Abstract
In clinical trials, the percentage of patients discontinuing treatment with ocrelizumab due to adverse events was low. However, real-world populations are often more diverse than randomized controlled trials (RCTs), therefore it is important to assess discontinuation rates in real-world studies. This systematic literature review (SLR) was conducted to identify real-world discontinuation and persistence data for ocrelizumab in studies of patients with relapsing remitting multiple sclerosis (RRMS) and primary progressive multiple sclerosis (PPMS). Searches were conducted in MEDLINE and Embase to identify relevant real-world studies that met pre-determined Population, Intervention, Comparison, Outcomes, and Study (PICOS) criteria. Only articles published in English were included, but the study country was not restricted. A total of 30 studies were included, with the majority reporting real-world persistence data that appear to be similar to or better than in the pivotal clinical trials, with only 1 study reporting higher discontinuation rates due to adverse events compared with the clinical trials. Other studies identified reported that the risk of discontinuation was higher for other disease-modifying therapies (DMTs) compared with ocrelizumab, and adherence was also higher for ocrelizumab versus other DMTs. These findings have clinical relevance, as other studies have reported improved clinical outcomes and lower care costs for patients that are persistent or adherent to other DMTs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-024-00667-w.
Keywords: Ocrelizumab, Persistence, Primary progressive multiple sclerosis, Real-world, Relapsing remitting multiple sclerosis
Key Summary Points
| •In clinical trials, the percentage of patients discontinuing treatment with ocrelizumab due to adverse events was low; however, real-world populations are often more diverse than clinical trials. |
| •Therefore, a systematic literature review (SLR) was conducted to identify discontinuation and persistence data for ocrelizumab in real-world studies of patients with relapsing remitting multiple sclerosis (RRMS) and primary progressive multiple sclerosis (PPMS). |
| •The majority of identified studies reported real-world persistence data that appear to be similar to or better than in the pivotal clinical trials, with only 1 study reporting higher discontinuation rates due to adverse events compared with the clinical trials. |
| •These findings have clinical relevance, as improved clinical outcomes and lower care costs are associated with better persistence or adherence in studies assessing other disease-modifying therapies (DMTs). |
Introduction
Multiple sclerosis (MS) is an autoimmune neurodegenerative disease that was estimated to affect 2.9 million people globally in 2023 [1]. Ocrelizumab is a humanized monoclonal antibody that selectively depletes CD20 + B-cells and has been approved for the treatment of relapsing remitting multiple sclerosis (RRMS) and primary progressive MS (PPMS), based on demonstrated safety and efficacy in Phase III clinical trials (OPERA I and II and ORATORIO)[2, 3]. Across these clinical trials, the percentage of patients discontinuing treatment with ocrelizumab due to adverse events was also low (3.2–4.1%) [2, 3].
Real-world populations are often more diverse than those included in randomized controlled trials (RCTs) [4], and assessment of real-world evidence is therefore important to gain a deeper understanding of the impact of ocrelizumab. Real-world studies suggest that the effectiveness of ocrelizumab in clinical practice is consistent with the results reported in the pivotal clinical trials [5], although, to date, no study has systematically collated real-world data on ocrelizumab persistence. Persistence and adherence to therapy is associated with better clinical outcomes in MS [6, 7], highlighting the importance of assessing persistence with therapies in clinical practice. Therefore, this systematic literature review (SLR) was conducted to identify real-world studies reporting on ocrelizumab persistence in patients with RRMS and PPMS.
Methods
This study is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The methods used to conduct this SLR have been previously published by Montalban et al. [5]. Appendix A in the electronic supplementary material shows the search strategy used in MEDLINE and Embase databases to find relevant real-world studies reporting data pertaining to persistence, discontinuation, and/or adherence to ocrelizumab in patients with RRMS or PPMS. Only studies published in English were identified for inclusion, but the study country was not restricted. The search was conducted on 7 October 2022, and both full peer-reviewed articles and conference abstracts were identified for inclusion. Where possible, identified studies were assessed for the reporting of data from the same study population.
