Abstract
Pyrroloquinoline Quinone (PQQ) is a redox-active quinone molecule with significant implications for human health. Originally identified as a bacterial cofactor, PQQ has since been lauded for its diverse biological and therapeutic activities. It serves as an essential cofactor for oxidative enzymes that are vital for mitochondrial function and ATP synthesis. PQQ exhibits superior antioxidant properties that protect against ROS-mediated oxidative stress, aging, neurodegenerative diseases, certain cancers, diabetes, and metabolic disorders. It also enhances cognitive abilities and reduces insulin sensitivity. PQQ's antioxidant nature helps mitigate oxidative stress, which is implicated in many diseases. It has been shown to target cancer cells selectively, suggesting its potential as a therapeutic agent. Clinical studies have indicated the potential benefits of PQQ supplementation, including improvements in cardiovascular health, cognitive function, weight management, insulin sensitivity, and the prevention of metabolic syndromes. The safety of PQQ has been established, with no reported toxicity or genotoxicity in various studies, and it is considered a safe nutritional supplement. Future research directions should focus on determining the optimal dosages of PQQ for specific health outcomes and assessing its long-term effectiveness and safety. The translation of PQQ research into clinical practice could offer new strategies for managing metabolic disorders, enhancing cognitive health, and potentially extending lifespan. In summary, PQQ is a promising molecule with broad potential health benefits, impacting human health from cellular metabolism to disease prevention and treatment, positioning it as a key player in nutritional science and public health.
Keywords: Pyrroloquinoline quinone, Cellular metabolism, Antioxidant, Neuroprotection, Metabolic disorders, Cardiovascular health
Graphical abstract
Highlights
-
•
PQQ is a potent antioxidant that supports redox balance and mitochondrial function, vital for energy and health.
-
•
PQQ contributes to lipid metabolism regulation, indicating potential benefits for energy management.
-
•
PQQ supplementation is linked to weight control, improved insulin sensitivity, and may help prevent metabolic disorders.
-
•
PQQ may attenuate inflammation, bolster cognitive and cardiovascular health, and potentially assist in cancer therapies.
-
•
Future research should investigate PQQ dosages, long-term outcomes, and its potential for metabolic and cognitive health.
1. Introduction
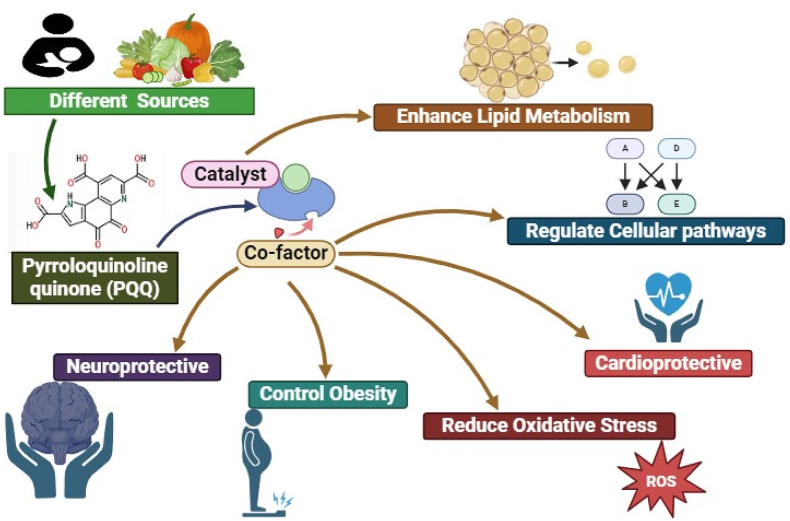
Methoxatin or pyrroloquinoline quinone (PQQ) is a novel cofactor that has garnered significant attention from nutritionists and health professionals recently. During 1970s, PQQ was identified as an unfamiliar cofactor for certain bacterial enzymes (Hauge, 1964; Rucker et al., 2009), which subsequently reported for its remarkable versatility and potential benefits for human health. PQQ is a redox-active quinone molecule that possess a distinct molecular structure distinct from other established cofactors like vitamins and minerals (Kasahara and Kato, 2003; Cordell and Daley, 2022). With its unique pyrroloquinoline ring system, PQQ occurs naturally in various food sources including fermented foods, vegetables, and human breast milk (Kumazawa et al., 1992). Its identification as a bacterial cofactor has spurred further exploration for its potential roles in mammalian physiology.
Numerous studies have consistently shown that PQQ plays a crucial role in various physiological processes, encompassing cellular energy metabolism, antioxidant defense mechanisms, and even neuroprotection (Zhu et al., 2006; Stites et al., 2000). Serving as a vital component of mitochondrial functions and ATP synthesis, PQQ functions as a cofactor for several redox activated enzymes (Cores et al., 2023). Given its potent antioxidant properties, including free radical scavenging and reduction of oxidative stress, PQQ holds promise for potential applications in both the treatment and prevention of aging associated neurodegenerative diseases (Cheng et al., 2020). PQQ impacts on cognition and neuroprotection, suggest its potential as a treatment for neurodegenerative diseases (Cheng et al., 2020; Kim et al., 2010a). Moreover, PQQ regulates multiple cellular metabolic pathways simultaneously, offering potential benefits for lifestyle associated conditions such as diabetes and obesity (Ishak and Ikemoto, 2023; Hwang and Willoughby, 2018). The potential role of PQQ in cardiovascular health is also evidenced by certain studies which indicates its ability to modulates endothelial function, mitigate inflammations, and provision of protection against ischemic injury (Zhu et al., 2004; Shafiq et al., 2022).
In addition, PQQ can effectively impede tumor growth along with the enhancement of efficacy of chemotherapeutic agents and is discussed in details in the following heads. But still needs discussion over its potential applications in cancer prevention and treatment (Zhu et al., 2004; Zhang et al., 2015). This review provides a comprehensive understanding of the current available literature of PQQ research, potential applications in human health and well-being (Fig. 1). Furthermore, recent advancements in PQQ research, consolidating influential studies elucidating its diverse biological functions and therapeutic applications shall also be highlighted.
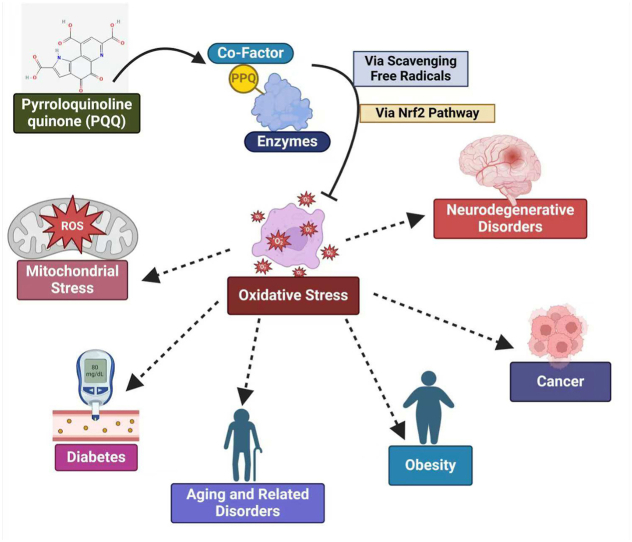
Fig. 1.
Naturally sourced PQQ as cofactor enhance human health via multiple physiological processes. By regulating numerous cellular signaling metabolic pathways help reduce fat deposits to lessen the chances of obesity, minimizing oxidative stress to boost neurological and cardiac health.
2. Biochemical functions of PQQ
Early in this century, PQQ was discovered as redox cofactor from human milk (He et al., 2003). Both in vitro and in vivo studies involving various animal models for human ailments revealed that PQQ is a strong antioxidant regulating mitochondrial physiology and biogenesis, and being an essential cofactor in various biological processes (Fig. 2). PQQ exhibits potent antioxidant properties by protecting the mitochondria against oxidative stress mediated lipid peroxidation (LPO), protein carbonyl formation, and avoid damages incurred to the respiratory chain (He et al., 2003; Gao et al., 2022). During in vitro studies, the cultured cells showed a notable death rate when exposed to PQQ. This unexpected effect of PQQ was reversed by catalase, indicating that it completely eradicated the production of H2O2 during PQQ autoxidation in the culture medium. Moreover, it is assumed that the reactivity of PQQ is based on concentration used, and it can either mitigate or exacerbate oxidation in different biological systems (He et al., 2003).
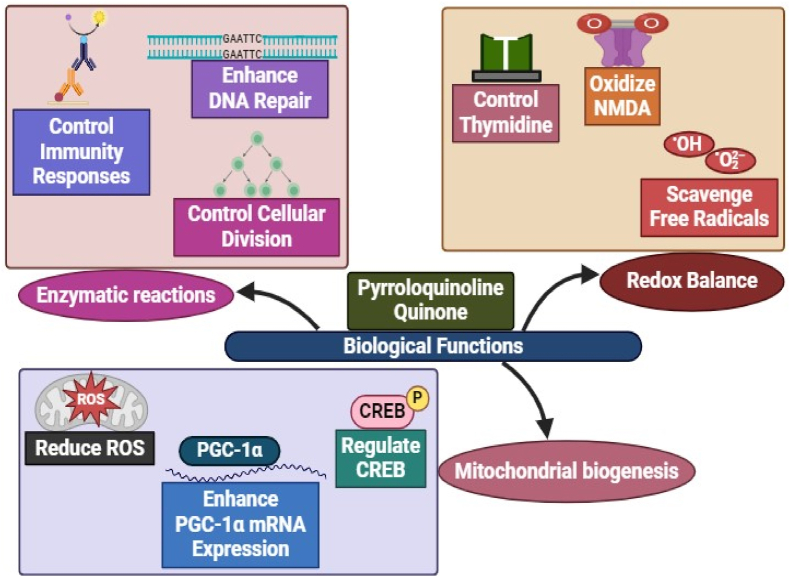
Fig. 2.
Diversified biological properties of PQQ. PQQ maintains cellular immunity by quick repairing of DNA damage, overcome cellular oxidative stress and its associated pathologies by regulation of metabolic cell signaling pathways.
2.1. Antioxidant properties of PQQ and role in cellular redox balance
The mitochondrial metabolism-oriented production of reactive oxygen species (ROS) is a significant contributor to cellular oxidative stress and damage to the biological macromolecules. Being a potent antioxidant, PQQ plays a crucial role in neutralizing these ROS burst and protect mitochondrial membranes and metabolic enzyme activities. This protective effect of PQQ helps mitigate aging associated mitochondrial dysfunction, thereby maintaining optimal metabolic activities (Nunome et al., 2008).
PQQ is a redox-active o-quinone that can be reversibly reduced to pyrroloquinoline quinol, also known as PQQH2, through a semiquinone intermediate, as described in reference (McIntire, 1998). This compound is highly stable and serves as an efficient electron transfer catalyst, facilitating the reaction of numerous organic substrates with molecular oxygen (O2). PQQ is also considered a key component in constructing quinoprotein model reactions. In the presence of ascorbate, along with NAD(P)H and glutathione (GSH), PQQ undergoes a two-electron reduction to form PQQH2 (He et al., 2003; Zhang et al., 2009; Kong et al., 2013). The reduced form, PQQH2, is capable of oxidizing back to its original quinone state by reducing two equivalents of O2 to O2−. This superoxide anion (O2−) can either spontaneously or enzymatically (via superoxide dismutase, SOD) dismutate into hydrogen peroxide (H2O2) (Akagawa et al., 2016a). PQQ demonstrates superiority over known cellular redox enzymes due to its ability to catalyze continuous redox cycling. This property allows a small amount of PQQ, in the picomolar range, to convert a micromolar quantity of product, as evidenced in references (Stites et al., 2000; Liu et al., 2021).
PQQ is highly stable but an efficient electron transfer catalyst from numerous organic substrates to molecular oxygen (O2). PQQ is regarded as constructing quinoprotein model reactions. If ascorbate is present, NAD(P)H, and thiol compounds such as glutathione (GSH), PQQ undergoes a two-electron reduction to form PQQH2.
