Abstract
Features of the extracellular matrix, along with biochemical factors, have a momentous impress in making genes on and/or off. The interaction of cells and the extracellular matrix is mediated by integrins. Therefore, these molecules have pivotal roles in regulating cell behaviors. Integrins include a group of molecules with a variety of characteristics that can affect different molecular cascades. Considering the importance of these molecules in tissue regeneration after injury, it is necessary to know well the integrins involved in the process of connecting cells to the extracellular matrix in each tissue.
With the increase in life expectancy, bone tissue engineering has received more attention from researchers. Integrins are critical components in osteoblast differentiation, survival, and bone mechanotransduction. During osteogenic differentiation in stem cells, specific integrins facilitate multiple signaling pathways through their cytoplasmic domain, leading to the induction of osteogenic differentiation. Also, due to the importance of using biomaterials in bone tissue engineering, efforts have been made to design and use biomaterials with maximum interaction with integrins. Notably, the use of RGD peptide or fibronectin for surface modification is a well-established and commonly employed approach to manipulate integrin activity.
This review article looks into integrins’ role in bone development and regeneration. It then goes on to explore the complex mechanisms by which integrins contribute to these processes. In addition, this review discusses the use of natural and synthetic biomaterials that target integrins to promote bone regeneration.
Keywords: Integrins, Bone regeneration, Tissue engineering, Cell adhesion, Biomaterials
1. Introduction
Cell adhesion to the extracellular matrix (ECM) and other cells is a fundamental process that establishes the groundwork for the development, maintenance, and optimal functioning of various cells and tissues within multicellular organisms 1. Cell adhesion molecules (CAMs) are typically categorized into five distinct groups, distinguished by their unique structures, ligands, and functions. Among them, integrins stand out as a prominent class of cell adhesion molecules. Integrins are characterized by their ability to bind to the ECM components, and the term “integrin” was initially coined to describe their role in integrating the ECM with the cellular cytoskeletal network 2. Integrins are crucial in mediating interactions between the cytoskeleton and the ECM, enabling cells to adhere to the matrix firmly. However, it is important to note that integrins are not solely limited to binding to the ECM. Immune cell integrins, in particular, can attach to both soluble ligands and ligands present in other cells 3, 4.
Integrins, as a superfamily of heterodimeric transmembrane adhesion receptors, play a pivotal role in various essential cellular functions. These functions encompass crucial aspects such as embryonic development, cell proliferation, adhesion, migration, leukocyte adhesion, and platelet aggregation. Integrins serve as key regulators in coordinating these diverse cellular processes 5, 6. It should be noted that adenovirus, echovirus, hantavirus, poliovirus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are some examples of viruses that use cell surface integrin receptors to enter and infect their host cells 4.
With increasing life expectancy and changing lifestyles in diverse populations, the incidence of orthopedic problems has also risen. Consequently, numerous studies have concentrated on investigating the potential of adhesion molecules, including integrins, to directly or indirectly influence cell behavior, considering their crucial involvement in development and differentiation 7–9. Studies show that the behavior and metabolism of bones are regulated in interaction with the surrounding environment and mechanical stresses. The role of integrins is very important in the transmission of environmental mechanical stimuli to cells 10. On the other hand, integrins affect bones not only by influencing the behavior and differentiation of osteoblasts but also by playing a role in the behavior and fate of osteoclasts 10, 11. Osteoclasts respond to various mechanical stresses through integrins and cytoskeletal components 12. In tissue engineering studies, there is growing recognition that it holds a promising strategy for the repair and regeneration of bone.
In this review, we aim to provide a summary of the intricate signaling pathways and interactions mediated by integrins that play a crucial role in bone regeneration. We specifically focus on recent advances that have significantly contributed to our understanding of the involvement of integrins in this intricate process. Furthermore, the current article endeavors diverse biomaterials that have been used for the reconstruction of bone ECM. Consequently, investigating the downstream signaling pathways of integrins and identifying integrins as pivotal adhesion molecules that facilitate binding between osteocytes, or stem cells and the ECM has garnered considerable interest. By elucidating the multifaceted functions of integrins, this review aims to shed light on their therapeutic potential and pave the way for innovative approaches in the field of bone regeneration.
2. Integrin Structure, classification and function
Despite their great diversity, the amino acid sequence in integrins is highly conserved. Their typical structure consists of a large extracellular segment (700–1100 aa), a short transmembrane segment (20–30 aa), and a cytoplasmic segment (20–75 aa). Both α and β subunits bind to their ligands through the large extracellular domain (Figure 1) 1, 13. Integrin molecules resemble the human body in terms of structure. The head is built on two legs that form the transmembrane domain. Interactions between the α and β subunits always occur in the head region 4.
Fig 1. Schematic illustration of integrin structure and activation.
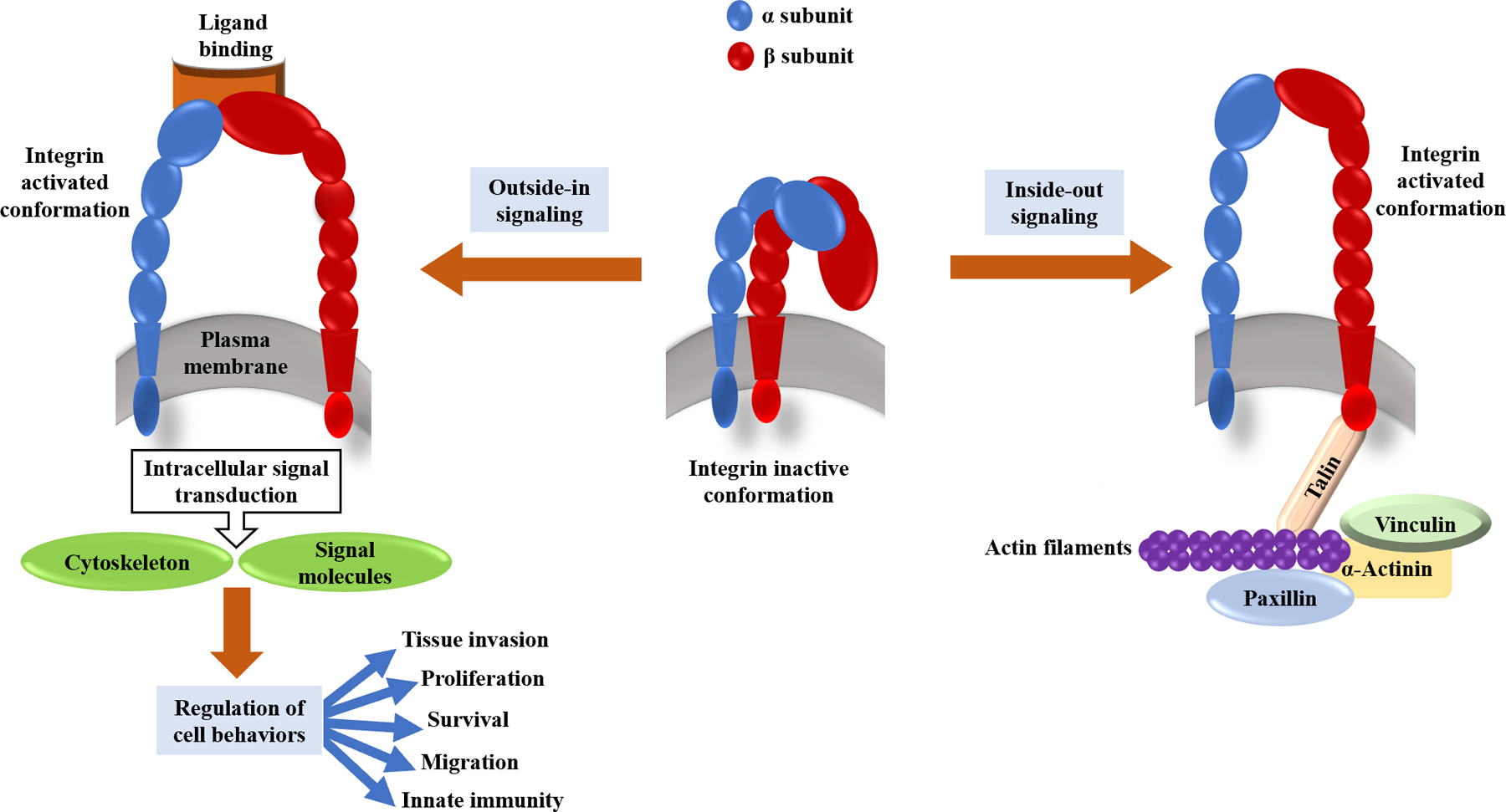
In Integrin heterodimers, α and β subunits are linked non-covalently. This structure on one side engages with ECM proteins and on the other side couples to cytoskeletal complexes and intracellular molecules which play roles in signal transduction. Some conformational transitions in integrin extracellular domains can be the result of a signaling cascade inside the cells (inside-out signaling); On the other hand, outside-in signaling includes the process in which ligand binding leads to ligand-induced integrin clustering and finally integrin-mediated intracellular signaling.
As previously mentioned, integrins are heterodimeric molecules with α and β subunits. To create a unique subfamily of integrins, each α subunit pairs up with other β subunits. Integrins are divided into eight subfamilies, and each has different specific ligands, tissue distribution, and other biochemical characteristics 14, 15. The vertebrates’ integrins are mainly divided into three major groups: β1-integrins, β2-integrins, and αv-integrins 16. The β1 subunit (CD29) can form heterodimers with α subunit types 1–11 and v to create the β1-integrins subfamily (Table 1). They have a crucial role in engaging the tumor microenvironment owing to their power to bind a broad range of ECM and cell adhesion molecules. Current investigations reveal that increased level of β1-integrins has a direct relationship with tumor progression, and metastasis, and as a result, many cancer types show treatment resistance 13, 17. The β2-integrins are the most predominant integrins in the leukocytes, also known as leukointegrins 18, 19. The β2-integrins are indicated CD11/CD18, because this subgroup is composed of routine β chain (CH18) and one of four unique α chains, including CD11a (αL subunit), CD11b (αM subunit), CD11c (αX subunit), and CD11d (αD subunit). β2-integrins subfamily involves different biological processes such as induction activation of some tyrosine kinases in leukocytes, regulation of some small GTD-binding proteins (including Ras and RhoA), etc. 18. The αv-integrin subfamily is formed when the αv integrin assembles with five different β subunits (β1, 3, 5, 6, or 8). The αv-integrin subfamily comprises five members, recognizing ECM components and other peptides via their Arg-Gly-Asp (RGD) motif 20, 21. The αv-integrins bind with high affinity and selectivity to the RGD motif in many ECM proteins, including vitronectin, collagen IV, TGFβ, etc. 22. Recent investigations reveal that there is a direct relationship between abnormal regulation of αv-integrins, especially αvβ3, and αvβ5, with poor patient outcomes and higher prevalence of tumor invasion and metastasis for many epithelial cancers 20.
Table 1.