Covidence software (www.covidence.org) was used for study screening. Two reviewers (GJ and DS) screened each study independently against the Population, Intervention, Comparison, Outcomes, and Study (PICOS) criteria [8] shown in Appendix B in the electronic supplementary material. A further reviewer (JLP) assessed studies where consensus was not reached by reviewers 1 and 2.
Results
Identified Studies
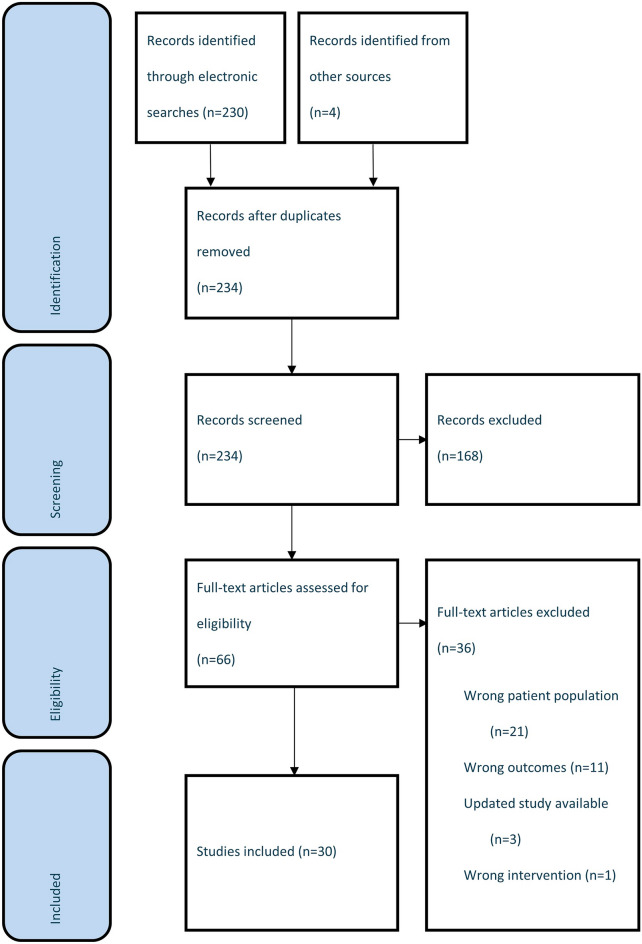
Overall, 30 studies were identified that met the PICOS criteria (Fig. 1) [4, 9–37], 14 (47.4%) of which were full text articles. Included studies were identified across various countries and regions, including Europe (n = 15), the US (n = 8), the Middle East (n = 2), and South America (n = 1) (Table S1 in the electronic supplementary material provides a detailed overview of study characteristics). Table S2 in the electronic supplementary material provides an overview of study baseline characteristics; 13 of the included studies did not categorize patients by MS type (RRMS, PPMS, or secondary progressive MS (SPMS)).
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram
Ocrelizumab Discontinuation and Persistence
All identified studies evaluated the number or proportion of patients who discontinued ocrelizumab treatment over varying treatment periods, ranging from 6 to 34 months, where reported (see Table S3 in the electronic supplementary material). The majority of studies (n = 24, 80.0%) reported discontinuation rates of 10% or lower. Seven studies, with total sample sizes ranging from 18 to 439 and follow-up times ranging from 6 to 18 months, reported discontinuation rates of 0% [12, 21, 24, 27, 31, 33, 36]. Discontinuation rates of greater than 20% were reported by 3 studies [25, 32, 34];however, in 1 of these studies, the majority of discontinuing patients did so due to insurance issues or being lost to follow-up [34]. In the other 2 studies, infections/adverse effects and MS progression were the most common reasons for discontinuation [25, 32].
Two studies performed survival analysis to assess ocrelizumab persistence at 12 and/or 24 months [4, 12], including 1 study reporting results from the CONFIDENCE study, a large real-world cohort of more than 2000 MS patients in Germany [4]. Persistence with ocrelizumab treatment was high in both studies: 12-month persistence ranged from 96.0% (n = 1702 RRMS patients and 398 PPMS patients) to 98.4% (n = 82 PPMS patients) and 24-month persistence rates ranged from 91.2% (n = 82 PPMS patients) to 92.0% (n = 1702 RRMS patients and 398 PPMS patients) [4, 12].
