et al. Diving deeper into individual foraging specializations of a large marine predator, the southern sea lion. Oecologia179, 1053–1065 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Camprasse, E. C. M., Cherel, Y., Arnould, J. P. Y., Hoskins, A. J. & Bost, C.-A. Combined bio-logging and stable isotopes reveal individual specialisations in a benthic coastal seabird, the Kerguelen shag. PLoS One12, e0172278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Speakman, C. N. et al. Intertrip consistency in hunting behaviour improves foraging success and efficiency in a marine top predator. Ecology and evolution11, 4428–4441 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Araújo, M. S., Bolnick, D. I. & Layman, C. A. The ecological causes of individual specialisation. Ecology letters14, 948–958 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Meyer-Gutbrod, E. L. et al. Redefining North Atlantic right whale habitat-use patterns under climate change. Limnology and Oceanography68, S71–S86 (2023). [Google Scholar]
- 82.Boyd, C. et al. Effects of variation in the abundance and distribution of prey on the foraging success of central place foragers. Journal of Applied Ecology54, 1362–1372 (2017). [Google Scholar]
- 83.Wassmann, P., Duarte, C. M., Agusti, S. & Sejr, M. K. Footprints of climate change in the Arctic marine ecosystem. Global change biology17, 1235–1249 (2011). [Google Scholar]
- 84.Hughes, L. Biological consequences of global warming: is the signal already apparent?. Trends in ecology & evolution15, 56–61 (2000). [DOI] [PubMed] [Google Scholar]
- 85.Hays, G. C., Richardson, A. J. & Robinson, C. Climate change and marine plankton. Trends in ecology & evolution20, 337–344 (2005). [DOI] [PubMed] [Google Scholar]
- 86.Hoegh-Guldberg, O. & Bruno, J. F. The impact of climate change on the world’s marine ecosystems. Science328, 1523–1528 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Sandery, P. A. & Kämpf, J. Transport timescales for identifying seasonal variation in Bass Strait, south-eastern Australia. Estuarine, Coastal and Shelf Science74, 684–696 (2007). [Google Scholar]
- 88.Arnould, J. P. Y. & Hindell, M. A. Milk consumption, body composition and pre-weaning growth rates of Australian fur seal (Arctocephalus pusillus doriferus) pups. Journal of Zoology256, 351–359 (2002). [Google Scholar]
- 89.Gittleman, J. L. & Oftedal, O. T. Comparative growth and lactation energetics in carnivores. Symposia of the Zoological Society of London57, 41–77 (1987). [Google Scholar]
- 90.Oftedal, O. T. Milk composition, milk yield and energy output at peak lactation: a comparative review. (1985).
- 91.Casper, R. M. Guidelines for the instrumentation of wild birds and mammals. Animal Behaviour10.1016/j.anbehav.2009.09.023 (2009). [Google Scholar]
- 92.Sumner, M. D., Wotherspoon, S. J. & Hindell, M. A. Bayesian estimation of animal movement from archival and satellite tags. PLoS One4, e7324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sumner, M.D. & Luque, S. trip: Spatial analysis of animal track data. R package version, 1.1–10. (2011).
- 94.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. (2020).
- 95.Luque, S. P. Diving Behaviour Analysis in R: An Introduction to the diveMove Package. (2009).
- 96.Tremblay, Y. & Cherel, Y. Benthic and pelagic dives: a new foraging behaviour in rockhopper penguins. Marine Ecology Progress Series204, 257–267 (2000). [Google Scholar]
- 97.Calenge, C., Dufour, A. B. & Maillard, D. K-select analysis: a new method to analyse habitat selection in radio-tracking studies. Ecological modelling186, 143–153 (2005). [Google Scholar]
- 98.Thomas, D. L. & Taylor, E. J. Study designs and tests for comparing resource use and availability. The Journal of wildlife management10.2307/3809050 (1990). [Google Scholar]
- 99.Calenge, C. The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecological modelling197, 516–519 (2006). [Google Scholar]
- 100.Martin, J. et al. Coping with human disturbance: spatial and temporal tactics of the brown bear (Ursus arctos). Canadian Journal of Zoology88, 875–883 (2010). [Google Scholar]
- 101.Jenkins, C. Building offshore soils databases. Sea Technology38, 25–28 (1997). [Google Scholar]
- 102.Volpov, B. L. et al. Dive characteristics can predict foraging success in Australian fur seals (Arctocephalus pusillus doriferus) as validated by animal-borne video. Biology open5, 262–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fisher, R., Wilson, S. K., Sin, T. M., Lee, A. C. & Langlois, T. J. A simple function for full-subsets multiple regression in ecology with R. Ecology and Evolution8, 6104–6113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harvey, E. S. et al. The BRUVs workshop–An Australia-wide synthesis of baited remote underwater video data to answer broad-scale ecological questions about fish, sharks and rays. Marine Policy127, 104430 (2021). [Google Scholar]
- 105.Symonds, M. R. & Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral ecology and sociobiology65, 13–21 (2011). [Google Scholar]
- 106.Oviatt, S. User-centered modelling and evaluation of multimodal interfaces. Proceedings of the IEEE91, 1457–1468 (2003). [Google Scholar]
- 107.Zuur, A. & Hilbe, J. & Ieno, E (Highland Statistics Ltd., 2013). [Google Scholar]
- 108.Wood, S. N. Generalized additive models: an introduction with R. CRC Press.10.1201/9781315370279 (2017). [Google Scholar]
- 109.QGIS Development Team, A. E. QGIS geographic information system. Open source geospatial foundation project, 504-507 (2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the Zenodo repository.