Abstract
An increasing number of newer targeted oncologic therapies approved for clinical use can cause drug-related pneumonitis. Drug-related pneumonitis can be difficult to diagnose and requires a high index of suspicion. This review serves as an update to a prior review in this journal about pneumonitis with precision oncology therapies. In this review, we focus on the incidence, timing of onset, and imaging patterns of pneumonitis associated with a number of newly approved precision oncologic agents, with a particular focus on new antibody-drug conjugate therapies.
Keywords: pneumonitis, precision oncology, pulmonary adverse event, antibody-drug conjugate
INTRODUCTION
The expansion of targeted oncologic drug therapies has led to substantial therapeutic advances for malignant disease. However, these treatments can result in significant toxicities, including drug-related pneumonitis (DRP).[1] Although symptoms may be ameliorated with drug cessation, the potential risk for developing fatal pneumonitis exists. DRP is difficult to diagnose and requires a high index of clinical suspicion.[1] Often the diagnosis is generally one of exclusion, and therefore requires careful clinical and radiologic evaluation, consideration of the timing of drug exposure in relation to symptom onset, and exclusion of other causes of pulmonary impairment (including infection). The diagnosis of DRP is even more challenging given the emergence of newer targeted oncologic therapies. As an update to our prior review of DRP, this review highlights DRP in newly approved precision oncologic therapies.[2]
CLINICAL EVALUATION
The diagnosis of DRP requires a high index of suspicion because presentation can be subtle and nonspecific. Concerning symptoms such as new or worsening shortness of breath, cough, or pleurisy in the appropriate clinical setting should prompt a computed tomography (CT) scan of the chest. Consultation with a pulmonary specialist may be helpful to evaluate for alternative diagnosis, including infection, thromboembolic disease, cardiac dysfunction, or progression of malignant disease. In select cases, bronchoscopy may be warranted.
LUNG INJURY PATTERNS ON IMAGING
Imaging plays a crucial role in the diagnosis of DRP. The radiographic changes seen in DRP are reflective of a number of potential insults, including cytotoxic injury, oxidative stress, and immune-mediated effects.[3] Because DRP radiographically mimics patterns seen in interstitial lung diseases (ILDs) in the general population, the radiographic injury patterns are often described and categorized similarly.[2] Despite similarities in imaging patterns, the clinical presentation, mechanisms of injury, response to treatment, and long-term prognosis in DRP differ.[4]
CT findings can vary substantially from case to case (Fig. 1). Common findings include ground-glass or consolidative opacities in a bilateral or multilobar distribution with possible interlobular septal thickening. The most common patterns seen on imaging in DRP include organizing pneumonia (OP), nonspecific interstitial pneumonia (NSIP) pattern, hypersensitivity pneumonitis (HP), and acute interstitial pneumonia pattern or acute respiratory distress syndrome (ARDS).[4,5] These radiologic patterns do not necessarily imply a similar clinical course, as compared with when these patterns are seen with sporadic ILDs. A combination of features from these radiographic injury patterns can occur in a single case of DRP.[2,5] For example, Delaunay and colleagues[6] found that up to 10.9% of DRP from immune checkpoint inhibitors had mixed radiographic injury patterns. A lack of a distinctive radiographic DRP pattern can also be observed in 15–35.9% of patients.[6,7]
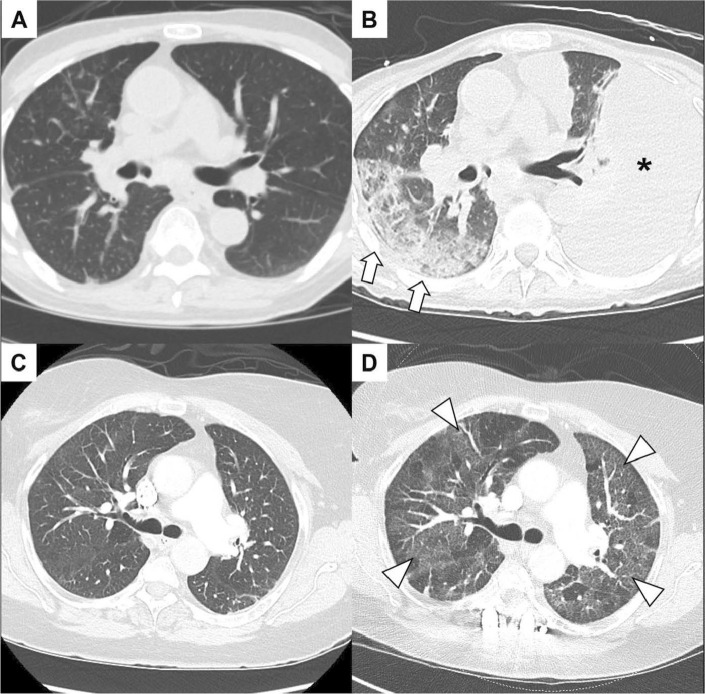
Figure 1.
Examples of different drug-related pneumonitis computed tomography imaging findings. A patient with metastatic lung cancer treated with off-label use of fam-trastuzumab deruxtecan after two cycles (A) and after six cycles (B) with new peripheral infiltrates (arrow) and pleural effusion (asterisk). A patient with metastatic breast cancer on treatment with exemestane and ribociclib with imaging prior to ribociclib (C) and after 4 months (D). Bilateral diffuse ground glass changes noted (arrowheads) with hypoxic respiratory insufficiency.
CLASSIFICATION OF SEVERITY
The severity of drug-induced pneumonitis is classified based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5 (Table 1).[2,8,9] These classifications have significant implications on how therapies are managed before and after DRP resolves. For example, a severe pneumonitis may preclude a drug rechallenge or even a dose reduction, but a grade 1 or 2 pneumonitis may allow for continuation of therapy after dose reduction or drug holiday. In general, guidelines are lacking, in part due to the significant variability in the features of DRP seen with different targeted oncologic therapies. Furthermore, the CTCAE categories are broad. For example, a given patient may have grade 2 DRP whether they clinically have mild, almost imperceptible symptoms or substantial dyspnea with activities of daily living. Similarly, a given patient may have grade 3 DRP whether they have borderline hypoxemia on exertion or have substantial hypoxemia at rest, but do not require intensive care unit monitoring. As such, recommendations that are based on CTCAE grading may be generally inclusive, and clinical decisions should be made on a case-by-case basis as needed with a multidisciplinary approach.