Reasons for Discontinuation
Of the 30 studies reporting discontinuation rates, 18 provided reasons for discontinuation (see Table S3 in the electronic supplementary material). The discontinuation reasons most frequently reported by the included studies were adverse effects (11 studies), lack of efficacy/clinical progression (8 studies), and pregnancy/family planning (6 studies). Four studies evaluated time to discontinuation [14, 18, 28, 32], with 3 providing a median time that ranged from 0.6 to 1.8 years (see Table S3 in the electronic supplementary material).
Comparative Studies
Only 1 study conducted statistical analysis to compare discontinuation rates between patients treated with ocrelizumab and another therapy; no statistically significant difference was found when comparing the percentage of patients treated with ocrelizumab and rituximab who stopped treatment within the first year (15% and 10%, respectively, p = 0.11) [19]. Significantly more patients treated with ocrelizumab discontinued treatment due to adverse events (9.3% vs. 2.6%, p < 0.01), although sample sizes of patients discontinuing due to adverse events were very small (n = 15 and n = 8 for ocrelizumab and rituximab, respectively) [19]. Only 2.5% and 1.6% of patients treated with ocrelizumab and rituximab, respectively, discontinued due to lack of efficacy, a difference that was not statistically significant [19]. However, assumptions on possible differences between ocrelizumab and rituximab identified in this study should be inferred carefully, as the cohort receiving rituximab consisted of patients in Sweden with RRMS and SPMS, whereas the cohort receiving ocrelizumab consisted of patients in the US with RRMS only (due to ocrelizumab not currently being approved for SPMS in the US). Patterns of discontinuation may be different between RRMS and SPMS, and the study highlighted that there may be differences in the classification of secondary progressive between the 2 countries. Additionally, there were differences between the percentages of patients receiving ocrelizumab and rituximab who were treatment-naïve and who had switched from natalizumab [19].
Three studies presented discontinuation data for ocrelizumab and other therapies without conducting statistical analyses or stating reasons for discontinuation [11, 21, 36]. Discontinuation rates varied between studies: 1 study reported no discontinuations amongst patients treated with ocrelizumab, rituximab, or cladribine for 18, 17, and 16 months, respectively [36]; another study reported fewer discontinuations in patients treated with ocrelizumab (0 patients) versus fingolimod (8 patients), dimethyl fumarate (18 patients), and teriflunomide (5 patients) [21]; whereas the third study found a numerically higher discontinuation rate in ocrelizumab-treated patients (10.2%) versus patients treated with fingolimod (0.7%), dimethyl fumarate (5.8%), and natalizumab (6.8%) [11]. However, in this study, a greater proportion of patients treated with ocrelizumab were in the “New” treatment group. This consisted of patients starting therapy less than 6 months ago, although the precise average treatment duration was not stated. The average number of prior disease-modifying therapies (DMTs) received was also higher in the ocrelizumab group [11]. These characteristics may account for the numerically higher discontinuation rate in patients receiving ocrelizumab.
Discussion
Although numerous real-world studies to date have reported discontinuation data for ocrelizumab, no study, to our knowledge, has systematically collated and evaluated these data. This SLR identified relevant data on real-world discontinuation of ocrelizumab across 30 studies in patients with RRMS and PPMS.
