Abstract
The historical restriction of magnetic resonance imaging (MRI) for patients with cardiac implantable electronic devices (CIEDs) has been lifted by certified MRI-conditional systems in recent years. Mixed-brand CIED systems consisting of a generator from one manufacturer and at least one lead from another manufacturer are not certified for MRI. We evaluated the temporal trend in the prevalence of mixed-brand systems in the era of MRI-conditional systems. Data were analyzed on 5853 CIEDs implanted de novo between 2012 and 2022 in 81 Italian centers linked to the nationwide Home Monitoring Expert Alliance network. The percentage of mixed-brand implants was calculated by device type (pacemaker, implantable cardioverter-defibrillator [ICD], cardiac resynchronization therapy [CRT] device) and over time. A mixed-brand system was implanted in 4.1% (95% CI, 3.6-4.6%) of analyzed patients or, by device type, in 4.5% (3.5-5.7%) of pacemaker patients, 1.1% (0.7-1.7%) of ICD patients, and 6.8% (5.7-7.9%) of CRT pacemaker/defibrillator patients (p < 0.001). Prevalence of mixed-brand implants exhibited significant temporal fluctuations, first declining from 6.6% (2012–2014) to 1.3% (2019), and then increasing to 5.1% (2022). Temporal changes were statistically significant for pacemakers (p < 0.001) and CRT devices (p = 0.001), but not for ICDs (p = 0.438). In the decade between 2012 and 2022, mixed-brand CIED systems were more prevalent in patients treated with pacemakers and CRT devices than in ICD recipients. A decline in the prevalence of mixed-brand systems was observed after the introduction of MRI-conditional systems, reaching a minimum in 2019, followed by a progressive increase in the subsequent years.
Keywords: Magnetic resonance imaging, Pacemaker, Implantable cardioverter-defibrillator, MRI-conditional, Cardiac implantable electronic devices
Subject terms: Cardiology, Medical research
Introduction
Patients with cardiac implantable electronic devices (CIEDs) often require magnetic resonance imaging (MRI), with an estimated 50–75% of patients requiring at least one MRI examination during CIED lifetime1,2. Currently, most CIED manufacturers offer MRI-conditional devices certified for scanning under specific conditions. The primary condition is that all components of the CIED system (generator and leads) come from the same manufacturer.
Due to the standardization of lead connectors, the implantation of system components from different manufacturers (“mixed-brand systems”) has become common practice for various reasons3. Current guidelines support performing MRI in patients with mixed-brand systems as an off-label use, based on the evidence of low risk of device failure or damage4,5. It is uncertain whether the evolution of MRI-conditional devices and emerging evidence of MRI safety even with non-MRI-conditional devices have influenced the prevalence of mixed-brand systems in clinical practice.
The aim of this analysis was to assess physicians’ preferences for single-brand or mixed-brand systems in normal clinical practice since 2012 in relation to the availability of MRI-conditional options and new evidence.
Methods
Sample selection
The first author conducted the investigation within the framework of the Italian Home Monitoring Expert Alliance (HMEA) project6. The HMEA project serves as an ongoing independent and permanent data repository from the routine care of patients with CIEDs monitored through the Home Monitoring system (BIOTRONIK SE & Co. KG, Berlin, Germany). Ethics approval for the HMEA project was obtained and all patients provided informed consent for data processing.
The present analysis included patients from the HMEA database who received a de novo conventional pacemaker, implantable cardioverter-defibrillator (ICD), or cardiac resynchronization therapy pacemaker/defibrillator (CRT-P/D). Patients with an unknown lead or generator manufacturer were excluded. Patients with devices requiring specific leads from the same manufacturer for technical reasons, such as single-lead ICDs with atrial sensing capacity (the DX ICD systems) were also excluded. Patient characteristics and electrical parameters of the implanted leads were recorded at the time of device implantation.
Analysis design and statistics
Patients were divided into two groups: a single-brand group (generator and leads from the same manufacturer) and a mixed-brand group (generator from one manufacturer and one or more leads from another manufacturer). Baseline clinical characteristics and electrical parameters of the implanted leads are presented as counts (percentages) or medians (interquartile ranges [IQRs]) and were compared between groups using the χ² test or Wilcoxon rank sum test, as appropriate.
