Introduction
Immune checkpoint inhibitors (ICIs) have substantially advanced the treatment of patients with malignant melanoma. However, improving therapeutic efficacy requires identifying drug combinations that elicit durable responses without inducing intolerable toxicity. Within that context, selinexor emerges as a possible combination option that has been shown in preclinical studies to enhance the efficacy of ICI therapy. Methods: In this phase 1b study, we investigated selinexor in combination with pembrolizumab in 25 patients with advanced non-uveal melanoma. Patients received selinexor at a dosage of 60 mg taken orally twice weekly, and pembrolizumab intravenously at a dosage of 200 mg every 3 weeks. Results: Despite the high incidence of adverse events (96%), most treatment-related toxicities were manageable with supportive care and dose reductions. The most common adverse events of any grade were nausea (n = 20; 80%), decreased white blood cell count (n = 15; 60%), vomiting (n = 14; 56%), anemia (n = 12; 48%), fatigue (n = 12; 48%), and decreased platelet count (n = 12; 48%). The 10 patients with treatment-naïve evaluable disease had an objective response rate (ORR) of 70% (n = 7, including three patients with complete response), which was significantly higher than that of the 14 patients with prior anti–programmed cell death protein 1 (anti-PD-1) therapy, whose ORR was 7% (n = 1; p = 0.002). Stable disease was observed in two patients (20%) with treatment-naïve disease and seven patients (50%) with prior anti-PD-1 therapy. Conclusion: Selinexor combined with pembrolizumab showed promising antitumor activity in patients with treatment-naïve metastatic melanoma. The toxicity profile of the combination was consistent with that reported for individual agents, with no additional safety concerns.
Keywords: Selinexor, immune checkpoint inhibitors, melanoma
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of patients with unresectable or metastatic malignancies. In patients with treatment-naïve melanoma, single-agent anti–programmed cell death protein 1 (PD-1) therapy with pembrolizumab or nivolumab has yielded response rates of approximately 35%.[1,2] The combination of the anti-CTLA-4 agent ipilimumab with nivolumab can achieve response rates exceeding 50%, but more than 50% of patients develop grade 3 or 4 toxicity requiring permanent discontinuation of the ICI.[2,3] Ongoing clinical trials are determining whether combinations of ICIs with other agents can either increase the response rate in patients with ICI-naïve disease or be used as salvage therapy for patients whose disease progressed on prior ICI therapy.[4–6]
Selinexor (KPT-330) is an investigational first-in-class orally available irreversible potent inhibitor of exportin (XPO1), a nuclear export protein that blocks the export of tumor suppressor proteins and growth regulatory proteins from the nucleus to cytosol and restores tumor-suppressive pathways in vitro.[7,8] This allows cells to undergo selective elimination via apoptosis and potentially helps restore sensitivity to other anti-cancer agents, including chemotherapeutic agents and ICIs.[9] In addition, selinexor has been shown to induce programmed death-ligand 1 (PD-L1) expression in tumor cell lines and have beneficial additive effects when given with anti-PD-1 therapy in syngeneic melanoma mouse models.[10] To the best of our knowledge, the combination of selinexor with ICIs in patients with advanced melanoma has not previously been clinically tested.
In this phase 1 trial, we investigated the safety and clinical outcomes of selinexor in combination with other agents in patients with advanced cancers. Herein, we report the results for patients with advanced melanoma who received selinexor in combination with pembrolizumab.
METHODS
Study Design
We designed an investigator-initiated, single-center phase 1b study of selinexor in combination with chemotherapy or ICIs in patients with advanced cancers (ClinicalTrials.gov Identifier: NCT02419495). The study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center and was conducted in accordance with the Declaration of Helsinki. All participants signed an informed consent before starting the study procedures. The study had multiple arms; patients with malignant melanoma who received treatment with selinexor plus pembrolizumab were enrolled in arm L between June 2016 and March 2020. The primary objective was to establish the safety and tolerability of selinexor in combination with pembrolizumab. The secondary objective was to determine the preliminary efficacy of the combinations.