Summary of the β1-integrin subfamily features
| Name | Distribution | Ligands | Functions | Ref |
|---|---|---|---|---|
| α1β1 (VLA-1) | • Many | • Col I • Col IV • Col IX • Col XVI • Laminin 111,112 • Matrilin I • Galectin 1,3,8 |
• Establishes human salivary glands • Roles in VEGF-driven angiogenesis |
23–25 |
| α2β1 (VLA-2) | • Many | • Collagens • Laminins |
• Regulates cell migration, proliferation and survival • Platelet adhesion • Epithelial differentiation • Morphogenesis • Promotes wound healing • VEGF-driven angiogenesis |
26, 27 |
| α3β1 (VLA-3) | • Col I • Col VI • Laminin-1 • Fibronectin • Entactin • Nidogen |
• Kidney development • Brain development • Liver development • Lung development • Maintains epithelial morphology |
28, 29 | |
| α4β1 (VLA-4) | • HSCs • Several types of tumor cells |
• VCAM-1 • MAdCAM-1 • Fibronectin • JAM-B • CD40L |
• Stem cell mobilization • Mediates homing of cells • Cell survival |
30, 31 |
| α5β1 (VLA-5) | • Many | • Fibronectin • CD40L • Irisin |
• Roles in migration and differentiation, especially during fetal development • Platelet adhesion to fibronectin under shear flow • During the invasion, facilitates the generation of adhesion forces, focal adhesion assembly, stress fiber formation, and contractile forces, respectively |
1, 32–36 |
| α6β1 (VLA-6) | • Many | • Laminins | • Involves in inflammation • Apoptosis regulation • Mediates cell adhesion • Cancer invasion • Axonal sorting in Schwann cells |
37, 38 |
| α7β1 | • Skeletal muscle localizes at the neuromuscular and myotendinous junctions | • Laminin | • Mediates axonal sorting in Schwann cells • Regulators of myofiber remodeling • Adheres outer ECM to the inner actin cytoskeleton • Serves as an intrinsic metalloprotein • Protects muscle structure • Promotes skeletal muscle hypertrophy in response to a mechanical stimulus |
37 |
| α8β1 | • Epithelial cells • Smooth muscle cells |
• Fibronectin • Vitronectin • Nephronectin |
• Mediates FA complex integrity • Involves in intestinal crypt cell homeostasis • Regulates spreading, adhesion, and growth of neuronal and mesenchymal-derived cells • Modulate actin stress fiber assembly • Promotes RhoA GTPase activation in VSMCs • Play a vital role in kidney morphogenesis |
1, 32, 39 |
| α9β1 | unknown | • TSP-1 • VCAM-1 • Tenascin-C • Osteopontin • VGEF-C • VEGF-D • ADAMs |
• Developments of the lymphatic system • Roles in neovascularization as a receptor for TSP-1 • Promotes Angiogenesis via interaction of TSP-1 • Plays an Instrumental role in human myogenic cells Differentiation |
40–42 |
| α10β1 | • In the cartilage-containing tissues • Chondrocytes in the growth plate • Bone lining cells in the trabecular bone |
• fibril-forming Col II/XI • FACIT Col IX in vitro |
• Chondrogenic differentiation and potency marker • Involves in skeletal development |
43 |
| α11β1 | • Many | • Collagen • Osteolectin |
• Mediates DGF-stimulated cell migration on collagen I • Mediates the contraction of collagen lattices in the regulation of ions and the reorganization of collagen matrices • Roles in myofibroblast differentiation • Involves in the regulation of bone homeostasis • Mediates cell spreading on a collagen XIII-coated surface |
44–47 |
Integrins serve as a critical component in many physiological and even some pathological processes, such as wound-healing processes 48, 49. The inflammation depends on the expression of sufficient adhesion molecules facilitating leukocyte migration 4. Furthermore, integrins promote cell proliferation, migration, and survival by transmitting different mechanical and biochemical signals across the cell membrane. The affinity of integrins for different ligands is regulated by their cytoplasmic tails 46. Integrin molecules transmit signals bidirectionally across the cell’s membrane. ‘Inside-out’ signals control integrin molecule’s affinity for their adhesive ligands, and ligand-dependent ‘outside-in’ signals control cellular responses to adhesion (Figure 1) 50.
3. Integrin roles in bone development
As the main adhesion receptors that link cells to the ECM, integrins are essential for embryonic development. As mentioned in Table 1, some types of integrins such as α1β1, α2β1, α3β1, α5β1, and αvβ3, are widely expressed for collagen, fibronectin (FN), and vitronectin (VN) in matrix ECM. In vivo experiments have shown that interactions between integrins and ECM regulate cell organization in tissue and organ development 51. As mentioned above, integrins participate in bone metabolism through the effect on bone precursor cells, mesenchymal stem cells (especially bone marrow mesenchymal stem cells), osteoclasts, osteoblasts, and also osteocytes 52. Integrins are required for osteoblast development, survival, and mechanotransduction in bone structure. Likewise, integrins can affect various aspects of cell behavior in tissue development, disease, and function. Intramembranous ossification is a process in which bones can form directly from mesenchymal precursors during the development of the skeletal system in mammalian cells and is a critical process during bone healing 53–55. Some evidence suggests that α2β1 integrin can play an essential role in developmental processes and differentiation 56. A study conducted on the role of integrin and fibronectin interaction in rat calvarial osteoblasts shows that interfering with α5β1 activity blocks bone mineralization and osteoblast differentiation. The α3β1 and α8β1 integrins also regulate osteogenesis through binding to FN like other ligands that are present in the ECM while perturbing the interactions between FN and integrin receptors αvβ3 and αvβ5 did not suppress mineralized nodule formation 57. In another study by Valat et al., it has been shown that β5 integrin acts as a negative regulator of osteogenesis. Their results showed that integrin β5 is a negative regulator of osterix and RUNX2, while integrin β1 is important for controlling ALP activity, RUNX2, and fibronectin organization in the cell environment 58. Also, The role of β5 integrin in bone homeostasis and mechanotransduction is revealed 59.
Cell-ECM interaction via integrins leads to several signaling pathways and induces osteogenic differentiation in human mesenchymal stem cells (hMSCs) 60, 61. hMSCs can be characterized into multilineage cells such as bone marrow stromal cells, chondrocytes, muscle cells, myocytes, and adipocytes 62, 63. Bone sialoprotein (BSP) and collagen I are the most known earlier molecular markers of differentiation of hMSCs into osteoblasts. However, there are various individual integrin subunits including αV, α5, α2β1, and β3 associated with osteogenic differentiation, which interact with different ligands such as vitronectin, FN, collagen I, and BSP 61, 64.
According to a study by Kundu et al., osteogenic differentiation can be promoted by α2β1 or α5β1 integrins, which usually bind to collagen I and FN in MSC adhesion, respectively. However, αvβ3 integrins, which bind to VN are not required for cell adhesion. Furthermore, It has been demonstrated that the majority of ECM molecules in bone tissues are α2β1 which is a major receptor for collagen I, and that it is necessary for differentiation and cell adhesion in MSCs 9, 65. These findings support the idea that β1 integrins assist the osteogenic differentiation of MSCs. Also, the signaling pathway through integrin α5β1 plays a critical role in the differentiation of hMSCs by inducing MSCs into osteoblasts, which is necessary for cellular differentiation 66. So, in bone differentiation, the balance between the expression of β1 and β5 should change in favor of β1.
It was shown that αvβ3- and α5β1-integrins differentially regulate cell adhesion and differentiation by mediating the adhesion of ECM glycoproteins such as FN, VN, and osteopontin (contain the RGD motif) in the differentiation of human bone marrow stromal cells 67. In an in vitro study on MC3T3-E1 preosteoblast, cells were exposed to the Noggin, a specific bone morphogenetic proteins (BMP) inhibitor, that was used to prevent ascorbic acid-induced gene expression that plays critical roles in osteoblastic differentiation. The results revealed the relationship between the BMP and ERK/MAPK signal transduction pathways through α2β1 integrin. The results also showed the necessity of BMP signaling and integrin-mediated cell-collagen interactions for osteoblastic development 68.
3.1. Integrin signaling in osteocyte differentiation and bone repair
Integrin clustering induces and activates focal adhesion complexes under the plasma membrane following ligand attachment to the integrin. With these conformational changes, focal adhesion kinase (FAK) activates and phosphorylates several essential signaling proteins such as phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and ERK1/2 69, 70. Numerous studies suggest that different ECM ligands regulate MAPK in various ways 64, 71, 72. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 are the most studied members among the MAP kinase family 73. The differentiation of hMSC into osteoblast is associated with the response of ERK/MAPK signaling to the ECM ligands and consequently phosphorylates transcription factor RUNX2/CBFA-1 at specific sites through translocation to the nucleus 74, 75. One of the crucial factors for regulating osteoblast differentiation is Runt-related transcription factor 2 (RUNX2), which can upregulate other osteoblast marker genes as well 76, 77.
According to several studies, after the culture of osteoblasts on a Connective Tissue Growth Factor (CTGF) matrix, Rac1 becomes activated and the expression of FAK and ERK are increased through αvβ1 integrin. This is then followed by increased osteocalcin expression by RUNX2. The RUNX2 subsequently regulates the expression of osteogenic markers and induces osteogenic differentiation. The RUNX2, also called Cbfa1, is the master gene of osteoblast differentiation and bone formation and also plays an important role in skeletal development, mesenchymal condensation, and osteoblast maturation. Furthermore, RUNX2 is upregulated during the early osteoblast differentiation of hMSC and decreased in mature osteoblasts. Additionally, some studies suggest that RUNX2 is sufficient to initiate the osteoblast differentiation program 61, 78–80.
Anti-integrin blocking experiments inhibit the FAK/ERK signaling transduction and prevent osteogenic differentiation in osteoblast cells attached to a CTGF matrix 81. In a study on mouse osteoblastic (MC3T3-E1) and fibroblastic (NIH3T3) cell line, phosphophoryn (PP) as a non-collagenous ligand, bound to αvβ3 integrin through the RGD domain and activated MAPK signaling cascade (specifically activated p38, ERK1/2, and JNK) that characterized by expression of differentiative marker genes like RUNX2, Osx, etc. 82. Although the function of PI3Ks is less understood, the information that is now available indicates that PI3Ks signaling cascade plays a significant role in osteoblast cell differentiation, survival, and mechanotransduction 83. Additionally, molecular studies showed that integrin α5β1, a cell surface receptor for FN, can trigger PI3K signaling pathways, which can promote some critical characteristics of hMSCs including survival, proliferation, and migration as well as osteogenic differentiation84–87.
It was shown that hMSCs have a critical role in healing bone defects and bone regeneration through integrins that convert mechanical forces into biochemical signals 88. According to the study done by Chung et al., artificial ECMs and biomimetic polymer networks can significantly enhance bone repair and regeneration caused by integrin, which provides a physical linkage between cytoskeletal matrix and nuclear elements 89. In addition, BSP which binds type I collagen (Col1) plays an essential role in bone tissue repair and can promote the expression of genes related to early osteoblast differentiation. Also, Col1 is a useful matrix for bone regeneration that binds to integrins through RGD and non-RGD domains 90. According to previous studies, synthetic peptides that can distinguish the RGD sequence completely prevent the interaction between some cell types (including rat osteoblasts, human osteosarcoma, and chick bone cells) with Col1, FN, and VN in vitro 91.
In osteopenic mice, the α5β1 integrin can promote osteoblast differentiation in MSCs and can enhance bone formation and repair through FAK-ERK activation. Additionally, a complex consisting of peptidomimetic ligands that link to a factor with a strong affinity for the α4β1 integrin improves MSCs bone homing, promotes osteoblast differentiation and increases bone mass 92. Studies that were done by blocking the integrin receptor-using antibodies to the α2, α3, α4, α5, α6, and β1 subunits and the adhesion ligand peptides (RGD and RGE sequence) suggest that initial osteoblastic cell adhesion to polymeric materials is regulated via integrin binding which is used in bone tissue engineering 93.
As previously mentioned, integrins play a critical role in osseous mechanobiology; however, when FAK was provisionally inactivated, the osteogenic response to the mechanical induction was abolished 94. In a study, the function of FAK in skeletal regeneration was evaluated by inducing small skeletal injuries in transgenic and wild-type mice. It was discovered that the osteogenic differentiation in FAK mutant cells was not inhibited. However, the mineral deposition by osteoblast was repressed in mice with mutant FAK 94. These observations clearly showed that cells can promote osteoblast differentiation in the lack of FAK-mediated integrin cascade 95, 96. Moreover, during distraction osteogenesis, BMP 2/4 is located in the ECM surrounding cells and seems to be the essential upregulating element for ERK 1/2 expression in mesenchymal precursor cells 88, 97. Rhee et al.’s analysis also indicated that an integrin-mediated mechanotransduction cascade can directly result in upregulating osseous regeneration by mechanical forces. This data shows that mesenchymal precursor cells’ ERK 1/2 expression increased during osteogenesis distraction 97.
