Abstract
Introduction
Galectin-3 plays critical roles in the adhesion, proliferation, and differentiation of tumor cells. Recent data have suggested that galectin-3 plays a role in the development of hepatocellular carcinoma (HCC); however, its prognostic value has not been validated. The aim of our study was to evaluate the clinical and prognostic value of galectin-3 in patients with HCC.
Methods
We prospectively enrolled and collected clinicopathologic data and serum samples from 767 patients with HCC between 2001 and 2014 at The University of Texas MD Anderson Cancer Center. Two hundred patients without HCC were also enrolled and had data collected. The Kaplan-Meier method was used to estimate overall survival (OS) distributions.
Results
The median OS in this cohort was 14.2 months (95% CI, 12–16.1). At the time of analysis, the 1-year OS rate was 45% (95% CI, 0.4–0.51) among patients with high galectin-3 levels and 59% (95% CI, 0.54–0.63) among patients with low galectin-3 levels. OS was significantly inferior in patients with high galectin-3 levels than in patients with lower galectin-3 levels (median OS: 10.12 vs. 16.49 months; p = 0.0022). Additionally, the multivariate model showed a significant association between high galectin-3 level and poor OS (hazard ratio [HR] = 1.249; 95% CI, 1.005–1.554). Comparison between low ( n = 464 patients) and high ( n = 302 patients) galectin-3 levels showed that mean serum galectin-3 levels were significantly higher in patients with HCC who had hepatitis C virus (HCV) infection ( p = 0.0001), higher Child-Pugh score (CPS) ( p = 0.0009), and higher Cancer of the Liver Italian Program (CLIP) score ( p = 0.0015).
Conclusion
Our study shows that serum galectin-3 level is a valid prognostic biomarker candidate.
Keywords: galectin-3, hepatocellular carcinoma, biomarker, overall survival, prognostic biomarkers
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer, contributing to around 90% of cases. [ 1 ] HCC is the third leading cause of cancer-related death worldwide, accounting for 906,000 new cases and approximately 830,000 deaths in 2020. [ 2 ] The main risk factors include chronic infection, such as chronic infection with hepatitis B and C virus (HBV and HCV). [ 2–4 ] Other risk factors include heavy alcohol intake and excess body weight, which contribute to nonalcoholic fatty liver disease, the most common liver disease with increasing obesity rates worldwide. [ 5 ] Most patients with HCC present with advanced disease and underlying cirrhosis, and thus are not amenable to curative-intent treatments. [ 6 ] Treatment options for patients with locally advanced and metastatic disease are increasing, with systemic targeted and immunotherapy agents such as atezolizumab with bevacizumab and tyrosine kinase inhibitors such as lenvatinib, sorafenib, regorafenib, and cabozantinib demonstrating an overall survival (OS) benefit. [ 1 ] Despite these multiple therapy options, the prognosis for advanced HCC remains poor with a 5-year survival rate of less than 10%. [ 7 ] The poor prognosis may be partly attributed to the lack of biomarkers that can provide a potential prognostic value and therapeutic target. Alpha-fetoprotein (AFP) remains the most commonly used HCC biomarker despite the lack of validation as an independent predictive or prognostic factor. [ 8 ]
Galectin-3 is a member of the galectin family and plays multiple roles in different biological functions such as the adhesion, proliferation, and differentiation of cancer cells; tumor progression; and metastasis. [ 9 ] It has been reported to suppress tumor cell apoptosis, with high levels of galectin-3 present in patients with solid cancers, such as breast and gastric cancer. [ 10 ] Galectin-3 plays a critical role in inflammation and fibrosis-related liver disease [ 11 ] and has been reported to be significantly elevated in patients with advanced hepatic fibrosis and chronic liver diseases. [ 12 ] Notably, serum galectin-3 levels were significantly higher in patients with HCC than in patients with chronic hepatitis and healthy volunteers, [ 13–17 ] highlighting its potential association with HCC development. Additionally, galectin-3 expression was reported to be significantly higher in the tumor versus adjacent hepatic tissues. [ 10 , 18–21 ]
This study aimed to evaluate the association between serum galectin-3 and clinicopathologic features, HCC staging systems, and OS in patients with HCC to determine its potential utility as a prognostic biomarker. Additionally, this study looked at the potential use of galectin-3 in the diagnosis of HCC.