Table 1.
Common Terminology Criteria for Adverse Events classification of drug-induced pneumonitis adverse events[8]
| Grade | Severity | Clinical Findings |
|---|---|---|
| 1 | Asymptomatic | Asymptomatic, only radiographic findings |
| 2 | Symptomatic | Symptomatic, able to perform activities of daily living |
| 3 | Severe symptoms | Symptomatic, unable to perform activities of daily living or requiring oxygen supplementation |
| 4 | Life-threatening respiratory compromise | Life-threatening or ventilator support required |
| 5 | Death related to adverse event |
SPECIFIC TARGETED ONCOLOGY THERAPIES
The following sections discuss the presentations of DRP associated with specific targeted oncologic therapies (Fig. 2).
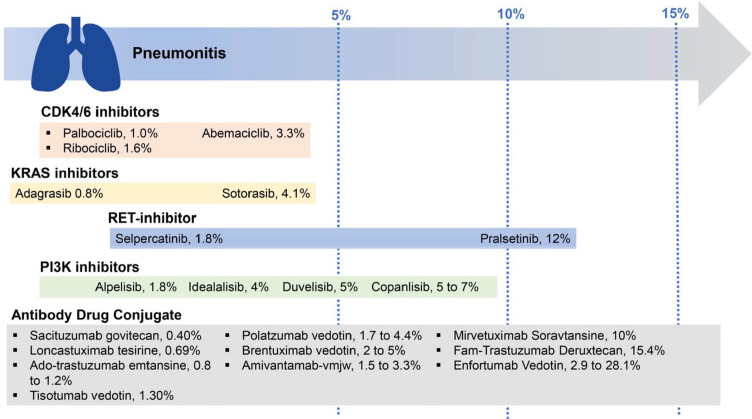
Figure 2.
Rates of drug-related pneumonitis in multiple drug classes reported throughout literature. CDK: cyclin-dependent kinase; KRAS: Kirsten rat sarcoma; PI3K: phosphatidylinositol 3-kinase; RET: rearrangement during transfection.
Antibody Drug Conjugates
Antibody drug conjugates (ADCs) combine the benefits of a potent drug paired with a selective antibody designed to bind a specific tumor antigen target.[10] Highly expressed tumor antigens are often selected to direct on-target toxicity.[10] Pharmacologically, ADCs are similar to prodrugs, requiring cell internalization prior to the release of the cytotoxic drug payload.[11] The creation of ADCs and their early clinical effect have resulted in a shift in the development of novel oncologic therapies.[11]
In our prior review, we discussed two of the earliest US Food and Drug Administration (FDA)–approved ADCs still on the market: brentuximab vedotin, approved in 2011, and ado-trastuzumab emtansine, approved in 2013.[2,11] In the phase 3 AETHERA trial, brentuximab vedotin showed significantly improved progression-free survival compared with placebo treatment in relapsed or primary refractory Hodgkin lymphoma.[12,13] Pneumonitis secondary to brentuximab vedotin has been well described in the literature, and occurrence rate is 2%.[14,15] However, when used in conjunction with bleomycin-containing regimens, pneumonitis rates have been reported in up to 44% of cases.[16]
Ado-trastuzumab emtansine was approved after the phase 3 EMILIA trial showed improvement in progression-free survival and overall survival in patients with HER2-positive metastatic breast cancer.[17,18] Ado-trastuzumab emtansine–induced pneumonitis occurred in 0.8–1.2% of cases, and deaths due to DRP occurred in 0.1–0.2% of cases.[19,20]
Within the last decade, several new ADCs have been approved by the FDA for treatment of various oncologic diseases.[21] Below, we will discuss all current FDA-approved ADCs that have been noted to cause DRP in patients with solid cancers (including fam-trastuzumab deruxtecan, sacituzumab govitecan, enfortumab vedotin, tisotumab vedotin, mirvetuximab soravtansine, and amivantamab-vmjw) and ADCs directed towards hematologic malignancies (including loncastuximab tesirine-lpyl and polatuzumab vedotin-piiq).
Fam-trastuzumab deruxtecan
Fam-trastuzumab deruxtecan was approved in 2022 to treat recurrent unresectable or metastatic HER2-positive breast cancer, unresectable or metastatic non–small cell lung cancer (NSCLC) with HER2 mutations, and gastric or gastroesophageal junction adenocarcinoma previously treated with a trastuzumab-based regimen.[22] A pooled analysis of phase 1 and 2 studies found that DRP due to trastuzumab deruxtecan occurred in 15.4% of treated participants.[23] Pneumonitis occurred within 12 months for 87% of the patients, and the median time of onset was 5.8 months after treatment initiation.[23] Identified factors associated with increased pneumonitis risk included a drug dose of >6.4 mg/kg every 3 weeks, oxygen saturation as measured by pulse oximetry (SpO2) <95% at baseline, moderate to severe renal impairment, lung comorbidities (not otherwise defined), younger age, and Japanese descent.[23] In the phase 3 trials DESTINY-Breast03 and DESTINY-Breast04, pneumonitis occurred in 10.5% and 12.1% of patients respectively.[24] In DESTINY-Breast03, 0.7% of patients had a grade 3 event, with no grade 4 or 5 events.[25] In DESTINY-Breast04, 1.3% of patients had a grade 3 event, and 0.8% of patients had a grade 5 event.[26] Similar to other DRP, reported radiologic patterns after DRP due to trastuzumab deruxtecan can mimic OP, HP, or ARDS.[27] Unlike immune checkpoint inhibitors, it is recommended to permanently discontinue use in patients who develop grade 2 or higher pneumonitis from trastuzumab deruxtecan due to the risk for severe pneumonitis.[22] At grade 1 DRP, it is recommended for drug interruption and consideration for steroid treatment.[27] If DRP resolves to grade 0, then drug rechallenge can be considered.[27] Early identification of DRP secondary to fam-trastuzumab deruxtecan is of high interest due to a high risk of evolution to serious illness or fatality; additionally, early identification (at grade 1) can allow for ongoing treatment with fam-trastuzumab deruxtecan.[28] Further work is needed to determine the safety of restarting fam-trastuzumab deruxtecan with low-grade DRP.