Eligibility
Arm L included patients with treatment-naïve or ICI-refractory melanoma who were at least 18 years old, had Eastern Cooperative Oncology Group (ECOG) performance status scores of 0 or 1, and had histologically or cytologically confirmed metastatic melanoma. Patients with history of any prior grade 3 or 4 immune-related adverse event, any grade ocular immune-related adverse event from prior immunotherapy, or prior treatment-related toxicity (with the exception of alopecia) that had not resolved to grade 1 or lower were excluded from this study.
Study Procedures
For arm L, the starting selinexor dose was 60 mg taken orally with a light meal twice weekly for 3 weeks in 21-day cycles. Pembrolizumab was administered intravenously at a fixed dose of 200 mg every 3 weeks.[1,11] Patients were treated until disease progression or intolerable toxicity.
Objective response was assessed using Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1).[12] Safety was monitored continuously throughout treatment and for 1 month after the final study drug dose. All adverse events occurring during this time were documented. Toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.[13]
Statistical Analysis
Statistical analyses were performed using SAS version 9.4 and R version 4.0.4. Descriptive data for categorical and continuous variables included frequencies, percentages, medians, and ranges, as appropriate. The objective response rate (ORR) was defined as the proportion of patients who had complete response (CR) or partial response (PR), and the disease-control rate was defined as the proportion of patients with CR, PR, or at least 6 months of stable disease (SD). The Fisher exact test was used to compare best response between patients with treatment-naïve melanoma and those with ICI-refractory disease. Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. OS was defined as the time between the treatment start date and the date of death or last contact. PFS was defined as the time between the treatment start date and the date of disease progression or death or the date of last contact in patients censored for event.
RESULTS
Twenty-five patients with metastatic non-uveal melanoma were enrolled in arm L of the study and received selinexor plus pembrolizumab. The patients’ characteristics are summarized in Table 1. The median age was 65.8 years (range, 39.5–83.0 years). Most patients were male (n = 17; 68%), and most were White (n = 23; 92%). The most common melanoma subtype was cutaneous melanoma (n = 15; 60%), and more than half the patients (n = 13; 52%) had mutations in BRAF (n = 7), NRAS (n = 4), or KIT (n = 2). At treatment initiation, 11 patients (44%) were treatment-naïve, whereas 14 (56%) had received prior anti-PD1 therapy, which was the immediate prior therapy in nine (64%) of these patients. The median number of prior therapies was one (range, 0–10). At the time of data cutoff, 24 patients (96%) had discontinued treatment because of disease progression (n = 13; 54%), toxicity (n = 4; 17%), or consent withdrawal (n = 7; 29%).
Table 1.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 8 (32) |
| Male | 17 (68) |
| Race | |
| White | 23 (92) |
| Other | 2 (8) |
| Melanoma subtype | |
| Acral lentiginous | 3 (12) |
| Cutaneous | 15 (60) |
| Mucosal | 5 (20) |
| Unknown primary | 2 (8) |
| No. of prior systemic therapies | |
| 0 | 11 (44) |
| 1 | 2 (8) |
| 2 | 3 (12) |
| 3 | 3 (12) |
| 4 | 1 (4) |
| 5 | 2 (8) |
| 6 | 1 (4) |
| 8 | 1 (4) |
| 10 | 1 (4) |
| Prior chemotherapy | |
| No | 19 (76) |
| Yes | 6 (24) |
| Prior targeted therapy | |
| No | 21 (84) |
| Yes | 4 (16) |
| Prior anti-PD1 therapy | |
| No | 11 (44) |
| Yes | 14 (56) |
Safety
Most patients (n = 24; 96%) had at least one treatment-related adverse event of any grade, and more than half (n = 15; 60%) had a grade 3 treatment-related adverse event. The most common treatment-related adverse events of any grade were nausea (n = 20; 80%), decreased white blood cell count (n = 15; 60%), vomiting (n = 14; 56%), anemia (n = 12; 48%), fatigue (n = 12; 48%), and decreased platelet count (n = 12; 48%). The most common grade 3 or 4 treatment-related adverse events were decreased white blood cell count (n = 2; 8%), anemia (n = 2; 8%), fatigue (n = 2; 8%), hyponatremia (n = 2; 8%), and decreased neutrophil count (n = 2; 8%) (Table 2). Fourteen patients (56%) required dose reduction, and four patients (17%) discontinued treatment because of toxicity. No dose-limiting toxicity was observed.