3.2. Integrins, Osteoclasts and Bone Regeneration
Osteoclasts are the second major type of bone cells that can play a crucial role in bone resorption and regeneration. So knowing the mechanisms that affect the osteoclast proliferation and activity is crucial. Osteoclasts and osteoblasts crosstalk through RANK/RANKL signaling and the active osteoclasts positively affect osteoblasts precursors therefore osteoclast’s activity leads to bone formation and regeneration indirectly. However, the imbalance between osteoblasts and osteoclasts activity may result in osteoporosis. Some studies target osteoclasts to find a way to address some disorders that threaten the integrity of bone structure. Integrins and their ligands could be involved in osteoclast activity.
Various studies have shown the role of αvβ3 integrin in osteoclast differentiation98, 99. Some studies have attempted to disrupt the αvβ3 downstream signaling to increase osteogenesis via suppressing osteoclast activity100. In a study by Qiu and colleagues, Puerarin (a known isoflavone) was used to target osteoclasts and the results showed that the isoflavone can inhibit bone loss by interrupting integrin-based signaling in osteoclasts of an ovariectomized rat model. Their results showed that the expression of the β3-integrin protein was lower in the group that was treated with Puerarin101. Also, in another similar study, it was shown that Phloretin (a natural phenol) suppresses the activity of osteoclasts by disrupting the αvβ3 integrin-c-Src-Pyk2/Syk pathway. These results suggest that some plant compounds can be used to suppress the activity of osteoclasts in the tissue engineering structures that are produced for use in patients with osteoporosis102.
The use of glycosaminoglycans in the structure of scaffolds has been considered due to their role in cell-ECM interaction and growth factor storage. Studies have shown that chondroitin sulfate E prevents the differentiation of osteoclasts by binding to αvβ3 integrin103. The combination of both strategies, that is, the use of chondroitin sulfate and compounds that can block the activity of osteoclasts, is an approach that can be considered in future studies.
4. Integrin-targeting biomaterials
There is still a growing demand for biomaterials that support the replacement, repair, or restoration of both soft and hard tissues 104. A growing difficulty in tissue engineering is the design of biomaterials that provide favorable conditions for cells 105. Tissue engineering scaffolds have recently become widely used to treat bone injuries. Scaffolds can be classified as either natural or synthetic, depending on their source 106. Hyaluronic acid, collagen, chitosan, fibrin glue, agarose, and alginic acid are examples of natural materials. In addition to having appropriate biocompatibility, degradation products, and cell adhesion, they also exhibit physiological traits that are non-toxic. Besides these advantages, they also have several drawbacks, such as complex processing, limited sources, the potential for disease transmission, and poor mechanical strength 107.
To reduce these challenges, researchers have made various efforts in synthetic materials. Typically, bioglasses, alloys, hydrogels, metals, bone cement, Poly polyethylene glycol (PEG) polymers, and polycaprolactone (PCL) are utilized as synthetic materials in musculoskeletal tissue engineering. Synthetic materials address some challenges of natural materials, but they usually have limited cellular adhesion properties 108. In bone regeneration, cell integrin expression and protein adsorption of the substrate are influenced by several characteristics of different biomaterials 109. It appears that differences in protein adsorption will affect differences in the interaction of biomaterial with integrins, which will then trigger different signals, influencing the control of cell survival, proliferation, and differentiation 110, 111.
Another crucial aspect of biomaterials is their chemical and physical makeup, which affects how cells behave and express certain proteins like integrins 109. A recent study showed that some physical properties of biomaterials such as pore size and porosity of scaffolds can influence cell differentiation, ECM secretion, cell proliferation, and tissue regeneration 112. Therefore, smart biomaterials are constructed by altering the physical characteristics of the scaffolds. These modifications can be conducted using peptide sequences and, most significantly, by developing materials that can improve cell-ECM interactions through integrins 113. Peptides, as a family of bioactive agents, are used frequently for biomaterial functionalization. To regulate a broad spectrum of physiological functions, the human body contains about 7000 naturally occurring peptides. These Peptides have been categorized as structural agents, neurotransmitters, growth factors and hormones, ion channels, receptors, and ligands, or anti-infective agents 114. Peptides can also be easily modified to improve their properties and mix with biomaterials. These characteristics make them particularly promising in the field of tissue engineering 115. Unquestionably the most often used bioactive sequence for the functionalization of biomedical materials is the RGD motif 116. RGD, a cell-binding peptide, is a short sequence that is found in the ECM proteins FN and laminin 117 and has been shown to improve cell adhesion, growth, proliferation, and differentiation 118. The following is a summary of several peptides that work like the RGD sequence or other ECM peptides. Figures 2 and 4 demonstrate how certain ligands mediate the interaction between biomaterials and integrin.
Fig 2. β1 Integrins; the appropriate target for bone tissue engineering.

In bone regeneration, surface characteristics of the biomaterials and their effects on integrins determine the importance of integrins for cell and tissue behavior concerning bone-repairing materials. By modifying the physical properties of the scaffolds using peptide sequences Smart biomaterials could be produced. Also, smart materials with improved cell adhesion could be achieved by creating materials that can promote integrin interactions. Biomaterial can be functionalized by specific integrin ligands such as collage type I (Col-mBGn-hydrogel PP/COL-I-Pda-Ca Alg/PVA/Mg/Cu), Collagen type IV (Col-mBGn-hydrogel) and Fibronectin (Chit-GBMP1-a) or natural peptide sequence such as RGD (Chit-HVP, mPEG-PCL and Titanium). Some biomaterials linked integrin via synthetic peptide sequences, such as GFOGER and HVP.
The term “bioactive glasses” (BG) refers to a class of reactive materials with appealing properties, including bioactivity and biocompatibility, or the capacity to construct bonds with mineralized bone in the body. Hench created the first BG starting in 1969 119. All the basic components, such as sodium dioxide, calcium oxide, and phosphorus, can be combined or altered to produce various BG. The resulting glasses exhibit composition-dependent promising properties like anti-inflammatory effects, degradability, antibacterial properties, the ability to form bones, and even soft tissue regeneration and wound healing 120. Some trace elements such as Mg2+, Cu2+, Zn2+, Sr2+, Fe3+/Fe2+, Co2+/3+, and Mn2+ are critical for the growth, development, and maintenance of bones and are involved in numerous metabolic processes 121–123. Their incorporation into biomaterials can potentially promote osteogenesis and angiogenesis 124. Furthermore, Ag+ and Cu2+ doping in the structure of bioceramics and BGs have been repeatedly observed. These ions are important in biomedical processes such as protecting against microbial infections 125.
Recent studies on BG incorporated into biological polymers, such as alginate, collagen, and chitosan, have shown improved biological properties, such as the stimulation of osteoblast proliferation and MSC differentiation into osteogenic lineages 126. Using divalent cations in biomaterials can be a potential strategy to improve the integration of implants 127. The primary role of BGs in tissue healing processes is to begin and promote cellular activity through the release of ions and the induction of biomineral precipitation 128. Depending on the kind and concentration of the discharged ions, osteogenesis, angiogenesis, antibacterial, and anti-inflammatory effects can occur 129. α1, α2, α5, α10, α11, and β1 integrins are all involved in cell adhesion and cell surface-mediated signaling. The I-domain collagen-binding subunits α1, α2, α10, and α11 are crucial for binding and intercellular adhesion, and the integrins α1β1, α2β1, α10β1 ligand, and α11β1 act as collagen receptors 130, and are well known for mediating adhesion to Col1 and/or Col2. In more precise terms, collagen type VI prefers receptor α1β1, while collagen type II prefers receptor α2β1 131. The FN receptor is α5β1 and the preferred receptor for Col1 is α11β1 132. The α1β1, α2β1, α11β1, and α10β1 are collagen receptors and they also can mediate adhesion to Col2. It is also known that α1β1 and α2β1 mediate adhesion to laminin 133.
All integrin β1 subunits have three metal-binding sites known as Synergistic Metal ion Binding Site (SyMBS), metal ion-dependent adhesion site (MIDAS), and Adjacent to MIDAS (ADMIDAS) 134. Certain integrin functions interact with divalent cations including calcium, magnesium, and manganese, and need MIDAS and MIDAS-like motives 134, 135. Magnesium boosts the integrins’ affinity for ligands, including ECM, in a millimolar concentration, making it one of the divalent cations that are frequently utilized as therapeutic agents in clinical practice 136. This ion attaches to a specific site at Col1 and facilitates integrin α2β1-mediated adhesion; Mg2+ can enhance osteoblast cell adhesion and differentiation. This adhesion activates the FAK-ERK pathway, which inspires the production of genes that promote osteogenesis. Through the Wnt signaling, Mg2+ can efficiently increase -catenin and encourage osteoblast differentiation 137. Magnesium also improves the adhesion of human synovial MSCs through integrin α3β1. Magnesium dose-dependently increased the amount of synovial MSCs adherence to collagen-coated slides 136. It is known that manganese as a divalent cation has a significant impact on integrin avidity and affinity. This interaction can influence cell adhesion by interacting with ECM proteins 138. The organization of focal adhesions and the formation of actin stress fibers are associated with the maturation of αvβ1 integrin affinity, leading to improved cell migration 127, 139. Copper (Cu2+) has received considerable attention in the design of biomaterials due to its multifunction, such as angiogenic ability, osteogenic ability, and antibacterial properties 140, 141.
4.1. How biomaterials can target integrins to induce bone regeneration
Several important natural biomaterials such as collagen/gelatin, alginate, chitosan, etc., are employed to mend broken bones via integrins 142. In contrast, synthetic biomaterials commonly utilized in bone regeneration scaffolds mainly contain poly(DL-lactide-co-glycolide) (PLGA), polyvinyl alcohol (PVA), polycaprolactone (PCL), and BG 143 (Table 2).
Table 2.