The prevalence of mixed-brand systems was calculated with 95% confidence interval (CI) for the entire population and by device type (pacemaker, ICD, or CRT) and year of implantation. Differences among device types were assessed using the χ² test. Variations over time were tested using generalized estimating equations with mixed-brand implant as the dependent variable and a linear combination of periodic terms over years as the independent terms. A binomial distribution was assumed for the dependent variable.
Additionally, literature was reviewed to identify the most significant publications on MRI safety in patients with non-conditional CIEDs and then evaluate the potential impact of these publications on clinical practice.
Results
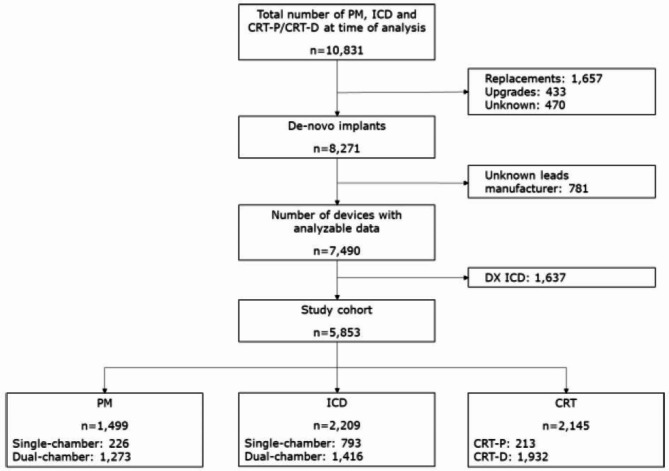
A total of 10,831 devices implanted in 81 Italian clinics between 2012 and 2022 had been registered in the HMEA project at the time of database freezing for the present study. After excluding device replacements or upgrades, DX ICD systems, and cases with incomplete device data, 5,853 patients with a de novo CIED were identified. One-quarter of them (25.6%) received a pacemaker, 37.7% a conventional ICD, and 36.6% a CRT device (Fig. 1). Most implants were single-brand systems (95.9%, n = 5,615). Mixed-brand systems (4.1%, n = 238) were implanted in 43 (53.1%) centers. Characteristics of the study cohort for the overall population and by group are compared in Table 1. The mixed-brand group had a significant higher prevalence of female sex, ischemic cardiomyopathy, and comorbidities (Table 1) compared to the single-brand group which may be related to the different prevalence of CRT systems for treating heart failure.
Fig. 1.
Sample selection. CRT, cardiac resynchronization therapy; CRT-D, CRT defibrillator; CRT-P, CRT pacemaker; DX ICD, single-lead ICD with atrial sensing capability and no atrial pacing capability; ICD, implantable cardioverter-defibrillator; PM, pacemaker.
Table 1.
Patient characteristics at implantation.
| Characteristic | All patients (n = 5,853) |
Single-brand group (n = 5,615) |
Mixed-brand group (n = 238) |
P-value |
|---|---|---|---|---|
| Age (years) | 72 (64–79) | 70 (65–79) | 73 (63–81) | 0.68 |
| Female | 1424 (24.4%) | 1351 (24.2%) | 73 (30.7%) | 0.027 |
| LVEF (%) | 33 (30–45) | 33 (30–45) | 35 (28–55) | 0.087 |
| Cardiomyopathy | 0.006 | |||
| Ischemic | 1996 (39.0%) | 1928 (39.3%) | 68 (31.5%) | |
|
Non-ischemic None |
1849 (36.1%) 1274 (24.9%) |
1749 (35.7%) 1226 (25.0%) |
100 (46.3%) 48 (22.2%) |
|
| Devices | < 0.001 | |||
| Single-chamber PM | 226 (3.9%) | 215 (3.8%) | 11 (4.6%) | |
| Dual-chamber PM | 1273 (21.7%) | 1216 (21.7%) | 57 (23.8%) | |
| Single-chamber ICD | 793 (13.5%) | 783 (13.9%) | 10 (4.2%) | |
| Dual-chamber ICD | 1416 (24.2%) | 1216 (21.7%) | 17 (7.1%) | |
| CRT-P | 213 (3.6%) | 193 (3.4%) | 20 (8.3%) | |
| CRT-D | 1932 (33.0%) | 1807 (32.2%) | 125 (52.1%) | |
| Comorbidities | ||||
| Hypertension | 3336 (63.8%) | 3189 (63.7%) | 147 (67.4%) | 0.29 |
| Diabetes | 1353(26.6%) | 1293 (26.5%) | 60 (27.6%) | 0.77 |
| Stroke/TIA | 196 (3.9%) | 190 (3.9%) | 6 (2.8%) | 0.48 |
| Renal insufficiency | 665 (13.1%) | 626 (12.9%) | 39 (17.9%) | 0.043 |
| History of AF | 1571 (30.5%) | 1485 (30.1%) | 86 (38.2%) | 0.012 |
| History of VF | 260 (5.1%) | 255 (5.3%) | 5 (2.3%) | 0.069 |
| History of VT | 497 (9.8%) | 485 (10.0%) | 12 (5.5%) | 0.036 |
Data are median (interquartile range) or number (% of available data).