Interestingly, the risk for pneumonitis among trastuzumab-based ADCs may vary based upon the payload. For example, we previously noted that ado-trastuzumab-emtansine causes DRP in about 1% of treated patients.[2] Emerging data from trials of trastuzumab duocarmazine, which currently does not have an FDA-approved indication or treatment, suggest that DRP occurs in about 8% of participants.[29] Both have comparatively lower rates of DRP than fam-trastuzumab deruxtecan. Because HER2 is ubiquitously expressed by numerous lung cells, it is not surprising that trastuzumab-based ADCs often result in DRP.[30] However, the variable rate of DRP despite the common targeting mechanism suggest that the mechanism of DRP may be primarily driven by the payload and not the HER2 target, particularly because HER2 inhibition may actually ameliorate lung inflammation in preclinical models.[30] However, the primary underlying mechanism driving DRP in ADCs is incompletely understood and further work is needed to understand the mechanism of DRP with trastuzumab-based ADCs. In particular, preclinical models may be helpful to understand the specific effects of the target (e.g., HER2) and the payload (e.g., deruxtecan) on the risk for lung epithelial injury across several different ADCs.
Sacituzumab govitecan
Sacituzumab govitecan was approved for use in recurrent unresectable locally advanced or metastatic triple negative breast cancer and recurrent locally advanced or metastatic urothelial cancer.[31] This ADC targets human trophoblast cell-surface antigen 2 (Trop-2), which is expressed on a majority of breast cancers.[32] In the phase 3 ASCENT trial, one patient with previous underlying lung comorbidities, including lung metastases and previous radiation-induced lung fibrosis, was found to have grade 3 pneumonitis (0.4%).[32] No grade 1 or 2 pneumonitis events were noted.[32] Interestingly, both govitecan and deruxtecan are topoisomerase I inhibitors, and pneumonitis was commonly seen in regimens involving the topoisomerase I inhibitor irinotecan, particularly in patients with preexisting ILD.[33] However, perhaps because Trop-2 expression is limited to alveolar epithelial cells and the primary function seems to be to drive fetal lung growth,[34] it is possible that the degree of targeting toward normal lung tissues is much lower than with HER-2 inhibitors. The lower rate of pneumonitis with sacituzumab govitecan highlights the complex mechanisms that may underpin the risk for DRP.
Enfortumab vedotin
Enfortumab vedotin, approved for use in recurrent locally advanced or metastatic urothelial cancer, is an ADC directed towards the protein Nectin-4 combined with the tubulin inhibitor vedotin.[35] In phase 2 and 3 trials (EV-201 and EV-301), pneumonitis occurred in 2.9% of patients with 0.8% grade 3–4.[35–38] In cohort 2 of the multicenter phase 2 trial EV-201, 1 of 89 patients had a grade 5 fatal pneumonitis, whereas none were reported in EV-201 cohort 1.[35,37] Median time of onset from drug exposure was 2.7 months.[36] However, in a subanalysis of the South Korean patient population who participated in the EV-201 and EV-301 trials, the incidence of pneumonitis was reported to be much higher at 28.1%.[39] Most DRP events were grade 1 and 2, but 3.1% of patients had grade 3 pneumonitis and 3.1% had grade 5 fatal pneumonitis.[39] The most common imaging pattern was OP (66.7%), followed by NSIP (16.7%).[39] When combined with pembrolizumab, incidence of pneumonitis occurred in 8.9% of patients (3.3% grade 3 and 0.8% grade 5 event) as found in the phase 1 EV-103 trial.[40] The median time of onset after combination exposure was 6 months.[40] It is recommended to permanently discontinue use if a ≥ grade 3 pneumonitis adverse event occurs.[36]
The experience with enfortumab vedotin highlights two nuances. First, the higher incidence of DRP in patients of Asian descent mirrors the observations from patients treated with fam-trastuzumab-deruxtecan,[23] immune checkpoint inhibitors,[24,41] and other targeted oncologic therapies.[42] However, whether differences in DRP by race are seen in all targeted oncologic therapies and the specific mechanisms that may drive these differences in DRP risk are unknown. Because race is a social and not a biological construct, studying the risk of DRP among groups with varying self-reported racial identities will be challenging, because racial identity is not a perfect correlate of biology.[43] Second, the risk of DRP may vary based upon the receipt and timing of other concurrent agents. As noted above, the risk of DRP with enfortumab vedotin was notably higher when combined with pembrolizumab. Similar observations have been noted with osimertinib, in which the risk for DRP was highest in those who underwent osimertinib therapy within 3 months of immune checkpoint blockade.[44]
Tisotumab vedotin
Tisotumab vedotin, a microtubule-disrupting agent linked to the cell-surface–expressed tissue factor, has an FDA-approved indication to treat recurrent or metastatic cervical cancer.[45] In pooled patient safety data across phase 1 and 2 trials for patients treated with tisotumab vedotin monotherapy for cervical cancer, 1.3% of patients developed DRP, including one patient with a grade 5 fatal event.[46] Phase 3 trials are ongoing. It is recommended to permanently discontinue use if a ≥ grade 3 pneumonitis adverse event occurs.[46]
Mirvetuximab soravtansine
Mirvetuximab soravtansine is an ADC of a folate receptor alpha-binding antibody combined with the tubulin targeting agent DM4 and is indicated to treat patients with recurrent FRα-positive epithelial ovarian, fallopian tube, or primary peritoneal cancer.[47] In a pooled patient population across pivotal phase 1 and 3 trials, pneumonitis from mirvetuximab occurred in 10% of patients, with 0.8% of patients developing grade 3 events and 0.2% of patients developing grade 4 events.[48] In the phase 3 FORWARD 1 trial, pneumonitis occurred in 2.9% of patients, with all events graded 1–3,[49] In the phase 3 SORAYA study (NCT04296890) 10% of patients developed pneumonitis.[48] Early studies using mirvetuximab soravtansine in combination therapy appear to have similar pneumonitis rates. A phase 1b study combining mirvetuximab and bevacizumab found that 9% of patients had pneumonitis events, which were all either grade 1 or 2.[50] A small phase 1 study using standard payload mirvetuximab soravtansine combined with gemcitabine in FRα-positive endometrial and breast cancer noted 2 pneumonitis events out of 20 patients, one grade 2 and one grade 3 (10%).[51] The mechanism of DRP with mirvetuximab soravtansine is not known. It is recommended to permanently discontinue use if a ≥ grade 3 pneumonitis adverse event occurs.[48]
Amivantamab-vmjw
