Abstract
The rapid growth (1-6 days) of the functionally overloaded soleus muscle, in response to tenotomy of the synergist gastrocnemius, was found to correlate with increases in both the protein synthetic and degradative rates, the change in the former being greater than that of the latter. These conclusions were drawn from two different methods used to measure (in vivo and in vitro) the average rates of protein synthesis and protein breakdown in these soleus muscles. Although the basal rates of synthesis were higher when measured in vivo, and the degradative rates higher in isolated muscle preparations incubated in vitro, both methods gave good agreement concerning the changes in protein turnover induced by tenotomy of the gastrocnemius. The possible involvement of passive stretch in inducing this additional growth is discussed. As an antagonist to the soleus, growth of the extensor digitorum longus muscle was decreased under the same conditions, presumably because of less usage. At 3 days after the cutting of the sciatic nerve, the previously normal or overloaded soleus muscles underwent rapid atrophy. Although in both cases RNA and protein were lost, while protein synthesis decreased and protein breakdown increased, denervation induced larger changes within these parameters of the formerly overloaded muscle. The slowing of growth in the tenotomized gastrocnemius, and its subsequent rapid atrophy after additional denervation, were explained by large increases in protein breakdown, with little or no change in the synthetic rate.
Full text
PDF

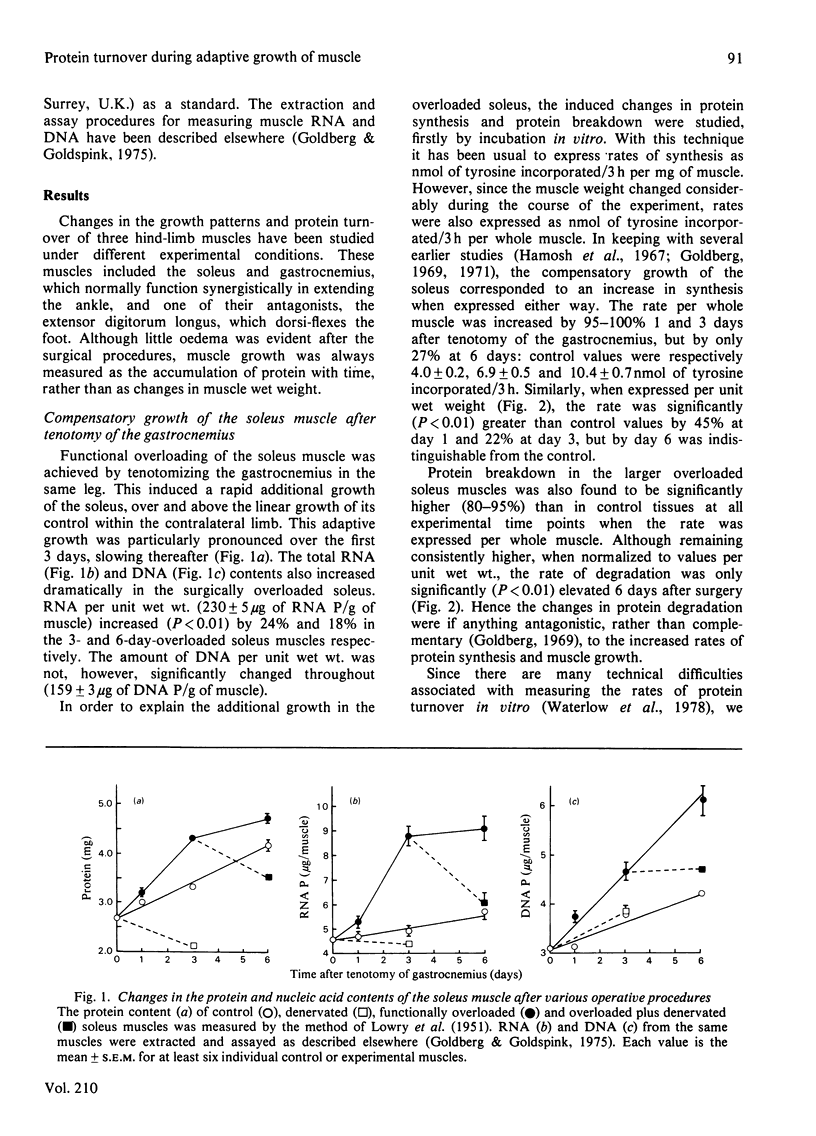






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin K. M., Cheadle W. G., Martinez O. M., Cooke D. A. Effect of functional overload on enzyme levels in different types of skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1977 Feb;42(2):312–317. doi: 10.1152/jappl.1977.42.2.312. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Goldspink D. F. Influence of food deprivation and adrenal steroids on DNA synthesis in various mammalian tissues. Am J Physiol. 1975 Jan;228(1):310–317. doi: 10.1152/ajplegacy.1975.228.1.310. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem. 1969 Jun 25;244(12):3223–3229. [PubMed] [Google Scholar]
- Goldspink D. F. The effects of denervation on protein turnover of rat skeletal muscle. Biochem J. 1976 Apr 15;156(1):71–80. doi: 10.1042/bj1560071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of activity on muscle size and protein turnover. J Physiol. 1977 Jan;264(1):283–296. doi: 10.1113/jphysiol.1977.sp011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977 Jan;264(1):267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink D. F. The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochem J. 1978 Aug 15;174(2):595–602. doi: 10.1042/bj1740595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Hájek I., Vítek V. Compensatory hypertrophy of the latissimus dorsi posterior muscle induced by elimination of the latissimus dorsi anterior muscle of the chicken. Physiol Bohemoslov. 1970;19(6):483–489. [PubMed] [Google Scholar]