Summary of the biomaterials in bone regeneration and its integrin relationship.
| The component | Biomaterial | Integrin interaction | Function | Signaling pathway &transcription factor | Reference |
|---|---|---|---|---|---|
| Natural biomaterials | |||||
| Col–mBGn hydrogels (Collagen mesoporous bioactive glass nanoparticles) | Collagen (prokaryotic collagen-like protein) | α1 and/or α2 subunit | Stimulation of osteoblast proliferation Osteogenic differentiation |
FAK-ERK pathway BMP-2/4 |
86, 96, 126, 169 |
| Chit-HVP Chit-GBMP1a |
Chitosan | A nonapeptide called HVP (Human vitronectin protein) RGD GBMP1a (BMP-2 peptide) indirect interaction by stimulating vitronectin protein |
Enhanced expression of α1β1integrins Bone regeneration |
BMP/Smad signaling pathway RUNX2 |
118, 170 |
| Synthetic biomaterials | |||||
| GFOGER-modified PEG hydrogel | PEG | A triple helical, α2β1 integrin-specific peptide (GFOGER) as a BMP-2 delivery vehicle | Promote bone regeneration in challenging defects with low delivered BMP-2 doses | BMP-2/Smad signaling pathway | 152 |
| Thermosensitive mPEG-PCL composite hydrogel | PCL PEG |
Enhanced expression of integrins (α2, α5, and β1) RGD-conjugated |
Facilitated the formation of focal adhesions | FAK-ERK signaling pathway | 155 |
| PP/COL-I-pDA-Ca | PLGA PCL |
Enhanced expression α10, α11 and β1 Collagen | Adhesion Proliferation Differentiation |
Osx BMP2 RUNX2 |
133 |
| Alg/PVA/MgB2NS scaffolds | PVA Alg MgB2NS |
Col1 | Promoting integrin α2β1-mediated adhesion Enhance osteogenesis |
FAK-ERK signaling pathway | 137 |
| Bioactive glasses (BGs) | |||||
| Functionalized Ti with either the αvβ3- or α5β1-selective peptidomimetics | Titanium | Anchoring αvβ3 or α5β1 integrin-selective RGD peptidomimetics | Bone growth Orthopedic applications |
RUNX2 OCN |
167 |
| (CMC/Alg/Cu) anionic carboxymethyl chitosan (CMC) and alginate (Alg | Copper Alginate Chitosan |
Enhance expression of Col1 | Promoted cell adhesion and osteogenesis by the release of Cu2+ ions Osteogenic differentiation Facilitate angiogenesis Higher expression of genes related to focal adhesion and actin cytoskeleton signaling pathway |
(FAK, PXN, and VCL) signaling pathway BMP-2 (ERK1/2) signaling pathway |
164 |
| MnCl2 Mn2+ |
Mn2+ | Integrin activity Enhance expression of Col1 |
Cell adhesion Spreading Proliferation The Mn2+ effects on the cell functions is strongly depend on their concentration |
P-Akt Signaling proteins p-ERK1/2 NFkB |
127 |
| Magnesium | Mg2+ | Enhance expression of Col1 | Improve osteoblasts Adhesion Differentiation |
FAK-ERK pathway | 137 |
| Boron-incorporated calcium silicate (Ca11Si4B2O22, B-CS) coating | Ca2+ Si4+ |
BMP-2 (Indirect role) Ca2+ in this component lade to BMP2 upregulated by bone mesenchymal stem cells |
Improving osteogenesis in orthopedic applications by coating decreased macrophages and change to the M2 phenotype | BMP2 signaling pathway Restraining the TLR signaling pathway, reduction in pro-inflammatory cytokines Moreover, the B-CS coating inhibited osteoclastogenesis and osteoclastic activities by downregulating osteoclastogenic genes and inhibiting the RANKL/RANK system |
153, 171 |
Other studies have shown that chitosan as a core of bioactive inorganic nano units shell can play a therapeutic role in tissue regeneration. Implanted 3D-printed scaffolds from therapeutic nanohybrids have been demonstrated to enhance new bone formation. This nano unit is biomimetic based on an inorganic nanoparticle core (BG, mesoporous silica, or hydroxyapatite) and a chitosan shell, namely Chit@IOC. The nano-roughened surface topography of these nanohybrids can speed up cellular reactions through promoted integrin-mediated focal adhesions 144. This nanohybrid in comparison to chitosan demonstrated that the modified primitive adhesion circumstances of MSCs were mainly mediated through the integrin (specifically, α2β1-and α3β1)-dependent focal adhesions. Because this nanohybrid sample increased the gene expression of integrin clusters (α2, α3, and β1) as well as adaptor proteins (Talin and FAK) that connect integrins to actin filaments 144.
One of the breakthroughs in bone tissue regeneration is biomimetic-based materials such as collagen. It was shown that collagen is an applicable biomaterial because of its Great features such as adhesion, degradability, biocompatibility, osteogenic induction properties, and low immunogenicity 145. As mentioned earlier, integrins including α1β1, α2β1, α3β1, α5β1, and αvβ3 are widely expressed for collagen, and this integrin activity has an essential function in bone differentiation and repair. Prior studies have shown that collagen-based matrices are biologically activated and stimulate cell migration in scaffolds 126. Several types of biomimetic grafts are the combination of collagen and different materials. In such structures like collagen-based hydrogels (CBS), organic materials like chitosan, hyaluronic acid, and alginate, or inorganic materials such as metal, hydroxyapatite (HA), and BG are used 146–151.
To address the impacts of mBGn addition, collagen was mixed with mesoporous bioactive glass nanoparticles (mBGns) with surface amination. The aminated mBGn was added to the Col-mBGn hydrogels to produce highly viable MSCs with improved cytoskeletal extensions 126.
For BMP-2 delivery, Shekaran et al. developed a synthetic PEG hydrogel that is protease-degradable and functionalized with the triple helical, α2β1 integrin-specific peptide (GFOGER). In the absence of BMP-2, GFOGER-functionalized hydrogels significantly ameliorated bone healing in a critical-sized bone lesion compared to RGD-binned hydrogels or untreated defects. GFOGER functionalization was crucial for the BMP-2-dependent healing reaction 152. In another work, Posa et al. used surface nanopatterning in association with BMP-2 covalently attached and integrin selective ligands to control cell adhesion and focal adhesion assembly. Block-copolymer micellar nanolithography was utilized to create gold nanoparticle arrays carrying single BMP-2 dimers, and click chemistry was used to immobilize the azide-functionalized integrin ligands (cyclic-RGD peptides or α5β1 integrin peptidomimetics) on the polyethylene glycol alkyne in the vicinity. These dual-functionalized platforms provide the interaction between integrins and BMP receptors. More generally, they may address the spatial regulation of growth factor and adhesion receptor interactions on biomimetic surfaces 153.
PCL is a common polymer for designing scaffolds for bone regeneration 154. By conjugation of RGD sequence with thermosensitive PCL composite hydrogels, Kim and colleagues studied the adhesion-dependent cellular behavior. In vitro studies showed that RGD-conjugated thermosensitive PCL composite hydrogel extensively enhanced the expression of α2, α5, and β1 as well as the increased expression of FAK in BMSCs. Consequently, the formation of focal adhesions leads to enhanced cytoskeletal reorganization via integrin-mediated cell signaling 155 (Figure. 3). Another study reported that due to the bio-inertness of primarily synthetic biomaterials, the cell adhesion peptide RGD is generally used as a surface-functionalized biomaterial to get better cell adhesion. RGD combined with calcium phosphate cement (CPCs), titanium alloy, and PCL was also reported 156–158.
Fig 3. Functionalized biomaterials influence bone regeneration via integrin interaction.

1. A GBMP1a a peptide that mimics BMP-2, and bound to chitosan with covalent bond. GBMP1a improved the osteogenic differentiation of MSC significantly compared to BMP-2. Cells seeded on Chit-GBMP1a demonstrated amplified mRNA expression levels of vitronectin (VN) and osteopontin (SPP1) coding ECM proteins essential in osteogenic differentiation. In cells cultivated on GBMP1a chitosan matrices, RUNX2 mRNA transcripts have been strongly induced. 2. Methoxy polyethylene glycol-b-polycaprolactone (MP) and RGD-conjugated MP (MP-RGD) with different ranges of RGD content (MP/MP-RGD) are two types of thermosensitive hydrogel systems, which improved expression of integrin (α2, α5, and β1) and elevated the expression of FAK, that leads to cytoskeletal reorganization through integrin-mediated molecular signaling. Receptor tyrosine kinase (RTK) signaling cascades such as mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) for growth factors and phosphoinositide 3-kinase (PI3K) and AKT (protein kinase B) for survival factors. Increased RGD content in MP/MP-RGD hydrogels caused an expressive upregulate in the rate of BMSC proliferation. Microscopic analyses also revealed that the hydrogels ameliorated FAK phosphorylation and subsequently, the organization of the actin filaments of BMSCs is enhanced. Also, MP/MP-RGD hydrogels positively impacted cell proliferation through the FAK- ERK and FAK-AKT signaling cascade (These signaling pathways are shown in part 4 in this figure by integrin α2β1). 3. Hydrogel combining GFOGER and BMP-2, even in low doses, improved bone growth and healing. PEG hydrogels which were cross-linked using protease-degradable bi-cysteine peptides were functionalized with either α2β1 integrin-specific peptide (GFOGER) or an αvβ3 integrin-targeting peptide (RGD). BMP-2 receptor and β3 integrin signaling work together to regulate Smad pathway activation as well as focal adhesion dynamics to manage cell migration and fate commitment. 4. Calcium surface-anchored Collagen-I polydopamine -PLGA/PCL scaffolds (PP/COL I-pDA-Ca). This bioactive synthetic scaffold maintained a 3D porous architecture with interconnected pores that were produced by randomly oriented filamentous fibers. PP/COL I-pDA-Ca meaningfully upregulated some transcription factors such as RUNX2 expression and some integrin subunits such as α10, α11, and β1 integrin expression. The pDA-based Ca chelation, COL I incorporation, and 3D bionic structure supported differentiation, and cell adhesion of MC3T3-E1 osteogenic cells. 5. Protease-degradable poly (ethylene glycol) (PEG) synthetic hydrogel functionalized with a triple helical, α2β1 integrin-specific peptide (GFOGER) as a BMP-2 delivery vehicle. Hydrogels which were functionalized using GFOGER, directed stem cell differentiation in the absence of BMP-2, and caused significant improvement in bone repair within a critical-sized defect compared to empty defects or RGD hydrogels.
PLGA has been widely used in tissue engineering owing to its tunable degradation, good biocompatibility, and exceptional mechanical stiffness and strength 159. In a study by Zhou, et al., the osteogenic differentiation was enhanced through Ca ions chelation, collagen incorporation, and 3D bionic PLGA/PCL nanofiber scaffold architecture. This study has shown the upregulation of α10, α11, and β1 integrins expressively. α1β1, α2β1, α10β1, and α11β1 integrins work as receptors for collagens and they mediate adhesion to collagen type I and/or type II. Zhou, et al., reported that their scaffold provides a promising microenvironment for MC3T3-E1 cells’ adhesion, differentiation, and proliferation that were well created by chelating Ca2+ ions onto the surface of the scaffold. The results revealed that the modified scaffold had excellent cytocompatibility 133. The significant increase in integrin expression suggests that Ca2+ ions assumably favored attaching to collagen I binding sites and cooperated with collagen I to promote cell adhesion 133. This polymeric structure and triggered signaling pathways are shown in Figure 3.
Polyvinyl alcohol (PVA) is a non-toxic, synthetic polymer that is commonly used as a scaffold in bone regeneration. In combination with other polymers, including alginate, gelatin, and polyvinyl pyrrolidone, PVA makes up for its insufficient hydrophilicity and enhances the composite’s mechanical characteristics 160. Abhinandan et al. produced MgB2 nanosheets (MgB2NS), by the ultrasonication exfoliation approach and combined them into a polymeric mix of PVA and alginate (Alg) by the freeze-drying technique. Studies have revealed the important role of metal ions in increasing osteogenesis through the activity of integrins 161, 162. This study revealed that MgB2NS in Al/PVA scaffolds can enhance osteogenesis. Mg2+ ions increase the adhesion and differentiation of osteoblasts through integrin α2β1. Integrin α2β1-mediated cell adhesion activates the FAK-ERK pathway and leads to increased osteogenic gene expression. So the Mg2+ ions enhance osteogenesis via increasing α2β1-mediated cell adhesion 137. Barrioni, et al. showed that Mn2+-containing BGs are not cytotoxic for hMSCs. Also, BGs enhance sustained ion release in the culture medium. They also showed that BGs stimulate osteogenesis, and impact on mineralization. Mn2+ combined BGs can present superior benefits for bone regeneration. Their study also revealed that Mn2+ influences integrin activity in cultured human osteoblasts. The integrin activity mediates osteoblasts interactions with the ECM and it is crucial for cell proliferation, adhesion, and expansion 163. Repairing infected bones is more difficult than normal bones and has a higher risk of failure. Lu et al. created a novel copper (Cu2+) containing natural polymeric scaffold that can enhance fracture healing in infected bone. To avoid uncontrolled polymer cross-linking, they used Cu2+ nanoparticles in the mixture of anionic carboxymethyl chitosan (CMC) and alginate 164. In vitro investigations showed that cell adhesion in the CMC/Alg/Cu scaffolds is more satisfactory compared to the CMC/Alg scaffolds. Cell adhesion is initiated by integrin subunits. The known metal ions can be bound to the integrin receptors and their ligand 165. Few studies have been done on how Cu2+ ions affect cell adherence in scaffolds. Therefore, researchers investigated the expression of the adhesion-related genes in the cells in both scaffolds (with Cu2+ ions and without Cu2+ ions). Excitingly, they discovered that the MC3T3-E1 cells’ cytoskeleton and adherence were better in the CMC/Alg/Cu scaffolds than in the CMC/Alg scaffolds as the attached cells started to multiply and migrate 164.