AF, atrial fibrillation; CRT-D, cardiac resynchronization therapy defibrillator; CRT-P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; PM, pacemaker; TIA, transient ischemic attack; VF, ventricular fibrillation; VT, ventricular tachycardia.
Significance values are bold.
In patients with 238 mixed-brand implants, a lead from a different manufacturer was connected to atrial (7.1%, n = 17), right ventricular (13.0%, n = 31), or left ventricular (40.3%, n = 96) port of the CIED, or to two or more of these three ports (39.5%, n = 94). A total of 129 mixed-brand leads were connected to the left ventricular port (see Table 2 for lead models).
Table 2.
List of lead models not manufactured by Biotronik and connected to the left ventricular port in cardiac resynchronization therapy devices.
| Lead model | N (%) |
|---|---|
| Attain Stability Quad (Medtronic) | 94 (72.9%) |
| 4968 CapSure Epi* (Medtronic) | 17 (13.2%) |
| QuickFlex Micro (Abbott) | 10 (7.8%) |
| 3830 lead (Medtronic) | 8 (6.2%) |
| Total | 129 (100%) |
*epicardial lead.
Table 3 summarizes the acute electrical parameters for non-mixed and mixed combinations of generator and lead. Statistically significant but clinically neglectable differences between the two groups were found in atrial pacing threshold and impedance, and in left ventricular sensing amplitude and pacing impedance.
Table 3.
Electrical parameters for single-brand and mixed-brand combinations of generator-lead.
| Parameter | Single-brand atrial lead (n = 3899, 3105, 3411) |
Mixed-brand atrial lead (n = 74, 65, 69) |
P-value |
|---|---|---|---|
| Atrial sensing amplitude (mV) | 3.1 (2.0-4.7) | 3.3 (2.3–4.6) | 0.87 |
| Atrial pacing threshold (V) @0.4 ms | 0.7 (0.5-1.0) | 0.5 (0.4–0.8) | < 0.001 |
| Atrial pacing impedance (Ω) | 600 (527–700) | 505 (448–612) | < 0.001 |
|
Single-brand RV lead
(n = 4892, 4925, 4994) |
Mixed-brand RV lead
(n = 76, 78, 81) |
P-value | |
|---|---|---|---|
| RV sensing amplitude (mV) | 12.4 (9.0–18.0) | 12.0 (8.2–15.3) | 0.16 |
| RV pacing threshold (V) @0.4 ms | 0.6 (0.4–0.7) | 0.65 (0.4-1.0) | 0.11 |
| RV pacing impedance (Ω) | 618 (538–721) | 585 (488–760) | 0.28 |
|
Single-brand LV lead
(n = 1639, 1674, 1739) |
Mixed-brand LV lead
(n = 97,95,105) |
P-value | |
|---|---|---|---|
| LV sensing amplitude (mV) | 13.6 (9.6–20.0) | 12.8 (7.6–18.6) | 0.043 |
| LV pacing threshold (V) @0.4 ms | 1.1 (0.8–1.6) | 1.0 (0.8–1.7) | 0.38 |
| LV pacing impedance (Ω) | 696 (559–870) | 678 (492–810) | 0.036 |
Data are median (interquartile range). The number of data points is indicated as n=, for sensing amplitude, pacing threshold, and pacing impedance, respectively.
RV, right ventricular; LV, left ventricular.