Extensive literature demonstrates that PQQ efficiently scavenges ROS particularly superoxide anion (O2−) and hydroxyl ions (HO●) in order to inhibit LPO and provide cytoprotection. PQQ reduces the oxidative stress to safeguard heart muscle cells, oxidizes the redox site of N-methyl-D-aspartate (NMDA) receptor, enhances thymidine integration into fibroblasts, inhibits melanin production in murine B16-F10 melanoma cells while boosting the production of nerve growth factors (NGF) (He et al., 2003; McIntire, 1998; Zhang et al., 2009). High doses of PQQ shown preventive effects against hypoxic/ischemic damages, seizures induced by chemical agents, inflammation from carrageenan, ethanol or carbon tetrachloride (CCl4)-induced liver damage, cataract formation, glutathione depletion in glucocorticoid-treated chicken embryos, and endotoxin-induced death in in vivo models (He et al., 2003; Kong et al., 2013). Generally it is believed that PQQ have strongest antioxidant properties which drives these health benefits, but exact underlying cellular or molecular mechanisms are still not fully known (Akagawa et al., 2016a). Some preliminary authentic mechanisms for its diverse biological activities of PQQ mainly involves modulation of respiratory metabolic mechanisms in mitochondria. The increased oxidative stress may lead to mitochondrial LPO and other oxidative damages which exacerbate cell death, contributing to multiple degenerative disorders. Mitochondria are major contributors to cellular oxidative stress which is acerbated by PQQ to reduce cell death by preserving mitochondrial functions (Hwang and Willoughby, 2018; Liu et al., 2021).
Certain reducing agents such as NADPH, sodium borohydride, glutathione, and cysteine may readily reduce PQQ (PQQH2), which making it strong antioxidant in vitro (He et al., 2003; Miyauchi et al., 1999). PQQH2 scavenges ROS (O2− and HO●) quite efficiently almost multi-fold time potently than ascorbic acid which is thought as a gold standard water soluble antioxidant (Ouchi et al., 2013; Mukai et al., 2011). In case of O2−, PQQH2 acts as a catalyst in O2−-quenching processes and quickly transform α-tocopheroxyl radicals into α-tocopherol (Ouchi et al., 2013) by mitigating pro-oxidant effects of α-tocopheroxyl radicals (Liu et al., 2021). In in vitro studies, PQQ disodium (PQQ•Na2) has been shown to protect neurons from oxidative stress-induced mortality. In a study where two dopamine producing neuroblastoma cell lines (SH-SY5Y and raw rat neurons) were exposed to neurotoxic 6-hydroxydopamine (6-OHDA), and PQQ help avoid and rescued cellular death, even this effect was significantly superior than ascorbic acid and vitamin E does. Like H2O2, the neurotoxic 6-OHDA also damages mitochondrial membrane protein complex I, leading to burst ROS (HO● and H2O2) generation (Cores et al., 2023; Akagawa et al., 2016b; Hara et al., 2007).
In vivo studies using rat models for cardiovascular or cerebral ischemia demonstrated significant reductions in ischemic damage because PQQ quickly scavenges cellular free radicals and hence shield mitochondria against oxidative stress-induced damages (Zhu et al., 2004; Gong et al., 2012; Wen et al., 2020). Levels of thiobarbituric acid reacting substances (TBARS) are indicative of malondialdehyde (MDA) formation from lipid hydroperoxides, markedly decreased in cells following a single dosage of PQQ•Na2 (0.2 mg/kg body weight). The highest plasma concentration (Cmax) of PQQ•Na2 was strongly correlated with the actual decrease in TBARS levels indicating strong antioxidant properties of PQQ (Akagawa et al., 2016a; Harris et al., 2013).
Majority of the bacterial dehydrogenases relies on the dissociable cofactor PQQ. Astonishingly PQQ efficiently catalyze redox cycling events and promising catalyst for various carbonyl reagents (semicarbazides, hydrazine, & hydroxylamine) and amine containing molecules to make stable complexes (Stites et al., 2000). Naturally occurring methylotrophic bacteria are able to metabolically condense L-Tyrosine and L-Glutamic acid to PQQ. While the role of PQQ as a cofactor in animals and higher plants remains debated for a long time, but recently there are compelling evidences that PQQ along with its associated quinoids have nutritional and pharmacological significance (Smidt et al., 1991a; Bugg, 2001). PQQ is added to chemically modified amino acids, and high nutrition diets to promote animal body growths. Whereas, the deficiency of PQQ in the diet seems to impede the maturation of connective tissues, particularly during fetal development throughout pregnancy, and subsequently affects overall development (Smidt et al., 1991a; Mandala et al., 2022; Mattern et al., 2023).
2.2. PQQ facilitates mitochondrial biogenesis
Huge literature demonstrates that the bioactive compounds which promote mitochondrial biogenesis are linked with several health benefits including delayed aging, improved energy utilization, and enhanced immunity against ROS mediated oxidative stress. In an in vitro study, PQQ induces mitochondrial biogenesis in mice hepatocytes, while in in vivo studies where the diets were deficient in PQQ reduces mitochondrial content consequently less energy production (Stites et al., 2006; Bauerly et al., 2011). Mitotracker labeling showed an increased mitochondrial DNA quantity, enhanced cellular aerobic respiration, citrate synthase and cytochrome c oxidase activities when the cells were exposed to small concentrations (10–30 μm) of PQQ for 24–48 h (Chowanadisai et al., 2009, 2010). The induction of mitochondrial biogenesis is mediated by the activation of cAMP responsive element-binding protein (CREB) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) cell signaling pathways. Application of PQQ led to an enhanced expression of PGC-1α both at transcriptional and translational level, activation of the inducer of PGC-1α, and phosphorylation at serine 133 of CREB. Knockdown of either PGC-1α or CREB genes expression through small RNA interference, mediates the decrease in PQQ-induced mitochondrial biogenesis (Chowanadisai et al., 2010). PQQ also enhance the activation of nuclear respiratory factors (NRF-1, NRF-2) and transcriptional activation of Tfam, TFB1M, and TFB2M, which are linked with the activation of the PGC-1α pathway. Furthermore, PQQ protect cells from damage induced by inhibitors of mitochondrial function such as rotenone, 3-nitropropionic acid, antimycin A, and sodium azide (NaN3). Diseases associated with mitochondrial dysfunction may benefit from PQQ due to its ability to enhance mitochondrial biogenesis (Chowanadisai et al., 2010).
2.3. Regulation of enzymatic activities by PQQ
PQQ functions as a biocatalyst, enhancing NADH generation through pyrroloquinoline quinone synthase C (PQQC). It plays a crucial role in various essential processes especially m-ATPase energy transduction in bacteria, biocontrol substance synthesis, promotion of growth activities, and DNA damage repair (Naveed et al., 2016). Its strong antioxidant properties enable PQQ to scavenge ROS, making it effective anti-neurodegenerative, anti-melanogenic, and anti-cancer agent. PQQ help diminish ROS-mediated inflammations, maintains the PGC-1α pathway, impact overall populations of CD4 cells, and modulates immunological responses (Naveed et al., 2016). In addition to these, PQQ also contributes to plant growth promotion, biocontrol to different pests, antifungal activities, and induced systemic resistance (ISR) through mineral phosphate solubilization. PQQ nanoparticles are utilized in the production of sulfonated polymers and for biofuel cells. PQQ regulate cell growth, cell differentiation, apoptosis, Box translocation, and metabolic processes through ADP phosphorylation and reducing ROS burst. In addition, PQQ also modulates multiple signaling pathways such as STAT, MAPK, JAK, JNK, PI3K/Akt, mTOR, EGFR, and Raps in order to regulate cellular redox homeostasis (Naveed et al., 2016; Anthony and Williams, 2003). PQQ prevents oxidative stress by regulating metabolic pathways associated with lysine, selenium, low-density lipoprotein, energy, carbohydrates, and ATP synthesis. Potential future applications of PQQ include its use in molecular modeling and docking studies for quantitative drug design based on bioinformatics and structural characterization of the novel drug molecules.
3. Mechanism of action within cells and cellular pathways
PQQ plays a significant role in various cellular pathways and metabolic processes (Fig. 3, Table 1). Primarily, PQQ functions by supporting mammalian dehydrogenases such as lactate dehydrogenase (LDH) for enhanced activity. When PQQ complexes with LDH, the former enhances the oxidation of NADH to NAD+ and facilitates the excessive generation of pyruvate. Metabolic regulation, particularly oxidative processes, is mediated by sirtuins, a family of protein transmitters (Akagawa et al., 2016a, 2016b). Optimal antioxidant defense and immunological functions are inherently linked to oxidative metabolism as sirtuins 1 (Sirt-1) and sirtuins 3 (Sirt-3) depend on NAD+ as a substrate for their deacylation activity.
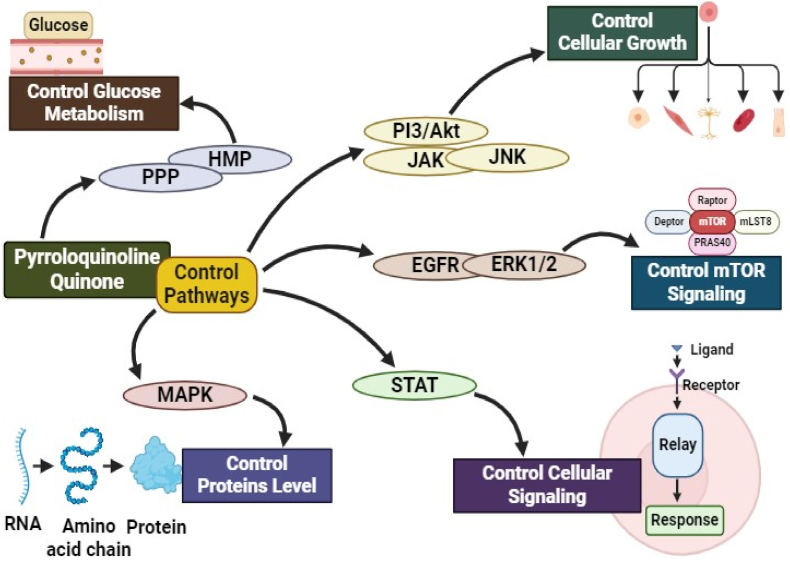
Fig. 3.
Involvement of PQQ in various cell signaling cascades. Primarily PQQ controls cell growth mechanisms, modulates glucose metabolism to minimize onset of T2D, and protein turn over in the cells.
Table 1.