- Gutmann E., Schiaffino S., Hanzliková V. Mechanism of compensatory hypertrophy in skeletal muscle of the rat. Exp Neurol. 1971 Jun;31(3):451–464. doi: 10.1016/0014-4886(71)90248-2. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs E. C. The longitudinal division of fibres in overloaded rat skeletal muscle. J Anat. 1970 Nov;107(Pt 3):459–470. [PMC free article] [PubMed] [Google Scholar]
- Hamosch M., Lesch M., Baron J., Kaufman S. Enhanced protein synthesis in a cell-free system from hypertrophied skeletal muscle. Science. 1967 Aug 25;157(3791):935–937. doi: 10.1126/science.157.3791.935. [DOI] [PubMed] [Google Scholar]
- Hník P., Macková E. V., Syrový I., Holas M., Krishna-Reddy V. Contractile properties of muscle undergoing "compensatory" hypertrophy and its increased susceptibility to denervation and reflex atrophy. Pflugers Arch. 1974 Jun 11;349(2):171–181. doi: 10.1007/BF00586627. [DOI] [PubMed] [Google Scholar]
- Ianuzzo C. D., Chen V. Metabolic character of hypertrophied rat muscle. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):738–742. doi: 10.1152/jappl.1979.46.4.738. [DOI] [PubMed] [Google Scholar]
- Jablecki C. K., Heuser J. E., Kaufman S. Autoradiographic localization of new RNA synthesis in hypertrophying skeletal muscle. J Cell Biol. 1973 Jun;57(3):743–759. doi: 10.1083/jcb.57.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolesz F., Sreter F. A. Development, innervation, and activity-pattern induced changes in skeletal muscle. Annu Rev Physiol. 1981;43:531–552. doi: 10.1146/annurev.ph.43.030181.002531. [DOI] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Millward D. J. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J. 1978 Nov 15;176(2):407–417. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. B., Fulks R. M., Goldberg A. L. Evidence that the intracellular pool of tyrosine serves as precursor for protein synthesis in muscle. J Biol Chem. 1973 Oct 25;248(20):7272–7275. [PubMed] [Google Scholar]
- Macková E., Hník P. Compensatory muscle hypertrophy induced by tenotomy of synergists is not true working hypertrophy. Physiol Bohemoslov. 1973;22(1):43–49. [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- McNurlan M. A., Tomkins A. M., Garlick P. J. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979 Feb 15;178(2):373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkin E., Kimata S., Skillman J. J. Myosin synthesis and degradation during development of cardiac hypertrophy in the rabbit. Circ Res. 1972 Jun;30(6):690–702. doi: 10.1161/01.res.30.6.690. [DOI] [PubMed] [Google Scholar]
- Turner L. V., Garlick P. J. The effect of unilateral phrenicectomy on the rate of protein synthesis in rat diaphragm in vivo. Biochim Biophys Acta. 1974 Apr 27;349(1):109–113. doi: 10.1016/0005-2787(74)90013-6. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]