High mechanical strength and biocompatibility have made Titanium (Ti) substrates suitable basic biomaterials in dental implantology or orthopedics. Surface characteristics of implants are key factors that can significantly affect the osseointegration of Ti implants. Accordingly, the development of chemical surface modification techniques is required to gain more satisfactory implants 166. According to a study by Fraioli et al., titanium can be effectively used to regulate MSC response in vitro and osteogenesis in rat calvarial defects by attaching αvβ3 or α5β1 integrin-selective RGD peptidomimetics to titanium 167.
In another study, Zhang, et al. designed a bone-engineered scaffold through a three-dimensional (3D) printing method. They utilized poly (lactic-co-glycolic acid)/β-calcium phosphate (PLGA/TCP) ink that incorporated Icaritin (ICT) and secretome that was derived from human fetal mesenchymal stem cells (HFS). The designed structure enhances the integrin–FAK–ERK1/2– RUNX2 axis, as well as the recruitment of MSCs, and finally promotes osteogenesis. The combination of ICT and HFS led to the upregulation of the integrin subunit β1 expression and the integrin α3 subunit expression 168. Enhancement osteogenesis using the combination of small molecules (including plant extracts such as Icaritin) together with appropriate scaffolds that support mechanical strength is a smart approach in bone tissue engineering.
5. Discussion
Emphasizing the significance of surface adhesion molecules in tissue engineering and regenerative medicine is highly valuable when devising strategies for the reconstruction of bone and cartilage tissue. Previous studies have often utilized biomaterials with limited cell attachment properties, yielding suboptimal outcomes. In contrast, the extensive body of research explored in this review article underscores the pivotal role of integrins, a subset of cell surface adhesion molecules, in cell signaling and the differentiation of stem cells into osteocytes. Moreover, targeting integrins to induce osteogenic differentiation in stem cells presents an intriguing approach that may obviate the need for biochemical agents or enhance the efficiency of differentiation and tissue repair. By leveraging integrins as key targets, the use of biochemical cues could potentially be minimized, while simultaneously improving the overall efficacy of differentiation processes and tissue regeneration.
β1 integrins play a significant role in the repair and regeneration of the musculoskeletal system, by starting the main signaling pathways that lead to the differentiation, proliferation, movement, and migration of stem cells. They are also important in establishing crosstalk with other receptors, especially growth factor receptors. The result of this crosstalk may have different effects on the intracellular signaling pathways, which can be controlled by different types of interactions. These results give researchers ideas for designing scaffolds. Integrin molecules have special sites where divalent ions can attach. When these ions attach to their binding site, the integrin molecule is converted into an active form, and the ligands can bind to their site. Numerous studies have been sparked by this property of the integrin molecule. Regarding this unique feature of integrins, they could be considered the key targets for designing and fabricating scaffolds for bone regeneration. The effect of Mg2+, Mn2+, and Ca2+ on the activation of the integrin molecule has been proven by several studies, but the precise mechanisms have not yet been determined and require more studies. In addition, many divalent ions haven’t been thoroughly researched but could have new impacts on this molecule. Of course, like many approaches in cell biology, using integrins must be done with caution, and knowing the molecular pathways and processes is vital. Nowadays, it is known that overexpression of integrins may lead to some undesired outcomes such as tumorigenesis. An increased level of β1-integrin has a direct relationship with tumor progression. Figure 3 shows the COX-2 transcription factor that serves as a rate-limiting enzyme in the biosynthesis of Prostaglandins and is induced in inflammatory, tumorigenic, and hypoxic conditions. Prostaglandin E2 (PGE2) increases the migration of chondrosarcoma cells by upregulating α2β1 integrin expression through the EP1/PLC/PKCα/c-Src/NF-κB signal transduction pathway. As mentioned above, the laminin-binding integrin α3β1 which is important for tissue development and function is highly expressed in epithelial tissues. The expression of α3β1 may be determinative in COX2 expression in various types of cancers, and some subtypes of COX2-positive carcinomas might be vulnerable to approaches that target α3β1. Studies show that this transcription factor may lead the cells to become cancerous through its effect on α2β1 and α3β1 integrins.
Taking into account the various advantages and disadvantages of integrins discussed throughout this article, it becomes evident that the significance of these adhesion molecules in the design of novel strategies for tissue engineering and regeneration cannot be overlooked. Their crucial role in cellular functions, ECM interactions, and signaling pathways emphasizes the need for further research to ascertain the most effective utilization of integrins in regenerative techniques, with a particular focus on bone regeneration. By gaining a deeper understanding of integrin-mediated processes and exploring their potential applications, we can pave the way for enhanced approaches to tissue engineering and regenerative medicine. Continued investigation and innovation in this field are essential for unlocking the full potential of integrins in promoting successful tissue regeneration.
Acknowledgment:
L.T. acknowledges the partial support from the National Institute of Dental & Craniofacial Research of the National Institutes of Health under award numbers R56 DE029191 and R15DE027533.
Abbreviation:
- ECM
Extracellular matrix
- FA
Focal adhesion
- VSMCs
Vascular smooth muscle cells
- TSP-1
Thrombospondin 1
- FACIT
Fibril-associated collagen with interrupt in the triple helix
- LFA-1
Lymphocyte function-associated antigen 1
- PMN
Polymorphonuclear neutrophil
- LAPs
Latency-associated peptides
- ICAM-1
Intercellular adhesion molecule-1
- MAdCAM-1
Mucosal addressin cell-adhesion molecule-1
- VWF
Von Willebrand factor
- AAV2
Adeno-associated virus type 2
- AC
Apoptotic-cell
- VSMCs
Vascular smooth muscle cells
- LPAM-1
lymphocyte Peyer’s patch adhesion molecules
- BSP
Bone sialoprotein
- FAK
Focal adhesion kinase
- FN
Fibronectin
- VN
Vitronectin
- BG
Bioactive glasses
Footnotes
Conflicts of interest:
All authors declare that they have no conflicts of interest.
References
- 1.Ludwig BS, Kessler H, Kossatz S, Reuning U. Rgd-binding integrins revisited: How recently discovered functions and novel synthetic ligands (re-) shape an ever-evolving field. Cancers 2021:13(7): 1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong J, Yan L, Zou C, Wang K, Chen M, Xu B, Zhou Z, Zhang D. Integrins regulate stemness in solid tumor: An emerging therapeutic target. Journal of Hematology & Oncology 2021:14(1): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Frontiers in immunology 2019:10(1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mrugacz M, Bryl A, Falkowski M, Zorena K. Integrins: An important link between angiogenesis, inflammation and eye diseases. Cells 2021:10(7): 1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan GS, Salti S, Mourad W. Novel functions of integrins as receptors of cd154: Their role in inflammation and apoptosis. Cells 2022:11(11): 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soe ZY, Park EJ, Shimaoka M. Integrin regulation in immunological and cancerous cells and exosomes. International journal of molecular sciences 2021:22(4): 2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haramshahi SMA, Bonakdar S, Moghtadaei M, Kamguyan K, Thormann E, Tanbakooei S, Simorgh S, Brouki-Milan P, Amini N, Latifi N. Tenocyte-imprinted substrate: A topography-based inducer for tenogenic differentiation in adipose tissue-derived mesenchymal stem cells. Biomedical Materials 2020:15(3): 035014. [DOI] [PubMed] [Google Scholar]
- 8.Kamguyan K, Moghaddam SZ, Nazbar A, Haramshahi SMA, Taheri S, Bonakdar S, Thormann E. Cell-imprinted substrates: In search of nanotopographical fingerprints that guide stem cell differentiation. Nanoscale Advances 2021:3(2): 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih YRV, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. Journal of bone and mineral research 2011:26(4): 730–738. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Chen H, Yang C, Hu Z, Jiang Z, Meng S, Liu R, Huang L, Yang K. Research progress on the regulatory mechanism of integrin-mediated mechanical stress in cells involved in bone metabolism. Journal of Cellular and Molecular Medicine 2024:28(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Ji L, Wang J, Liu C. Matrix stiffness regulates osteoclast fate through integrin-dependent mechanotransduction. Bioactive Materials 2023:27(138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Di C-x, Wang N-n, Chen F, Zhao F, Peng P, Qiu Z-H, Chen Z, Zhang L, Hu L. Mechanobiology of osteoclast. In. Mechanobiology of osteoclast. Bone cell biomechanics, mechanobiology and bone diseases: Elsevier; 2024. [Google Scholar]
- 13.Boppart MD, Mahmassani ZS. Integrin signaling: Linking mechanical stimulation to skeletal muscle hypertrophy. American Journal of Physiology-Cell Physiology 2019:317(4): C629–C641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachmann M, Kukkurainen S, Hytönen VP, Wehrle-Haller B. Cell adhesion by integrins. Physiological reviews 2019:99(4): 1655–1699. [DOI] [PubMed] [Google Scholar]
- 15.Beta Dib K. 2 integrin signaling in leukocytes. Frontiers in Bioscience-Landmark 2000:5(3): 438–451. [DOI] [PubMed] [Google Scholar]
- 16.Hughes ALJJome. Evolution of the integrin α and β protein families. 2001:52(1): 63–72. [DOI] [PubMed] [Google Scholar]
- 17.Jahangiri A, Aghi MK, Carbonell WS. Β1 integrin: Critical path to antiangiogenic therapy resistance and beyond. Cancer research 2014:74(1): 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dib K, Andersson T. Beta 2 integrin signaling in leukocytes. Front Biosci 2000:5(D438–451. [DOI] [PubMed] [Google Scholar]
- 19.Gahmberg CG, Fagerholm SC, Nurmi SM, Chavakis T, Marchesan S, Grönholm M. Regulation of integrin activity and signalling. Biochimica et Biophysica Acta (BBA)-General Subjects 2009:1790(6): 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong J, Yan L, Zou C, Wang K, Chen M, Xu B, Zhou Z, Zhang D. Integrins regulate stemness in solid tumor: An emerging therapeutic target. Journal of Hematology & Oncology 2021:14(1): 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiesser JV, Loudovaris T, Thomas HE, Elefanty AG, Stanley EG. Integrin αvβ5 heterodimer is a specific marker of human pancreatic beta cells. Scientific reports 2021:11(1): 8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarty JH. Αvβ8 integrin adhesion and signaling pathways in development, physiology and disease. Journal of cell science 2020:133(12): jcs239434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remson A, Gabriele S, Surin M. Rôle de la chiralité moléculaire et supramoléculaire sur la migration de cellules épithéliales. 2020. [Google Scholar]