Different metabolisms and their related pathways under the influence of PQQ.
| Sr. No. | Substrate or stage of metabolism | Effect of PQQ on metabolism and related pathways | Ref. |
|---|---|---|---|
| 1 | Lysine | PQQ might indirectly affect lysine metabolism by influencing cellular processes regulated by STAT, MAPK, and JAK signaling pathways, potentially alter requirements for lysine or its derivatives through the modulation of cell proliferation, differentiation, and survival. | Naveed et al. (2016) |
| 2 | Selenium (TrxR1) | PQQ could potentially influence selenium metabolism by regulating the expression or activity of TrxR1 through MAPK, PI3K/Akt, and Ras pathways, thereby impacting cellular redox balance and associated functions | Naveed et al. (2016) |
| 3 | Mitochondrial-related metabolism | PQQ may impact mitochondrial biogenesis and functions via PGC-1α, JNK, and mTOR pathways, affect levels of inflammatory biomarkers (plasma C-reactive protein and IL-6) and mitochondrial dysfunction | Harris et al. (2013) |
| 4 | Lipids | PQQ help regulate lipid metabolism through MAPK, PI3K/Akt, and PGC-1α. Impact lipolysis, lipogenesis, lipid transport, and maintains levels of HDLs, LDLs, and TGs. |
Supruniuk et al. (2020) |
| 5 | Energy | Affect metabolism by modulating PGC-1α, MAPK, and PI3K/Akt. Regulate mitochondrial biogenesis, oxidative phosphorylation, and glucose/lipid metabolism. This could result in enhanced energy production and utilization. | Harris et al. (2013) |
| 6 | Glucose | PQQ affect glucose/sugar metabolism directly through pathways like the Entner-Doudoroff pathway and indirectly through ERK1/2 and mTOR. Modulate insulin signaling or glycolytic enzymes. Promote conversion of glucose into gluconic acid, thus indirectly impacting cellular processes involved in glucose utilization or storage. | Fliege et al. (1992) |
| 8 | Aromatic compounds | PQQ indirectly affect metabolism of aromatic compounds by influencing cellular metabolic activities and energy status, which could impact the availability of substrates and cofactors required for this pathway. | Matsumura et al. (2014) |
| 9 | TCA cycle and intracellular metabolism (Regulatory and bioenergetics role) | PQQ could modulate mitochondrial metabolism (TCA cycle) by involving JNK and mTOR pathways. Affect levels of inflammatory biomarkers (plasma C-reactive protein and IL-6), reflecting changes in cellular metabolism and energy homeostasis. |
Hwang et al. (2020) Hwang and Willoughby (2018) |
There are several cellular pathways that depend on PQQ for normal physiological processes and responses to the environmental stressors. PQQ is a booster molecule for Sirt-1, and Sirt-3, which are NAD+-dependent protein deacetylases and have crucial role in modulation of mitochondria or its associated functions (Zhang et al., 2015). In vitro studies involving HepG2 cells, PQQ enhances the expression of Sirt-1 and Sirt-3 at transcriptional and translational levels by targeting PGC-1α, NRF-1/-2, and mitochondrial transcription factor A (MTFA). Such cellular and biological activities of PQQ make it a potential and attractive therapeutic drug molecule to cure certain aging associated and metabolism linked diseases (Zhang et al., 2015).
PQQ safeguard cells against nitric oxide (NO)-induced proliferation and promotes DNA synthesis suppression in the Ras regulatory pathway (Zhang et al., 2015). Furthermore, PQQ influences the STAT pathway, a transcriptional and signal transduction system crucial in carcinogenesis (Anthony and Williams, 2003). PQQ impacts MAPK signaling pathway to affect CREB activation, ROS production, ATP levels, matrix metalloproteinase (MMP), and Bcl-2 protein levels (Min et al., 2014a). Through the JAK, JNK, and PI3K/Akt pathways, PQQ influences cell proliferation and differentiation, cell survival, death, intracellular ROS suppression, and the production and release of NGF (Ameyama et al., 1984; Yamaguchi et al., 1993; Min et al., 2022). Additionally, PQQ manages mitochondrial dysfunction and biogenesis, impacts glucose metabolism and glycolytic activities by interacting with osteoclast-forming pathways such as RANKL, hexose monophosphate, and pentose phosphate pathways (Odkhuu et al., 2012). Moreover, by regulating intracellular ROS generation, the cell cycle, and tyrosine dephosphorylation via ERK1/2 and EGFR, PQQ shields cancer patients against radiations (Decker, 1995; Suriyo et al., 2015). The involvement of PQQ in both its own biosynthetic routes and the Entner-Doudoroff pathway underscores its broad influence on various cellular processes and activities (Fliege et al., 1992).
4. Role of PQQ in energy metabolism
PQQ has garnered significant attention due to its potential role in energy metabolism, and associated health benefits including anti-aging impacts. PQQ is believed to exert its effects on energy metabolism primarily through modulation of lipid metabolism and enhanced mitochondrial activity. Mitochondria are the powerhouses of cells, produce cellular energy molecules (adenosine triphosphate or ATP) through oxidative phosphorylation. There is compelling evidence indicating that PQQ enhances energy generation and cellular efficiency by bolstering and optimizing mitochondrial physiology.
In addition, PQQ also promote mitochondrial biogenesis in order to increase the quantity and functional capability of mitochondria for enhanced metabolic activity to generate excessive energy rich ATPs. In a study, mice supplemented with PQQ demonstrated improved mitochondrial function and increased biogenesis (Chowanadisai et al., 2010). PQQ modulate cellular respiration during electron transport chain (ETC), which is a series of redox processes that occur on the inner mitochondrial membrane. PQQ enhances ATP synthesis by acting as a cofactor for several enzymes in the ETC. Investigations have revealed that PQQ serves as a redox cofactor for bacterial glucose dehydrogenase, which is involved in the ETC (Stites et al., 2006; Ghosh et al., 2013; Misra et al., 2012).
4.1. PQQ and lipid metabolism
Lipid metabolism plays a crucial role in energy generation and overall metabolic health. Numerous studies shown that PQQ influence both mitochondrial activity and enhanced lipid breakdown (lipolysis). Experimental evidence suggests that PQQ promotes lipolysis inside mitochondrial matrix through β-oxidation to release free fatty acids could be a potential source of energy evidenced in an in vivo model (Jonscher and Rucker, 2019). PQQ is seen to enhance this fatty acid oxidation process to maintain the cellular energy balance, and aid in weight management in mice (Hwang and Willoughby, 2018). Moreover, lipid biosynthesis from smaller precursor molecules and lipolysis is regulated by PQQ-mediated gene expression linked with lipids metabolism in mice (Jonscher and Rucker, 2019; Supruniuk et al., 2020). PQQ may secure metabolic homeostasis by regulating lipogenesis and lipolysis.
5. Effects of PQQ supplementation on health
5.1. Weight management
Supplementation of PQQ is found to aid in weight loss, improve insulin sensitivity, and help prevent metabolic syndrome (Mohamad Ishak et al., 2021; Charrier et al., 2024). These findings are especially noteworthy given the increasing prevalence of obesity and metabolic disorders worldwide. PQQ impact lipid metabolism and energy expenditure, which can play a role in weight loss management (Charrier et al., 2024). In an in vivo study, PQQ supplementation in mice resulted in increased fatty acid oxidation and energy expenditure, indicating its potential for promoting weight loss or maintaining a weight to body mass index (BMI) (Hwang and Willoughby, 2018). Additionally, mice supplemented with PQQ exhibited altered gene expression patterns linked with lipid processing i.e., lipolysis and further fatty acid oxidation (Jiang et al., 2020). This regulation of lipid metabolism could potentially aid in weight management by promoting lipolysis to utilize stored fats for the generation of ATPs.
5.2. Insulin sensitivity
Metabolic disorders like type 2 diabetes (T2D) and metabolic syndromes are primarily characterized by insulin resistance. PQQ has shown promising results in increasing insulin sensitivity, potentially aiding in the prevention or management of metabolic disorders. Supplementing mice with PQQ, enhanced their insulin sensitivity and glucose tolerance, even when fed a high-fat diet (Ishak and Ikemoto, 2023). Moreover, PQQ may exert these effects by influencing the transcriptional activity associated to glucose metabolism and insulin signaling. In diabetic mice, PQQ supplementation resulted in improved insulin sensitivity and reduced hyperglycemia. It is further suggested that PQQ beneficial effects on insulin sensitivity may partly be attributed to antioxidant and anti-inflammatory capabilities of this drug molecule (Qu et al., 2022).
5.3. Prevention of metabolic syndromes
Obesity around the middle ages, abnormal lipid profiles, high blood pressure, and poor glucose tolerance are all components of metabolic syndromes, which increases the risk of cardiovascular disease and T2D (Powell-Wiley et al., 2021). PQQ may aid in the management or prevention of metabolic syndrome due to its potential effects on diminishing oxidative stress, insulin sensitivity, and lipid metabolism. Certain in vivo studies investigating metabolic syndromes, where mice were fed with high-fat and high-sucrose diets to examine the implications of PQQ supplementation shown improved glucose tolerance, lipid profiles, and oxidative stress indicators suggested that it may help prevent or reduce the severity of metabolic syndrome (Zhang et al., 2022; Ishak et al., 2021). Though much of the research has been conducted in animal models, but there are encouraging evidences for the positive effects of PQQ administration on weight control, insulin sensitivity, and metabolic syndrome prevention. Further human clinical trials are needed to confirm these findings and determine the optimal dosage and duration of PQQ supplementation to reap the benefits, as previous clinical trials have reported in Table 2.
Table 2.
Previously reported different clinical studies to examine pharmacological potential of PQQ.
| Sr. No. | Objective | Experiment Model | Produced effects | Ref. |
|---|---|---|---|---|
| 1 | Learning and memory function | Rats | Significantly improved learning ability and prevent neuron-degeneration caused by oxidative stress | (Takatsu et al., 2009; Ohwada et al., 2008) |
| 2 | Neuropsycho-logical status based on RBANS | Human | People whose RBANS scores are lower may see a more pronounced improvement after taking the PQQ•Na2-supplements | (Tamakoshi et al., 2023; Shiojima et al., 2022; Itoh et al., 2016) |
| 3 | Cognitive functions | Human | Significant improvement in task performance | Itoh et al. (2016) |
| 4 | Impacts on anxiety, fatigue, and sleeping | Human | A marked improvement in sleepiness upon awakening, the beginning of sleep, and the maintenance of sleep length | Nakano et al. (2012) |
| 5 | The impact on dry skin hydration levels, elasticity, and transepidermal water loss (TEWL) | Mice and Human | Benefits impaired skin barrier function as well as dry-skinned human female individuals and in mice model | Nakano et al. (2015a) |
| 6 | Impact on cholesterol and serum TGs levels | Human | PQQ reduce LDL-cholesterol levels | Nakano et al. (2015b) |
| 7 | Impacts on development, fertility, and markers of collagen expression and maturation | Balb/c mice | By modulating mRNA levels for Type I procollagen α1-chains, PQQ supplementation might improve growth, reproductive success, and possibly regulate indicators of the synthesis and maturation of the extracellular matrix (ECM) in neonates | Steinberg et al. (2003) |
| 8 | Nutritional essentiality of PQQ | Rats and mice | Animals fed with PQQ meals showed consistent signs of increased growth. A decrease in tendon benzylamine oxidase and an increase in cutaneous collagen solubility were seen in subjects deficient in PQQ | Rucker et al. (1989) |
| 9 | Effects on growth performance and small intestine characteristics | Weaned pigs | Reduced levels of cytokines (IL-1β, IL-2), and interferon-γ, along with improved activity of antioxidant enzymes (SOD, GSH-Px, CAT, and MDA) in the small intestine mucosa. The jejunum also showed an increase in the expression of occludin and zonula occluden protein-1 (ZO-1). Weaned piglets' growth performance and intestinal health were both significantly enhanced |
(Jiang et al., 2020; Yin et al., 2019) |
| 10 | Challenged intestinal morphology, oxidative stress, and inflammatory responses to lipopolysaccharide | Broiler chickens | Incorporating PQQ•Na2 into the diet reduce IL-1β and IL-10 levels at transcriptional level in the duodenum mucosa and enhanced GSH-Px activity in serum and T-SOD and CAT activities in liver. In addition, it reduces the effects of LPS on the duodenal villus height to bulb depth ratio in broilers. |
(Jiang et al., 2020; Zheng et al., 2020) |
6. Other potential benefits of PQQ
In addition to its effects on energy metabolism, PQQ has been associated with various other potential benefits for overall health and well-being (Akagawa et al., 2016a). In this section, we will highlight various health conditions and the potential role of PQQ.
6.1. Anti-inflammatory properties
PQQ have been reported to possess strong anti-inflammatory properties that may help mitigate chronic inflammation and its associated health risks such as coronary artery disease and metabolic disorders (Charrier et al., 2024). A study demonstrated that PQQ suppressed inflammatory responses in human monocytes and macrophages (Han et al., 2021). TNF-α induces damages to chondrocytes leading to matrix degradation and cell death in osteoarthritis, whereas PQQ help diminish this inflammatory burst to dissipate this condition (Han et al., 2021). PQQ exposed mice showed a significant decline in neuroinflammations, and expression levels of NO, PGE2, pro-inflammatory factors (iNOS, COX-2, TNF-a, IL-1b, IL-6, MCP-1 and MIP-1a) in LPS treated primary microglia cells was declined to a notable extent (Yang et al., 2014a).
