Abstract
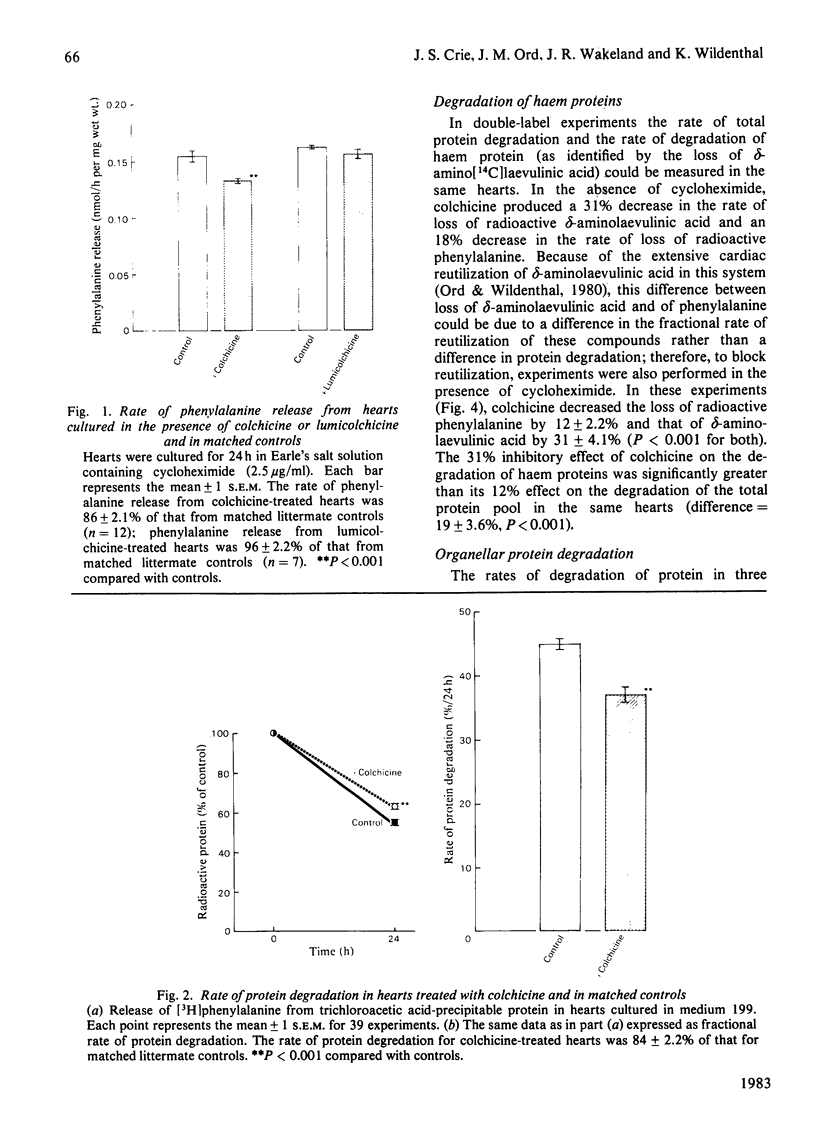
1. The effect of colchicine (2.5 microM) on cardiac protein turnover was tested with foetal mouse hearts in organ culture. 2. Colchicine had no effect on protein synthesis, but inhibited total protein degradation by 12-18%. Lumicolchicine, which lacks colchicine's ability to disaggregate microtubules, but shares its non-specific effects, did not alter protein degradation. 3. The colchicine-induced inhibition of protein degradation was accompanied by significant changes in cardiac lysosomal enzyme activities and distribution. 4. Colchicine inhibited the degradation of organellar proteins, including mitochondrial cytochromes, more than that of cytosolic proteins. 5. Colchicine decreased the rate of myosin degradation and the rate of proteolysis of the total protein pool to a similar extent. Since the regulation of myosin degradation does not involve lysosomes, this suggests that colchicine affects non-lysosomal as well as lysosomal pathways. 6. Release of branched-chain amino acids from colchicine-treated hearts was disproportionately decreased, suggesting that colchicine increased their metabolism. 7. It is concluded that colchicine, via its actions on microtubules, exerts important inhibitory effects on cardiac proteolysis. Colchicine is especially inhibitory to the degradation of organellar proteins, including mitochondrial cytochromes. Its inhibitory effects may be mediated in part via lysosomal mechanisms, but non-lysosomal mechanisms are probably involved as well.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Baccino F. M., Sargus M. J. Cell protein degradation in cultured rat embryo fibroblasts. Suppression by vinblastine of the enhanced proteolysis by serum-deficient media. Biochim Biophys Acta. 1976 Dec 21;451(2):511–516. doi: 10.1016/0304-4165(76)90146-x. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Baccino F. M. Effect of microtubular or translational inhibitors on general cell protein degradation. Evidence for a dual catabolic pathway. Biochem J. 1977 Nov 15;168(2):223–227. doi: 10.1042/bj1680223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arstila A. U., Nuuja I. J., Trump B. F. Studies on cellular autophagocytosis. Vinblastine-induced autophagy in the rat liver. Exp Cell Res. 1974 Aug;87(2):249–252. doi: 10.1016/0014-4827(74)90477-7. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967 Aug;34(2):525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Friedkin M., Rozengurt E. Colchicine inhibits epidermal growth factor degradation in 3T3 cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):480–484. doi: 10.1073/pnas.77.1.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Friderici K. H., Buse J. F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972 Dec 25;247(24):8085–8096. [PubMed] [Google Scholar]
- Decker R. S., Decker M. L., Poole A. R. The distribution of lysosomal cathepsin D in cardiac myocytes. J Histochem Cytochem. 1980 Mar;28(3):231–237. doi: 10.1177/28.3.6986433. [DOI] [PubMed] [Google Scholar]
- Freed J. J., Lebowitz M. M. The association of a class of saltatory movements with microtubules in cultured cells. J Cell Biol. 1970 May;45(2):334–354. doi: 10.1083/jcb.45.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinde B., Seglen P. O. Role of microtubuli in the lysosomal degradation of endogenous and exogenous protein in isolated rat hepatocytes. Hoppe Seylers Z Physiol Chem. 1981 May;362(5):549–556. doi: 10.1515/bchm2.1981.362.1.549. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M., Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3863–3866. doi: 10.1073/pnas.75.8.