METHODS
Patients and Specimens
This cohort study enrolled patients with HCC treated at MD Anderson Cancer Center between 2001 and 2014, and the study was approved by the institutional review board. A control group of patients without HCC were also enrolled. Informed written consent was obtained from all patients before study commencement. The clinicopathologic data and serum samples were collected on the first clinic visit before any treatment was given.
Standard characteristics of contrast-enhanced cross-sectional imaging or pathologic examination via biopsy were the two accepted means for the diagnosis of HCC in our cohort. The following patient characteristics were recorded at the time of blood collection: HCC risk factors, liver nodules, size of tumors, tumor grade and differentiation, the presence of macrovascular invasion, and extrahepatic metastasis. Several widely used classification systems for HCC staging were used: (1) the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) system [ 22 ] ; (2) the Barcelona Clinic Liver Cancer (BCLC) [ 23 ] ; and (3) the Cancer of the Liver Italian Program (CLIP) [ 24 ] . Additionally, several biomarker scoring systems were established by our group and used, such as insulin-like growth factor 1 (IGF-1) level, [ 25 ] IGF-1 score, [ 26 ] and HepatoScore-14, [ 7 ] which have been able to dramatically refine patient prognostic assessments and therapeutic decision-making and enrollment in clinical trials. [ 7 ]
Measurement of Serum Galectin-3
Serum galectin-3 (ng/mL) was measured by Myriad RBM (Austin, TX), a Clinical Laboratory Improvement Amendments–certified biomarker testing laboratory. A multiplexed immunoassay panel (DiscoveryMAP v.3.3; Myriad RBM) was used to quantitate galectin-3 on an automated, Luminex xMAP-based platform (Austin, TX). All results are given in ng/mL.
Statistical Analysis
Descriptive statistics and oncologic outcomes
Chi-square test was used to evaluate the correlation between galectin-3 and patients’ characteristics. The Kaplan-Meier method was used to estimate OS distributions. A p < 0.05 was considered statistically significant. R software 4.1.1 was used for analysis.
Prognosis analysis
There is no agreed upon cutoff value for galectin-3 as a prognostic biomarker. As such, both the 50th percentile (median) and the 60th percentile (slightly higher than median) of galectin-3 levels were tested for their prognostic significance. The former and the latter correspond to values of 6 and 6.6 ng/mL, respectively, and each value was tested as a cutoff between high- and low-galectin level groups. Log-rank test, univariate, and multivariate Cox models were applied to evaluate the association between galectin-3 and OS. We evaluated whether galectin-3 could provide additional prognostic value of OS to each of the existing HCC staging or biomarker scoring systems by fitting Cox models including galectin-3 and each of the existing score systems in each Cox model.
Diagnosis analysis
An independent two-sample t test was used to test whether mean galectin-3 levels were different between patients with HCC and healthy controls. Additionally, receiver operating characteristic (ROC) analysis and Youden index were used to identify the ideal cutoff value for galectin-3 as a diagnostic biomarker.
RESULTS
Our study included 767 patients with HCC, of whom 766 had OS data available for analysis. Table 1 summarizes the patients’ demographic and clinicopathologic characteristics. Fifty-seven percent of patients in the study were older than 60 years, with a male to female ratio of 2.8:1. Vascular invasion was present in 31.4% of patients, and 24.6% had distant metastasis. Cirrhosis was present in 63.7% of patients, and 76.6% had either BCLC stage C or D disease. The median OS for the 766 patients was 14.2 months (95% CI, 12–16.1) with 586 patients having died at the time of analysis.
Table 1.