6.2. Longevity and anti-aging potential
Due to its antioxidant properties, ability to maintain mitochondrial activity, and support for overall cellular health, some studies suggested that PQQ may play a role in prolonging lifespan and slow the aging process (Mohamad Ishak et al., 2024). In another study, nematodes were protected against oxidative stress and experienced an increase in lifespan when given PQQ supplements (Yang et al., 2021). While many studies on PQQ and its effects on energy metabolism and mitochondrial function have been conducted on animal models or in vitro, their applicability to humans may be limited. To gain a better understanding of PQQ therapeutic applications and its potential benefits, further human clinical trials are needed based on in vivo studies. It is essential to consult with healthcare professionals before incorporating PQQ or any other dietary supplements into a person's daily intake, as they may interact with existing medications or have unintended side effects, especially when uses at high doses.
6.3. PQQ in neuroprotection and cognitive health
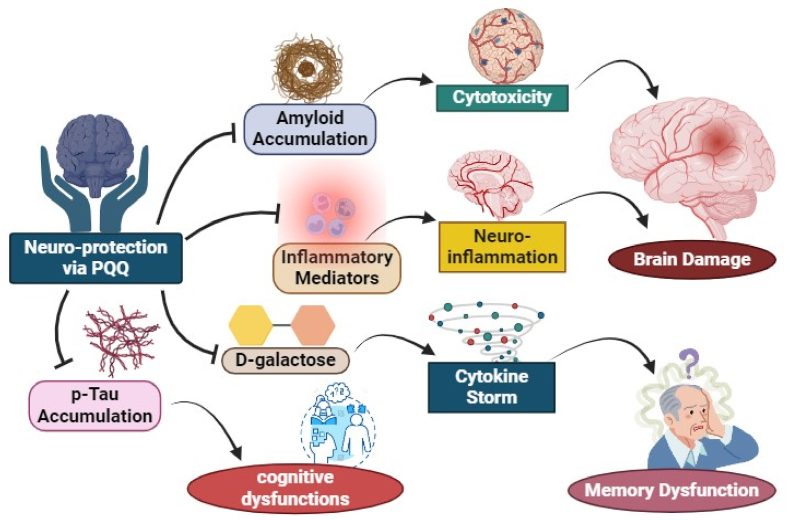
PQQ plays a significant role in neuroprotection due to its involvement in various neuroregulatory mechanisms (Fig. 4). Amyloid oligomer proteins which are formed through fibrilization, accumulate and can selectively damage neurons, is a condition common in numerous neurodegenerative disorders. Given the cytotoxicity induced by amyloid oligomer production, identifying pharmacologic agents capable of penetrating through blood-brain barrier (BBB) to prevent conversion into oligomers and/or fibrils is crucial for treating neurodegenerative diseases (Wells et al., 2021; Tsukakoshi et al., 2018). PQQ inhibits the aggregation and fibrillation of Amyloid β1-42 (Aβ1-42), α-synuclein, and mouse prion protein (Kim et al., 2010a, 2010b). In vitro studies have shown that PQQ-TME, an esterified form of PQQ, exhibits twice the BBB permeability of PQQ and demonstrates higher inhibitory activity against the fibrillation of α-synuclein, Aβ1-42, and prion protein. These findings suggest that esterifying PQQ could be a promising strategy for developing novel amyloid inhibitors based on PQQ (Tsukakoshi et al., 2018).
Fig. 4.
Mechanism of action of PQQ in aging associated syndromes. The neuroprotective action of PQQ is solely via inhibition of the inflammatory factors, amyloid accumulation and reduction in oxidative stress.
PQQ is an essential vitamin like molecule having strong antioxidant properties could act as redox cofactor, and exhibits potent immunosuppressive effects (Jonscher et al., 2021). In a study focusing on primary microglia cells treated with LPS, the anti-inflammatory PQQ pretreatment significantly reduced the production of NO, PGE2, and inflammatory cytokines (iNOS, COX-2, TNF-a, IL-1b, IL-6, MCP-1, and MIP-1a) (Brodzikowska et al., 2022). Additionally, in LPS-activated primary microglia cells, PQQ decreased the nuclear trafficking of NF-κB and dephosphorylate p65, p38, and JNK in MAPK pathways. To assess the inhibitory effects of PQQ against neurological inflammation in vivo, a systemic treatment with LPS in an acute inflammatory murine brain model was employed. Immunoblotting and immunohistochemical analyses of brain tissue, specifically using Iba1 antibody, revealed a significant reduction in neuroinflammation in mice treated with PQQ (Yang et al., 2014a). Furthermore, evidence indicated that PQQ prevented microglia-induced neurotoxicity in primary cortical neurons (Peng et al., 2020). Taken together, these findings suggest that PQQ could serve as a potential therapeutic agent for neurodegenerative diseases associated with microglia activation, potentially slowing their progression (Peng et al., 2020; Yang et al., 2014b).
Some studies have suggested that D-galactose (D-gal) induces memory loss by triggering an oxidative stress response, making it a model for senescence (Zhou et al., 2018; Tamakoshi et al., 2023). Previously, it was shown that PQQ has the potential to form derivatives by bonding with amino acids, which could be beneficial in treating various pathological conditions. It is suggested that a combination of PQQ with glutamate may alleviate D-galactose (D-gal)-induced neurotoxicity resulting from oxidative stress induced damage in mice (Zhou et al., 2018; Guan et al., 2015). Additionally, the findings indicate that PQQ may influence the production of cytokines and prostaglandins, two proinflammatory mediators linked with the ageing process. Supplementation of PQQ may reversed the alterations observed in the hippocampus of D-gal-induced mice, including elevated levels of MDA, ROS, as well as reduced T-AOC activity (Zhou et al., 2018).
The expression of superoxide dismutase 2 (SOD-2) at transcriptional level was significantly improved by the exposure to PQQ (Zhou et al., 2018). By binding with excess glutamate, PQQ may alleviate the memory impairments and neurotoxicity caused by D-gal (Guan et al., 2015; Xu et al., 2020). This establishes a connection between inflammation, oxidative stress, and glutamate-mediated neurotoxicity. Moreover, PQQ sustained the enzyme activity of GSK-3β and decreased the up-regulated production of p-Akt by D-gal, resulting a decline in the level of p-Tau in the hippocampus. The Akt/GSK-3β pathway was partially regulated by PQQ in terms of memory ability (Zhou et al., 2018).
PQQ is abundantly present in plant foods and human breast milk (Chongrong et al., 2021). When reacting with an amino acid at an elevated ratio, PQQ reportedly undergoes a structural transition to an imidazole pyrroloquinoline, indicating its strong reactivity and bioavailability (Yamada et al., 2020). A comparative study was conducted using a human neuroblastoma cell line and a hepatic carcinoma cell line to elucidate the physiological effects of PQQ and imidazole pyrroloquinoline (IPQ). These effects included neuroprotection, growth promotion, antioxidant activity, and stimulation of biogenesis of mitochondria (Yamada et al., 2020). Furthermore, the expression levels of COX4/1, an indicator of cellular mitochondrial abundance, were evaluated in cells exposed to PQQ, PQQH2, and IPQ. This gene is associated with human cytochrome c oxidase subunit IV isoform I. The comparative study revealed that, except for antioxidative activity, IPQ demonstrated nearly identical biological activities to PQQ. Additionally, it is further shown that PQQ enhances language-related brain function in humans, while IPQ enhances memory learning and improvements in aged mice (Tamakoshi et al., 2023; Yamada et al., 2020; Ikemoto et al., 2024).
The prevalence of cognitive dysfunctions is increasing globally, leading scientists to explore ways to enhance brain function in the future. PQQ is a food-based coenzyme with potent antioxidant properties that enhances various functions which includes increasing the activity of NGF and its receptors, which protect nerve cells and suppressing the formation of fibrils and aggregation of amyloid-β (Jonscher et al., 2021). Supplementing with PQQ•Na2 may benefit individuals in middle-ages and in elderly who are concerned about aging-related cognitive decline, addressing issues such as attention span, memory, decision-making, and cognitive function (Shiojima et al., 2022). Apoptotic cell death, adverse external environments, and the limited regenerative capacity of neurons all contribute to the central nervous system (CNS) inability to recover from traumatic brain injury (TBI) and the effect is multi-fold when exposed to PQQ (Zhang et al., 2012). In a systematic administration of the PQQ upregulated the expression of β-1,4-GalT-I, -V, galactosidase β-1 (Galβ-1), 4-galactosyltransferase N-acylsphingosine (4-GlcNAc) in microglial and neuron cells in cortex and hippocampal CA2 region of the brain (Zhang et al., 2012). Therefore, a viable therapeutic strategy could involve promoting the innate capacity for regeneration and reducing the rate of neuronal cell death.
The neuroprotective and regenerative properties of PQQ on peripheral neurons have been extensively documented. Given that one of the severely damaged regions following TBI is the cerebellar circuit, PQQ could stimulates neurite regeneration in a wound recovery model using cultured cerebellar granular neurons (CGNs) (Shanan et al., 2019). Additionally, PQQ pose neuroprotective effect in CGNs using a model of K+/FCS deprivation-induced apoptosis. Resveratrol (RVT) alone and or in combination with PQQ may possibly have synergistic impact for neuroprotection and regeneration (Shanan et al., 2019). RVT (5.0 μM) and PQQ (0.5 μM) on neurite re-growth in the wound recovery experiment was not much obvious, but the efficacy increased when PQQ concentrations (1–2 μM) was increased (Shanan et al., 2019). In CGNs culture, resveratrol had no effect on neurite length but greatly enhanced the quantity of viable CGNs. As for the neuroprotective impact, after K+/FCS deprivation of culture, both PQQ and RVT significantly increased the survival of CGNs. Hence, both of these molecules demonstrated a strong neuroprotective impact and a propensity to promote neurite outgrowth, but no evidence of a synergistic effect was seen (Shanan et al., 2019).
6.4. PQQ and cardiovascular health
The development of pulmonary hypertension (PH) is influenced by inflammation, an overabundance of pulmonary artery smooth muscle cells (PASMCs) and endothelial cells (PAECs), and disruptions in mitochondrial and metabolic functions (Perez, 2016; Katseff et al., 2021). PQQ being a recognized natural antioxidant promotes mitochondrial biogenesis, strong anti-diabetic potential, neuroprotection, and cardioprotective effects (Qu et al., 2022). Treatment with PQQ resulted in decreased insulin resistance, enhanced mitochondrial bioenergetics with preserved respiratory complexes, and prevention of metabolic and mitochondrial dysfunctions in human pulmonary artery smooth muscle cells (HPASMCs). Both preventive and curative treatments with PQQ significantly reduced endothelial dysfunction and pulmonary artery remodeling, right ventricular hypertrophy, and elevated right ventricle pressure. Additionally, PQQ reduced inflammations, improved heart function, and prevent cardiac fibrosis. Given its ability to inhibit mitochondrial and metabolic dysfunction in PASMCs and its effectiveness in mitigating PH development, PQQ holds therapeutic potential as a treatment for PH (Shafiq et al., 2022).
A major contributor to chronic heart failure (CHF) is mitochondrial dysfunction in the myocardium. This dysfunction is characterized by increased levels of ROS, interference with mitochondrial biogenesis and imbalance in Ca2+ homeostasis, and decreased mitochondrial membrane potential (ΔΨm) (Knowlton et al., 2014; Sabbah, 2020). Pressure overload leads to myocardial remodeling and cell hypertrophy, processes improved by PQQ pretreatment both in vivo and in vitro, thereby preventing CHF. In addition to preserving mitochondrial morphology, PQQ attenuated the downregulation of PGC-1α and TFAM induced by angiotensin II (Ang II) or transaortic constriction (TAC). Moreover, PQQ modulated ROS levels and enhanced ΔΨm in neonatal rat left ventricular myocytes (NRVMs) treated with Ang II + PQQ. PQQ also maintained [Ca2+]m homeostasis and prevented [Ca2+]m overload by increasing the expression of the Na+/Ca2+ exchanger (NCLX). These findings demonstrate that PQQ protects ΔΨm against [Ca2+]m overload by upregulating NCLX expression, thereby reducing ROS generation. Furthermore, PQQ simultaneously enhances the expression of PGC-1α and TFAM to regulate mitochondrial biogenesis. By mitigating myocardial damage from pressure overload and minimizing CHF development, PQQ plays a crucial role in limiting mitochondrial dysfunction (Xu et al., 2020).