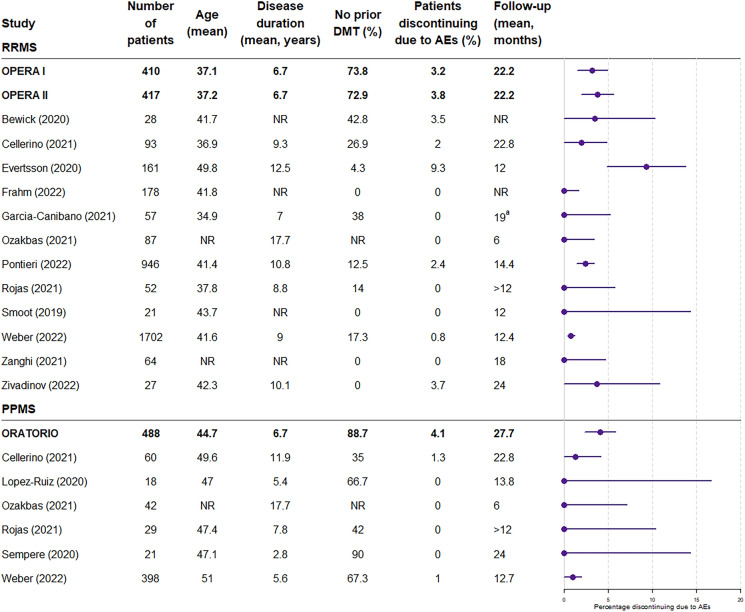
The pivotal phase 3 clinical trials supporting the use of ocrelizumab in patients with RRMS (OPERA I/II) and PPMS (ORATORIO) reported discontinuation due to adverse events in 3.2%, 3.8%, and 4.1% of patients in the ocrelizumab treatment arms, respectively, at the end of the controlled periods (96 weeks for OPERA I/II and median duration of 2.9 years in the ORATORIO trial) [2, 3]. In the vast majority of real-world studies included in this SLR, the proportion of patients discontinuing ocrelizumab treatment was similar to or lower than in the clinical trials (Fig. 2). Only 1 study reported higher discontinuation rates due to adverse events compared with the clinical trials [19]; mean disease duration in this study was higher than in the OPERA I and II clinical trials (12.5 years vs. 6.7 years), as was the mean age of the patients (49.8 vs. 37.1 and 37.2, respectively), which may be factors in the seemingly high discontinuation rate. The study by Weber et al. reported numerically higher discontinuation rates in patients aged 55 years or older compared to the overall cohort (7.0% vs. 4.7%), which provides some support for older age being a factor [4]. The variability in discontinuation rates in these studies is somewhat to be expected, given the inherent heterogeneity of populations across different real-world studies.
Fig. 2.
Patients discontinuing due to adverse events: clinical trials versus real-world studies. 95% binomial confidence intervals were calculated for the proportion of patients discontinuing due to adverse events, and the rule of three was used in the case of zero counts. Note that the rule of three is a very good approximation when n > 30, and is a good approximation, although slightly less accurate, when n < 30. AE adverse event, DMT disease-modifying therapy, PPMS primary progressive multiple sclerosis, RRMS relapsing remitting multiple sclerosis
Only 1 study performing comparative statistical analysis of ocrelizumab with another DMT was identified, which found no overall difference in discontinuation rates between ocrelizumab and rituximab, but a significantly higher treatment discontinuation rate due to adverse events in patients treated with ocrelizumab [19]. However, sample sizes of patients discontinuing due to adverse events in this study were very small (n = 15 and n = 8 for ocrelizumab and rituximab, respectively) and there was variability in the study cohorts, with the rituximab cohort consisting of patients with RRMS and SPMS, and the ocrelizumab cohort consisting of patients with RRMS only [19]. Three additional studies were identified that reported comparative analysis of ocrelizumab discontinuation and adherence with other therapies, but these studies did not specify the MS patient population and therefore did not meet the inclusion criteria of this SLR [38–40]. Nevertheless, 1 of these studies found that the risk of discontinuation was higher for other DMTs versus ocrelizumab, across all types of administration (oral, injectable, and infusion). Adherence (defined as proportion of days covered) was also higher for ocrelizumab versus low/medium efficacy DMTs [39]. The other 2 identified studies determined that discontinuation rates were lower and adherence was higher for ocrelizumab-treated patients compared with patients receiving injectable, oral, and other intravenous (IV) therapies [38, 40]. In the study by Engmann et al., which analyzed a total of 4587 patients receiving either ocrelizumab, injectable DMT, oral DMT, or other IV DMT, patients receiving ocrelizumab had the lowest discontinuation rate at 12 months compared to patients receiving other IV, oral, or injectable therapies [38]. Patients receiving ocrelizumab also had the highest mean proportion of days covered compared to patients receiving other therapies. Discontinuation rates for patients undergoing continuous treatment for 18 months were also lower in the ocrelizumab cohort compared with the other treatment groups [38]. Studies have reported better clinical outcomes and lower care costs in patients that are persistent and adherent to other DMTs [6, 7], therefore, further long-term comparative studies assessing these outcomes in ocrelizumab-treated patients could provide important insights into factors influencing clinical outcomes.