Demographic and clinicopathologic characteristics and risk factors of 767 patients with hepatocellular cancer
| Variables | Patients with HCC, n (%) |
|---|---|
| Age at diagnosis | |
| ≤ 60 y | 327 (42.6) |
| > 60 y | 440 (57.4) |
| Sex | |
| Male | 567 (73.9) |
| Female | 200 (26.1) |
| Race | |
| White | 514 (67.0) |
| Non-White | 253 (33.0) |
| Hepatitis status | |
| HCV only | 301 (39.2) |
| HBV only | 88 (11.5) |
| HCV and HBV | 111 (14.5) |
| History of cigarette smoking | 498 (64.9) |
| History of alcohol consumption | 560 (73.0) |
| History of diabetes | 271 (35.3) |
| AFP level ≥ 400 ng/dL | 251 (32.7) |
| Presence of vascular invasion | 241 (31) |
| > 50% tumor involvement | 180 (23.5) |
| Distant metastasis | 189 (24.6) |
| Lymph node metastasis | 157 (20.4) |
| Adjacent organ invasion | 27 (3.5) |
| Multinodularity | 474 (61.8) |
| Tumor differentiation | |
| Well differentiated | 193 (25.2) |
| Moderately differentiated | 211 (27.5) |
| Poorly differentiated | 120 (13.0) |
| Fibrolamellar | 13 (1.6) |
| Clear cell | 7 (0.9) |
| Presence of cirrhosis | 489 (63.7) |
| Child-Pugh class | |
| A | 412 (53.7) |
| B | 299 (39.0) |
| C | 56 (7.3) |
| CLIP staging | |
| Stage 0–2 | 485 (63.2) |
| Stage 3–6 | 282 (36.8) |
| BCLC staging | |
| Stage 0–B | 172 (22.4) |
| Stage C–D | 588 (76.6) |
| TNM staging | |
| Stage I–II | 253 (33) |
| Stage IIIA–IIIB | 225 (29.3) |
| Stage IIIC–IVB | 266 (34.7) |
AFP: alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; CLIP: The Cancer of the Liver Italian Program; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; TNM: tumor, node, metastasis.
Patients with HCC and HCV-positive status had significantly higher galectin-3 levels than HCV-negative patients (p < 0.001) (Table 2). Significantly higher galectin-3 levels were also observed in patients with an Eastern Cooperative Oncology Group (ECOG) score of 2 or higher than in those with an ECOG score of 0–1 (p = 0.017). Similarly, this was noted among CLIP stages, with stages 4–6 having higher levels than stages 0–2 and stage 3 (p = 0.0015) (Table 2). The levels of galectin-3 were significantly higher in patients with poorer HepatoScore-14, insulin growth factor–Child-Pugh score (IGF-CPS), and IGF-1 performance scores. Galectin-3 levels did not significantly differ between patients with HCC with and without vascular invasion, metastasis, lymph node involvement, or across the TNM and BCLC scoring systems (Table 2).
Table 2.
Comparisons of galectin-3 levels between subgroups by patient clinical factors
| Characteristic | n | Galectin-3 Levels: Mean ± SD, Median (Range) | p-value |
|---|---|---|---|
| Cirrhosis | |||
| HCC with cirrhosis | 489 | 6.41 ± 2.986, 6 (0.18–22) | 0.6286 |
| HCC without cirrhosis | 278 | 6.259 ± 2.819, 5.9 (0.044–20) | |
| HCV | |||
| Negative | 466 | 6.026 ± 2.695, 5.7 (0.044–22) | 0.0001 |
| Positive | 301 | 6.865 ± 3.188, 6.6 (0.18–20) | |
| Hepatitis | |||
| HBV only | 88 | 6.159 ± 2.55, 5.8 (0.2–16) | 0.0008 |
| HCV and HBV | 111 | 6.744 ± 3.163, 6.6 (0.23–17) | |
| HCV only | 190 | 6.935 ± 3.209, 6.4 (0.18–20) | |
| No virus | 378 | 5.995 ± 2.731, 5.6 (0.044–22) | |