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D. S., Bache R. J. Transmural distribution of myocardial blood flow during systole in the awake dog. Circ Res. 1976 Jan;38(1):5–15. doi: 10.1161/01.res.38.1.5. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., McGrath P. A., Hale P. T. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978 Nov;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Kolset S. O., Tolleshaug H., Berg T. The effects of colchicine and cytochalasin B on uptake and degradation of asialo-glycoproteins in isolated rat hepatocytes. Exp Cell Res. 1979 Aug;122(1):159–167. doi: 10.1016/0014-4827(79)90570-6. [DOI] [PubMed] [Google Scholar]
- Marzella L., Glaumann H. Increased degradation in rat liver induced by vinblastine. I. Biochemical characterization. Lab Invest. 1980 Jan;42(1):8–17. [PubMed] [Google Scholar]
- Marzella L., Glaumann H. Inhibitory effects of vinblastine on protein degradation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;34(2):111–122. doi: 10.1007/BF02892412. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Mori M., Enomoto K., Satoh M., Onoé T. Translocation of hepatocyte lysosomes following partial hepatectomy and its inhibition by colchicine. Exp Cell Res. 1981 Jan;131(1):25–30. doi: 10.1016/0014-4827(81)90401-8. [DOI] [PubMed] [Google Scholar]
- Ord J. M., Wildenthal K. Increased release of delta-aminolevulinic acid from protein during inhibition of protein synthesis in heart: evidence for the extensive reutilization of heme in cardiac protein metabolism. Biochem Biophys Res Commun. 1980 Mar 28;93(2):577–582. doi: 10.1016/0006-291x(80)91116-x. [DOI] [PubMed] [Google Scholar]
- Ose L., Røken I., Norum K. R., Berg T. The effect of ammonia, chloroquine, leupeptin, colchicine and cytochalasin B on degradation of high density lipoproteins in isolated rat hepatocytes. Exp Cell Res. 1980 Nov;130(1):127–135. doi: 10.1016/0014-4827(80)90049-x. [DOI] [PubMed] [Google Scholar]
- Ostlund R. E., Jr, Pfleger B., Schonfeld G. Role of microtubules in low density lipoprotein processing by cultured cells. J Clin Invest. 1979 Jan;63(1):75–84. doi: 10.1172/JCI109281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr M. A morphometric analysis of microtubules in relation to the inhibition of lysosome movement caused by colchicine. Eur J Cell Biol. 1979 Dec;20(2):189–194. [PubMed] [Google Scholar]
- Schatte C. Congressional budget politics: implications for funding of biomedical research. Fed Proc. 1980 Jan;39(1):3–5. [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E., Grinde B., Gordon P. B., Schwarze P. E., Gjessing R., Poli A. Amino acid control of protein synthesis and degradation in isolated rat hepatocytes. Ann N Y Acad Sci. 1980;349:1–17. doi: 10.1111/j.1749-6632.1980.tb29510.x. [DOI] [PubMed] [Google Scholar]
- Szasz G., Busch E. W., Farohs H. B. Serum-Kreatinkinase. I. Methodische Erfahrungen und Normalwerte mit einem neuen handelsüblichen Test. Dtsch Med Wochenschr. 1970 Apr 10;95(15):829–835. doi: 10.1055/s-0028-1108548. [DOI] [PubMed] [Google Scholar]
- Wildenthal K. Long-term maintenance of spontaneously beating mouse hearts in organ culture. J Appl Physiol. 1971 Jan;30(1):153–157. doi: 10.1152/jappl.1971.30.1.153. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Poole A. R., Dingle J. T. Influence of starvation on the activities and localization of cathepsin D and other lysosomal enzymes in hearts of rabbits and mice. J Mol Cell Cardiol. 1975 Nov;7(11):841–855. doi: 10.1016/0022-2828(75)90135-2. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Wakeland J. R., Ord J. M., Stull J. T. Interference with lysosomal proteolysis fails to reduce cardiac myosin degradation. Biochem Biophys Res Commun. 1980 Sep 30;96(2):793–798. doi: 10.1016/0006-291x(80)91424-2. [DOI] [PubMed] [Google Scholar]