This SLR has several limitations, namely the disparate nature of study designs and populations in the included studies, the inclusion of conference abstracts, some of which lacked detailed descriptions of study results and data interpretation, and the limited number of studies comparing ocrelizumab with other DMTs (three additional comparative studies were identified but not included in the main analysis due to lack of information on the included MS patient populations). Due to the lack of detail in some included studies, it is possible that data from the same patient populations may have been reported in more than 1 included study. Despite these limitations, it is clear that the discontinuation rate seen with ocrelizumab in real-world studies is consistently low.
Conclusion
This SLR has found that current data pertaining to real-world discontinuation rates for ocrelizumab are lower or similar to discontinuation rates in the pivotal clinical trials. Analyses of patients who received ocrelizumab in clinical trials suggest that long-term discontinuation rates remain low (3.2% over a period of up to 7 years) [41]. However, it will be important to continue to assess persistence, discontinuation, and adherence to ocrelizumab in longer term real-world studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing and Editorial Assistance: Medical writing and editorial assistance in the preparation of this article was provided by John L Petrie and Donna Fountain of Putnam. Support for this assistance was funded by F Hoffmann-La Roche, Basel, Switzerland.
Author Contributions
Sreeram Ramagopalan (employee of F Hoffmann-La Roche), Cormac Sammon (employee of Putnam), John Petrie, and Donna Fountain designed the study. John L Petrie, Daniel Shaw (employee of Putnam), and Gabriel Jones (employee of Putnam) ran searches, screened studies against PICOS criteria, and performed data extraction of studies meeting inclusion criteria. Emily Robertshaw (employee of Putnam) produced Fig. 2. John L Petrie wrote the manuscript. All authors reviewed, edited, and approved the final manuscript.
Funding
Sponsorship for this study and the Rapid Service Fee were funded by F Hoffmann-La Roche, Basel, Switzerland.
Data Availability
No original data were generated for this review. Data utilized from studies included in this review are presented in the supplementary files.
Declarations
Conflict of Interest
Charlie A Smith and Gerardo Machnicki are employees of F Hoffmann-La Roche. John L Petrie and Donna Fountain are employees of Putnam, which received financial support from F Hoffmann-La Roche for the work, including the development of the literature review and preparation of the manuscript.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.MS International Federation. Atlas of MS Factsheet. 2023.
- 2.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34. [DOI] [PubMed] [Google Scholar]
- 3.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–20. [DOI] [PubMed] [Google Scholar]
- 4.Weber MS, Buttmann M, Meuth SG, et al. Safety, adherence and persistence in a real-world cohort of German MS patients newly treated with ocrelizumab: first insights from the CONFIDENCE study. Front Neurol. 2022;13: 863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montalban X, Matthews PM, Simpson A, et al. Real-world evaluation of ocrelizumab in multiple sclerosis: a systematic review. Ann Clin Transl Neurol. 2023. 10.1002/acn3.51732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lizan L, Comellas M, Paz S, Poveda JL, Meletiche DM, Polanco C. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literature. Patient Prefer Adherence. 2014;8:1653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQueen RB, Livingston T, Vollmer T, et al. Increased relapse activity for multiple sclerosis natalizumab users who become nonpersistent: a retrospective study. J Manag Care Spec Pharm. 2015;21(3):210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Counsell C. Formulating questions and locating primary studies for inclusion in systematic reviews. Ann Intern Med. 1997;127(5):380–7. [DOI] [PubMed] [Google Scholar]
- 9.Baghestani AA, Al-Sharif GA, Krieger DW. Preliminary clinical experience with ocrelizumab, a New recombinant humanized monoclonal antibody for multiple sclerosis. Mult Scler Relat Disord. 2020; Conference: The Fifth MENACTRIMS Congress. Intercontinental Hotel.