| Pathology | |||
| Poorly | 188 | 6.203 ± 2.863, 5.8 (0.044–19) | 0.5318 |
| Well-moderate | 404 | 6.386 ± 2.917, 6.05 (0.18–22) | |
| ECOG | |||
| 0–1 | 665 | 6.272 ± 2.859, 5.8 (0.044–20) | 0.0174 |
| 2+ | 102 | 6.899 ± 3.289, 6.75 (0.18–22) | |
| Evidence of cirrhosis | |||
| No | 278 | 6.259 ± 2.819, 5.9 (0.044–20) | 0.6286 |
| Yes | 489 | 6.41 ± 2.986, 6 (0.18–22) | |
| Vascular invasion | |||
| No | 524 | 6.317 ± 2.783, 6.1 (0.044–20) | 0.8717 |
| Yes | 241 | 6.442 ± 3.227, 5.7 (0.18–22) | |
| Metastasis | |||
| None | 576 | 6.338 ± 2.939, 5.9 (0.044–22) | 0.7289 |
| Present | 189 | 6.414 ± 2.904, 6.1 (0.12–17) | |
| Lymph node involvement | |||
| None | 608 | 6.356 ± 2.986, 5.9 (0.044–22) | 0.4948 |
| Present | 157 | 6.359 ± 2.705, 6.2 (0.12–16) | |
| AFP, ng/dL | |||
| < 400 | 516 | 6.26 ± 2.795, 5.85 (0.044–20) | 0.2066 |
| ≥ 400 | 251 | 6.55 ± 3.175, 6.3 (0.18–22) | |
| Child-Pugh score | |||
| A | 412 | 6.014 ± 2.632, 5.7 (0.044–17) | 0.0009 |
| B | 299 | 6.56 ± 2.97, 6.3 (0.18–20) | |
| C | 56 | 7.771 ± 4.063, 6.95 (2.1–22) | |
| CLIP | |||
| Stage 0–2 | 485 | 6.081 ± 2.693, 5.8 (0.044–20) | 0.0015 |
| Stage 3 | 147 | 6.468 ± 2.885, 6.3 (0.35–16) | |
| Stage 4–6 | 109 | 7.402 ± 3.775, 6.8 (0.18–22) | |
| TNM group | |||
| Stage I–II | 253 | 6.174 ± 2.6, 5.9 (0.2–14) | 0.416 |
| Stage III–IV | 491 | 6.449 ± 3.123, 6.1 (0.044–22) | |
| BCLC group | |||
| Stage 0–B | 172 | 5.96 ± 2.383, 5.7 (0.2–14) | 0.1074 |
| Stage C–D | 588 | 6.486 ± 3.067, 6.1 (0.044–22) | |
| HepatoScore-14 | |||
| Low | 135 | 5.101 ± 1.786, 4.9 (1.4–13) | < 0.0001 |
| Medium | 238 | 6.094 ± 2.485, 5.65 (0.044–14) | |
| High | 394 | 6.943 ± 3.308, 6.7 (0.18–22) | |
| IGF-CPS | |||
| A | 391 | 5.922 ± 2.493, 5.6 (0.044–17) | < 0.0001 |
| B | 151 | 7.138 ± 3.435, 6.6 (0.97–19) | |
| C | 57 | 7.602 ± 3.929, 7 (2.1–22) | |
| IGF1a | |||
| < 26 | 87 | 7.332 ± 3.587, 6.8 (0.97–20) | 0.0075 |
| ≥ 26 | 519 | 6.243 ± 2.843, 5.9 (0.044–22) |
AFP: alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; CLIP: The Cancer of the Liver Italian Program; ECOG: Eastern Cooperative Oncology Group; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; IGF1a: insulin growth factor 1; IGF-CPS: insulin growth factor–Child-Pugh score; TNM: tumor, node, metastasis.
The Prognostic Significance of Serum Galectin-3
When using the 50th percentile median level, 6 ng/mL, as a cutoff value, there was no significant difference in OS across the high– and low–galectin-3 level groups, using both the univariate (p = 0.092) and multivariate (p = 0.054) analysis. When using the 60th percentile level, 6.6 ng/mL, as a cutoff value, the univariate Cox model analysis showed that OS was significantly lower for patients with high galectin-3 levels than for patients with low galectin-3 levels (median OS: 10.12 vs. 16.49 months; p = 0.0022). This is further emphasized when assessing the relationship over time, as patients in the high galectin-3 group had worse survival rates at both 24 and 48 months (Fig. 1).
Figure 1.
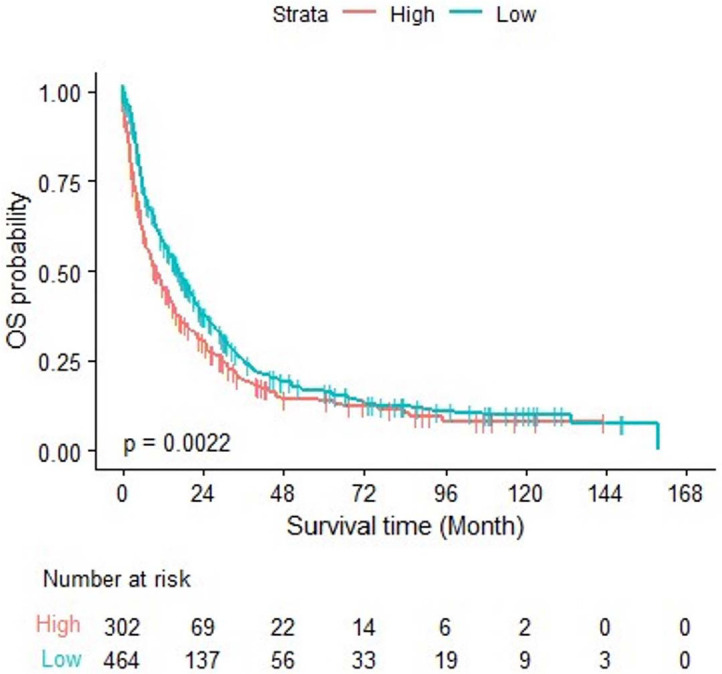
OS of the two galectin-3 groups over time. OS: overall survival.