- 24.Wu D, Witt RL, Harrington DA, Farach-Carson MC. Dynamic assembly of human salivary stem/progenitor microstructures requires coordinated α1β1 integrin-mediated motility. Frontiers in Cell and Developmental Biology 2019:7(224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcinkiewicz C, Weinreb PH, Calvete JJ, Kisiel DG, Mousa SA, Tuszynski GP, Lobb RR. Obtustatin: A potent selective inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer research 2003:63(9): 2020–2023. [PubMed] [Google Scholar]
- 26.Madamanchi A Α2β1 integrin in retinopathy and sprouting angiogenesis [dissertation]. 2016. [Google Scholar]
- 27.Wright S, Malinin NL, Powell KA, Yednock T, Rydel RE, Griswold-Prenner I. Α2β1 and αvβ1 integrin signaling pathways mediate amyloid-β-induced neurotoxicity. Neurobiology of aging 2007:28(2): 226–237. [DOI] [PubMed] [Google Scholar]
- 28.Kreidberg JA. Functions of α3β1 integrin. Current opinion in cell biology 2000:12(5): 548–553. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji T. Physiological and pathological roles of α3β1 integrin. The Journal of membrane biology 2004:200(115–132. [DOI] [PubMed] [Google Scholar]
- 30.Fang F, Feng T, Li J, Zhang H, Wang Q, Chen Y, Wang G, Shen Y, Liu X. Cathepsin k contributed to disturbed flow-induced atherosclerosis is dependent on integrin-actin cytoskeleton–nf–κb pathway. Genes & Diseases 2023:10(2): 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baiula M, Spampinato S, Gentilucci L, Tolomelli A. Novel ligands targeting α4β1 integrin: Therapeutic applications and perspectives. Frontiers in Chemistry 2019:7(489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell and tissue research 2010:339(269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myint PK, Ito A, Appiah MG, Obeng G, Darkwah S, Kawamoto E, Gaowa A, Park EJ, Shimaoka M. Irisin supports integrin-mediated cell adhesion of lymphocytes. Biochemistry and Biophysics Reports 2021:26(100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slack R, Macdonald S, Roper J, Jenkins R, Hatley R. Emerging therapeutic opportunities for integrin inhibitors. Nature Reviews Drug Discovery 2022:21(1): 60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Lu F, Chen Y, Plow E, Qin J. Integrin mediates cell entry of the sars-cov-2 virus independent of cellular receptor ace2. Journal of Biological Chemistry 2022:298(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janus-Bell E, Yakusheva A, Scandola C, Receveur N, Ahmed UM, Mouriaux C, Bourdon C, Loubière C, Eckly A, Senis YA. Characterization of the role of integrin α5β1 in platelet function, hemostasis, and experimental thrombosis. Thrombosis and haemostasis 2022:122(05): 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pellegatta M, De Arcangelis A, d'Urso A, Nodari A, Zambroni D, Ghidinelli M, Matafora V, Williamson C, Georges-Labouesse E, Kreidberg J. Α6β1 and α7β1 integrins are required in schwann cells to sort axons. Journal of Neuroscience 2013:33(46): 17995–18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferraz FB, Cunha PM, Fraga HM, de Almeida Filho JL, Hernandez Fernandez J. Α6β1-antagonist peptide downregulates laminin-dependent adhesion and migration in j774a1 macrophages. Available at SSRN 4173658. [Google Scholar]
- 39.Benoit YD, Lussier C, Ducharme PA, Sivret S, Schnapp LM, Basora N, Beaulieu JF. Integrin α8β1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant rhoa/rock-dependent mechanism. Biology of the Cell 2009:101(12): 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kon S, Uede T. The role of α9β1 integrin and its ligands in the development of autoimmune diseases. Journal of Cell Communication and Signaling 2018:12(333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafuste P, Sonnet C, Chazaud B, Dreyfus PA, Gherardi RK, Wewer UM, Authier F-Jm. Adam12 and α9β1 integrin are instrumental in human myogenic cell differentiation. Molecular Biology of the Cell 2005:16(2): 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staniszewska I, Zaveri S, Valle LD, Oliva I, Rothman VL, Croul SE, Roberts DD, Mosher DF, Tuszynski GP, Marcinkiewicz C. Interaction of α9β1 integrin with thrombospondin-1 promotes angiogenesis. Circulation Research 2007:100(9): 1308–1316. [DOI] [PubMed] [Google Scholar]
- 43.Lundgren-Åkerlund E, Aszòdi A. Integrin α10β1: A collagen receptor critical in skeletal development. I Domain Integrins 2014: 61–71. [DOI] [PubMed] [Google Scholar]
- 44.Zeltz C, Lu N, Gullberg D. Integrin α11β1: A major collagen receptor on fibroblastic cells. I Domain Integrins 2014: 73–83. [DOI] [PubMed] [Google Scholar]
- 45.Koivunen J, Tu H, Kemppainen A, Anbazhagan P, Finnilä MA, Saarakkala S, Käpylä J, Lu N, Heikkinen A, Juffer AH. Integrin α11β1 is a receptor for collagen xiii. Cell and tissue research 2021:383(1135–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolte MA, Nolte EN, Margadant C. Integrins control vesicular trafficking; new tricks for old dogs. Trends in biochemical sciences 2021:46(2): 124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz J-N, Zeltz C, Sørensen IW, Barczyk M, Carracedo S, Hallinger R, Niehoff A, Eckes B, Gullberg D. Reduced granulation tissue and wound strength in the absence of α11β1 integrin. Journal of Investigative Dermatology 2015:135(5): 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, Cross B, Nye E, Hart IR, DiPersio CM. Α3β1 integrin–controlled smad7 regulates reepithelialization during wound healing in mice. The Journal of clinical investigation 2008:118(3): 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnittert J, Bansal R, Storm G, Prakash J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Advanced drug delivery reviews 2018:129(37–53. [DOI] [PubMed] [Google Scholar]
- 50.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Current opinion in cell biology 2005:17(5): 509–516. [DOI] [PubMed] [Google Scholar]
- 51.Bouvard D, Brakebusch C, Gustafsson E, Aszódi A, Bengtsson T, Berna A, Fässler R. Functional consequences of integrin gene mutations in mice. Circulation research 2001:89(3): 211–223. [DOI] [PubMed] [Google Scholar]
- 52.Mao L, Wang L, Xu J, Zou J. The role of integrin family in bone metabolism and tumor bone metastasis. Cell death discovery 2023:9(1): 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Docheva D, Popov C, Alberton P, Aszodi A. Integrin signaling in skeletal development and function. Birth Defects Research Part C: Embryo Today: Reviews 2014:102(1): 13–36. [DOI] [PubMed] [Google Scholar]
- 54.Erik H, Arnoud S. Erratum: Integrins in regulation of tissue development and function. J pathol; 200: 471–480. The Journal of Pathology 2003:201(4): 632–641. [DOI] [PubMed] [Google Scholar]
- 55.Tarone G, Hirsch E, Brancaccio M, De Acetis M, Barberis L, Balzac F, Retta S, Botta C, Altruda F, Silengo L. Integrin function and regulation in development. International Journal of Developmental Biology 2004:44(6): 725–731. [PubMed] [Google Scholar]
- 56.Wu JE, Santoro SA. Complex patterns of expression suggest extensive roles for the α2β1 integrin in murine development. Developmental dynamics 1994:199(4): 292–314. [DOI] [PubMed] [Google Scholar]
- 57.Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. Journal of cell science 1997:110(18): 2187–2196. [DOI] [PubMed] [Google Scholar]
- 58.Valat A, Fourel L, Sales A, Machillot P, Bouin A-P, Fournier C, Bosc L, Arboléas M, Bourrin-Reynard I, Wagoner Johnson AJ. Interplay between integrins and cadherins to control bone differentiation upon bmp-2 stimulation. Frontiers in cell and developmental biology 2023:10(1027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin L, He T, Yang D, Wang Y, Li Z, Yan Q, Zhang P, Chen Z, Lin S, Gao H. Osteocyte β1 integrin loss causes low bone mass and impairs bone mechanotransduction in mice. Journal of Orthopaedic Translation 2022:34(60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: The shape of things to come. Biochemical Journal 1999:339(3): 481–488. [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng H, Li X, Chen Y, Zhou R, Zhao H, Qian C. Integrin subunits αv and β3 promote the osteogenic differentiation of umbilical cord blood mesenchymal stem cells. International journal of clinical and experimental pathology 2018:11(4): 2008. [PMC free article] [PubMed] [Google Scholar]
- 62.Hamid HA, Sarmadi VH, Prasad V, Ramasamy R, Miskon A. Electromagnetic field exposure as a plausible approach to enhance the proliferation and differentiation of mesenchymal stem cells in clinically relevant scenarios. J Zhejiang Univ Sci B 2022:23(1): 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarmadi VH, Heng FS, Ramasamy R. The effect of human mesenchymal stem cells on tumour cell proliferation. Med J Malaysia 2008:63 Suppl A(63–64. [PubMed] [Google Scholar]
- 64.Kundu AK, Khatiwala CB, Putnam AJ . Extracellular matrix remodeling, integrin expression, and downstream signaling pathways influence the osteogenic differentiation of mesenchymal stem cells on poly (lactide-co-glycolide) substrates. Tissue Engineering Part A 2009:15(2): 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kundu AK, Khatiwala CB, Putnam AJJTEPA. Extracellular matrix remodeling, integrin expression, and downstream signaling pathways influence the osteogenic differentiation of mesenchymal stem cells on poly (lactide-co-glycolide) substrates. 2009:15(2): 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huveneers S, Danen EH. Adhesion signaling–crosstalk between integrins, src and rho. Journal of cell science 2009:122(8): 1059–1069. [DOI] [PubMed] [Google Scholar]
- 67.Schwab EH, Halbig M, Glenske K, Wagner A-S, Wenisch S, Cavalcanti-Adam EA. Distinct effects of rgd-glycoproteins on integrin-mediated adhesion and osteogenic differentiation of human mesenchymal stem cells. International journal of medical sciences 2013:10(13): 1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in mc3t3-e1 cells. Journal of bone and mineral research 2002:17(1): 101–110. [DOI] [PubMed] [Google Scholar]
- 69.Marie PJ, Haÿ E, Saidak Z. Integrin and cadherin signaling in bone: Role and potential therapeutic targets. Trends in Endocrinology & Metabolism 2014:25(11): 567–575. [DOI] [PubMed] [Google Scholar]
- 70.Dhavalikar P, Robinson A, Lan Z, Jenkins D, Chwatko M, Salhadar K, Jose A, Kar R, Shoga E, Kannapiran A. Review of integrin-targeting biomaterials in tissue engineering. Advanced healthcare materials 2020:9(23): 2000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. Ecm compliance regulates osteogenesis by influencing mapk signaling downstream of rhoa and rock. Journal of bone and mineral research 2009:24(5): 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim S-H, Turnbull J, Guimond S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. Journal of Endocrinology 2011:209(2): 139–151. [DOI] [PubMed] [Google Scholar]