- 10.Bewick C, Das E, Collins J, Nash C, Fisniku L. The incidence of infusion associated reactions in ocrelizumab-treated relapsing-remitting multiple sclerosis patients. Eur J Neurol. 2020;27(Supplement 1):684. [Google Scholar]
- 11.Bossart J, Kamm CP, Kaufmann M, et al. Real-world disease-modifying therapy usage in persons with relapsing-remitting multiple sclerosis: cross-sectional data from the Swiss multiple sclerosis registry. Mult Scler Relat Disord. 2022;60: 103706. [DOI] [PubMed] [Google Scholar]
- 12.Braune S, Bluemich S, Bruns C, et al. Real world experience with ocrelizumab in patients with primary progressive multiple sclerosis: insights from the German neuro trans data registry. Mult Scler J. 2021;27(2 SUPPL):210–1. [Google Scholar]
- 13.Braune S, Heer Y, Tozzi V et al. Real-World experience with ocrelizumab in the German NeuroTransData Registry. 8th joint ACTRIMS-ECTRIMS meeting; September 11–13, 2020.
- 14.Butzkueven H, Spelman T, Ozakbas S, et al. Real-world experience with ocrelizumab in relapsing multiple sclerosis: Insights from the MSOCR-R cohort, an MS base registry sub-study. Mult Scler J. 2021;27(2 SUPPL):104–6. [Google Scholar]
- 15.Butzkueven H, Spelman T, Ozakbas S, et al. Real-world experience with ocrelizumab in the MS base registry. Mult Scler J. 2019;26:550–1. [Google Scholar]
- 16.Cellerino M, Boffa G, Lapucci C, et al. Predictors of ocrelizumab effectiveness in patients with multiple sclerosis. Neurother. 2021;18(4):2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coban H, Germaine S, Dimaandal I, et al. Real-world experience of ocrelizumab initiation in a diverse multiple sclerosis population. Mult Scler Relat Disord. 2021;53: 103021. [DOI] [PubMed] [Google Scholar]
- 18.Ellwardt E, Rolfes L, Klein J, et al. Ocrelizumab initiation in patients with MS: a MULTICENTER observational study. Neurol Neuroimmunol Neuroinflamm. 2020;7(4): e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evertsson B, Hoyt T, Christensen A, Nimer FAL, Foley J, Piehl F. A comparative study of tolerability and effects on immunoglobulin levels and CD19 cell counts with ocrelizumab vs low dose of rituximab in multiple sclerosis. Mult Scler J Exp Transl Clin. 2020;6(4):2055217320964505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Diaz E, Perez-Vicente JA, Villaverde-Gonzalez R, et al. Real-world experience of ocrelizumab in multiple sclerosis in a Spanish population. Ann Clin Transl Neurol. 2021;8(2):385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finder S, Patel A, Smith S, Rahn R, Fagan S. Ocrelizumab infusions preferred over oral therapies in an MS clinic. JACCP J Am Coll Clin Pharm. 2020;3(1):249. [Google Scholar]
- 22.Frahm N, Fneish F, Ellenberger D, et al. Therapy Switches in fingolimod-treated patients with multiple sclerosis: long-term experience from the German MS registry. Neurol Ther. 2022;11(1):319–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Canibano B, Ouanes S, Ganesan GS, et al. Real-world experience of ocrelizumab in multiple sclerosis in an Arab population. J Drug Assess. 2021;10(1):106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez Ruiz R, Dotor Garcia-Soto J, Hiraldo JDG, et al. Real world experience with ocrelizumab in patients with primary progressive multiple sclerosis. Mult Scler J. 2020;26(3 SUPPL):301–2. [Google Scholar]
- 25.Luxenberg E, Von Geldern G, Wundes A. Analysis of ocrelizumab treatment in aged patients at an academic MS center. Mult Scler J. 2021;27(1 SUPPL):48–9. [Google Scholar]
- 26.Magyari M, Pontieri L, Blinkenberg M, et al. Early experience with ocrelizumab in Denmark. A population-based registry study. Mult Scler J. 2020;26(3 SUPPL):530–1. [Google Scholar]