Similarly, the multivariate model showed a significant association between high galectin-3 level and poor OS (hazard ratio [HR] = 1.249; 95% CI, 1.005–1.554; p = 0.0455). The association of several demographic and clinicopathologic characteristics with OS is summarized in Table 3. The association of AFP level of ≥ 400 with poor OS provides the potential for using both AFP and galectin-3 for prognostication in HCC.
Table 3.
Multivariate analysis: galectin-3 with overall survival after adjusting for the effects of patient clinical factors
| Parameter | HR | 95% CI |
p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Sex: male vs. female | 1.378 | 1.079 | 1.759 | 0.0101 |
| Pathology: poorly vs. well/moderate | 1.301 | 1.029 | 1.644 | 0.028 |
| ECOG: 2+ vs. 0–1 | 2.156 | 1.576 | 2.95 | < 0.0001 |
| Metastasis: present vs. none | 1.919 | 1.467 | 2.511 | < 0.0001 |
| Tumor nodule: multinodular vs uninodular | 1.636 | 1.285 | 2.083 | < 0.0001 |
| Tumor involvement: > 50% vs. ≤ 50% | 1.423 | 1.107 | 1.829 | 0.0059 |
| AFP: ≥ 400 vs. < 400 ng/dL | 1.779 | 1.399 | 2.262 | < 0.0001 |
| TNM group: stage III–IV vs. stage I–II | 1.509 | 1.146 | 1.986 | 0.0033 |
| CPS: B vs. A | 1.42 | 1.118 | 1.802 | 0.004 |
| CPS: C vs. A | 6.1 | 3.554 | 10.469 | < 0.0001 |
| IGF-1: ≥ 26 vs. < 26 | 0.992 | 0.988 | 0.997 | 0.0003 |
| Galectin-3: high vs. low | 1.249 | 1.005 | 1.554 | 0.0455 |
AFP: alpha-fetoprotein; CPS: Child-Pugh score; ECOG: Eastern Cooperative Oncology Group; HR, hazard ratio; IGF-1: insulin-like growth factor 1; TNM: tumor, node, metastasis.
In our analysis, we evaluated whether galectin-3 could provide additional prognostic value to each of the existing HCC staging or scoring systems. Patients with BCLC stages C and D disease had a higher HR than patients with BCLC stage A disease (p < 0.001) (Table 4). After adjusting for the effect of the BCLC classification system, galectin-3 remained significantly associated with OS (HR = 1.219; 95% CI, 1.03–1.443 (Table 4). Likewise, higher CLIP stages (1–6) were associated with significantly higher HRs than stage 0 (Table 4). High galectin-3 levels remained significantly associated with poor OS, after adjusting for the effect of the CLIP classification system (HR = 1.216; 95% CI, 1.029–1.438) (Table 4). Both Child-Pugh score (CPS) B and C had a higher HR than CPS A (p = 0.0016 and p < 0.001, respectively), and high levels of galectin-3 were significantly associated with worse OS (HR = 1.188; 95% CI, 1.004–1.405) after adjusting for CPS (Table 4).
Table 4.