Mixed-brand prevalence by device type and over time
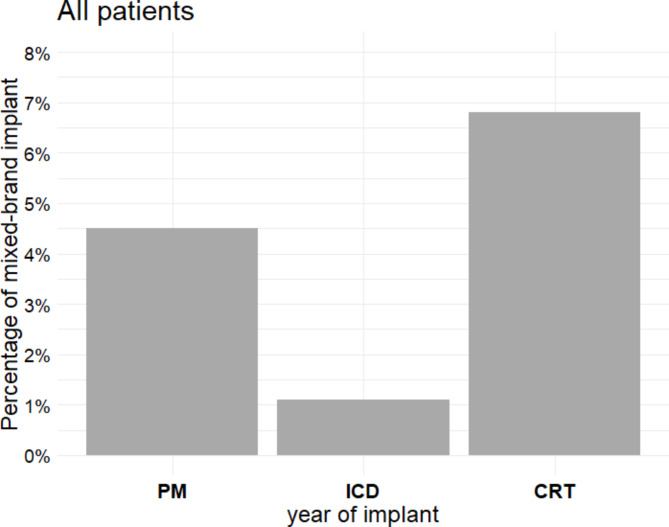
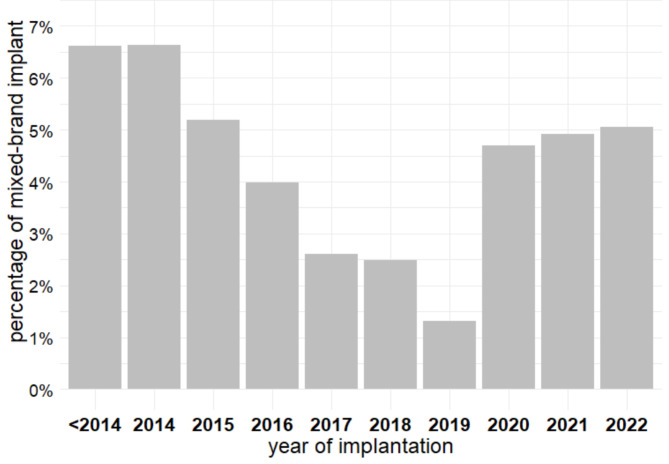
As illustrated in Fig. 2, the prevalence of mixed-brand CIED systems differed significantly by device type: 4.5% for pacemakers (95% CI, 3.5-5.7%), 1.1% for ICDs (95% CI, 0.7-1.7%), and 6.8% for CRT devices (95% CI, 5.7-7.9%) (p < 0.001). In addition, the prevalence of patients with mixed-brand implants varied significantly over the years (p < 0.001), with a notable decrease from 6.6% before 2015 to 1.3% in 2019, followed by a subsequent increase to 5.1% in 2022 (Fig. 3).
Fig. 2.
Proportion of patients with a mixed-brand implant by device type (χ² test p < 0.001). CRT, cardiac resynchronization therapy device; ICD, implantable cardioverter-defibrillator; PM, pacemaker.
Fig. 3.
Proportion of patients with mixed-brand implants over time.
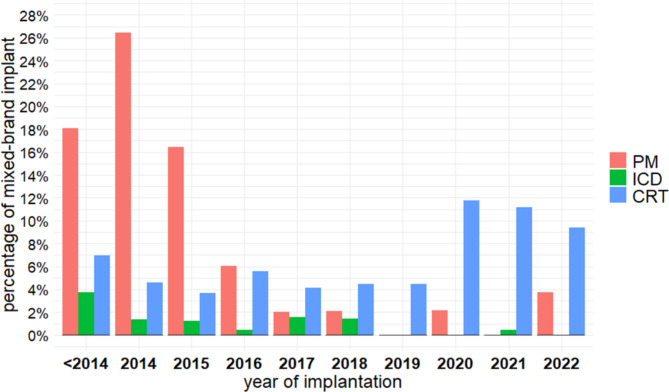
Significant changes over time were observed for pacemaker and CRT mixed-brand implants (Fig. 4). For example, the proportion of mixed-brand pacemaker systems was ≥ 16% until 2015, to drop to 0% in 2019–2020 and increase again to 3.7% in 2022 (p < 0.001 for variation over time, using generalized estimating equations). The proportion of mixed-brand CRT systems ranged from 3.8 to 6.9% in the period 2014–2019 and increased to 9.3-11.9% in 2020–2022 (p = 0.001). The proportion of mixed-brand ICD systems was low during the whole decade, with a maximum of 3.7% before 2014 and a minimum of 0% in 2019–2020 and 2022 (p = 0.438).
Fig. 4.
Proportion of patients with mixed-brand implants over time by device model. CRT, cardiac resynchronization therapy device; ICD, implantable cardioverter-defibrillator; PM, pacemaker.