- 27.Ozakbas S, Ozcelik S, Kaya E, Ozdogar AT, Sagici O, Baba C. Early treatment response of ocrelizumab in persons with multiple sclerosis: six-month results. Mult Scler J. 2021;27(2 SUPPL):278. [Google Scholar]
- 28.Pontieri L, Blinkenberg M, Bramow S, et al. Ocrelizumab treatment in multiple sclerosis: a Danish population-based cohort study. Eur J Neurol. 2022;29(2):496–504. [DOI] [PubMed] [Google Scholar]
- 29.Rojas JI, Patrucco L, Fruns M, et al. Real-world experience of ocrelizumab in multiple sclerosis patients in Latin America. Arq Neuropsiquiatr. 2021;79(4):305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sempere A, Berenguer-Ruiz L, Burgos-San Jose A, Aragones M, Navarro-Lopez MD. Safety and effectiveness of ocrelizumab in multiple sclerosis: a real-world study from Spain. Mult Scler J. 2020;26(3 SUPPL):553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smoot K, Chen C, Gervasi-Follmar T, et al. Evaluating the efficacy and safety of transitioning patients from natalizumab to ocrelizumab (OCTAVE). Neurology. 2019;92(15_supplement):P3.2–056. [DOI] [PubMed] [Google Scholar]
- 32.Smoot K, Stuchiner T, Grote L, Chen C, Cohan S. Utilization, safety, and tolerability of ocrelizumab: data from the providence ocrelizumab registry. Mult Scler J. 2022;28:386. [Google Scholar]
- 33.Tsantes E, Curti E, Fiore A, Bazzurri V, Granella F. Ocrelizumab as exit strategy from natalizumab: results from a clinical series. Mult Scler J. 2020;26(3 SUPPL):294. [Google Scholar]
- 34.Vollmer B, Ijadi N, Declusin A, et al. Two-year real-world experience with ocrelizumab in the treatment of patients with multiple sclerosis. Mult Scler J. 2021;27(2 SUPPL):595–6. [Google Scholar]
- 35.Vollmer B, Nair K, Sillau S, Corboy J, Vollmer T, Alvarez E. Ocrelizumab real-world safety and effectiveness in the one year treatment of multiple sclerosis compared to other disease modifying therapies. Mult Scler J. 2019;25(Supplement 2):747–8. [Google Scholar]
- 36.Zanghi A, Gallo A, Avolio C, et al. Exit strategies in natalizumab-treated RRMS at high risk of progressive multifocal leukoencephalopathy: a multicentre comparison study. Neurother. 2021;18(2):1166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zivadinov R, Jakimovski D, Ramanathan M, et al. Effect of ocrelizumab on leptomeningeal inflammation and humoral response to Epstein-Barr virus in multiple sclerosis. A pilot study. Mult Scler Relat Disord. 2022;67:104094. [DOI] [PubMed] [Google Scholar]
- 38.Engmann NJ, Sheinson D, Bawa K, Ng CD, Pardo G. Persistence and adherence to ocrelizumab compared with other disease-modifying therapies for multiple sclerosis in U.S. commercial claims data. J Manag Care Spec Pharm. 2021;27(5):639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moccia M, Affinito G, Berera G, et al. Persistence, adherence, healthcare resource utilization and costs for ocrelizumab in the real-world of the Campania Region of Italy. J Neurol. 2022;269(12):6504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardo G, Pineda ED, Ng CD, Bawa KK, Sheinson D, Bonine NG. Adherence to and persistence with disease-modifying therapies for multiple sclerosis over 24 months: a retrospective claims analysis. Neurol Ther. 2022;11(1):337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauser SL, Kappos L, Montalban X, et al. Safety of ocrelizumab in patients with relapsing and primary progressive multiple sclerosis. Neurology. 2021;97(16):e1546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No original data were generated for this review. Data utilized from studies included in this review are presented in the supplementary files.