Association between galectin-3 levels and overall survival after adjusting for the effects of each of the HCC scoring systems
| Parameter | HR | 95% CI |
p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| BCLC | ||||
| Stage 0 vs. Stage A | 0.35 | 0.105 | 1.166 | 0.0874 |
| Stage B vs. Stage A | 1.346 | 0.861 | 2.105 | 0.1919 |
| Stage C vs. Stage A | 2.475 | 1.65 | 3.713 | < 0.0001 |
| Stage D vs. Stage A | 7.628 | 4.486 | 12.97 | < 0.0001 |
| Galectin-3 after adjusting for BCLC | ||||
| High vs. low | 1.219 | 1.03 | 1.443 | 0.0211 |
| CLIP | ||||
| 1 vs. 0 | 1.741 | 1.292 | 2.345 | 0.0003 |
| 2 vs. 0 | 2.122 | 1.582 | 2.846 | < 0.0001 |
| 3 vs. 0 | 4.183 | 3.081 | 5.679 | < 0.0001 |
| 4 vs. 0 | 6.317 | 4.443 | 8.983 | < 0.0001 |
| 5 vs. 0 | 22.902 | 14.217 | 36.894 | < 0.0001 |
| 6 vs. 0 | 36.728 | 16.303 | 82.74 | < 0.0001 |
| Galectin-3 after adjusting for CLIP | ||||
| High vs. low | 1.216 | 1.029 | 1.438 | 0.0221 |
| CPS | ||||
| B | 1.331 | 1.114 | 1.591 | 0.0016 |
| C | 4.453 | 3.261 | 6.079 | < 0.0001 |
| Galectin-3 after adjusting for CPS | ||||
| High vs. low | 1.188 | 1.004 | 1.405 | 0.045 |
BCLC: Barcelona Clinic Liver Cancer; CLIP: Cancer of the Liver Italian Program; CPS: Child-Pugh score; HCC: hepatocellular carcinoma; HR, hazard ratio.
The Diagnostic Significance of Serum Galectin-3
The serum galectin-3 level in patients with HCC was significantly higher (6.355 ± 2.925 ng/mL; n = 767) than that of healthy controls (4.254 ± 1.506 ng/mL; n = 200; p < 0.0001). A ROC curve analysis was performed (Fig. 2) to identify the ideal diagnostic cutoff value, and the area under the curve was found to be 0.753. The Youden index was calculated from this finding, and 5.15 ng/mL was recognized as the most optimal diagnostic cutoff value, with a sensitivity of 63.5% and specificity of 80%.
Figure 2.
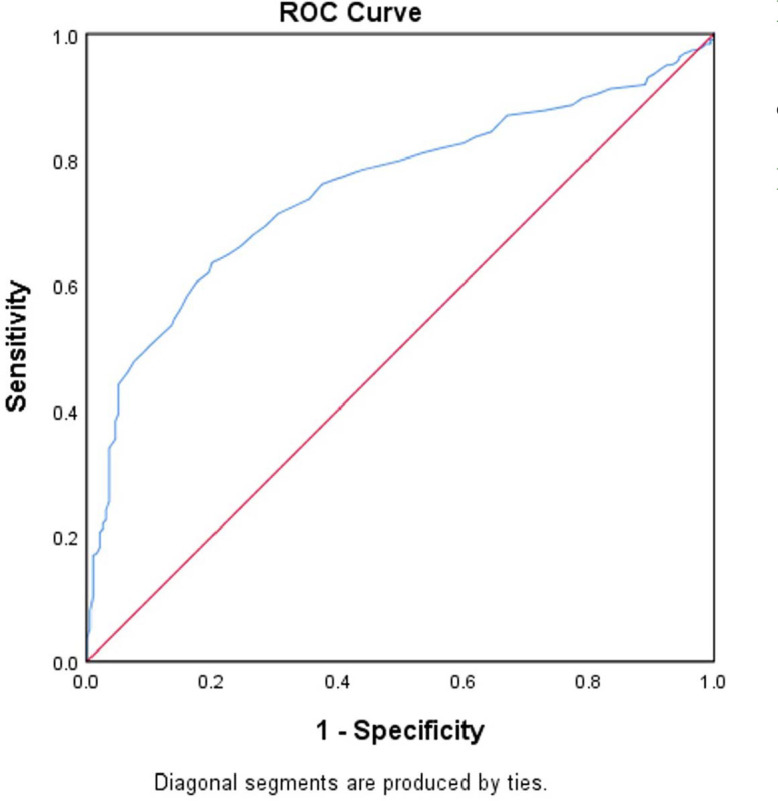
Diagnostic performance of serum galectin-3 level generated by ROC curve values. ROC: receiver operating characteristic.
DISCUSSION
To our knowledge, this study is the largest prospective study to date to investigate the clinical and prognostic significance of serum galectin-3 levels in patients with HCC. Our findings indicated that higher serum levels of galectin-3 are associated with shorter OS and advanced clinicopathologic features. In agreement with these findings, previous studies have shown that high galectin-3 levels are significantly associated with worse OS.[10,14,18,19,27,28] High levels of galectin-3 have been shown to be associated with poor progression-free survival after resection,[27] although we did not evaluate this survival metric. Galectin-3 remained significantly associated with OS after adjusting for other clinical factors in our study. Additionally, galectin-3 could provide independent additional prognostic value to several renowned staging systems, such as BCLC, CLIP, and CPS. Future studies can aim to validate galectin-3 in independent cohorts to ensure its reproducibility and reliability. No other studies have demonstrated this potential benefit.