MDA serves as a marker of LPO (Cał et al., 2016), and PQQ has been shown to decrease its levels in cardiac tissues. In an in vivo rat model study of ischemia and ischemia/reperfusion, PQQ demonstrates dose-related improvement in heart function and reduction in infarct size when administered either as pretreatment or at the onset of reperfusion. PQQ emerges as an excellent cardioprotective agent, potentially scavenging free radicals in ischemic myocardium (Hwang and Willoughby, 2018).
Isolated adult male rat cardiomyocytes were given PQQ, and found to be protected against acute oxidative stress injury. Fluorescence microscopy detected the excessive generation of ROS and the depolarization of the ΔΨm induced by H2O2. Pre-incubation with PQQ significantly reduced cell viability loss caused by H2O2. Fluorescent markers CM-H2XRos and dihydroethidium revealed an increase in cellular ROS levels in response to H2O2, which were markedly diminished upon the administration of PQQ (Gao et al., 2022; Tao et al., 2007). These findings demonstrate that PQQ maintains redox homeostasis, mitigates mitochondrial dysfunction, and reduces cell viability loss in isolated adult rat cardiomyocytes, shedding light on PQQ mechanisms of action in the heart (Tao et al., 2007).
PQQ improved adipose tissue quality and viability while decreasing fibrosis. Under hypoxic stress, PQQ scavenges excessive ROS burst, restoring ΔΨm, enhancing adipocyte viability, alleviating tissue apoptosis, and promote angiogenesis (Zhang et al., 2023). In the mitochondrial apoptotic pathway, PQQ decreased the expression of pro-apoptotic proteins e.g., Bax and cytochrome c, while antiapoptotic protein Bcl-2 level was upregulated. By reducing hypoxic stress and promoting timely angiogenesis in cardiac health, PQQ may enhance the overall survival of adipocytes (Zhang et al., 2024).
Pre-eclampsia (PE), an elevated blood pressure condition that can develop during pregnancy, currently lack any accepted clinical treatment despite widespread belief that the placenta is its etiological agent. In rats with PE, clinical symptoms and pregnancy outcomes were significantly improved by PQQ (Wang et al., 2022a). Correspondingly, there was a reversal in the levels of antioxidant and inflammatory markers. PQQ may achieve these therapeutic targets by blocking NF-κB and enhancing Nrf2 antioxidant pathways. Ultimately, the NF-κB-Nrf2 pathway amplifies PQQ anti-inflammatory and antioxidative effects, thereby protecting animals with a PE-like model (Wang et al., 2022b).
6.5. PQQ role in cancer treatment
PQQ shows promise as a potential anti-tumor agent with minimal or no toxicity to normal cells by activating mitochondrial-dependent apoptosis pathways. It triggers both caspase-dependent and caspase-independent apoptotic pathways in human malignant chondrosarcomas, a cancer type known for its resistance to radio- and chemotherapy (Wu et al., 2018). PQQ induces apoptosis in chondrosarcoma SW1353 cells and inhibits tumor growth in vivo in a dose-dependent manner, without activating apoptotic proteins in normal cells (Wu et al., 2018). In SW1353 cells, PQQ facilitates the binding of Smac to the X-linked inhibitor-of-apoptosis protein (XIAP), thereby preventing XIAP from interacting with caspase-3. This results in a reduction of procaspase-3 and caspase-1 expression levels. Additionally, PQQ promotes the cytoplasmic accumulation of apoptosis-inducing factor (AIF) and cytochrome c, depleting their presence within the mitochondria. This energy deficit ultimately drives caspase-dependent apoptotic pathways in cancer cells (Wu et al., 2018). PQQ induces tumor cell apoptosis and death by disrupting cell proliferation and triggering mitochondrial-dependent apoptosis in three solid tumor cell lines (A549, Neuro-2A, and HCC-LM3). Treatment with low (15 μM) to medium (1200 μM) doses of PQQ exhibited strong anti-tumor activity in A549 and Neuro-2A cells, with no observed effects on normal human cells (HRPTEpiC and HUVEC) (Min et al., 2014b). It is believed that increasing PQQ concentrations may lead to a burst of reactive oxygen species (ROS), a reduction in ATP production, disruption of mitochondrial membrane potential (MMP), decreased production of the cytoprotective Bcl-2 protein, activation of caspase-3, and irregular MAPK phosphorylation patterns (Min et al., 2014b). In human promonocytic leukemia U937 cells, PQQ induces apoptotic cell death by depleting intracellular antioxidant defenses and triggering a ROS burst (Shankar et al., 2010). Interestingly, when combined with glutathione (GSH) or N-acetyl-L-cysteine (NAC), PQQ induces 2–5 times higher apoptosis in U937 cells. This suggests that modulating cellular GSH levels could be a novel approach to enhancing PQQ's cytotoxic effects (Shankar et al., 2010).
7. Future directions and clinical implications
While current research on PQQ has yielded valuable insights into its potential benefits for metabolic health, there remain several gaps and areas requiring further investigation. One critical gap in current research is the absence of clear dose-response relationships for PQQ supplementation. Many studies have employed varying doses, complicating the establishment of optimal dosage for specific metabolic outcomes (Devasani and Majumdar, 2019). The U.S. Food and Drug Administration has approved various PQQ•Na2 dietary supplements, with no reported side effects (Akagawa et al., 2016a). In one study, rats were given PQQ•Na2 (11.5 mg/kg of body weight) intraperitoneally for four consecutive days, resulting in physiological and morphological changes in the kidneys (Watanabe et al., 1989). Acute and subchronic toxicity studies indicate that the oral LD50 value of PQQ disodium salt (BioPQQ™) ranges from 1000 to 2000 mg/kg of body weight in male rats and 500–1000 mg/kg in female rats. In a 14-day study, high doses of PQQ resulted in increased relative kidney weight and associated pathological changes in female rats only. Similarly, in a 28-day study, female animals showed elevated urine protein levels and the presence of crystals, although these effects were reversible during the recovery period (Nakano et al., 2014). In a thirteen-week study, notable biochemical and histopathological changes were observed, but they were not of toxicological significance as PQQ levels remained within safe ranges, showing no dose-dependent toxicity (Liang et al., 2015). Genetic toxicity studies revealed weakly positive results in vitro in chromosome aberration tests using Chinese hamster lung cells, but no genetic toxicity was observed in human peripheral blood lymphocytes. In a micronucleus test on mice, PQQ disodium salt exhibited no genetic toxicity at doses up to 2000 mg/kg of body weight (Nakano et al., 2013). Overall, PQQ disodium salt demonstrates low toxicity in animal models, and no significant genetic toxicity has been observed. While in humans, following one single dosage of PQQ•Na2 (0.2 mg/kg of body weight), thiobarbituric acid reactive products (TBARS) were quantified by the levels of MDA production from lipid hydroperoxides, showed a significant decline in a time-dependent manner (Harris et al., 2013). In addition, the change of TBARS values correlated significantly with the maximum plasma concentration (Cmax) for PQQ•Na2 and hence confirmed its antioxidant properties. Furthermore, the absorption (62%) of the PQQ in the intestinal tract and excretion (81%) from the kidneys is quite similar to vitamin B-complex system within 24 h (Smidt et al., 1991b). A similar pattern to urine PQQ level rise and clearance, a highest serum levels (10 nM) was observed after one time application of 0.2 mg PQQ•Na2/kg of body weight (Harris et al., 2013). Like vitamins B-complex molecules, pharmacokinetic studies confirmed that no accumulation of PQQ•Na2 in the body was seen (Harris et al., 2013).
Future research should concentrate on systematically assessing the effects of different PQQ doses on parameters like weight management, insulin sensitivity, and markers of metabolic syndrome. This review underscored the necessity for well-designed dose-response studies to ascertain the most effective and safe dosages of PQQ for various applications.
Data regarding the safety and long-term effectiveness of PQQ supplementation are scarce, with recent research primarily focusing on short-term effects. Based on the literature, we concluded 100–200 mg/kg of body weight PQQ administration is a safer dosage range for various conditions. In addition, it is further emphasized to conduct large sample sized studies to determine the potential hazards, side effects, and influence on metabolic health associated with long-term PQQ administration. As PQQ garners interest as a potential therapeutic agent, it is crucial to investigate its interactions with commonly prescribed medications and dietary factors. These interactions could affect the efficacy, safety, and metabolic outcomes of PQQ supplementation. Future research should probe into potential interactions between PQQ and medications used for metabolic disorders such as metformin, insulin, and lipid-lowering drugs, as well as the impact of dietary components like fiber, polyphenols, and other natural bioactive compounds.
8. Translating current PQQ research into clinical practice
While the translation of current PQQ research into clinical practice is still in its early stages, several potential avenues for its application in managing metabolic disorders exist. Given the promising findings regarding PQQ effects on insulin sensitivity, glucose metabolism, and lipid profiles, it may serve as a supplementary treatment for metabolic syndromes and T2D, demands further in-depth research. PQQ supplementation could complement existing treatments like lifestyle modifications and pharmacological interventions, potentially improving metabolic control and reducing risks associated with complications. Additionally, PQQ potential to influence energy expenditure and lipid metabolism could enhance weight management strategies, making it a valuable addition to calorie-restricted diets and exercise programs. Nevertheless, comprehensive clinical trials are essential to determine PQQ safety, effectiveness, and appropriate dosage recommendations for managing weight in overweight or obese individuals. Due to individual's specific responses to PQQ supplementation, there may arise opportunities to integrate PQQ into personalized nutrition and precision medicine strategies. By pinpointing biomarkers, genetic factors, or other individual traits that impact the metabolic response to PQQ, healthcare providers could customize supplementation regimens for maximal efficacy and safety. Close collaboration among researchers, clinicians, and regulatory authorities are pivotal to responsibly translate PQQ research into clinical practice, maintaining stringent standards for safety, efficacy, and evidence-based decision-making.
9. Conclusions
PQQ has been demonstrated to be a ubiquitous molecule that influences a wide range of biochemical and physiological activities. In this compilation, we present the latest research on PQQ impact on human health and wellness. Supplementing with PQQ may aid in cholesterol and glucose regulation, help prevent cardiovascular and neurodegenerative diseases, enhance cognitive abilities, and potentially play a crucial role in anti-aging interventions. Emerging evidence suggests that PQQ can modulate cell signaling pathways, scavenge radicals, and exhibit redox activity, all contributing to its potential health benefits. Recent findings also indicate that oral intake of PQQ does not cause toxicity or genotoxicity, suggesting its safety as a supplement. However, despite these advancements, the precise molecular mechanisms underlying PQQ's therapeutic effects warrant further exploration. Further mechanistic investigations are needed to fully elucidate PQQ role and potential additional benefits for human health, particularly in the context of anti-aging applications.
CRediT authorship contribution statement
Tingdong Yan: Investigation, Writing – original draft, Funding acquisition, Conceptualization, Supervision, Resources, Writing – review & editing, All authors have read and agreed to the final manuscript draft. Muhammad Farrukh Nisar: Investigation, Writing – original draft. Xiaomeng Hu: Resources, Investigation. Jieming Chang: Resources, Investigation. Yichen Wang: Resources, Investigation, Resources, Investigation. Zhaoguo Liu: Investigation. Yi Cai: Investigation. Jia Jia: Funding acquisition, Resources, Supervision. Yanming Xiao: Funding acquisition, Resources, Supervision. Chunpeng Wan: Funding acquisition, Conceptualization, Supervision, Resources, Writing – review & editing, All authors have read and agreed to the final manuscript draft.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 31972889), the Program for Professor of Special Appointment of Jiangsu Province.