Timing of main publications on non-MRI-conditional CIEDs
A non-systematic literature review was conducted to identify main publications on MRI safety in non-conditional CIEDs. These publications, summarized in Table 4, consistently reported a low adverse event rate in recipients of non-MRI-conditional pacemakers and ICDs undergoing MRI. Already in 2017, the Heart Rhythm Society expert consensus paper made a class IIa recommendation for patients with a non-MRI-conditional CIED system to undergo MRI in the absence of fractured, epicardial, or abandoned leads if the MRI examination is the best test for the condition and there is an institutional protocol in which a designated responsible MRI physician and a CIED physician are involved in the procedure7. This recommendation was reinforced in the 2019 American College of Radiology guidance document on MRI safe practices (Class IIa recommendation)8. More recently, the 2021 European Society of Cardiology guidelines extended the endorsement of MRI to pacemaker patients with abandoned transvenous leads when no alternative imaging modality is available and assigned it a Class IIb recommendation4.
Table 4.
Main publications on the safety of MRI in non-conditional pacemakers and ICDs.
| Year / study | Population | Study type | Conclusions |
|---|---|---|---|
| 2017 MagnaSafe11 | N = 1,000 (848 pts) PM and N = 500 (428 pts) ICD cases. Inclusion criteria: non-thoracic MR scans at 1.5 T. Exclusion criteria: CIED implanted before 2002, PM-dependency, abandoned or inactive lead, thoracic MR scans. |
Multicenter prospective |
Device or lead failure did not occur in any patient with a non-MRI-conditional PM or ICD who underwent clinically indicated non-thoracic MRI at 1.5 T, who was appropriately screened, and who had the device reprogrammed in accordance with the prespecified protocol. |
| 2017 Okamura et al.13 | N = 442 pts with non-MRI-conditional CIEDs, 569 MR scans. Inclusion criteria: 13 scans performed with a nearly depleted battery in 9 pts. Exclusion criteria: PM-dependency. |
Single center prospective |
Patients with PMs and ICDs with a nearly depleted battery can safely undergo MRI when pts are not PM-dependent. In old devices, PoR or ERI during MRI may lead to oversensing and inhibition of pacing. |
| 2017 Nazarian et al.14 | N = 1,509 pts with PM (58%) or ICD (42%) non-MRI-conditional, 2,103 thoracic and non-thoracic MRI scans. Exclusion criteria: device-dependency, implanted < 4 weeks, pts with permanent surgical epicardial leads or permanent nonfunctional leads. |
Single center prospective |
No long-term clinically significant adverse events were reported. |
| 2017 Padmanabhan et al.15 | N = 952 pts, 80 (8.4%) underwent 97 MRI scans with CIEDs in situ with abandoned leads in place. Inclusion criteria: pts with abandoned leads. |
Single center prospective |
No evidence of myocardial injury as measured by paired cardiac troponin assessment. The risk of MRI with abandoned leads appears low, suggesting a favorable risk-benefit profile in pts with CIEDs and abandoned leads who are considered for MRI. |
| 2017 Indik et al.7 | HRS Expert Consensus Statement on MRI and Radiation Exposure in Pts with CIEDs | It is reasonable for pts with a non-MRI-conditional CIED system to undergo MRI if there are no fractured, epicardial, or abandoned leads, the MRI is the best test for the condition, and there is an institutional protocol and a designated responsible MR physician and CIED physician (class IIa recommendation). | |
| 2018 Lupo et al.16 |
N = 120 pts with conventional PM or conventional ICD, n = 142 MRI 1.5 T (55 cardiac) 1.5 T MRI non-cardiac. Exclusion criteria: device-dependency, implanted < 6 weeks, implanted before 01/01/2000. |
Single center prospective |
A favorable risk-benefit ratio for MRI 1.5 T in conventional PM/ICD carriers was reported at MRI, immediately after MRI, and 3–12 months after MRI. |
| 2018 Shah et al.17 | 70 studies of non-MRI-conditional devices undergoing MRI were identified, allowing for analysis of 5,099 pts who underwent a total of 5,908 MRI studies (thoracic imaging in 773 pts) |
Systematic review and meta-analysis |
This review demonstrated low lead failure and clinical event rates in non-MRI-conditional PM and ICD recipients undergoing MRI. Observed changes were small and inter-study variance was low, suggesting that the composite event rates offer a reasonable estimate of true effect. The observed adverse events reinforce the need for ongoing vigilance and caution, particularly with older devices. |