- 73.Zhu X, Assoian RK. Integrin-dependent activation of map kinase: A link to shape-dependent cell proliferation. Molecular Biology of the Cell 1995:6(3): 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Ge C, Franceschi RT. Map kinase-dependent runx2 phosphorylation is necessary for epigenetic modification of chromatin during osteoblast differentiation. Journal of cellular physiology 2017:232(9): 2427–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klees RF, Salasznyk RM, Kingsley K, Williams WA, Boskey A, Plopper GE. Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an erk-dependent pathway. Molecular biology of the cell 2005:16(2): 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou Z, Wang Z, Tao Y, Bai J, Yu B, Shen J, Sun H, Xiao L, Xu Y, Zhou J. Klf2 regulates osteoblast differentiation by targeting of runx2. Laboratory Investigation 2019:99(2): 271–280. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Q, Zuo H, Yu S, Lin Y, Chen S, Liu H, Chen Z. Runx2 co-operates with egr1 to regulate osteogenic differentiation through htra1 enhancers. Journal of cellular physiology 2020:235(11): 8601–8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Long F, Ornitz DM. Development of the endochondral skeleton. Cold Spring Harbor perspectives in biology 2013:5(1): a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu TM, Lee EH. Transcriptional regulatory cascades in runx2-dependent bone development. Tissue Engineering Part B: Reviews 2013:19(3): 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner EF, Karsenty G. Genetic control of skeletal development. Current opinion in genetics & development 2001:11(5): 527–532. [DOI] [PubMed] [Google Scholar]
- 81.Hendesi H, Barbe MF, Safadi FF, Monroy MA, Popoff SN. Integrin mediated adhesion of osteoblasts to connective tissue growth factor (ctgf/ccn2) induces cytoskeleton reorganization and cell differentiation. PloS one 2015:10(2): e0115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jadlowiec J, Koch Hr, Zhang X, Campbell PG, Seyedain M, Sfeir C. Phosphophoryn regulates the gene expression and differentiation of nih3t3, mc3t3-e1, and human mesenchymal stem cells via the integrin/mapk signaling pathway. Journal of Biological Chemistry 2004:279(51): 53323–53330. [DOI] [PubMed] [Google Scholar]
- 83.Golden LH, Insogna KL. The expanding role of pi3-kinase in bone. Bone 2004:34(1): 3–12. [DOI] [PubMed] [Google Scholar]
- 84.Hamidouche Z, Fromigué O, Ringe J, Häupl T, Vaudin P, Pagès J-C, Srouji S, Livne E, Marie PJ. Priming integrin α5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proceedings of the National Academy of Sciences 2009:106(44): 18587–18591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watabe H, Furuhama T, Tani-Ishii N, Mikuni-Takagaki Y. Mechanotransduction activates α5β1 integrin and pi3k/akt signaling pathways in mandibular osteoblasts. Experimental cell research 2011:317(18): 2642–2649. [DOI] [PubMed] [Google Scholar]
- 86.Matter ML, Ruoslahti E. A signaling pathway from the α5β1 and αvβ3 integrins that elevatesbcl-2 transcription. Journal of Biological Chemistry 2001:276(30): 27757–27763. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z, Telci D, Griffin M. Importance of syndecan-4 and syndecan-2 in osteoblast cell adhesion and survival mediated by a tissue transglutaminase− fibronectin complex. Experimental cell research 2011:317(3): 367–381. [DOI] [PubMed] [Google Scholar]
- 88.Huang C, Ogawa R. Mechanotransduction in bone repair and regeneration. The FASEB Journal 2010:24(10): 3625–3632. [DOI] [PubMed] [Google Scholar]
- 89.Chung EH, Gilbert M, Virdi AS, Sena K, Sumner DR, Healy KE. Biomimetic artificial ecms stimulate bone regeneration. Journal of Biomedical Materials Research Part A 2006:79(4): 815–826. [DOI] [PubMed] [Google Scholar]
- 90.Kruger TE, Miller AH, Wang J. Collagen scaffolds in bone sialoprotein-mediated bone regeneration. The Scientific World Journal 2013:2013( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. Journal of Bone and Mineral Research 1997:12(8): 1189–1197. [DOI] [PubMed] [Google Scholar]
- 92.Marie PJ. Targeting integrins to promote bone formation and repair. Nature Reviews Endocrinology 2013:9(5): 288–295. [DOI] [PubMed] [Google Scholar]
- 93.El-Amin SF, Kofron MD, Attawia MA, Lu HH, Tuan RS, Laurencin CT. Molecular regulation of osteoblasts for tissue engineered bone repair. Clinical Orthopaedics and Related Research® 2004:427(220–225. [DOI] [PubMed] [Google Scholar]
- 94.Leucht P, Kim J-B, Currey JA, Brunski J, Helms JA. Fak-mediated mechanotransduction in skeletal regeneration. PLoS One 2007:2(4): e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim J-B, Leucht P, Luppen CA, Park YJ, Beggs HE, Damsky CH, Helms JA. Reconciling the roles of fak in osteoblast differentiation, osteoclast remodeling, and bone regeneration. Bone 2007:41(1): 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fan C, Wu Z, Cooper DM, Magnus A, Harrison K, Eames BF, Chibbar R, Groot G, Huang J, Genth H. Activation of focal adhesion kinase restores simulated microgravity-induced inhibition of osteoblast differentiation via wnt/β-catenin pathway. International Journal of Molecular Sciences 2022:23(10): 5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rhee ST, El-Bassiony L, Buchman SR. Extracellular signal-related kinase and bone morphogenetic protein expression during distraction osteogenesis of the mandible: In vivo evidence of a mechanotransduction mechanism for differentiation and osteogenesis by mesenchymal precursor cells. Plastic and reconstructive surgery 2006:117(7): 2243–2249. [DOI] [PubMed] [Google Scholar]
- 98.Tucci M, Lombardi L, Reshkin S, Cardone RA, Franco S. Alphavbeta3 (αvβ3) integrin drives the osteoclastogenesis through a osteoclast-like functional differentiation of myeloma cells. In. Alphavbeta3 (αvβ3) integrin drives the osteoclastogenesis through a osteoclast-like functional differentiation of myeloma cells: American Society of Hematology; 2007. [Google Scholar]
- 99.Kong L, Wang B, Yang X, He B, Hao D, Yan L. Integrin-associated molecules and signalling cross talking in osteoclast cytoskeleton regulation. Journal of Cellular and Molecular Medicine 2020:24(6): 3271–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo D-y, Chen Z-h, Fu Y-f, Li Y-y, Chen M-n, Wu J-j, Yuan Z-d, Ye J-X, Li X, Yuan F-l. Cilengitide inhibits osteoclast adhesion through blocking the αvβ3-mediated fak/src signaling pathway. Heliyon 2023:9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qiu Z, Li L, Huang Y, Shi K, Zhang L, Huang C, Liang J, Zeng Q, Wang J, He X. Puerarin specifically disrupts osteoclast activation via blocking integrin-β3 pyk2/src/cbl signaling pathway. Journal of Orthopaedic Translation 2022:33(55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee E-J, Kim J-L, Gong J-H, Park S-H, Kang Y-H. Inhibition of osteoclast activation by phloretin through disturbing αvβ3 integrin-c-src pathway. BioMed research international 2015:2015(1): 680145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyazaki T, Miyauchi S, Anada T, Tawada A, Suzuki O. Chondroitin sulfate-e binds to both osteoactivin and integrin αvβ3 and inhibits osteoclast differentiation. Journal of cellular biochemistry 2015:116(10): 2247–2257. [DOI] [PubMed] [Google Scholar]
- 104.Montoya C, Du Y, Gianforcaro AL, Orrego S, Yang M, Lelkes PI. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Research 2021:9(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Malcor J-D, Mallein-Gerin F Biomaterial functionalization with triple-helical peptides for tissue engineering. Acta Biomaterialia 2022. [DOI] [PubMed] [Google Scholar]
- 106.Wubneh A, Tsekoura EK, Ayranci C, Uludağ H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomaterialia 2018:80(1–30. [DOI] [PubMed] [Google Scholar]
- 107.Kowalczewski CJ, Saul JM. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Frontiers in pharmacology 2018:9(513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang M, Zhang Z-C, Yuan F, Chen Y, Deng R-H, Zhang Z-N, Liu Y, Yu J-k. Function and mechanism of rgd in bone and cartilage tissue engineering. Frontiers in Bioengineering and Biotechnology 2021: 1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anselme K Osteoblast adhesion on biomaterials. Biomaterials 2000:21(7): 667–681. [DOI] [PubMed] [Google Scholar]
- 110.Palomino-Durand C, Pauthe E, Gand A. Fibronectin-enriched biomaterials, biofunctionalization, and proactivity: A review. Applied Sciences 2021:11(24): 12111. [Google Scholar]
- 111.Raines AL, Berger MB, Schwartz Z, Boyan BD. Osteoblasts grown on microroughened titanium surfaces regulate angiogenic growth factor production through specific integrin receptors. Acta biomaterialia 2019:97(578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Zhang W, Yao Q. Copper-based biomaterials for bone and cartilage tissue engineering. Journal of Orthopaedic Translation 2021:29(60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fahmy MD, Shah B, Razavi M, Jazayeri H, Fahimipour F, White J, Masri R, Tayebi L. Smart biomaterials for tissue engineering of cartilage. In. Smart biomaterials for tissue engineering of cartilage. Smart materials for tissue engineering; 2017. [Google Scholar]
- 114.Lee YM, Park YJ, Lee SJ, Ku Y, Han SB, Klokkevold PR, Chung CP. The bone regenerative effect of platelet-derived growth factor-bb delivered with a chitosan/tricalcium phosphate sponge carrier. Journal of periodontology 2000:71(3): 418–424. [DOI] [PubMed] [Google Scholar]
- 115.Sawyer AA, Hennessy KM, Bellis SL. The effect of adsorbed serum proteins, rgd and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials 2007:28(3): 383–392. [DOI] [PubMed] [Google Scholar]
- 116.Ruoslahti E. Rgd and other recognition sequences for integrins. Annual review of cell and developmental biology 1996:12(1): 697–715. [DOI] [PubMed] [Google Scholar]
- 117.Massia SP, Hubbell JA. Covalently attached grgd on polymer surfaces promotes biospecific adhesion of mammalian cells a. Annals of the New York Academy of Sciences 1990:589(1): 261–270. [DOI] [PubMed] [Google Scholar]
- 118.Brun P, Zamuner A, Cassari L, D’Auria G, Falcigno L, Franchi S, Contini G, Marsotto M, Battocchio C, Iucci G. Chitosan covalently functionalized with peptides mapped on vitronectin and bmp-2 for bone tissue engineering. Nanomaterials 2021:11(11): 2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Profeta AC, Prucher GM. Bioactive-glass in periodontal surgery and implant dentistry. Dental materials journal 2015:34(5): 559–571. [DOI] [PubMed] [Google Scholar]
- 120.Gerhardt L-C, Boccaccini AR. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010:3(7): 3867–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maciejewska K, Drzazga Z, Kaszuba M. Role of trace elements (zn, sr, fe) in bone development: Energy dispersive x-ray fluorescence study of rat bone and tooth tissue. BioFactors 2014:40(4): 425–435. [DOI] [PubMed] [Google Scholar]
- 122.Hu J, Shao J, Huang G, Zhang J, Pan S. In vitro and in vivo applications of magnesium-enriched biomaterials for vascularized osteogenesis in bone tissue engineering: A review of literature. Journal of Functional Biomaterials 2023:14(6): 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ciaffaglione V, Rizzarelli E. Carnosine, zinc and copper: A menage a trois in bone and cartilage protection. International Journal of Molecular Sciences 2023:24(22): 16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu T, Shi H, Liang Y, Lu T, Lin Z, Ye J. Improving osteogenesis of calcium phosphate bone cement by incorporating with manganese doped β-tricalcium phosphate. Materials Science and Engineering: C 2020:109(110481. [DOI] [PubMed] [Google Scholar]
- 125.Schatkoski VM, do Amaral Montanheiro TL, de Menezes BRC, Pereira RM, Rodrigues KF, Ribas RG, da Silva DM, Thim GP. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceramics International 2021:47(3): 2999–3012. [Google Scholar]