Amivantamab-vmjw is a bispecific epidermal growth factor receptor (EGFR) and mesenchymal epithelial transition (MET) receptor directed ADC indicated for treatment in patients with disease progression in advanced non-small cell lung cancer (NSCLC) with EGFR exon 20 insertion mutations.[52,53] Based on the phase 1 dose-escalation and dose-expansion CHRYSALIS study, pneumonitis occurred in 3.3% of patients (0.7% grade 3 pneumonitis) who received amivantamab-vmjw as a single agent.[54] One percent of patients had to permanently discontinue amivantamab-vmjw due to a pneumonitis adverse event.[54] In the phase 3 PAPILLON trial, 2.6% of patients who received amivantamab-vmjw in combination with chemotherapy (carboplatin-pemetrexed) developed grade 3 pneumonitis and required permanent discontinuation of therapy.[52,53] In the phase 3 MARIPOSA-2 study, 1.5% of patients receiving amivantamab-chemotherapy combination developed a pneumonitis adverse event (0.77% ≥ grade 3 pneumonitis) and 2.7% of patients who received amivantamab-lazertinib-chemotherapy combination developed a pneumonitis adverse event (1.9% ≥ grade 3 pneumonitis).[55] It is recommended to immediately hold amivantamab-vmjw in patients with suspected pneumonitis and permanently discontinue if DRP is confirmed.[52]
Loncastuximab tesirine-lpyl
Loncastuximab tesirine-lpyl is an ADC linking anti-CD19 antibody with a pyrrolobenzodiazepine dimer.[56] It is indicated for use in patients with relapsed or refractory large B-cell lymphoma.[57] In the multicenter open-label phase 2 LOTIS-2 trial, one patient had a grade 3 pneumonitis event (0.69%).[56] On the other hand, infectious adverse events were more commonly seen, as might be expected in participants with hematologic malignancies. Distinguishing pneumonias from pneumonitis is challenging and requires a multidisciplinary evaluation,[58] though newer tools may streamline approaches.[59]
Polatuzumab vedotin-piiq
Polatuzumab vedotin-piiq combines an anti-CD79b and an antimitotic agent, MMAE.[60] The multicenter phase 3 POLARIX study compared polatuzumab vedotin-piiq and R-CHP (rituximab, cyclophosphamide, doxorubicin, and prednisone) combination therapy to R-CHOP in diffuse large B-cell lymphoma patients. Based on this trial, drug-induced pneumonitis occurred in 1.7% of patients.[61] A phase 1/2 clinical trial showed that when combined with rituximab, polatuzumab vedotin-piiq caused pneumonitis in 4.4% of patients,[60] again highlighting the effect of concurrent therapies on rates of DRP with targeted oncologic therapies.
Kirsten Rat Sarcoma Inhibitors
Kirsten rat sarcoma viral oncogene homologue (KRAS) is a common oncogene associated with a number of cancers, including NSCLC, pancreatic cancer, and colorectal cancer.[62] KRAS activates multiple downstream pathways including major pathways RAF-MEK-ERK and PI3K-AKT-mTOR.[62] Two KRAS inhibitors have been FDA approved for use in KRAS-mutated locally advanced or metastatic NSCLC who have received at least one prior systemic therapy: sotorasib in 2021 and adagrasib in 2022.[63,64] In pooled patient safety data, pneumonitis occurred in 0.8% and 4.1% of patients in sotorasib and adagrasib respectively.[63,64] All sotorasib-induced pneumonitis events were grade 3–4 at onset, with one fatal event.[63,65] The median time to pneumonitis onset after drug exposure was 2 weeks.[63] Comparatively, 1.4% of adagrasib-induced pneumonitis events were grade 3 or 4, with one fatal case.[64,66] Median time to DRP was 12 weeks after drug exposure in adagrasib.[64] The significant difference in time to DRP onset between the two KRAS inhibitors suggests that the mechanism of DRP may be independent of the target of inhibition (KRAS), but possibly due to differences in immunogenicity between the two agents. Drug therapy had to be discontinued in 0.6% to 0.8% of patients taking sotorasib and adagrasib respectively secondary to adverse pneumonitis events.[63,64] It is recommended to stop KRAS inhibitor therapy if pneumonitis is confirmed at any stage.[63,64]
Phosphatidylinositol 3-Kinase Inhibitors
The phosphatidylinositol 3-kinase (PI3K) pathway is vital for regulation of cell survival and proliferation.[67] Excess activation of this pathway is considered to be a hallmark of many different cancers.[67] Several PI3K medications have been approved by the FDA, including idelalisib, duvelisib, copanlisib, and alpelisib. Umbralisib received accelerated approval from the FDA but was subsequently withdrawn and removed from the U.S. market.[68,69]
Idelalisib
Idelalisib is a PI3Kδ inhibitor approved for use in relapsed chronic lymphocytic leukemia (CLL) in combination with rituximab.[69,70] Idelalisib carries an FDA black box warning for pneumonitis.[70] Across randomized clinical trials for CLL patients, idelalisib given with rituximab resulted in a rate of severe or fatal pneumonitis in 4% of patients.[71] Three fatalities were attributed to DRP due to idelalisib (<0.5%).[71] In a phase 2 trial studying idelalisib in indolent non-Hodgkin lymphoma, drug discontinuation was required in 2% of patients due to severe pneumonitis adverse effects.[72] Time from drug exposure to DRP ranged from <1 month to 15 months.[70] CT imaging findings consistent with HP and OP patterns have been reported.[73] The mechanism of action of DRP with idelalisib is unclear, though some have theorized that idelalisib preferentially inhibits T-regulatory cells, leading to a rise in effector T cells, which may induce injury to healthy organs.[74,75] Although other rituximab-containing regimens are not associated with a high rate of DRP, it is possible that the two agents synergistically increase the rate of DRP.[73] Such a synergy was seen in a phase 2 study of idelalisib given with the Syk inhibitor entospletinib, in which grade 3 or higher DRP occurred in 17% of patients, five of whom required mechanical ventilation and two of whom later died.[76,77] Because the clinical presentations of pneumonia and pneumonitis can be similar and pneumonia is a common complication of hematologic malignancy, a thorough evaluation for opportunistic infection is necessary before initiating treatment. It is recommended to interrupt idelalisib if pneumonitis is suspected until the cause is determined and to discontinue idelalisib at any grade of symptomatic pneumonitis.[71]
Duvelisib
Duvelisib is a dual PI3Kδ and PI3Kγ inhibitor that is approved for use in patients with relapsed or refractory CLL and small lymphocytic lymphoma (SLL) after at least two prior therapies.[67,78] Duvelisib also carries an FDA black box warning for pneumonitis due to serious, including fatal, pneumonitis adverse events occurring in 5% of patients.[78] Fatal pneumonitis occurred in <1% of patients.[78] On average the median time to pneumonitis event after drug exposure was 4 months.[78] In two phase 1 duvelisib trials, pneumonitis was reported in 4–9% of patients with no dose-dependent relationship.[79,80] In the phase 3 DUO trial, pneumonitis occurred in 3% of CLL and SLL patients with 2% of patients requiring discontinuation of therapy due to adverse pneumonitis effects.[81] As with idelalisib, the exact mechanism of toxicity is unclear.