Declaration of competing interest
The authors declare that there are no financial or other relationships that might lead to a conflict of interest of the present article. All authors have reviewed the final version of the manuscript and approved it for publication.
Handling Editor: Dr. Quancai Sun
Contributor Information
Tingdong Yan, Email: tingdong.yan@ntu.edu.cn.
Chunpeng Wan, Email: chunpengwan@jxau.edu.cn.
List of Abbreviation
- ΔΨm
Mitochondrial membrane potential
- 6-OHDA
6-hydroxydopamine
- BBB
Blood-brain barrier
- CAT
Catalse
- CGNs
Cerebellar granular neurons
- CREB
cAMP responsive element-binding protein (CREB)
- IPQ
Imidazole pyrroloquinoline
- LDH
Lactate dehydrogenase
- MMP
Matrix metalloproteinase
- MTFA
Mitochondrial transcription factor A
- NGF
Nerve growth factor
- NMDA
N-methyl-D-aspartate
- NO
Nitric oxide
- NRF-1
Nuclear respiratory factor 1
- NRF-2
Nuclear respiratory factor 2
- PASMCs
Pulmonary artery smooth muscle cells
- PGC-1α
Peroxisome proliferator-activated receptor-γ coactivator-1α
- PH
Pulmonary hypertension
- PQQ
Pyrroloquinoline Quinone
- PQQC
Pyrroloquinoline quinone synthase C
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- TBARS
Thiobarbituric acid reacting substances
- TBI
Traumatic brain injury
Data availability
No data was used for the research described in the article.
References
- Akagawa M., Nakano M., Ikemoto K., expand A. Recent progress in studies on the health benefits of pyrroloquinoline quinone. Biosci. Biotechnol. Biochem. 2016;80(1):13–22. doi: 10.1080/09168451.2015.1062715. [DOI] [PubMed] [Google Scholar]
- Akagawa M., Minematsu K., Shibata T., Kondo T., Ishii T., Uchida K. Identification of lactate dehydrogenase as a mammalian pyrroloquinoline quinone (PQQ)-binding protein. Sci. Rep. 2016;6(1) doi: 10.1038/srep26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameyama M., Shinagawa E., Matsushita K., Adachi O. Growth stimulating substance for microorganisms produced by Escherichia coli causing the reduction of the lag phase in microbial growth and identity of the substance with pyrroloquinoline quinone. Agric. Biol. Chem. 1984;48(12):3099–3107. doi: 10.1080/00021369.1984.10866645. [DOI] [Google Scholar]
- Anthony C., Williams P. The structure and mechanism of methanol dehydrogenase. Biochim. Biophys. Acta. 2003;1647(1–2):18–23. doi: 10.1016/S1570-9639(03)00042-6. [DOI] [PubMed] [Google Scholar]
- Bauerly K., Harris C., Chowanadisai W., Graham J., Havel P.J., Tchaparian E., et al. Altering pyrroloquinoline quinone nutritional status modulates mitochondrial, lipid, and energy metabolism in rats. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodzikowska A., Ciechanowska M., Kopka M., Stachura A., Włodarski P.K. Role of lipopolysaccharide, derived from various bacterial species, in pulpitis—a systematic review. Biomolecules. 2022;12(1):138. doi: 10.3390/biom12010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T.D.H. The development of mechanistic enzymology in the 20th century. Nat. Prod. Rep. 2001;18(5):465–493. doi: 10.1039/b009205n. [DOI] [PubMed] [Google Scholar]
- Całyniuk B., Grochowska-Niedworok E., Walkiewicz K.W., Kawecka S., Popiołek E., Fatyga E. Malondialdehyde (MDA)–product of lipid peroxidation as marker of homeostasis disorders and aging. Annales Academiae Medicae Silesiensis. 2016;2016(70):224–228. doi: 10.18794/aams/65697. [DOI] [Google Scholar]
- Charrier D., Cerullo G., Carpenito R., Vindigni V., Bassetto F., Simoni L., et al. Metabolic and biochemical effects of pyrroloquinoline quinone (PQQ) on inflammation and mitochondrial dysfunction: potential health benefits in obesity and future perspectives. Antioxidants. 2024;13(9):1027. doi: 10.3390/antiox13091027. https://www.mdpi.com/2076-3921/13/9/1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q., Chen J., Guo H., Lu J., Zhou J., Guo X., et al. Pyrroloquinoline quinone promotes mitochondrial biogenesis in rotenone-induced Parkinson's disease model via AMPK activation. Acta Pharmacol. Sin. 2020;42:665–678. doi: 10.1038/s41401-020-0487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chongrong K., Yang R., Ping G., Jian H., Yong T., Jianzhong H., et al. Separation and purification of pyrroloquinoline quinone from fermentation broth by pretreatment coupled with macroporous resin adsorption. Separ. Purif. Technol. 2021;257 doi: 10.1016/j.seppur.2020.117962. 2021. [DOI] [Google Scholar]
- Chowanadisai W., Bauerly K.A., Tchaparian E., Wong A., Cortopassi G.A., Rucker R.B. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through CREB phosphorylation and increased PGC-1α expression. J. Biol. Chem. 2009;285(1):142–152. doi: 10.1074/jbc.M109.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowanadisai W., Bauerly K.A., Tchaparian E., Wong A., Cortopassi G.A., Rucker R.B. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1α expression. J. Biol. Chem. 2010;285(1):142–152. doi: 10.1074/jbc.M109.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell G.A., Daley S.-K. Pyrroloquinoline quinone chemistry, biology, and biosynthesis. Chem. Res. Toxicol. 2022;35(3):355–377. doi: 10.1021/acs.chemrestox.1c00340. [DOI] [PubMed] [Google Scholar]
- Cores Á., Carmona-Zafra N., Clerigué J., Villacampa M., Menéndez J.C. Quinones as neuroprotective agents. Antioxidants. 2023;12(7):1464. doi: 10.3390/antiox12071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E.A. The role of phenolics, conjugated linoleic acid, carnosine, and pyrroloquinoline quinone as nonessential dietary antioxidants. Nutr. Rev. 1995;53(3):49–58. doi: 10.1111/j.1753-4887.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Devasani K., Majumdar A. Pyrroloquinoline quinone attenuates obesity associated low grade inflammation. Obesity Medicine. 2019;16 doi: 10.1016/j.obmed.2019.100134. [DOI] [Google Scholar]
- Fliege R., Tong S., Shibata A., Nickerson K.W., Conway T. The Entner-Doudoroff pathway in Escherichia coli is induced for oxidative glucose metabolism via pyrroloquinoline quinone-dependent glucose dehydrogenase. Appl. Environ. Microbiol. 1992;58(12):3826–3829. doi: 10.1128/aem.58.12.3826-3829.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Kamogashira T., Fujimoto C., Iwasaki S., Yamasoba T. Pyrroloquinoline quinone (PQQ) protects mitochondrial function of HEI-OC1 cells under premature senescence. NPJ Aging. 2022;8(1):3. doi: 10.1038/s41514-022-00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Chakraborty R., Raychaudhuri U. Pyrroloquinoline quinone a redox cofactor and its involvement in biological system. Int J Sci Nat. 2013;4:371–380. [Google Scholar]
- Gong D.Z., Geng C.Y., Jiang L.P., Aoki Y., Nakano M., Zhong L.F. Effect of pyrroloquinoline quinone on neuropathic pain following chronic constriction injury of the sciatic nerve in rats. Eur. J. Pharmacol. 2012;697(1–3):53–58. doi: 10.1016/j.ejphar.2012.09.052. [DOI] [PubMed] [Google Scholar]
- Guan S., Xu J.Q., Guo Y.F., Ge D., Liu T.Q., Ma X.H., et al. Pyrroloquinoline quinone against glutamate-induced neurotoxicity in cultured neural stem and progenitor cells. Int. J. Dev. Neurosci. 2015;42:37–45. doi: 10.1016/j.ijdevneu.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Han G.T., Cai W.S., Zhang Y.B., Zhou S.Q., He B., Li H.H. Protective effect of pyrroloquinoline quinone on TNF-α-induced mitochondrial injury in chondrocytes. Current Medical Science. 2021;41:100–107. doi: 10.1007/s11596-020-2248-3. [DOI] [PubMed] [Google Scholar]
- Hara H., Hiramatsu H., Adachi T. Pyrroloquinoline quinone is a potent neuroprotective nutrient against 6-hydroxydopamine-induced neurotoxicity. Neurochem. Res. 2007;32(3):489–495. doi: 10.1007/s11064-006-9257-x. [DOI] [PubMed] [Google Scholar]
- Harris C.B., Chowanadisai W., Mishchuk D.O., Satre M.A., Slupsky C.M., Rucker R.B. Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. J. Nutr. Biochem. 2013;24(12):2076–2084. doi: 10.1016/j.jnutbio.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Hauge J.G. Glucose dehydrogenase of Bacterium anitratum: an enzyme with a novel prosthetic group. J. Biol. Chem. 1964;239(11):3630–3639. jbc.org/article/S0021-9258(18)91183-X. [PubMed] [Google Scholar]
- He K., Nukada H., Urakami T., Murphy M.P. Antioxidant and pro-oxidant properties of pyrroloquinoline quinone (PQQ): implications for its function in biological systems. Biochem. Pharmacol. 2003;65(1):67–74. doi: 10.1016/s0006-2952(02)01453-3. [DOI] [PubMed] [Google Scholar]
- Hwang P., Willoughby D.S. Mechanisms behind pyrroloquinoline quinone supplementation on skeletal muscle mitochondrial biogenesis: possible synergistic effects with exercise. J. Am. Coll. Nutr. 2018;37(8):738–748. doi: 10.1080/07315724.2018.1461146. [DOI] [PubMed] [Google Scholar]
- Hwang P.S., Machek S.B., Cardaci T.D., Wilburn D.T., Kim C.S., Suezaki E.S., et al. Effects of pyrroloquinoline quinone (PQQ) supplementation on aerobic exercise performance and indices of mitochondrial biogenesis in untrained men. J. Am. Coll. Nutr. 2020;39(6):547–556. doi: 10.1080/07315724.2019.1705203. [DOI] [PubMed] [Google Scholar]
- Ikemoto K., Ishak N.S.M., Akagawa M. The effects of pyrroloquinoline quinone disodium salt on brain function and physiological processes. J. Med. Invest. 2024;71(1.2):23–28. doi: 10.2152/jmi.71.23. [DOI] [PubMed] [Google Scholar]
- Ishak N.S.M., Ikemoto K. Pyrroloquinoline-quinone to reduce fat accumulation and ameliorate obesity progression. Front. Mol. Biosci. 2023;10 doi: 10.3389/fmolb.2023.1200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak N.S.M., Ikemoto K., Kikuchi M., Ogawa M., Akutagawa K., Akagawa M. Pyrroloquinoline quinone attenuates fat accumulation in obese mice fed with a high-fat diet, daphnia magna supplied with a high amount of food, and 3T3-L1 adipocytes. ACS Food Science & Technology. 2021;1(10):1979–1989. doi: 10.1021/acsfoodscitech.1c00301. [DOI] [Google Scholar]
- Itoh Y., Hine K., Miura H., Uetake T., Nakano M., Takemura N., et al. Effect of the antioxidant supplement pyrroloquinoline quinone disodium salt (BioPQQ™) on cognitive functions. Randomized Controlled Trial. 2016;876:319–325. doi: 10.1007/978-1-4939-3023-4_40. [DOI] [PubMed] [Google Scholar]
- Jiang X.X., Zhou Y.F., Zhang Y., Tian D.Y., Jiang S., Tang Y.P. Hepatoprotective effect of pyrroloquinoline quinone against alcoholic liver injury through activating Nrf2-mediated antioxidant and inhibiting TLR4-mediated inflammation responses. Process biochemistry. 2020;92:303–312. doi: 10.1016/j.procbio.2020.01.023. [DOI] [Google Scholar]
- Jonscher K.R., Rucker R.B. Pyrroloquinoline quinone: its profile, effects on the liver and implications for health and disease prevention. Dietary Interventions in Liver Disease. 2019:157–173. [Google Scholar]