The significant correlation between higher serum galectin-3 levels and advanced CPS and HCV-positive status was consistent with other reports of the association between galectin-3 levels and advanced hepatic fibrosis and chronic liver diseases.[12] Interestingly, we also found a significant correlation between higher levels of serum galectin-3 and lower levels of IGF-1. Decreased IGF-1 levels have been previously reported to be strongly associated with advanced clinicopathologic features and poor outcomes of HCC,[25] and this was replicated in our study. The rationale behind this finding is that worse liver function would lead to decreased production of IGF-1, and as mentioned above, high levels of galectin-3 are positively associated with liver disease and poor hepatic reserve.
We found no significant correlation between galectin-3 levels and major vascular invasion, metastasis, or liver cirrhosis. In contrast, several studies have reported that high galectin-3 levels are significantly associated with these parameters.[9,10,14,17,18,27] This discrepancy might be attributed to the heterogeneity of the patient population, demographics, risk factors, and underlying degree of liver cirrhosis. Therefore, future prospective validation studies are needed to study these correlations.
Additionally, galectin-3 levels were not associated with the differentiation grade of HCC, with no significant difference found in mean galectin-3 levels between poorly differentiated and well-differentiated pathology. A controversy regarding this finding was noted in the literature; whereas some studies were in line with our findings,[14,19] others found that a higher galectin-3 expression was associated with poor histologic differentiation.[9,10,18,21]
Although the main objective of this study was to identify the prognostic significance of increased galectin-3 levels in patients with HCC, we also looked at its diagnostic potential. ROC analysis determined the optimal diagnostic cutoff value at 5.15 ng/mL, with a sensitivity and specificity of 63.5% and 80%, respectively. This was similar to the results reported by Matsuda et al,[18] where the sensitivity and specificity were 70.8% and 66.7%. However, unlike their study, we did not evaluate the combination of AFP and galectin-3 to attempt to improve the diagnostic performance in HCC. Their assessment showed an improvement of sensitivity to 93.8%, although the specificity decreased to 61.9% with AFP cotesting. The abovementioned sensitivities and specificities are comparable to those reported for the AFP value of 20 ng/mL.[29,30] Despite the similarities, AFP continues to be more commonly used in clinical settings owing to its widespread familiarity and extensive validation. Given the impeccable diagnostic performance of imaging, or the combination of imaging and AFP levels, in the diagnosis of HCC, biomarkers alone lack independent diagnostic capability.[30]
Our study has several strengths, the first of which is the large sample size, especially in comparison to the sample sizes used in other studies that assess the prognostic role of galectin-3 in HCC. Our results regarding the clinical and prognostic significance of serum galectin-3 levels, as opposed to galectin-3 expression in tissues, introduce the feasibility and the clinical advantage of validating the role of this minimally invasive and easily accessible biomarker. In future studies, galectin-3 levels could be followed serially after resection and local and systemic therapies to authenticate its predictive value. A notable strength of this study is the introduction of two different cutoff values: one for the diagnostic cutoff point and one for the prognostic cutoff point. This is not uncommon as biomarkers have distinct applications from screening to diagnosis and prognosis.[31] This study also has several limitations. A noteworthy limitation is the lack of comparison between circulating and tissue levels of galectin-3. Another limitation is that it is a single-institution study, which may pose inherent bias regarding the patient population and practice pattern of managing patients at our institution. Lastly, this study is cross-sectional, providing a snapshot of galectin-3 levels and patient outcomes at a single time point. Longitudinal data tracking galectin-3 levels over time and their correlation with disease progression and treatment response would provide more robust evidence of its prognostic value.
CONCLUSION
This study represents a study of circulating galectin-3 and showed that high serum levels of galectin-3 in patients with HCC are associated with worse OS, advanced clinicopathologic features, and poor hepatic reserve. In addition, our results support the exploration of targeting galectin-3 in HCC therapy.
Data Availability
The study data may be provided by contacting the corresponding author.