Copanlisib
Copanlisib is a pan–class I PI3K inhibitor approved for use in patients with follicular lymphoma who have received at least two prior systemic therapies.[69,82] Across pooled patient safety data, pneumonitis occurs in about 5% of patients treated with copanlisib monotherapy.[82] In the 2-year follow-up analysis of the phase 2 CHRONOS-1 study, pneumonitis occurred in 6.3% of patients, with 1.4% having a grade 3 event.[83] In the phase 3 CHRONOS-3 trial, copanlisib in combination with rituximab resulted in pneumonitis in 7% of patients with 2.9% ≥ grade 3 and one death (<1%).[84] Discontinuation of copanlisib was required in 6% of patients due to adverse pneumonitis events.[84] It is not clear whether the difference in DRP incidence between copanlisib and idelalisib is due to a difference in the PI3K isoforms that are inhibited, or whether it is a difference in immunogenicity of the drug itself. It is recommended to permanently discontinue therapy if grade 2 reoccurs or if ≥ grade 3 pneumonitis adverse event occurs.[82]
Alpelisib
Alpelisib is a selective PI3Kα inhibitor approved for use in HR+/HER2−, PIK3CA-mutated advanced or metastatic breast cancer.[84,85] In pooled patient safety data, pneumonitis occurred in 1.8% of patients.[85] In a phase 3 trial, 1.4% of HR+/HER2− breast cancer patients developed a serious adverse pneumonitis event requiring discontinuation of therapy.[73] It is recommended to permanently discontinue therapy in all patients with confirmed pneumonitis.[85]
Cyclin-Dependent Kinase 4 and 6 Inhibitors
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors prevent progression of cell cycle division by acting at the G1-to-S cell cycle checkpoint.[86] Currently three CDK4/6 inhibitors are approved for use in HR+/HER2− advanced or metastatic breast cancer in combination with endocrine therapy: palbociclib, ribociclib, and abemaciclib.[86] Currently, abemaciclib is the only CDK4/6 inhibitor approved for adjuvant setting in early breast cancer at high risk of recurrence.[87] Abemaciclib is preferred for patients with breast cancer that has primary endocrine resistance compared with ribociclib or palbociclib.[88]
Across phase 2 and 3 clinical trials, pneumonitis occurred in 1.0% (0.1% of grade 3–4 and no grade 5 events) in patients treated with palbociclib and endocrine combination therapy.[89] Across phase 3 trials, ribociclib in combination with endocrine therapy resulted in pneumonitis at 1.6% (0.4% grade 3–4 and 0.1% fatal adverse events).[90] Pneumonitis occurred in 3.3% of patients (0.6% grade 3–4 and 0.4% fatal adverse events) treated with abemaciclib for advanced or metastatic breast cancer across phase 2 and 3 trials.[91] It is notable, however, that in the phase 3 trials abemaciclib was used in combination with endocrine therapy but in the phase 2 trial abemaciclib was used as monotherapy.[86] In the phase 3 monarchE trial, 3% of early high-risk breast cancer patients treated with abemaciclib and endocrine therapy developed an adverse pneumonitis event (0.4% grade 3–4 and 0.1% fatal events).[91,92] In postmarketing analysis, CKD4/6-induced pneumonitis in breast cancer patients occurred at 2.1% for abemaciclib and 0.3% for both ribociclib and palbociclib.[93] Most cases occurred in Asia and in patients >65 years of age. Death occurred in 29% of pneumonitis cases. The median timing between drug exposure and DRP ranged from 50 to 250 days depending on the specific CDK4/6 drug.[93] In case reports, CDK4/6-induced pneumonitis generally appeared on CT imaging as bilateral ground glass opacities, but no specific pattern was associated.[94] In a pooled meta-analysis of phase 2 or 3 trials involving over 16,000 patients with breast cancer randomized to either CDK4/6 inhibitors or placebo, the overall incidence of DRP in all CDK4/6 inhibitors was 1.6%, compared with 0.7% in the placebo arms.[95] CDK4/6 inhibitors were associated with a twofold increase in any-grade DRP and a threefold increase in ≥ grade 3 DRP compared with placebo treatment.[95] The mechanism of action of DRP due to CDK4/6 inhibitors is unclear, but it is suspected that drugs in this class may induce cellular senescence and increase tissue inflammation.[96] Abemaciclib, in particular, may be associated with higher rates of DRP due to lower specificity for CDK4/6 and a broader inhibition of other CDKs.[97] In general, it is recommended to discontinue therapy in recurrent grade 2 symptomatic or ≥ grade 3 pneumonitis.[89–91] Converting treatment from abemaciclib to one with less risk of pneumonitis may be a clinical consideration but may also enhance permissive toxicity; thus, alternating to another agent in the same class would be deferred to the oncology team to discern on a case-by-case basis.
Trilaciclib
Trilaciclib is a CDK4/6 inhibitor approved for use prior to specific chemotherapy regimens in small cell lung cancer to help reduce chemotherapy-induced myelosuppression.[98] In clinical trials to date, there has been 1 patient with an adverse pneumonitis event (0.4%).[98] No specific CT imaging pattern has been associated with trilaciclib-induced pneumonitis.