- Jonscher K.R., Chowanadisai W., Rucker R.B. Pyrroloquinoline-quinone is more than an antioxidant: a vitamin-like accessory factor important in health and disease prevention. Biomolecules. 2021;11(10) doi: 10.3390/biom11101441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T., Kato T. Nutritional biochemistry: a new redox-cofactor vitamin for mammals. Nature. 2003;422(6934):832. doi: 10.1038/422832a. 832. [DOI] [PubMed] [Google Scholar]
- Katseff A., Alhawaj R., Wolin M.S. Redox and inflammatory signaling, the unfolded protein response, and the pathogenesis of pulmonary hypertension. Lung Inflammation in Health and Disease. 2021;2:333–373. doi: 10.1007/978-3-030-68748-9_17. [DOI] [PubMed] [Google Scholar]
- Kim J., Harada R., Kobayashi M., Kobayashi N., Sode K. The inhibitory effect of pyrroloquinoline quinone on the amyloid formation and cytotoxicity of truncated alpha-synuclein. Mol. Neurodegener. 2010;5:1–11. doi: 10.1186/1750-1326-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kobayashi M., Fukuda M., Ogasawara D., Kobayashi N., Han S., et al. Pyrroloquinoline quinone inhibits the fibrillation of amyloid proteins. Prion. 2010;4(1):26–31. doi: 10.4161/pri.4.1.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A.A., Chen L., Malik Z.A. Heart failure and mitochondrial dysfunction: the role of mitochondrial fission/fusion abnormalities and new therapeutic strategies. J. Cardiovasc. Pharmacol. 2014;63(3):196–206. doi: 10.1097/01.fjc.0000432861.55968.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L.B., Yang C.F., Yu L.F., Smith W.L., Zhu S., Zhu J.Y., et al. Pyrroloquinoline quinine inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos in mouse bone marrow cells and inhibits wear particle-induced osteolysis in mice. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Seno H., Urakami T., Matsumoto T., Suzuki O. Trace levels of pyrroloquinoline quinone in human and rat samples detected by gas chromatography/mass spectrometry. Biochim. Biophys. Acta Gen. Subj. 1992;1156(1):62–66. doi: 10.1016/0304-4165(92)90096-d. [DOI] [PubMed] [Google Scholar]
- Liang C., Zhang X., Wang W., Song Y., Jia X. A subchronic oral toxicity study on pyrroloquinoline quinone (PQQ) disodium salt in rats. Food Chem. Toxicol. 2015;75:146–150. doi: 10.1016/j.fct.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Liu L.X., Zhang Y.Y., Liu T., Ke C.R., Huang J.Z., Fu Y.J., et al. Pyrroloquinoline quinone protects against exercise‐induced fatigue and oxidative damage via improving mitochondrial function in mice. Faseb. J. 2021;35(4) doi: 10.1096/fj.202001977RR. [DOI] [PubMed] [Google Scholar]
- Mandala A., Dobrinskikh E., Janssen R.C., Fiehn O., D'Alessandro A., Friedman J.E., et al. Maternal pyrroloquinoline quinone supplementation improves offspring liver bioactive lipid profiles throughout the lifespan and protects against the development of adult NAFLD. Int. J. Mol. Sci. 2022;23(11):6043. doi: 10.3390/ijms23116043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H., Umezawa K., Takeda K., Sugimoto N., Ishida T., Samejima M., et al. Discovery of a eukaryotic pyrroloquinoline quinone-dependent oxidoreductase belonging to a new auxiliary activity family in the database of carbohydrate-active enzymes. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern J., Gemmell A., Allen P.E., Mathers K.E., Regnault T.R.H., Stansfield B.K. Oral pyrroloquinoline quinone (PQQ) during pregnancy increases cardiomyocyte endowment in spontaneous IUGR Guinea pigs. Journal of Developmental Origins of Health and Disease. 2023;14(3):321–324. doi: 10.1017/S2040174423000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire W.S. Newly discovered redox cofactors: possible nutritional, medical, and pharmacological relevance to higher animals. Annu. Rev. Nutr. 1998;18(1):145–177. doi: 10.1146/annurev.nutr.18.1.145. [DOI] [PubMed] [Google Scholar]
- Min Z.H., Wang L.Y., Jin J.J., Wang X.D., Zhu B.J., Chen H., et al. Pyrroloquinoline quinone induces cancer cell apoptosis via mitochondrial-dependent pathway and down-regulating cellular Bcl-2 protein expression. J. Cancer. 2014;5(7):609–624. doi: 10.7150/jca.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Z., Wang L., Jin J., Wang X., Zhu B., Chen H., et al. Pyrroloquinoline quinone induces cancer cell apoptosis via mitochondrial-dependent pathway and down-regulating cellular bcl-2 protein expression. J. Cancer. 2014;5(7):609–624. doi: 10.7150/jca.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Z.H., Zhou J.B., Mao R.L., Cui B., Cheng Y.F., Chen Z.H. Pyrroloquinoline quinone administration alleviates allergic airway inflammation in mice by regulating the JAK-STAT signaling pathway. Mediat. Inflamm. 2022;2022 doi: 10.1155/2022/1267841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H.S., Rajpurohit Y.S., Khairnar N.P. Pyrroloquinoline-quinone and its versatile roles in biological processes. J. Biosci. 2012;37(2):313–325. doi: 10.1007/s12038-012-9195-5. [DOI] [PubMed] [Google Scholar]
- Miyauchi K., Urakami T., Abeta H., Shi H., Noguchi N., Niki E. Action of pyrroloquinolinequinol as an antioxidant against lipid peroxidation in solution. Antioxidants Redox Signal. 1999;1(4):547–554. doi: 10.1089/ars.1999.1.4-547. [DOI] [PubMed] [Google Scholar]
- Mohamad Ishak N.S., Ikemoto K., Kikuchi M., Ogawa M., Akutagawa K., Akagawa M. Pyrroloquinoline quinone attenuates fat accumulation in obese mice fed with a high-fat diet, Daphnia magna supplied with a high amount of food, and 3T3-L1 adipocytes. ACS Food Science & Technology. 2021;1(10):1979–1989. doi: 10.1021/acsfoodscitech.1c00301. [DOI] [Google Scholar]
- Mohamad Ishak N.S., Kikuchi M., Ikemoto K. Dietary pyrroloquinoline quinone hinders aging progression in male mice and D-galactose-induced cells. Front. Aging. 2024;5 doi: 10.3389/fragi.2024.1351860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K., Ouchi A., Nakano M. Kinetic study of the quenching reaction of singlet oxygen by pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in micellar solution. J. Agric. Food Chem. 2011;59(5):1705–1712. doi: 10.1021/jf104420y. [DOI] [PubMed] [Google Scholar]
- Nakano M., Yamamoto T., Okamura H., Tsuda A., Kowatari Y. Effects of oral supplementation with pyrroloquinoline quinone on stress, fatigue, and sleep. Functional foods in health and disease. 2012;2(8):307–324. doi: 10.1590/S1679-45082012000300007. [DOI] [Google Scholar]
- Nakano M., Suzuki H., Imamura T., Lau A., Lynch B. Genotoxicity of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™) Regul. Toxicol. Pharmacol. 2013;67(2):189–197. doi: 10.1016/j.yrtph.2013.07.007. https://www.sciencedirect.com/science/article/pii/S0273230013001098 [DOI] [PubMed] [Google Scholar]
- Nakano M., Takahashi H., Koura S., Chung C., Tafazoli S., Roberts A. Acute and subchronic toxicity studies of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™) in rats. Regul. Toxicol. Pharmacol. 2014;70(1):107–121. doi: 10.1016/j.yrtph.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Nakano M., Kamimura A., Watanabe F., Kamiya T., Watanabe D., Yamamoto E., et al. Effects of orally administered pyrroloquinoline quinone disodium salt on dry skin conditions in mice and healthy female subjects. J. Nutr. Sci. Vitaminol. 2015;61(3):241–246. doi: 10.3177/jnsv.61.241. [DOI] [PubMed] [Google Scholar]
- Nakano M., Kawasaki Y., Suzuki N., Takara T. Effects of pyrroloquinoline quinone disodium salt intake on the serum cholesterol levels of healthy Japanese adults. J. Nutr. Sci. Vitaminol. 2015;61(3):233–240. doi: 10.3177/jnsv.61.233. [DOI] [PubMed] [Google Scholar]
- Naveed M., Tariq K., Sadia H., Ahmad H., Mumtaz A.S. The life history of pyrroloquinoline quinone (PQQ): a versatile molecule with novel impacts on living systems. Int J Mol Biology Open Access. 2016;1:29–46. doi: 10.15406/ijmboa.2016.01.00005. [DOI] [Google Scholar]
- Nunome K., Miyazaki S., Nakano M., Iguchi-Ariga S., Ariga H. Pyrroloquinoline quinone prevents oxidative stress-induced neuronal death probably through changes in oxidative status of DJ-1. Biol. Pharm. Bull. 2008;31(7):1321–1326. doi: 10.1248/bpb.31.1321. [DOI] [PubMed] [Google Scholar]
- Odkhuu E., Koide N., Haque A., Tsolmongyn B., Naiki Y., Hashimoto S., et al. Inhibition of receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclast formation by pyrroloquinoline quinine (PQQ) Immunol. Lett. 2012;142(1–2):34–40. doi: 10.1016/j.imlet.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Ohwada K., Takeda H., Yamazaki M., Isogai H., Nakano M., Shimomura M., et al. Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by oxidative stress in rats. J. Clin. Biochem. Nutr. 2008;42(1):29–34. doi: 10.3164/jcbn.2008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi A., Ikemoto K., Nakano M., Nagaoka S.-I., Mukai K. Kinetic study of aroxyl radical scavenging and α-tocopheroxyl regeneration rates of pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in dimethyl sulfoxide solution: finding of synergistic effect on the reaction rate due to the coexistence of α-tocopherol and PQQH2. J. Agric. Food Chem. 2013;61(46):11048–11060. doi: 10.1021/jf4040496. [DOI] [PubMed] [Google Scholar]
- Peng Y., Xu D., Mao S., Zhou X. Neurotoxicity and apoptosis induced by pyrroloquinoline quinone and its ester derivative on primary cortical neurons. Neurotoxicology. 2020;78:47–56. doi: 10.1016/j.neuro.2020.02.005. https://www.sciencedirect.com/science/article/pii/S0161813X20300279 [DOI] [PubMed] [Google Scholar]
- Perez V.A.d.J. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail. Rev. 2016;21(3):239–257. doi: 10.1007/s10741-015-9519-2. [DOI] [PubMed] [Google Scholar]
- Powell-Wiley T.M., Poirier P., Burke L.E., Després J.P., Gordon-Larsen P., Lavie C.J., et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X.F., Zhai B.Z., Hu W.L., Lou M.H., Chen Y.H., Liu Y.F., et al. Pyrroloquinoline quinone ameliorates diabetic cardiomyopathy by inhibiting the pyroptosis signaling pathway in C57BL/6 mice and AC16 cells. Eur. J. Nutr. 2022;61(4):1–14. doi: 10.1007/s00394-021-02768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker R., Killgore J., Duich L., Romero-Chapman N., Smidt C., Tinker D. PQQ and Quinoproteins; 1989. Nutritional Essentiality of Pyrroloquinoline Quinone; pp. 159–161. [DOI] [PubMed] [Google Scholar]
- Rucker R., Chowanadisai W., Nakano M. Potential physiological importance of pyrroloquinoline quinone. Alternative Med. Rev. 2009;14(2):268–277. doi: 10.1007/978-3-0348-8397-9_10. [DOI] [PubMed] [Google Scholar]