| 2020 Greenberg et al.8 | ACR Guidance Document on MR Safe Practices: Updates and Critical Information 2019 | Guidance regarding performing MRI examinations in pts with non-MRI-conditional CIEDs including PMs, ICDs, CRT pacemakers/defibrillators is deferred to current recommendations by the HRS [Indik et al.]. 7 | |
| 2020 PROMeNADe18 |
N = 532 pts with non-MRI-conditional CIEDs, n = 608 MR scans (61 cardiac) Inclusion criteria: PM-dependency (27%), abandoned leads (2%). |
Single center prospective |
MRI examinations (also thoracic) can be performed safely in pts with non-MRI-conditional devices, in PM-dependent patients with ICDs, and in pts with abandoned leads. These MRI examinations can have a substantial impact on patient care, justifying the extensive resources used to perform them. |
| 2020 Rahsepar et al.19 |
N = 1,464 pts with CIEDs, n = 2028 MRI examinations. Exclusion criteria: newly implanted leads, abandoned or epicardial leads, PM-dependence with an ICD without asynchronous pacing capability. |
Single center prospective |
There was no evidence of an association between MRI parameters that characterize patient exposure to radiofrequency energy and changes in device and lead parameters immediately after MRI. |
| 2020 Munawar et al.20 | About 35 cohort studies with a total of 5,625 pts and 7,196 MRI scans (0.5-3 T) in non-conditional CIEDs were included |
Systematic review and meta-analysis |
This meta-analysis affirms the safety of MRI in non-conditional CIEDs: no death or ICD shock, extremely low incidence of lead or device-related complications. |
| 2021 Glikson et al.4 | 2021 ESC Guidelines on Cardiac Pacing and CRT | In pts with non-MRI-conditional PM systems, MRI should be considered if no alternative imaging mode is available and if no epicardial leads, abandoned or damaged leads, or lead adaptors/extenders are present (Class IIa). MRI may be considered in PM pts with abandoned transvenous leads if no alternative imaging modality is available (Class IIb). | |
| 2021 Bhuva et al.21 |
N = 970 pts with CIEDs (54% non-MRI-conditional); n = 111 (18%) scans with ‘mismatch’ devices; n = 105 (17%) scans with abandoned, epicardial or very old leads (< 2001), or scanned < 6 weeks post-implant). N = 1148 MR scans (506 (44%) cardiac). Inclusion criteria: (15%) were PM-dependent, pts with abandoned, epicardial or very old leads or scanned < 6 weeks post-implant. |
Multicenter prospective |
There was no increased risk of MRI in pts with non-MRI-conditional PM or ICD leads when following recommended protocols. Standardizing MR conditions for all leads would significantly improve access to MRI by enabling pts to be scanned in non-specialist centres, with no discernible incremental risk. |
ACR, American College of Radiology; CIED, cardiovascular implantable electronic device; CRT, cardiac resynchronization therapy; ERI, elective replacement indicator; ESC, European Society of Cardiology; HRS, Heart Rhythm Society; ICD, implantable cardioverter-defibrillator; MR, magnetic resonance; MRI, MR imaging; PM, pacemaker; PoR, power-on resets; pts, patients.
Discussion
Main findings
This study analyzed 5,853 de-novo pacemaker, ICD, and CRT implantations from a large real-world database ongoing at 81 Italian sites, and revealed that the overall proportion of patients with a mixed-brand system (generator and at least one lead from different manufacturers) was 4.1%. Mixed-brand implants were more prevalent in CRT (6.8%) and pacemaker (4.5%) recipients than in patients with conventional single- or dual-chamber ICDs. The complexity of CRT procedures may lead operators to preferentially use left ventricular lead with which they feel more confident. For example, a left ventricular lead with an active fixation system, produced by a single manufacturer, might be preferred when a conventional lead with passive fixation is perceived to be unstable9. Furthermore, a minority of mixed-brand CRT implants (Table 2), distributed over years, involved a surgically implanted permanent epicardial lead, which may be necessary due to unsuitable coronary sinus anatomy for lead positioning. These systems are still not certified for MRI even in the case of a single-brand implant, but since their use is driven by clinical necessity it should not be influenced by the availability of MRI-conditional options10.
For pacemaker recipients, a higher proportion of mixed-brand implants in certain hospitals may be attributed to previous public tenders in Italy in which brady leads and generators were purchased from different lots.