Table 2.
Treatment-related adverse events occurring in more than 10% of patients
| Adverse Event | Total, n (%) | Grade 1 or 2, n (%) | Grade 3 or 4, n (%) |
|---|---|---|---|
| Nausea | 20 (80) | 19 (73) | 1 (4) |
| White blood cell count decreased | 15 (60) | 13 (50) | 2 (8) |
| Vomiting | 14 (56) | 14 (56) | – |
| Anemia | 12 (48) | 10 (38) | 2 (8) |
| Fatigue | 12 (48) | 10 (38) | 2 (8) |
| Platelet count decreased | 12 (48) | 11 (42) | 1 (4) |
| Hyponatremia | 11 (44) | 9 (35) | 2 (8) |
| Neutrophil count decreased | 10 (40) | 8 (32) | 2 (8) |
| Anorexia | 9 (36) | 8 (32) | 1 (4) |
| Weight loss | 8 (32) | 8 (32) | – |
| Alanine aminotransferase increased | 5 (20) | 4 (15) | 1 (4) |
| Blurred vision | 5 (20) | 5 (20) | – |
| Creatinine increased | 5 (20) | 5 (20) | – |
| Aspartate aminotransferase increased | 4 (16) | 4 (16) | – |
| Constipation | 4 (16) | 4 (16) | – |
| Dysgeusia | 4 (16) | 4 (16) | – |
| Diarrhea | 3 (12) | 2 (8) | 1 (4) |
| Lymphocyte count decreased | 3 (12) | 2 (8) | 1 (4) |
Efficacy
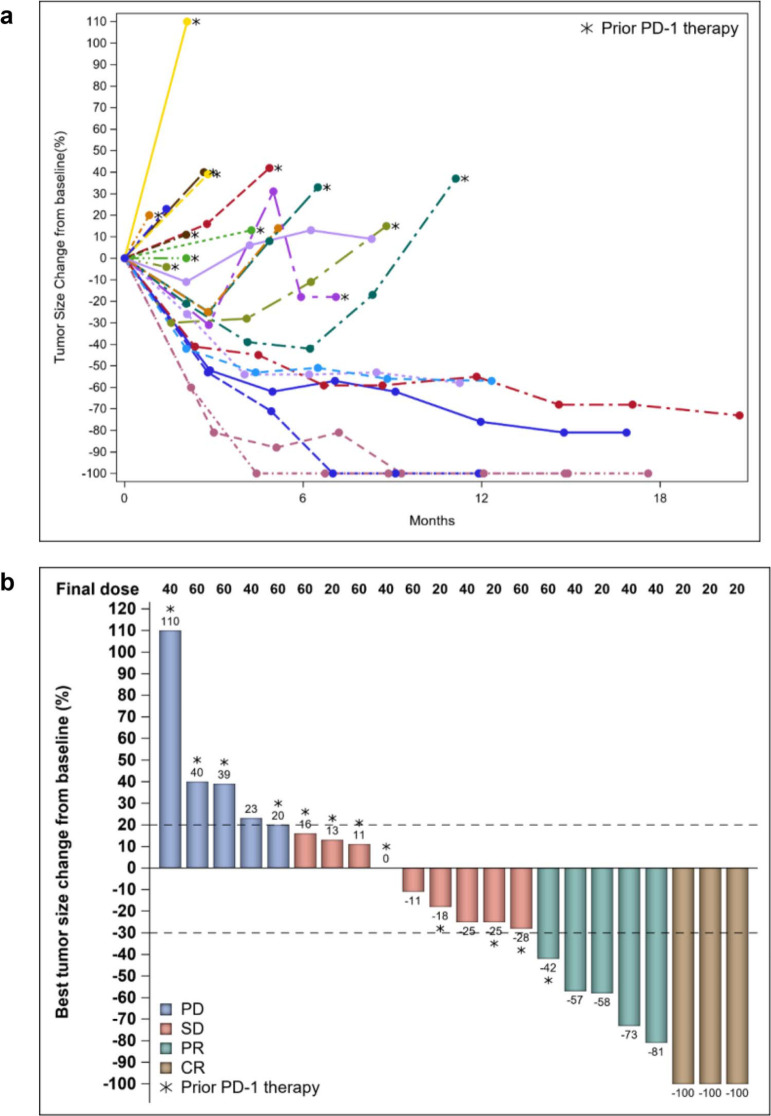
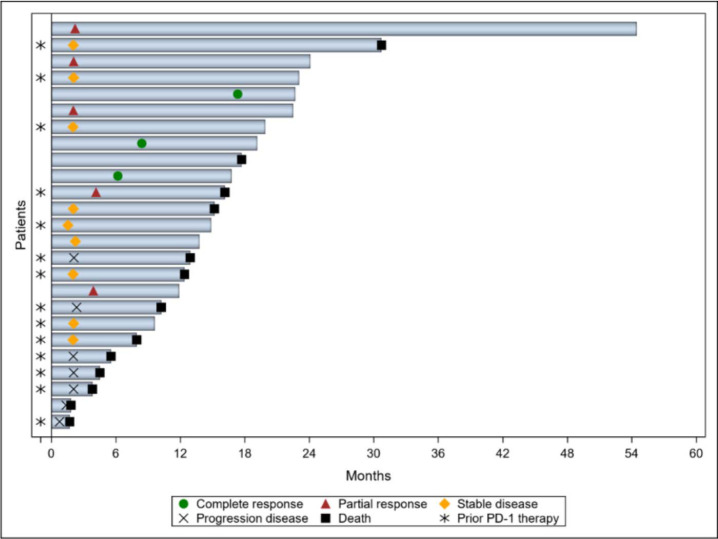
At the time of data cutoff, 24 patients were evaluable for response (Fig. 1). One treatment-naïve patient was not evaluable because of early consent withdrawal. Among the 10 patients with no prior anti-PD-1 therapy, four had PR and three had CR, yielding an ORR of 70%. Additional two patients (20%) had SD with a disease-control rate of 90%. The ORR of patients with no prior anti-PD-1 therapy was significantly higher than that of patients with prior anti-PD-1 therapy, whose ORR was 7% (n = 1; p = 0.002). Among the patients with prior anti-PD-1 therapy, seven (50%) had SD and six (43%) had progressive disease. Among all patients who had a response, the median time to response was 4.0 months (range, 2.0–17.3 months) (Fig. 2). The times to best response for the three patients with CR were 6.2, 8.4, and 17.3 months, respectively.
Figure 1.
(a) Changes in tumor diameters in patients with evaluable disease. (b) Waterfall plot showing best responses in the overall cohort. CR: complete response; PD: progressive disease; PD-1: programmed cell death protein 1; PR: partial response; SD: stable disease.
Figure 2.
Swimmer plot showing responses in patients with evaluable disease.