- 126.El-Fiqi A, Lee JH, Lee E-J, Kim H-W. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: Improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering. Acta biomaterialia 2013:9(12): 9508–9521. [DOI] [PubMed] [Google Scholar]
- 127.Lüthen F, Bulnheim U, Müller PD, Rychly J, Jesswein H, Nebe JB. Influence of manganese ions on cellular behavior of human osteoblasts in vitro. Biomolecular engineering 2007:24(5): 531–536. [DOI] [PubMed] [Google Scholar]
- 128.O’Neill E, Awale G, Daneshmandi L, Umerah O, Lo KW-H. The roles of ions on bone regeneration. Drug discovery today 2018:23(4): 879–890. [DOI] [PubMed] [Google Scholar]
- 129.Zheng K, Niu W, Lei B, Boccaccini AR. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomaterialia 2021:133(168–186. [DOI] [PubMed] [Google Scholar]
- 130.Siebers M, Ter Brugge P, Walboomers X, Jansen J. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials 2005:26(2): 137–146. [DOI] [PubMed] [Google Scholar]
- 131.Loeser R, Sadiev S, Tan L, Goldring M. Integrin expression by primary and immortalized human chondrocytes: Evidence of a differential role for α1β1 and α2β1 integrins in mediating chondrocyte adhesion to types ii and vi collagen. Osteoarthritis and cartilage 2000:8(2): 96–105. [DOI] [PubMed] [Google Scholar]
- 132.Tiger C-F, Fougerousse F, Grundström G, Velling T, Gullberg D. Α11β1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Developmental biology 2001:237(1): 116–129. [DOI] [PubMed] [Google Scholar]
- 133.Zhou X, Cheng X, Xing D, Ge Q, Li Y, Luan X, Gu N, Qian Y. Ca ions chelation, collagen i incorporation and 3d bionic plga/pcl electrospun architecture to enhance osteogenic differentiation. Materials & Design 2021:198(109300. [Google Scholar]
- 134.Arimori T, Miyazaki N, Mihara E, Takizawa M, Taniguchi Y, Cabañas C, Sekiguchi K, Takagi J. Structural mechanism of laminin recognition by integrin. Nature communications 2021:12(1): 4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Anderson JM, Li J, Springer TA. Regulation of integrin α5β1 conformational states and intrinsic affinities by metal ions and the admidas. Molecular Biology of the Cell 2022:33(6): ar56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shimaya M, Muneta T, Ichinose S, Tsuji K, Sekiya I. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthritis and cartilage 2010:18(10): 1300–1309. [DOI] [PubMed] [Google Scholar]
- 137.Abhinandan R, Adithya SP, Sidharthan DS, Balagangadharan K, Selvamurugan N. Synthesis and characterization of magnesium diboride nanosheets in alginate/polyvinyl alcohol scaffolds for bone tissue engineering. Colloids and Surfaces B: Biointerfaces 2021:203(111771. [DOI] [PubMed] [Google Scholar]
- 138.Xia D, Yang F, Zheng Y, Liu Y, Zhou Y. Research status of biodegradable metals designed for oral and maxillofacial applications: A review. Bioactive materials 2021:6(11): 4186–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dormond O, Ponsonnet L, Hasmim M, Foletti A, Rüegg C. Manganese-induced integrin affinity maturation promotes recruitment of αvβ3 integrin to focal adhesions in endothelial cells: Evidence for a role of phosphatidylinositol 3-kinase and src. Thrombosis and haemostasis 2004:92(07): 151–161. [DOI] [PubMed] [Google Scholar]
- 140.Xu C, Chen J, Li L, Pu X, Chu X, Wang X, Li M, Lu Y, Zheng X. Promotion of chondrogenic differentiation of mesenchymal stem cells by copper: Implications for new cartilage repair biomaterials. Materials Science and Engineering: C 2018:93(106–114. [DOI] [PubMed] [Google Scholar]
- 141.Alasvand N, Behnamghader A, Milan P, Mozafari M. Synthesis and characterization of novel copper-doped modified bioactive glasses as advanced blood-contacting biomaterials. Materials Today Chemistry 2023:29(101465. [Google Scholar]
- 142.Valente J, Valente TAM, Alves P, Ferreira P, Silva A, Correia I. Alginate based scaffolds for bone tissue engineering. Materials science and engineering: C 2012:32(8): 2596–2603. [Google Scholar]
- 143.Beattie JH, Avenell A. Trace element nutrition and bone metabolism. Nutrition research reviews 1992:5(1): 167–188. [DOI] [PubMed] [Google Scholar]
- 144.Kim H-S, Lee J-H, Mandakhbayar N, Jin G-Z, Kim S-J, Yoon J-Y, Jo SB, Park J-H, Singh RK, Jang J-H. Therapeutic tissue regenerative nanohybrids self-assembled from bioactive inorganic core/chitosan shell nanounits. Biomaterials 2021:274(120857. [DOI] [PubMed] [Google Scholar]
- 145.Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML. Collagen: A review on its sources and potential cosmetic applications. Journal of Cosmetic Dermatology 2018:17(1): 20–26. [DOI] [PubMed] [Google Scholar]
- 146.Vasile C, Pamfil D, Stoleru E, Baican M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020:25(7): 1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Credi C, Biella S, De Marco C, Levi M, Suriano R, Turri S. Fine tuning and measurement of mechanical properties of crosslinked hyaluronic acid hydrogels as biomimetic scaffold coating in regenerative medicine. Journal of the Mechanical Behavior of Biomedical Materials 2014:29(309–316. [DOI] [PubMed] [Google Scholar]
- 148.Jin G-Z, Kim H-W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. Journal of tissue engineering 2018:9(2041731418802438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Norris K, Mishukova OI, Zykwinska A, Colliec-Jouault S, Sinquin C, Koptioug A, Cuenot S, Kerns JG, Surmeneva MA, Surmenev RA. Marine polysaccharide-collagen coatings on ti6al4v alloy formed by self-assembly. Micromachines 2019:10(1): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen L, Hu J, Ran J, Shen X, Tong H. Synthesis and cytocompatibility of collagen/hydroxyapatite nanocomposite scaffold for bone tissue engineering. Polymer Composites 2016:37(1): 81–90. [Google Scholar]
- 151.Sarker B, Hum J, Nazhat SN, Boccaccini AR. Combining collagen and bioactive glasses for bone tissue engineering: A review. Advanced healthcare materials 2015:4(2): 176–194. [DOI] [PubMed] [Google Scholar]
- 152.Shekaran A, García JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, García AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a bmp-2 delivery vehicle. Biomaterials 2014:35(21): 5453–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Posa F, Baha-Schwab EH, Wei Q, Di Benedetto A, Neubauer S, Reichart F, Kessler H, Spatz JP, Albiges-Rizo C, Mori G. Surface co-presentation of bmp-2 and integrin selective ligands at the nanoscale favors α5β1 integrin-mediated adhesion. Biomaterials 2021:267(120484. [DOI] [PubMed] [Google Scholar]
- 154.Wang S, Kempen DH, De Ruiter GC, Cai L, Spinner RJ, Windebank AJ, Yaszemski MJ, Lu L. Molecularly engineered biodegradable polymer networks with a wide range of stiffness for bone and peripheral nerve regeneration. Advanced Functional Materials 2015:25(18): 2715–2724. [Google Scholar]
- 155.Kim HJ, You SJ, Yang DH, Chun HJ, Kim MS. Preparation of novel rgd-conjugated thermosensitive mpeg-pcl composite hydrogels and in vitro investigation of their impacts on adhesion-dependent cellular behavior. Journal of Industrial and Engineering Chemistry 2020:84(226–235. [Google Scholar]
- 156.Attur MG, Dave MN, Clancy RM, Patel IR, Abramson SB, Amin AR. Functional genomic analysis in arthritis-affected cartilage: Yin-yang regulation of inflammatory mediators by α5β1 and αvβ3 integrins. The Journal of Immunology 2000:164(5): 2684–2691. [DOI] [PubMed] [Google Scholar]
- 157.Almonte-Becerril M, Costell M, Kouri JB. Changes in the integrins expression are related with the osteoarthritis severity in an experimental animal model in rats. Journal of Orthopaedic Research 2014:32(9): 1161–1166. [DOI] [PubMed] [Google Scholar]
- 158.Alipour M, Baneshi M, Hosseinkhani S, Mahmoudi R, Jabari Arabzadeh A, Akrami M, Mehrzad J, Bardania H. Recent progress in biomedical applications of rgd-based ligand: From precise cancer theranostics to biomaterial engineering: A systematic review. Journal of Biomedical Materials Research Part A 2020:108(4): 839–850. [DOI] [PubMed] [Google Scholar]
- 159.Wu J, Xu S, Han CC, Yuan G. Controlled drug release: On the evolution of physically entrapped drug inside the electrospun poly (lactic-co-glycolic acid) matrix. Journal of Controlled Release 2021:331(472–479. [DOI] [PubMed] [Google Scholar]
- 160.Teodorescu M, Bercea M, Morariu S. Biomaterials of pva and pvp in medical and pharmaceutical applications: Perspectives and challenges. Biotechnology advances 2019:37(1): 109–131. [DOI] [PubMed] [Google Scholar]
- 161.Li J, Zheng Y, Yu Z, Kankala RK, Lin Q, Shi J, Chen C, Luo K, Chen A, Zhong Q. Surface-modified titanium and titanium-based alloys for improved osteogenesis: A critical review. Heliyon 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu M, Zhuang X, Tang K, Wu P, Guo X, Yin L, Cao H, Zou D. Intrinsic surface effects of tantalum and titanium on integrin α5β1/erk1/2 pathway-mediated osteogenic differentiation in rat bone mesenchymal stromal cells. Cellular Physiology and Biochemistry 2018:51(2): 589–609. [DOI] [PubMed] [Google Scholar]
- 163.Barrioni BR, Norris E, Li S, Naruphontjirakul P, Jones JR, Pereira MdM. Osteogenic potential of sol–gel bioactive glasses containing manganese. Journal of Materials Science: Materials in Medicine 2019:30(7): 1–15. [DOI] [PubMed] [Google Scholar]
- 164.Lu Y, Li L, Zhu Y, Wang X, Li M, Lin Z, Hu X, Zhang Y, Yin Q, Xia H. Multifunctional copper-containing carboxymethyl chitosan/alginate scaffolds for eradicating clinical bacterial infection and promoting bone formation. ACS applied materials & interfaces 2018:10(1): 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang G, Ye J, Wang M, Qi Y, Zhang S, Shi L, Fang Y, Tian Y, Ning G. Copper boron–imidazolate framework incorporated chitosan membranes for bacterial-infected wound healing dressing. Carbohydrate Polymers 2022:291(119588. [DOI] [PubMed] [Google Scholar]
- 166.Lee JS, Lee J-C, Heo JS. Polydopamine-assisted bmp-2 immobilization on titanium surface enhances the osteogenic potential of periodontal ligament stem cells via integrin-mediated cell-matrix adhesion. Journal of cell communication and signaling 2018:12(661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fraioli R, Neubauer S, Rechenmacher F, Bosch B, Dashnyam K, Kim J-H, Perez R, Kim H-W, Gil F, Ginebra M. Control of stem cell response and bone growth on biomaterials by fully non-peptidic integrin selective ligands. Biomaterials science 2019:7(4): 1281–1285. [DOI] [PubMed] [Google Scholar]
- 168.Zhang X, Wang X, Lee Y-w, Feng L, Wang B, Pan Q, Meng X, Cao H, Li L, Wang H. Bioactive scaffold fabricated by 3d printing for enhancing osteoporotic bone regeneration. Bioengineering 2022:9(10): 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Picker J, Lan Z, Arora S, Green M, Hahn M, Cosgriff-Hernandez E, Hook M. Prokaryotic collagen-like proteins as novel biomaterials. Frontiers in bioengineering and biotechnology 2022: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brun P, Zamuner A, Battocchio C, Cassari L, Todesco M, Graziani V, Iucci G, Marsotto M, Tortora L, Secchi V. Bio-functionalized chitosan for bone tissue engineering. International Journal of Molecular Sciences 2021:22(11): 5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu X, Li K, Xie Y, Qi S, Shen Q, Yu J, Huang L, Zheng X. Improved osteogenesis of boron incorporated calcium silicate coatings via immunomodulatory effects. Journal of Biomedical Materials Research Part A 2019:107(1): 12–24. [DOI] [PubMed] [Google Scholar]