References
- 1.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 3.Balogh J, Victor D, III,, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. [DOI] [PubMed] [Google Scholar]
- 5.Yuan JM, Wang Y, Wang R, et al. Serum IL27 in relation to risk of hepatocellular carcinoma in two nested case-control studies. Cancer Epidemiol Biomarkers Prev. 2021;30:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(suppl 4):14–22. [DOI] [PubMed] [Google Scholar]
- 7.Morris JS, Hassan MM, Zohner YE, et al. HepatoScore-14: measures of biological heterogeneity significantly improve prediction of hepatocellular carcinoma risk. Hepatology. 2021;73:2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan SL, Chan AT, Yeo W. Role of alpha-fetoprotein in hepatocellular carcinoma: prognostication, treatment monitoring or both? Future Oncol. 2009;5:889–899. [DOI] [PubMed] [Google Scholar]
- 9.Setayesh T, Colquhoun SD, Wan YY. Overexpression of Galectin-1 and Galectin-3 in hepatocellular carcinoma. Liver Res. 2020;4:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong F, Jin M, Cao D, et al. Galectin-3 not Galectin-9 as a candidate prognosis marker for hepatocellular carcinoma. PeerJ. 2020;8:e9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8831–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Turco S, De Simone P, Ghinolfi D, et al. Comparison between galectin-3 and YKL-40 levels for the assessment of liver fibrosis in cirrhotic patients. Arab J Gastroenterol. 2021;22:187–192. [DOI] [PubMed] [Google Scholar]
- 13.Nassar ES, Elkalbashawy YA, Kamal A, Zakaria NHE. Galectin-3 is not useful for hepatocellular carcinoma surveillance in cirrhotic patients but it may be a marker of cirrhosis development. Clin Exp Hepatol. 2021;7:74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An Y, Xu S, Liu Y, et al. Role of galectins in the liver diseases: a systematic review and meta-analysis. Front Med. 2021;8:744518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulu M, Alacacioglu A, Yuksel E, et al. Prognostic significance of serum galectin-3 levels in patients with hepatocellular cancer and chronic viral hepatitis. Saudi J Gastroenterol. 2015;21:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Çavuş B, Akyuz F, İliaz R, et al. Assessment of prognostic and diagnostic value of some biomarkers in hepatocellular carcinoma. Exp Oncol. 2020;42:208–214. [DOI] [PubMed] [Google Scholar]
- 17.Eisa NH, Ebrahim MA, Ragab M, et al. Galectin-3 and matrix metalloproteinase-9: Perspective in management of hepatocellular carcinoma. J Oncol Pharm Pract. 2015;21:323–330. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda Y, Yamagiwa Y, Fukushima K, et al. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol Res. 2008;38:1098–1111. [DOI] [PubMed] [Google Scholar]
- 19.Jiang SS, Weng DS, Wang QJ, et al. Galectin-3 is associated with a poor prognosis in primary hepatocellular carcinoma. J Transl Med. 2014;12:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu DK, Dowling CA, Jeng KC, et al. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519–526. [DOI] [PubMed] [Google Scholar]
- 21.Fang QQ, Ni RZ, Xiao MB, et al. Serum and tissue expressions of galectin-3 in hepatocellular carcinoma and the clinical significances [in Chinese]. Zhonghua Gan Zang Bing Za Zhi. 2011;19:527–531. [DOI] [PubMed] [Google Scholar]
- 22.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. [DOI] [PubMed] [Google Scholar]
- 23.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniele B, Annunziata M, Barletta E, et al. Cancer of the Liver Italian Program (CLIP) score for staging hepatocellular carcinoma. Hepatol Res. 2007;37(Suppl 2):S206–S209. [DOI] [PubMed] [Google Scholar]
- 25.Kaseb AO, Morris JS, Hassan MM, et al. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol. 2011;29:3892–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaseb AO, Xiao L, Hassan MM, et al. Development and validation of insulin-like growth factor-1 score to assess hepatic reserve in hepatocellular carcinoma. J Natl Cancer Inst. 2014;106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song M, Pan Q, Yang J, et al. Galectin-3 favours tumour metastasis via the activation of β-catenin signalling in hepatocellular carcinoma. Br J Cancer. 2020;123:1521–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao Q, He J, Chen Z, Wu C. Prognostic role of galectins expression in patients with hepatic cancer: a meta-analysis. Medicine. 2020;99:e19622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford ). 2005;7:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colli A, Nadarevic T, Miletic D, et al. Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev. 2021;4:Cd013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte PS. Cut point identification of continuous biomarkers: a challenge that goes beyond statistical aspects. J Nucl Med. 2021. Sep 2;62:1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data may be provided by contacting the corresponding author.