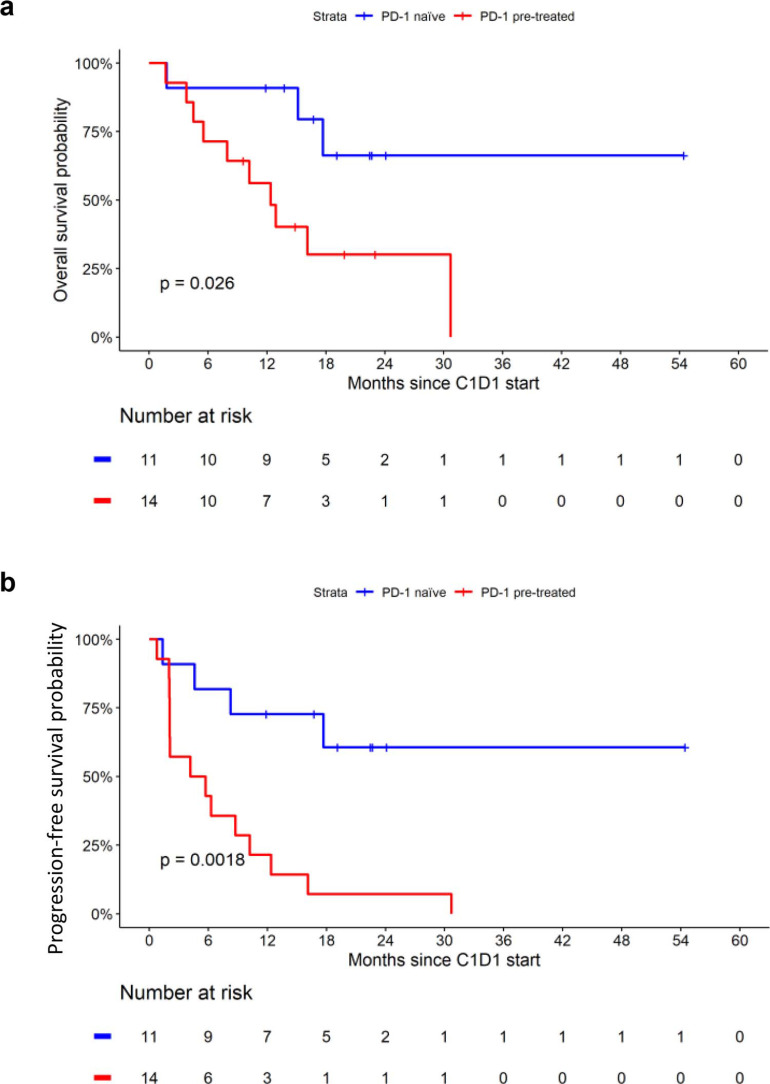
At a median follow-up duration of 22.5 months, the overall cohort had a median OS duration of 17.7 months (95% CI, 10.2–not reached). The 6- and 12-month OS rates were 80% (95% CI, 66%–97%) and 72% (95% CI, 56%–92%), respectively (Fig. 3). The median OS in the PD-1 naïve cohort was not reached compared with 12.4 months in the PD-1 pretreated cohort (p = 0.026). The median PFS duration was 8.8 months (95% CI, 4.2–17.7), and the 6- and 12-month PFS rates were 60% (95% CI, 44%–83%) and 44% (95% CI, 28%–69%), respectively. The median PFS was significantly longer in PD-1-naiive compared with PD-1 pretreated patients (not reached vs 8.8 months, p = 0.0018).
Figure 3.
Kaplan-Meier curves for overall survival (a) and progression-free survival (b) stratified by prior therapy. PD-1: programmed cell death protein 1.
DISCUSSION
ICIs have led to a paradigm change in the clinical management of patients with advanced melanoma.[14,15] The rate of response to pembrolizumab monotherapy is approximately 35% and to nivolumab plus ipilimumab combination approximately 55%.[16,17] However, because nearly half of patients do not derive a clinical benefit from current regimens, developing combinations of ICIs with other agents is needed for improving response rates and survival outcomes. Moreover, such combinations may overcome secondary resistance to anti-PD-1 therapies, which is not infrequent. Our findings demonstrate that a combination of elinexor and pembrolizumab has promising antitumor activity against metastatic melanoma, especially in patients with treatment-naïve disease, with no additional safety concerns to that known of individual drugs.