- Sabbah H.N. Targeting the mitochondria in heart failure: a translational perspective. Basic to Translational Science. 2020;5(1):88–106. doi: 10.1016/j.jacbts.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq M., Lone Z.R., Bharati P., Mahapatra S., Rai P., Khandelwal N., et al. Pyrroloquinoline quinone (PQQ) improves pulmonary hypertension by regulating mitochondrial and metabolic functions. Pulm. Pharmacol. Therapeut. 2022;76 doi: 10.1016/j.pupt.2022.102156. [DOI] [PubMed] [Google Scholar]
- Shanan N., GhasemiGharagoz A., Abdel-Kader R., Breitinger H.-G. The effect of pyrroloquinoline quinone and resveratrol on the survival and regeneration of cerebellar granular neurons. Neurosci. Lett. 2019;694:192–197. doi: 10.1016/j.neulet.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Shankar B.S., Pandey R., Amin P., Misra H.S., Sainis K.B. Role of glutathione in augmenting the anticancer activity of pyrroloquinoline quinone (PQQ) Redox Rep. 2010;15(4):146–154. doi: 10.1179/174329210X12650506623762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojima Y., Takahashi M., Takahashi R., Moriyama H., Bagchi D., Bagchi M., et al. Effect of dietary pyrroloquinoline quinone disodium salt on cognitive function in healthy volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. J. Am. Nutraceutical Assoc. 2022;41(8):796–809. doi: 10.1080/07315724.2021.1962770. [DOI] [PubMed] [Google Scholar]
- Smidt C.R., Steinberg F.M., Rucker R.B. Physiologic importance of pyrroloquinoline quinone. PSEBM (Proc. Soc. Exp. Biol. Med.) 1991;197(1):19–26. doi: 10.3181/00379727-197-43218. [DOI] [PubMed] [Google Scholar]
- Smidt C.R., Unkefer C.J., Houck D.R., Rucker R.B. Intestinal absorption and tissue distribution of [14C]pyrroloquinoline quinone in mice. Proc Soc Exp Biol Med. 1991;197(1):27–31. doi: 10.3181/00379727-197-43219. [DOI] [PubMed] [Google Scholar]
- Steinberg F., Stites T.E., Anderson P., Storms D., Chan I., Eghbali S., et al. Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp. Biol. Med. 2003;228(2):160–166. doi: 10.1177/153537020322800205. [DOI] [PubMed] [Google Scholar]
- Stites T.E., Mitchell A.E., Rucker R.B. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J. Nutr. 2000;130(4):719–727. doi: 10.1093/jn/130.4.719. [DOI] [PubMed] [Google Scholar]
- Stites T., Storms D., Bauerly K., Mah J., Harris C., Fascetti A., et al. Pyrroloquinoline quinone modulates mitochondrial quantity and function in mice. J. Nutr. 2006;136(2):390–396. doi: 10.1093/jn/136.2.390. [DOI] [PubMed] [Google Scholar]
- Supruniuk E., Mikłosz A., Chabowski A. Pyrroloquinoline quinone modifies lipid profile, but not insulin sensitivity, of palmitic acid-treated L6 myotubes. Int. J. Mol. Sci. 2020;21(21):8382. doi: 10.3390/ijms21218382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriyo T., Tachachartvanich P., Visitnonthachai D., Watcharasit P., Satayavivad J. Chlorpyrifos promotes colorectal adenocarcinoma H508 cell growth through the activation of EGFR/ERK1/2 signaling pathway but not cholinergic pathway. Toxicology. 2015;338:117–129. doi: 10.1016/j.tox.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Takatsu H., Owada K., Abe K., Nakano M., Urano S. Effect of vitamin E on learning and memory deficit in aged rats. J. Nutr. Sci. Vitaminol. 2009;55(5):389–393. doi: 10.3177/jnsv.55.389. [DOI] [PubMed] [Google Scholar]
- Tamakoshi M., Suzuki T., Nishihara E., Nakamura S., Ikemoto K. Pyrroloquinoline quinone disodium salt improves brain function in both younger and older adults. Food Funct. 2023;14(5):2496–2501. doi: 10.1039/d2fo01515c. [DOI] [PubMed] [Google Scholar]
- Tao R., Karliner J.S., Simonis U., Zheng J., Zhang J.Q., Honbo N., et al. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem. Biophys. Res. Commun. 2007;363(2):257–262. doi: 10.1016/j.bbrc.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukakoshi K., Yoshida W., Kobayashi M., Kobayashi N., Kim J., Kaku T., et al. Esterification of PQQ enhances blood-brain barrier permeability and inhibitory activity against amyloidogenic protein fibril formation. ACS Chem. Neurosci. 2018;9(12):2898–2903. doi: 10.1021/acschemneuro.8b00355. [DOI] [PubMed] [Google Scholar]
- Wang H., Li M., Chen P., Shi X. Anti-inflammatory and antioxidant effects of pyrroloquinoline quinone in L-NAME-induced preeclampsia-like rat model. Reprod. Sci. 2022;29(2):578–585. doi: 10.1007/s43032-021-00743-8. [pii] 10.1007/s43032-021-00743-8 [doi] [DOI] [PubMed] [Google Scholar]
- Wang H., Li M., Chen P., Shi X. Anti-inflammatory and antioxidant effects of pyrroloquinoline quinone in L-NAME-induced preeclampsia-like rat model. Reprod. Sci. 2022:1–8. doi: 10.1007/s43032-021-00743-8. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Hobara N., Ohsawa T., Higashi T., Tsuji T. Nephrotoxicity of pyrroloquinoline quinone in rats. Hiroshima J. Med. Sci. 1989;38(1):49–51. [PubMed] [Google Scholar]
- Wells C., Brennan S., Keon M., Ooi L. The role of amyloid oligomers in neurodegenerative pathologies. Int. J. Biol. Macromol. 2021;181:582–604. doi: 10.1016/j.ijbiomac.2021.03.113. [DOI] [PubMed] [Google Scholar]
- Wen J.R., Shen J.W., Zhou Y.J., Zhao X.H., Dai Z.S., Jin Y.L. Pyrroloquinoline quinone attenuates isoproterenol hydrochloride-induced cardiac hypertrophy in AC16 cells by inhibiting the NF-κB signaling pathway. Int. J. Mol. Med. 2020;45(3):873–885. doi: 10.3892/ijmm.2020.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Pan J., Shen M., Xing C. Apoptotic effect of pyrroloquinoline quinone on chondrosarcoma cells through activation of the mitochondrial caspase-dependent and caspase-independent pathways. Oncol. Rep. 2018;40(3):1614–1620. doi: 10.3892/or.2018.6569. [DOI] [PubMed] [Google Scholar]
- Xu X., Chen C., Lu W.J., Su Y.L., Shi J.Y., Liu Y.C., et al. Pyrroloquinoline quinone can prevent chronic heart failure by regulating mitochondrial function. Cardiovasc. Diagn. Ther. 2020;10(3):453. doi: 10.21037/cdt-20-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Nishii K., Kuwata K., Nakamichi M., Nakanishi K., Sugimoto A., et al. Effects of pyrroloquinoline quinone and imidazole pyrroloquinoline on biological activities and neural functions. Heliyon. 2020;6(1) doi: 10.1016/j.heliyon.2020.e03240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Sasano A., Urakami T., Tsuji T., Kondo K. Stimulation of nerve growth factor production by pyrroloquinoline quinone and its derivatives in vitro and in vivo. Biosci., Biotechnol., Biochem. 1993;57(7):1231–1233. doi: 10.1271/bbb.57.1231. [DOI] [PubMed] [Google Scholar]
- Yang C., Yu L., Kong L., Ma R., Zhang J., Zhu Q., et al. Pyrroloquinoline quinone (PQQ) inhibits lipopolysaccharide induced inflammation in part via downregulated NF-κB and p38/JNK activation in microglial and attenuates microglia activation in lipopolysaccharide treatment mice. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.F., Yu L.F., Kong L.B., Ma R., Zhang J.L., Zhu Q.S., et al. Pyrroloquinoline quinone (PQQ) inhibits lipopolysaccharide induced inflammation in part via downregulated NF-κB and p38/JNK activation in microglial and attenuates microglia activation in lipopolysaccharide treatment mice. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Ye Q., Zhang X.G., Li K., Liang X.S., Wang M., et al. Pyrroloquinoline quinone extends caenorhabditis elegans' longevity through the insulin/IGF1 signaling pathway-mediated activation of autophagy. Food Funct. 2021;12(22):11319–11330. doi: 10.1039/d1fo02128a. [DOI] [PubMed] [Google Scholar]
- Yin X.D., Ming D.X., Bai L.L., Wu F., Liu H., Chen Y.F., et al. Effects of pyrroloquinoline quinone supplementation on growth performance and small intestine characteristics in weaned pigs. J. Anim. Sci. 2019;97(1):246–256. doi: 10.1093/jas/sky387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Xu Y.P., Sun J.X., Li X.Y., Wang L.H., Jin L.J. Protection of pyrroloquinoline quinone against methylmercury-induced neurotoxicity via reducing oxidative stress. Free Radic. Res. 2009;43(3):224–233. doi: 10.1080/10715760802677348. [DOI] [PubMed] [Google Scholar]
- Zhang L.L., Liu J., Cheng C., Yuan Y., Yu B.Y., Shen A.G., et al. The neuroprotective effect of pyrroloquinoline quinone on traumatic brain injury. J. Neurotrauma. 2012;29(5):851–864. doi: 10.1089/neu.2011.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Meruvu S., Bedi Y.S., Chau J., Arguelles A., Rucker R., et al. Pyrroloquinoline quinone increases the expression and activity of Sirt1 and -3 genes in HepG2 cells. Nutr. Res. 2015;35(9):844–849. doi: 10.1016/j.nutres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang J., Powell C., Meruvu S., Sonkar R., Choudhury M. Pyrroloquinoline quinone attenuated benzyl butyl phthalate induced metabolic aberration and a hepatic metabolomic analysis. Biochem. Pharmacol. 2022;197 doi: 10.1016/j.bcp.2021.114883. [DOI] [PubMed] [Google Scholar]
- Zhang X., Gu T., Liu Y., Liu C., Lin Y., Li H., et al. Pyrroloquinoline quinone (PQQ) improves long-term survival of fat grafts by alleviating oxidative stress and promoting angiogenesis during the early phase after transplantation. Aesthetic Surg. J. 2023;44(1):NP104–NP118. doi: 10.1093/asj/sjad282. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Gu T.Y., Liu Y., Liu C., Lin Y., Li H.R., et al. Pyrroloquinoline quinone (PQQ) improves long-term survival of fat grafts by alleviating oxidative stress and promoting angiogenesis during the early phase after transplantation. Aesthetic Surg. J. 2024;44(1):NP104–NP118. doi: 10.1093/asj/sjad282. [DOI] [PubMed] [Google Scholar]
- Zheng Y.W., Zhang J.Y., Zhou H.B., Guo Y.P., Ma Q.G., Ji C., et al. Effects of dietary pyrroloquinoline quinone disodium supplementation on inflammatory responses, oxidative stress, and intestinal morphology in broiler chickens challenged with lipopolysaccharide. Poultry Sci. 2020;99(11):5389–5398. doi: 10.1016/j.psj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.Q., Yao Z.W., Peng Y., Mao S.S., Xu D., Qin X.F., et al. PQQ ameliorates D-galactose induced cognitive impairments by reducing glutamate neurotoxicity via the GSK-3β/Akt signaling pathway in mouse. Sci. Rep. 2018;8(1):8894. doi: 10.1038/s41598-018-26962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B.Q., Zhou H.Z., Teerlink J.R., Karliner J.S. Pyrroloquinoline quinone (PQQ) decreases myocardial infarct size and improves cardiac function in rat models of ischemia and ischemia/reperfusion. Cardiovasc. Drugs Ther. 2004;18:421–431. doi: 10.1007/s10557-004-6219-x. [DOI] [PubMed] [Google Scholar]
- Zhu B.Q., Simonis U., Cecchini G., Zhou H.Z., Li L.Y., Teerlink J.R., et al. Comparison of pyrroloquinoline quinone and/or metoprolol on myocardial infarct size and mitochondrial damage in a rat model of ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. Therapeut. 2006;11(2):119–128. doi: 10.1177/1074248406288757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.