Selective Rearrangement During Transfection Tyrosine Kinase Inhibitors
Rearrangement during transfection (RET) fusion mutations are present in 1–2% of NSCLC and 10–20% of thyroid cancers, resulting in downstream cell proliferation.[99,100] This makes them an interesting target for oncologic therapy. Within the last 5 years, two selective RET inhibitors have been approved for use based on early trials: pralsetinib and selpercatinib. Based on the phase 1/2 ARROW trial, 12% of patients with RET-fusion–positive NSCLC treated with pralsetinib monotherapy developed DRP (3.3% grade 3–4 and 0.2% fatal event),[101,102] and 2.5% of patients had to discontinue therapy as a result of a pneumonitis complication.[103] Based on the phase 1/2 LIBRETTO-001 trial, 1.8% of patients treated with selpercatinib monotherapy developed DRP (0.3% grade 3–4 and 0.3% fatal event).[104,105] No specific radiologic patterns have been associated with pneumonitis secondary to specific RET inhibitors. The mechanism of action of RET inhibitor–induced DRP is not clear. Phase 3 trials are ongoing. It is recommended to discontinue therapy in recurrent grade 2 symptomatic or ≥ grade 3 pneumonitis adverse events secondary to selective RET inhibitors.[101,104]
AREAS OF UNCERTAINTY
In cancers with targetable driver mutations, targeted therapies are preferably used over conventional chemotherapy or other nontargeted therapies, and therefore DRP related to targeted therapies poses a significant challenge because this may exclude certain therapies permanently in individuals. Several key questions remain unanswered. First, it is not clear when it is safe to reintroduce targeted therapies in low-grade DRP. Second, the optimal management of high-grade DRP is unclear because there are no prospective data to suggest that more intense immunosuppression or longer periods of immunosuppression may be beneficial in treating DRP. Third, it is not clear whether DRP related to a given agent increases the likelihood of DRP with an agent in the same therapeutic class, in part because it is not clearly defined whether DRP is due to the mechanism of action of certain drugs or rather a hypersensitivity reaction to the drug itself. These questions can be answered only with well-conducted prospective clinical trials, but are crucial to the understanding of optimal management of DRP.
CONCLUSION
An increasing number of targeted oncologic therapies are used in clinical practice and many of these are associated with DRP. DRP may range from mild to potentially severe and life-threatening respiratory complications. Clinicians should have a high index of suspicion in select patients on these novel agents with evidence of new pulmonary impairment. A multidisciplinary approach is crucial, and pulmonary subspecialists can aid in prompt diagnostic evaluation and initiation of therapies after exclusion of other disease processes. Further investigation into the mechanisms of action for targeted oncologic therapies that induce DRP is needed and likely will continue to evolve, and results from ongoing and upcoming trials will need to be integrated to improve current therapeutic algorithms.
References
- 1.Nishino M, Hatabu H, Hodi FS, Ramaiya NH. Drug-related pneumonitis in the era of precision cancer therapy. JCO Precis Oncol. 2017;1:PO.17.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain A, Shannon VR, Sheshadri A. Pneumonitis after precision oncology therapies: a concise review. J Immunother Precis Oncol. 2018;1:26–37. [Google Scholar]
- 3.Erasmus JJ, McAdams HP, Rossi SE. High-resolution CT of drug-induced lung disease. Radiol Clin North Am. 2002;40:61–72. [DOI] [PubMed] [Google Scholar]
- 4.Johkoh T, Lee KS, Nishino M, et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: a position paper from the Fleischner Society. Chest. 2021;159:1107–1125. [DOI] [PubMed] [Google Scholar]
- 5.Kalisz KR, Ramaiya NH, Laukamp KR, Gupta A. Immune checkpoint inhibitor therapy–related pneumonitis: patterns and management. Radiographics. 2019;39:1923–1937. [DOI] [PubMed] [Google Scholar]
- 6.Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50:1700050. [DOI] [PubMed] [Google Scholar]
- 7.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti–programmed death-1/programmed death ligand 1 therapy. JCO. 2017;35:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. U.S. Department of Health and Human Services, National Institutes of Health. Published Nov 27, 2017. Accessed Dec 3, 2023. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf [Google Scholar]
- 9.Skeoch S, Weatherley N, Swift AJ, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med. 2018;7:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducry L, ed. Antibody-Drug Conjugates. Vol 1045. Humana Press; 2013. 10.1007/978-1-62703-541-5 [DOI] [Google Scholar]
- 11.Olivier KJ, Hurvitz SA, eds. Antibody‐Drug Conjugates: Fundamentals, Drug Development, and Clinical Outcomes to Target Cancer. Wiley; 2016. 10.1002/9781119060727 [DOI] [Google Scholar]
- 12.Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862. [DOI] [PubMed] [Google Scholar]
- 13.Scott LJ. Brentuximab vedotin: a review in CD30-positive Hodgkin lymphoma. Drugs. 2017;77:435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adcetris (brentuximab vedotin) . Package insert. US Food and Drug Administration. Revised Nov 2022. Accessed Sep 1, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2022/125388s106lbl.pdf [Google Scholar]
- 15.Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14:1348–1356. [DOI] [PubMed] [Google Scholar]
- 16.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378:331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016;17:e254–e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadcyla (ado-trastuzumab emtansine). Package insert. US Food and Drug Administration. Revised May 2019. Accessed Sep 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/125427s105lbl.pdf [Google Scholar]
- 20.Hackshaw MD, Danysh HE, Singh J, et al. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2020;183:23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao MZ, Lu D, Kågedal M, et al. Model‐informed therapeutic dose optimization strategies for antibody–drug conjugates in oncology: what can we learn from US Food and Drug Administration–approved antibody–drug conjugates? Clin Pharmacol Ther. 2021;110:1216–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ENHERTU (fam-trastuzumab deruxtecan-nxki) . Package insert. US Food and Drug Administration. Revised Jan 2021. Accessed Sep 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761139s011lbl.pdf [Google Scholar]
- 23.Powell CA, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7:100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rugo HS, Crossno CL, Gesthalter YB, et al. Real-world perspectives and practices for pneumonitis/interstitial lung disease associated with trastuzumab deruxtecan use in human epidermal growth factor receptor 2–expressing metastatic breast cancer. JCO Oncol Pract. 2023;19:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–1154. [DOI] [PubMed] [Google Scholar]