In the present study, a large proportion of patients who received selinexor in combination with pembrolizumab (96%) had treatment-related adverse events, including a substantial number with grade 3 and 4 events (n = 15); however, most of these events were generally manageable with supportive care and dose reductions. The most common non-hematological adverse events were nausea, vomiting, and fatigue, and the most common hematological adverse events included decreased white blood cell count, anemia, and decreased platelet count, which is consistent with prior reports.[18,19] More than half of the patients (54%) had dose reductions to help with tolerance to treatment.
The treatment-naïve cohort’s ORR of 70% is higher than those reported in previous trials of pembrolizumab alone, nivolumab plus ipilimumab, or nivolumab plus relatlimab,[4,16,17] although this should be interpreted with caution given the small number of patients and trial design of our study. In vitro data suggest that selinexor sensitizes melanoma cell lines to anti-PD-1 and anti-PD-L1 antibodies.[10] This sensitization, in addition to the recognized synergistic antitumor activity of selinexor’s inhibition of XPO1, might explain the high response rate in the present study.[7–9,20–22] Our results are consistent with those reported for other combinations of ICIs and non-immunotherapy agents tested in clinical trials, although again heterogeneity in study designs and sample size may contribute to differences observed in outcomes. For example, in a study of pembrolizumab combined with dabrafenib and/or trametinib, patients receiving the triplet combination had an ORR of 63%, and those receiving the doublet combinations had an ORR of 72%.[23] Similar response rates were reported in the IMPemBra trial, which tested pembrolizumab with dual MAPK pathway inhibition.[24] Notably, those trials included only patients who had targetable molecular alterations, which would restrict clinical benefit to a molecularly selected population that is not inclusive of all patients with malignant melanoma. Given its mechanism of action, selinexor has antitumor activity even in patients with tumors that do not have a specific driver mutated pathway. Previous trials of combinations of nontargeted therapies in such patients had variable results. In one study, for example, patients receiving the combination of pembrolizumab and temozolomide had an ORR of 40%.[25] In another trial of all-trans retinoic acid plus an ICI, the ORR was 71%,[26] which is similar to that in the present study. As a phase 1b study, our trial had the primary objective of evaluating the safety of selinexor-containing combinations; validation of the combination’s efficacy in larger phase 2 trials is warranted. We also found that patients with prior anti-PD-1 therapy, whose treatment remains a therapeutic challenge, had a much lower ORR (7%) than did patients with treatment-naïve disease. In the LEAP-004 trial of the combination of lenvatinib with pembrolizumab, patients who had progressive disease on prior anti-PD-1 or anti-P-DL1 therapy had an ORR of 21.4%, which is also considerably low although relatively better than that reported with selinexor.[27]
Our study had several limitations. For example, the small numbers of patients with treatment-naïve or ICI-refractory disease necessitate a cautious interpretation of the data. In addition, this study was not randomized, and because it lacked a comparison group that did not receive selinexor, we could only compare our results with previously published data. The high response rate in treatment-naïve patients could be related to a favorable selected population.
CONCLUSION
Our study provides a proof-of-concept of the safety and efficacy of selinexor in combination with pembrolizumab for the treatment of patients with metastatic melanoma, particularly those who have treatment-naïve disease. Phase 2 trials of selinexor and pembrolizumab are recommended to validate the preliminary signs of efficacy observed in this study, especially in patients with treatment-naïve disease.