In contrast, the lower percentage of mixed-brand implants for conventional ICDs can be explained by a simpler procedure than for CRT devices, which typically does not require a change in the lead model. Additionally, operators may have a ‘psychological habit’ of viewing a single-brand system as safer with respect to right ventricular sensing and ICD therapies. This perception could contribute to a preference for a unified brand in the context of ICD implantation.
MRI is often the preferred imaging modality for neurological, musculoskeletal, oncological, and cardiovascular disorders. However, there was a historical contra-indication for CIED recipients. This limitation has been overcome with the development of MRI-conditional devices, with the first adoption in 2010 for pacemakers and 2012 for ICD and CRT devices in Europe. The pivotal requirement for MRI conditionality is that the entire CIED system, encompassing the generator and leads, must come from the same manufacturer. Our study showed that technological advances have had a notable impact on the use of mixed-brand implants over the last decade. Indeed, the rate of mixed-brand systems significantly decreased from 6.6% in 2015 to 1.3% in 2019. This reduction may be due to operators’ reluctance to provide patients with implants without MRI certification. In recent years, the proportion of mixed-brand implants has increased again to 5.1% in 2022. The increase may be explained by recent reports on the safety of MRI with non-MRI-conditional devices. These reports have likely led to less attention being paid to MRI conditionality4,5,7. The growing confidence in the safety of MRI scans using non-MRI-conditional devices may influence the decision-making process and contribute to the observed increase in the rate of mixed-brand implants.
The literature review revealed key publications on MRI safety in patients with non-MRI-conditional CIEDs, with the first larger studies published in 2017. These studies, including the multicenter MagnaSafe Registry11, consistently demonstrated a low adverse event rate in non-MRI-conditional pacemaker and defibrillator recipients undergoing MRI. Recommendations evolved over time, with a class IIa recommendation for this procedure established in the 2017 Heart Rhythm Society expert consensus7. This recommendation was subsequently reinforced by the American College of Radiology in 20198 and the European Society of Cardiology in 20214. Recent studies, specifically investigating mixed-brand CIED recipients undergoing MRI, found no increased risk of adverse events compared to patients with MRI-conditional CIED systems12.
Clinical implications
The growing evidence supporting the safety of MRI in patients with non-conditional CIEDs challenges the historical requirement of single-brand systems for MRI access. The selection of the generator and leads should be made independently and based on clinical characteristics to give the operator flexibility in selecting lead models from different manufacturers. This flexibility, particularly relevant for left ventricular lead positioning, was reflected in our results, which showed no clinically relevant changes in lead parameters between non-mixed and mixed generator-lead combinations. The long-term safety of mixed-brand systems, even after device replacement, has been demonstrated by the recent Detect Long-term Complications After ICD Replacement (DECODE) Registry3, further supporting the interchangeability of the technology. These findings underscore the need for a nuanced approach to CIED component selection that focuses on individual patient characteristics and preferences.
Study limitations
Several limitations should be acknowledged in our analysis. First, the study relies on data from the HMEA project, where CIED generators were obtained from a single manufacturer, which inevitably affects the generalizability of the results. Second, data were collected exclusively from Italian sites, preventing extrapolation to other contexts. Healthcare practices, device preferences, and patient demographics can vary significantly in different regions. Finally, while the study examined trends in implantation, it did not assess long-term clinical outcomes related to mixed-brand implants, such as device complications, patient outcomes, or physician’s choice of device brand at the time of elective replacement. Future research is warranted to address this aspect.
Conclusions
Our study provides insights into the prevalence of mixed-brand CIED systems over the past decade and its relationship to the evolving landscape of MRI accessibility knowledge and options. Mixed-brand systems were more prevalent in patients receiving CRT devices and pacemakers than in patients with conventional ICDs. Following the introduction of MRI-conditional devices, a decline in the prevalence of mixed-brand systems was observed until 2019, with a resurgence in recent years.
The changing paradigm of MRI safety in CIEDs challenges conventional practices and opens opportunities for more patient-centered and adaptable approaches to generator and lead selection.
Acknowledgements
The authors thank Alessandro Capucci for critical reading and suggestions and Dejan Danilovic for advice on medical writing. We are also thankful to Amanuel Lombardi, Samuele Meli, Elena Massai, Lorenzo Radi and the entire BIOTRONIK Italia technical support team.