- 26.Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swain SM, Nishino M, Lancaster LH, et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis—focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev. 2022;106:102378. [DOI] [PubMed] [Google Scholar]
- 28.Henning JW, Brezden-Masley C, Gelmon K, et al. Managing the risk of lung toxicity with trastuzumab deruxtecan (T-DXd): a Canadian perspective. Curr Oncol. 2023;30:8019–8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerji U, Van Herpen CML, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–1135. [DOI] [PubMed] [Google Scholar]
- 30.Mishra R, Foster D, Vasu VT, et al. Cigarette smoke induces human epidermal receptor 2–dependent changes in epithelial permeability. Am J Respir Cell Mol Biol. 2016;54:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trodelvy (sacituzumab govitecan-hziy) . Package insert. US Food and Drug Administration. Revised Jun 2022. Accessed Sep 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2022/761115s023lbl.pdf [Google Scholar]
- 32.Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa Y, Koda K, Akahori D, et al. Preexisting interstitial lung disease and lung injury associated with irinotecan in patients with neoplasms. Anticancer Res. 2018;38:5937–5941. [DOI] [PubMed] [Google Scholar]
- 34.McDougall ARA, Hooper SB, Zahra VA, et al. The oncogene Trop2 regulates fetal lung cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2011;301:L478-L489. [DOI] [PubMed] [Google Scholar]
- 35.Yu EY, Petrylak DP, O’Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:872–882. [DOI] [PubMed] [Google Scholar]
- 36.Padcev (enfortumab vedotin-ejfv). Package insert. US Food and Drug Administration. Revised Apr 2023. Accessed Sep 20, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761137s018.pdf [Google Scholar]
- 37.Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37:2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon S, Shin SJ, Kim HC, et al. Enfortumab vedotin-related pneumonitis is more common than expected and could lead to acute respiratory failure. Eur J Cancer. 2022;174:81–89. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Rosenberg JE, McKay RR, et al. Study EV-103 dose escalation/cohort A: long-term outcome of enfortumab vedotin + pembrolizumab in first-line (1L) cisplatin-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC) with nearly 4 years of follow-up. JCO. 2023;41(16_suppl):4505. [Google Scholar]
- 41.Liu T, Li S, Ding S, et al. Comparison of post-chemoradiotherapy pneumonitis between Asian and non-Asian patients with locally advanced non-small cell lung cancer: a systematic review and meta-analysis. EClinicalMedicine. 2023;64:102246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suh CH, Park HS, Kim KW, et al. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients. Lung Cancer. 2018;123:60–69. [DOI] [PubMed] [Google Scholar]
- 43.Braveman P, Parker Dominguez T. Abandon “race”: focus on racism. Front Public Health. 2021;9:689462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luu K, Chu A, Chang B. A review of the novel tissue factor antibody-drug conjugate: tisotumab vedotin. J Oncol Pharm Pract. 2023;29:441–449. [DOI] [PubMed] [Google Scholar]
- 46.Tivdak (tisotumab vedotin-tftv). Package insert. US Food and Drug Administration. Revised Sep 2021. Accessed Sep 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761208Orig1s000lbledt.pdf [Google Scholar]
- 47.Porter RL, Matulonis UA. Mirvetuximab soravtansine for platinum-resistant epithelial ovarian cancer. Expert Rev Anticancer Ther. 2023;23:783–796. [DOI] [PubMed] [Google Scholar]
- 48.Elahere (mirvetuximab soravtansine-gynx) . Package insert. US Food and Drug Administration. Revised Nov 2022. Accessed Sep 1, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2022/761310s000lbl.pdf [Google Scholar]
- 49.Moore KN, Oza AM, Colombo N, et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol. 2021;32:757–765. [DOI] [PubMed] [Google Scholar]
- 50.O’Malley DM, Matulonis UA, Birrer MJ, et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2020;157:379–385. [DOI] [PubMed] [Google Scholar]
- 51.Cristea MC, Stewart D, Synold T, et al. A phase I study of mirvetuximab soravtansine and gemcitabine in patients with FRα-positive recurrent ovarian, primary peritoneal, fallopian tube, or endometrial cancer, or triple negative breast cancer. Gynecol Oncol. 2024;182:124–131. [DOI] [PubMed] [Google Scholar]
- 52.Rybrevant (amivantamab-vmjw). Package insert. US Food and Drug Administration. Revised; Mar 2024. Accessed Mar 10, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2024/761210s003lbl.pdf [Google Scholar]
- 53.Zhou C, Tang KJ, Cho BC, et al. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N Engl J Med. 2023;389:2039–2051. [DOI] [PubMed] [Google Scholar]
- 54.Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion–mutated non–small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. JCO. 2021;39:3391–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Passaro A, Wang J, Wang Y, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol. 2024;35:77–90. [DOI] [PubMed] [Google Scholar]
- 56.Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22:790–800. [DOI] [PubMed] [Google Scholar]
- 57.Zynlonta (loncastuximab tesirine-lpyl). Package insert. US Food and Drug Administration. Revised Apr 2021. Accessed Sep 4, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/761196s000lbl.pdf [Google Scholar]
- 58.Sheshadri A, Goizueta AA, Shannon VR, et al. Pneumonitis after immune checkpoint inhibitor therapies in patients with acute myeloid leukemia: a retrospective cohort study. Cancer. 2022;128:2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aminu M, Daver N, Godoy MCB, et al. Heterogenous lung inflammation CT patterns distinguish pneumonia and immune checkpoint inhibitor pneumonitis and complement blood biomarkers in acute myeloid leukemia: proof of concept. Front Immunol. 2023;14:1249511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polivy (polatuzumab vedotin-piiq) . Package insert. US Food and Drug Administration. Revised Apr 2023. Accessed Sep 1, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761121s008lbl.pdf [Google Scholar]
- 61.Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022;386:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang L, Guo Z, Wang F, Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021;6:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lumakras (sotorasib). Package insert. US Food and Drug Administration. Revised Nov 2022. Accessed Sep 18, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2022/214665s002lbl.pdf [Google Scholar]
- 64.Krazati (adagrasib) . Package insert. US Food and Drug Administration. Revised Dec 2022. Accessed Sep 18, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2022/216340s000lbl.pdf [Google Scholar]