References
- 1.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. [DOI] [PubMed] [Google Scholar]
- 2.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40:127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlino MS, Sandhu S. Safety and efficacy implications of discontinuing combination ipilimumab and nivolumab in advanced melanoma. J Clin Oncol. 2017;35:3792–3793. [DOI] [PubMed] [Google Scholar]
- 4.Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diab A, Tykodi SS, Daniels GA, et al. Bempegaldesleukin plus nivolumab in first-line metastatic melanoma. J Clin Oncol. 2021;39:2914–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo DC, Luo PH, Huang SX, et al. Safety and efficacy of pembrolizumab plus lenvatinib versus pembrolizumab and lenvatinib monotherapies in cancers: a systematic review. Int Immunopharmacol. 2021;91:107281. [DOI] [PubMed] [Google Scholar]
- 7.Inoue H, Kauffman M, Shacham S, et al. CRM1 blockade by selective inhibitors of nuclear export attenuates kidney cancer growth. J Urol. 2013;189:2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salas Fragomeni RA, Chung HW, Landesman Y, et al. CRM1 and BRAF inhibition synergize and induce tumor regression in BRAF-mutant melanoma. Mol Cancer Ther. 2013;12:1171–1179. [DOI] [PubMed] [Google Scholar]
- 9.Senapedis WT, Baloglu E, Landesman Y. Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol. 2014;27:74–86. [DOI] [PubMed] [Google Scholar]
- 10.Farren MR, Hennessey RC, Shakya R, et al. The exportin-1 inhibitor selinexor exerts superior antitumor activity when combined with T-cell checkpoint inhibitors. Mol Cancer Ther. 2017;16:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamid O, Robert C, Daud A, et al. Long-term outcomes in patients with advanced melanoma who had initial stable disease with pembrolizumab in KEYNOTE-001 and KEYNOTE-006. Eur J Cancer. 2021;157:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 13.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. National Cancer Institute, U.S. Department of Health and Human Services, 2021. [Google Scholar]
- 14.Switzer B, Puzanov I, Skitzki JJ, et al. Managing metastatic melanoma in 2022: a clinical review. JCO Oncol Pract. 2022;18:335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curti BD, Faries MB. Recent advances in the treatment of melanoma. New Engl J Med. 2021;384:2229–2240. [DOI] [PubMed] [Google Scholar]
- 16.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med. 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 17.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. New Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavriatopoulou M, Chari A, Chen C, et al. Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials. Leukemia. 2020;34:2430–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.XPOVIO® (selinexor) tablets, for oral use: FDA Packaging Insert. 2022.
- 20.Azmi AS, Uddin MH, Mohammad RM. The nuclear export protein XPO1 - from biology to targeted therapy. Nat Rev Clin Oncol. 2021;18:152–169. [DOI] [PubMed] [Google Scholar]
- 21.Kim E, Mordovkina DA, Sorokin A. Targeting XPO1-dependent nuclear export in cancer. Biochemistry (Mosc). 2022;87(Suppl 1):S178-S70. [DOI] [PubMed] [Google Scholar]
- 22.Landes JR, Moore SA, Bartley BR, et al. The efficacy of selinexor (KPT-330), an XPO1 inhibitor, on non-hematologic cancers: a comprehensive review. J Cancer Res Clin Oncol. 2023;149:2139–2155. [DOI] [PubMed] [Google Scholar]
- 23.Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozeman EA, Versluis JM, Sikorska K, et al. IMPemBra: a phase 2 study comparing pembrolizumab with intermittent/short-term dual MAPK pathway inhibition plus pembrolizumab in patients with melanoma harboring the BRAFV600 mutation. J Immunother Cancer. 2023;11:e006821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu T, Sun W, Xu Y, et al. Combination of pembrolizumab plus temozolomide therapy in unresectable and advanced melanoma: a multicenter retrospective analysis in China. Ann Transl Med. 2021;9:1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobin RP, Cogswell DT, Cates VM, et al. Targeting MDSC differentiation using ATRA: a Phase I/II clinical trial combining pembrolizumab and all-trans retinoic acid for metastatic melanoma. Clin Cancer Res. 2023;29:1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arance A, de la Cruz-Merino L, Petrella TM, et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J Clin Oncol. 2023;41:75–85. [DOI] [PubMed] [Google Scholar]