Author contributions
DS and MG conceived the study design. DS, EC, CA, GZ, MB, GM, MB, VC, LS, AC, AC, ECP, GR, GS, FC, FN, MM, FS, MP, LT, LB, GN, LP, SP, MG, PP, ET GM, MG performed all the evaluations. IB, DG and AG performed statistical analyses and prepared figures / tables. DS, IB, and DG wrote the main manuscript text. AG and MG scientifically reviewed the manuscript. All the authors read and approved the final version.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Declarations
Competing interests
Irene Baldasserre, Daniele Giacopelli and Alessio Gargaro are employees of BIOTRONIK Italia.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Celentano, E. et al. Access to magnetic resonance imaging of patients with magnetic resonance-conditional pacemaker and implantable cardioverter-defibrillator systems: results from the really ProMRI study. Europace. 20 (6), 1001–1009 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Kalin, R. & Stanton, M. S. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin. Electrophysiol.28 (4), 326–328 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Biffi, M. et al. Manufacturer change and risk of system-related complications after implantable cardioverter defibrillator replacement: physicians’ survey and data from the Detect Long-Term complications after Implantable Cardioverter Defibrillator replacement Registry. J. Cardiovasc. Med. (Hagerstown). 18 (12), 968–975 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Glikson, M. et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J.42 (35), 3427–3520 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Greenberg, T. D. et al. ACR Guidance Document on MR Safe practices: updates and critical information 2019. J. Magn. Reson. Imaging. 51, 331–338 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Zanotto, G. et al. Organizational model and reactions to alerts in remote monitoring of cardiac implantable electronic devices: a survey from the Home Monitoring Expert Alliance project. Clin. Cardiol.42 (1), 76–83 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indik, J. H. et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 14 (7), e97–e153 (2017). [DOI] [PubMed] [Google Scholar]
- 8.ACR Committee on MR Safety: et al. ACR guidance document on MR safe practices: updates and critical information 2019. J. Magn. Reson. Imaging. 51 (2), 331–338 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Ziacchi, M. et al. Cardiac resynchronization therapy: a comparison among left ventricular bipolar, quadripolar and active fixation leads. Sci. Rep.8 (1), 13262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma, Y. D. et al. Safety of magnetic resonance imaging in patients with surgically implanted permanent epicardial leads. Heart Rhythm. 20 (8), 1111–1118 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Russo, R. J. et al. Assessing the risks Associated with MRI in patients with a pacemaker or defibrillator. N Engl. J. Med.376 (8), 755–764 (2017). [DOI] [PubMed] [Google Scholar]
- 12.König, C. A. et al. Is diversity harmful?-Mixed-brand cardiac implantable electronic devices undergoing magnetic resonance imaging. Wien Klin. Wochenschr. 134 (7–8), 286–293 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamura, H. et al. Magnetic Resonance Imaging in Nondependent Pacemaker patients with pacemakers and defibrillators with a nearly depleted battery. Pacing Clin. Electrophysiol.40 (5), 476–481 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Nazarian, S. et al. Safety of Magnetic Resonance Imaging in patients with Cardiac devices. N Engl. J. Med.377 (26), 2555–2564 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padmanabhan, D. et al. Safety of magnetic resonance imaging in patients with legacy pacemakers and defibrillators and abandoned leads. Heart Rhythm. 15 (2), 228–233 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Lupo, P. et al. An eight-year prospective controlled study about the safety and diagnostic value of cardiac and non-cardiac 1.5-T MRI in patients with a conventional pacemaker or a conventional implantable cardioverter defibrillator. Eur. Radiol.28 (6), 2406–2416 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Shah, A. D. et al. Magnetic resonance imaging safety in nonconditional pacemaker and defibrillator recipients: a meta-analysis and systematic review. Heart Rhythm. 15 (7), 1001–1008 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Gupta, S. K., Ya’qoub, L., Wimmer, A. P., Fisher, S. & Saeed, I. M. Safety and clinical impact of MRI in patients with Non-MRI-conditional Cardiac devices. Radiol. Cardiothorac. Imaging. 2 (5), e200086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahsepar, A. A. et al. The relationship between MRI Radiofrequency Energy and function of Nonconditional Implanted Cardiac devices: a prospective evaluation. Radiology. 295 (2), 307–313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munawar, D. A. et al. Magnetic resonance imaging in non-conditional pacemakers and implantable cardioverter-defibrillators: a systematic review and meta-analysis. Europace. 22 (2), 288–298 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Bhuva, A. N. et al. Evidence to support magnetic resonance conditional labelling of all pacemaker and defibrillator leads in patients with cardiac implantable electronic devices. Eur. Heart J.43 (26), 2469–2478 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.