- 65.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non–small-cell lung cancer harboring a KRAS G12C mutation. N Engl J Med. 2022;387:120–131. [DOI] [PubMed] [Google Scholar]
- 67.Yu M, Chen J, Xu Z, et al. Development and safety of PI3K inhibitors in cancer. Arch Toxicol. 2023;97:635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.FDA’s cancer advisory committee to evaluate safety of PI3K inhibitors. News release. BioSpace. Apr 20, 2022. Accessed Sep 19, 2023. bit.ly/3L7l4jA
- 69.Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K inhibitors in cancer: clinical implications and adverse effects. Int J Mol Sci. 2021;22:3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zydelig (idelalisib). Package insert. US Food and Drug Administration. Revised Feb 2022. Accessed Sep 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2022/205858s016lbl.pdf [Google Scholar]
- 71.Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56:2779–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haustraete E, Obert J, Diab S, et al. Idelalisib-related pneumonitis. Eur Respir J. 2016;47:1280–1283. [DOI] [PubMed] [Google Scholar]
- 74.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the phosphoinositide 3-kinase p110δ is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. [DOI] [PubMed] [Google Scholar]
- 75.Chellappa S, Kushekhar K, Munthe LA, et al. The PI3K p110δ isoform inhibitor idelalisib preferentially inhibits human regulatory T cell function. J Immunol. 2019;202:1397–1405. [DOI] [PubMed] [Google Scholar]
- 76.Hanlon A, Brander DM. Managing toxicities of phosphatidylinositol-3-kinase (PI3K) inhibitors. Hematology. 2020;2020:346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barr PM, Saylors GB, Spurgeon SE, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016;127:2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Copiktra (duvelisib) . Package insert. US Food and Drug Administration. Revised Jul 2019. Accessed Sep 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/211155s001lbl.pdf [Google Scholar]
- 79.Flinn IW, O’Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Brien S, Patel M, Kahl BS, et al. Duvelisib, an oral dual PI3K-δ,γ inhibitor, shows clinical and pharmacodynamic activity in chronic lymphocytic leukemia and small lymphocytic lymphoma in a phase 1 study. Am J Hematol. 2018;93:1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132:2446–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aliqopa (copanlisib) . Package insert. US Food and Drug Administration. Revised Mar 2023. Accessed Sep 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2023/209936s011lbl.pdf [Google Scholar]
- 83.Dreyling M, Santoro A, Mollica L, et al. Long‐term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2‐year follow‐up of the CHRONOS‐1 study. Am J Hematol. 2020;95:362–371. [DOI] [PubMed] [Google Scholar]
- 84.Matasar MJ, Capra M, Özcan M, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:678–689. [DOI] [PubMed] [Google Scholar]
- 85.Piqray (alpelisib). Package insert. US Food and Drug Administration. Revised May 2022. Accessed Sep 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2022/212526s006lbl.pdf [Google Scholar]
- 86.Shah M, Nunes MR, Stearns V. CDK4/6 inhibitors: game changers in the management of hormone receptor–positive advanced breast cancer? Oncology (Williston Park). 2018;32:216–222. [PMC free article] [PubMed] [Google Scholar]
- 87.Schlam I, Giordano A, Tolaney SM. Interstitial lung disease and CDK4/6 inhibitors in the treatment of breast cancer. Expert Opin Drug Saf. 2023;22:1149–1156. [DOI] [PubMed] [Google Scholar]
- 88.Wang X, Zhao S, Xin Q, et al. Recent progress of CDK4/6 inhibitors’ current practice in breast cancer. Cancer Gene Ther. Published online Feb 26, 2024. 10.1038/s41417-024-00747-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ibrance (palbociclib). Package insert. US Food and Drug Administration. Revised Nov 2019. Accessed Sep 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2019/212436lbl.pdf [Google Scholar]
- 90.Kisqali (ribociclib). Package insert. US Food and Drug Administration. Revised Dec 2022. Accessed Sep 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2022/209092s013,209935s021lbl.pdf [Google Scholar]
- 91.Verzenio (abemaciclib). Package insert. US Food and Drug Administration. Revised Oct 2021. Accessed Sep 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/208716s006s007s008lbl.pdf [Google Scholar]
- 92.Johnston SRD, Toi M, O’Shaughnessy J, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raschi E, Fusaroli M, Ardizzoni A, et al. Cyclin-dependent kinase 4/6 inhibitors and interstitial lung disease in the FDA adverse event reporting system: a pharmacovigilance assessment. Breast Cancer Res Treat. 2021;186:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sarkisian S, Markosian C, Ali Z, Rizvi M. Palbociclib-Induced Pneumonitis: A Case Report and Review of the Literature. Cureus. 2020;12:e8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Ma Z, Sun X, et al. Interstitial lung disease in patients treated with cyclin-dependent kinase 4/6 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Breast. 2022;62:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Birnhuber A, Egemnazarov B, Biasin V, et al. CDK4/6 inhibition enhances pulmonary inflammatory infiltration in bleomycin-induced lung fibrosis. Respir Res. 2020;21:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chong QY, Kok ZH, Bui NLC, et al. A unique CDK4/6 inhibitor: current and future therapeutic strategies of abemaciclib. Pharmacol Res. 2020;156:104686. [DOI] [PubMed] [Google Scholar]
- 98.Cosela (trilaciclib). Package insert. US Food and Drug Administration. Revised Feb 2021. Accessed Sep 19, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2021/214200s000lbl.pdf [Google Scholar]
- 99.Lu C, Wei XW, Zhang YC, et al. Selective RET inhibitors shift the treatment pattern of RET fusion-positive NSCLC and improve survival outcomes. J Cancer Res Clin Oncol. 2023;149:2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thein KZ, Velcheti V, Mooers BHM, et al. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer. 2021;7:1074–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gavreto (pralsetinib). Package insert. US Food and Drug Administration. Revised Aug 2023. Accessed Sep 20, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2023/213721s009lbl.pdf [Google Scholar]
- 102.Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22:959–969. [DOI] [PubMed] [Google Scholar]
- 103.Griesinger F, Curigliano G, Thomas M, et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. 2022;33:1168–1178. [DOI] [PubMed] [Google Scholar]
- 104.Retevmo (selpercatinib). Package insert. US Food and Drug Administration. Revised May 2020. Accessed Sep 20, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2020/213246s000lbl.pdf [Google Scholar]
- 105.Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion–positive non–small-cell lung cancer. N Engl J Med. 2020;383:813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]