Abstract
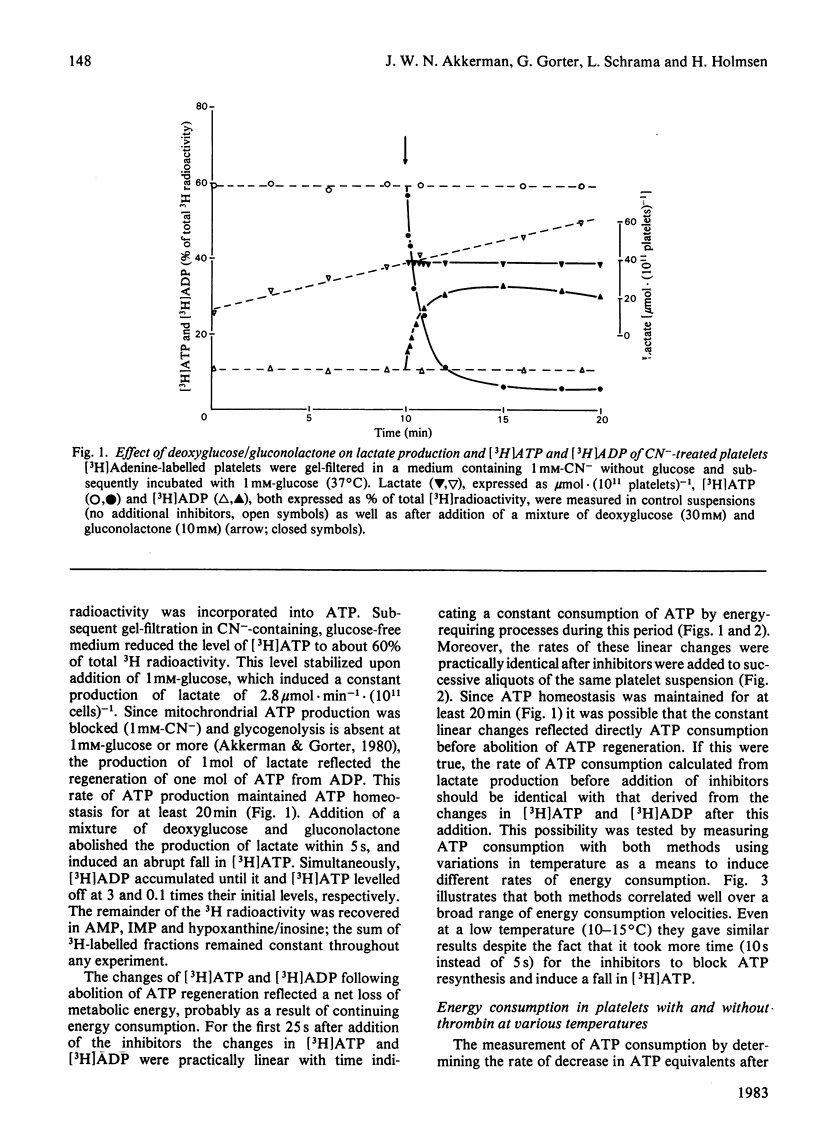
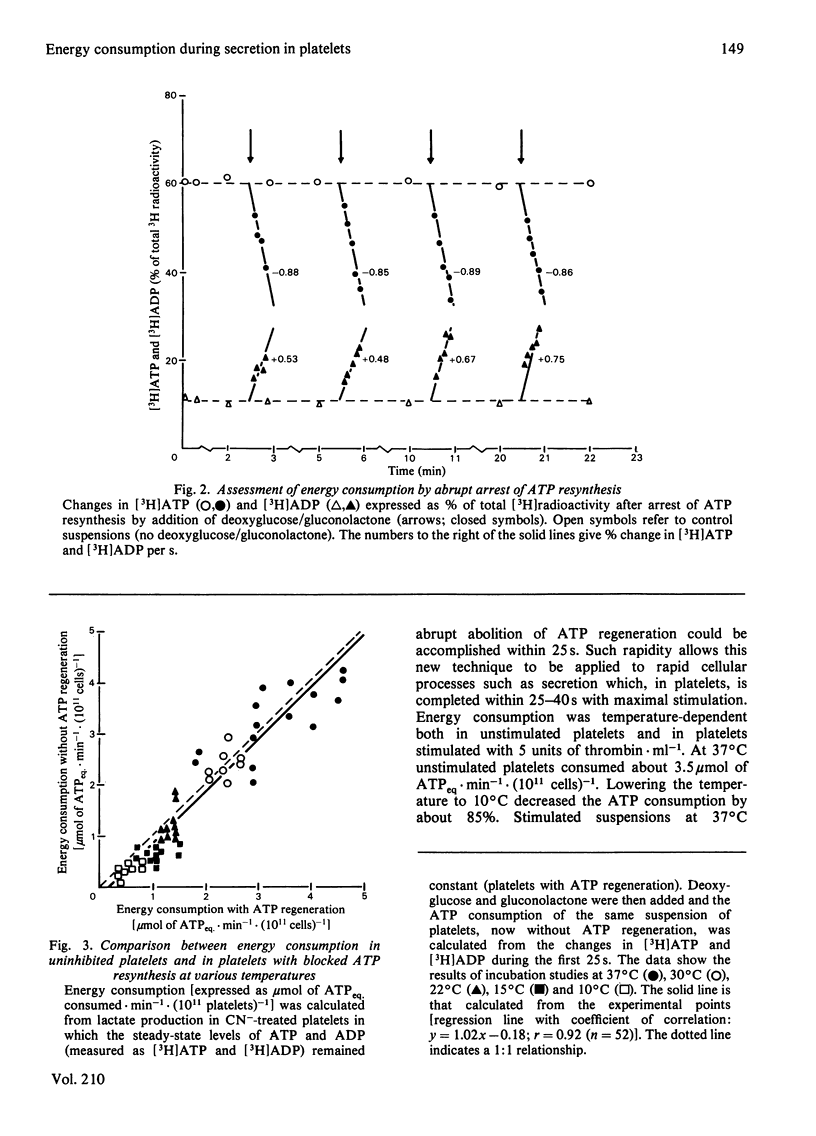
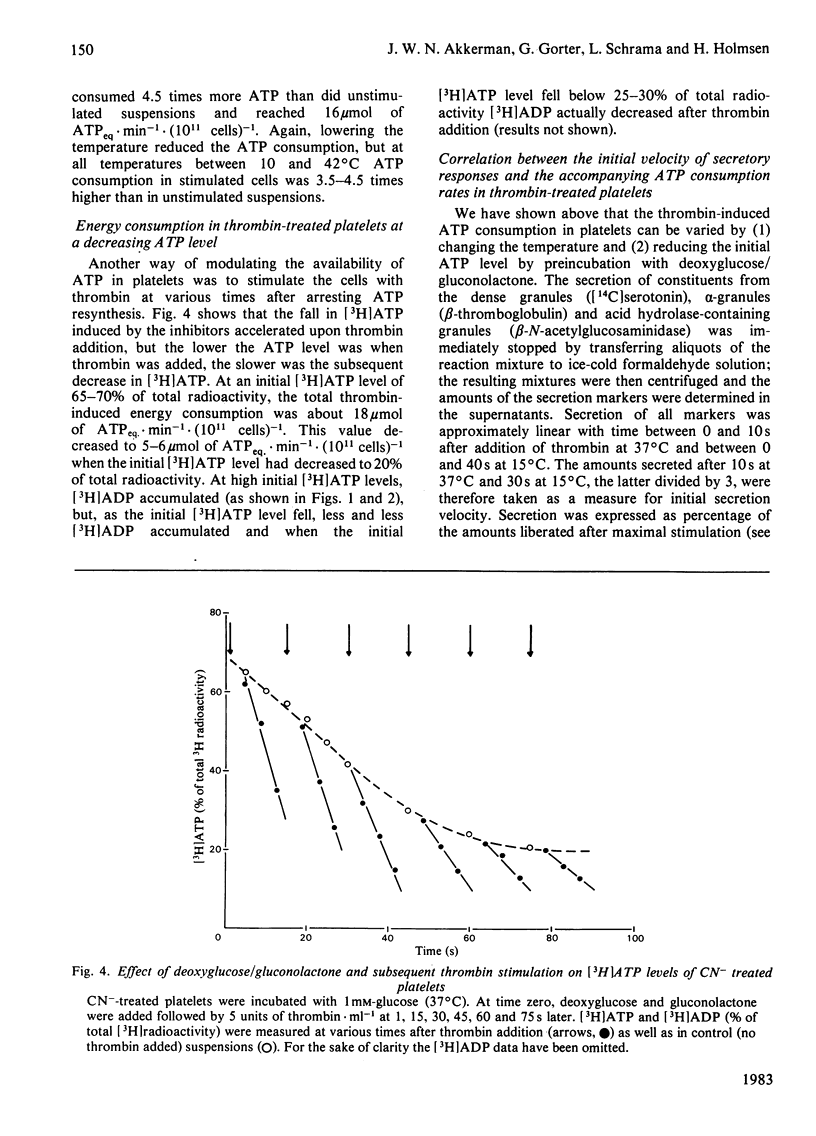
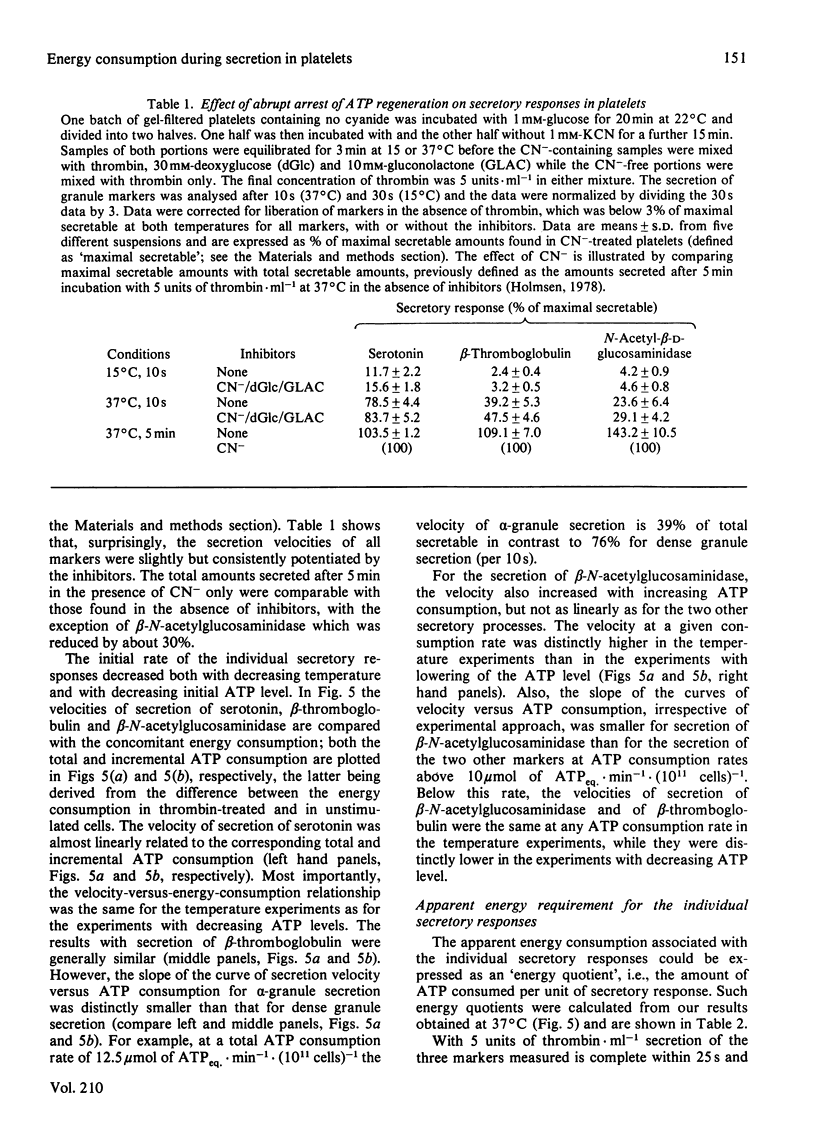
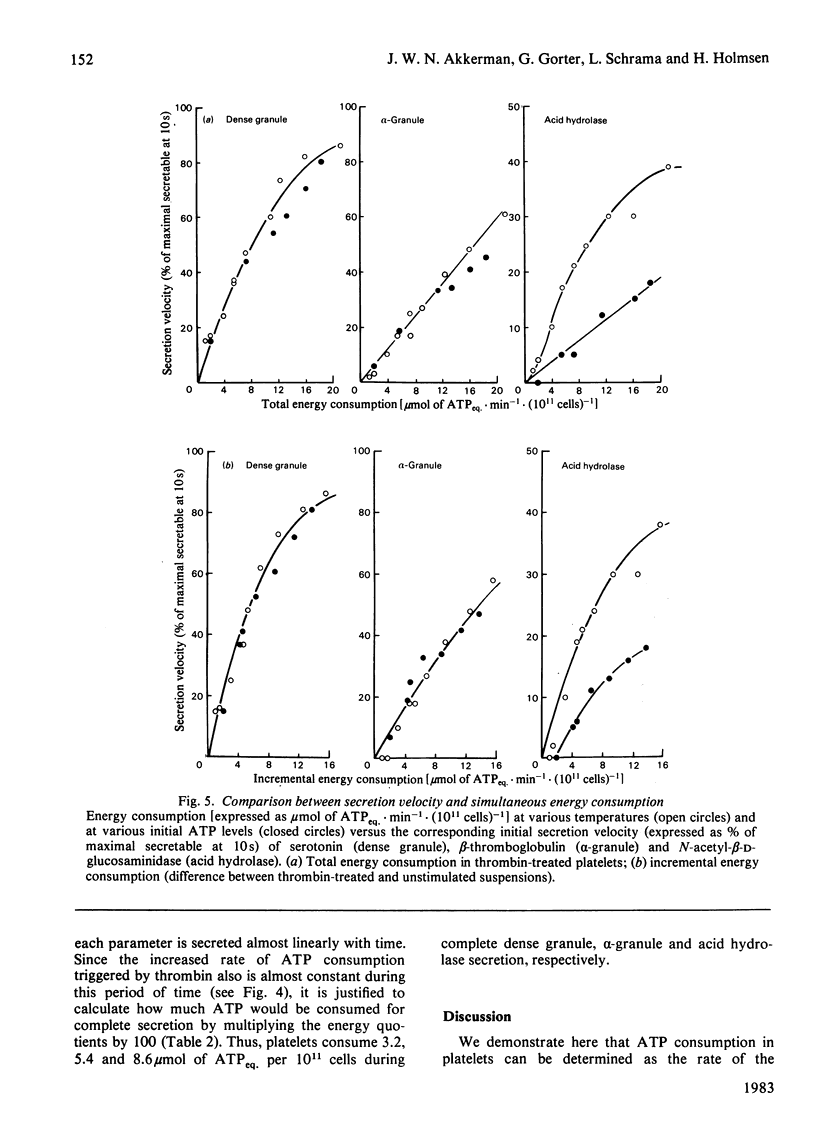
A novel method has been developed for rapid and quantitative determination of the rate of energy consumption in platelets. In platelets suspended in a cyanide-containing medium. ATP resynthesis is abruptly blocked by addition of 2-deoxyglucose and D-glucono-1,5-lactone. We demonstrate that the subsequent changes in the levels of cytoplasmic ATP and ADP reflect the velocity of energy consumption in the platelets immediately before addition of the inhibitors. Despite the arrest in ATP resynthesis the platelets remain responsive to stimulation by thrombin (5 units x ml-1) which triggers the secretion of the contents of dense, alpha- and acid hydrolase granules. Unstimulated platelets were found to consume about 3.5 and 0.5 mumol of ATP equivalents x min-1 x (10(11) cells)-1 at 37 degrees C and 15 degrees C, respectively; the thrombin-treated platelets consumed respectively 16 and 2 mumol of ATP equivalents x min-1 x (10(11) cells)-1 at these temperatures. When the velocity of energy consumption was varied by (a) changing the temperature and (b) preincubation with glyco(geno)lytic inhibitors, it was found to be linearly related to the initial rate of secretion from the three types of granules. The precise nature of this relationship differed between the three types of secretion responses and indicated an increasing requirement for metabolic energy for secretion from the three types of granules in the order: dense granule less than alpha-granule less than acid hydrolase granule. The results obtained with changes in temperature were superimposable on those obtained with the glyco(geno)lytic inhibitors for dense granule secretion and alpha-granule secretion, suggesting an apparent coupling between energy consumption and the rate of these secretion responses. The rate of secretion of acid hydrolase was always higher when energy consumption was varied by temperature changes than when glyco(geno)lytic inhibitors were used, probably as a result of metabolic changes prior to induction of secretion. On the basis of these experiments, we calculated an incremental energy consumption during complete secretion of dense, alpha- and acid hydrolase granule contents of 2.5, 4.2 and 6.7 mumol of ATP equivalents x (10(11) platelets)-1, respectively.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A., Anderson W., Jr Phosphorylation of human platelet myosin. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3115–3119. doi: 10.1073/pnas.70.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman J. W., Ebberink R. H., Lips J. P., Christiaens G. C. Rapid separation of cytosol and particle fraction of human platelets by digitonin-induced cell damage. Br J Haematol. 1980 Feb;44(2):291–300. doi: 10.1111/j.1365-2141.1980.tb01211.x. [DOI] [PubMed] [Google Scholar]
- Akkerman J. W., Gorter G., Kloprogge E. Kinetic analysis of alpha-granule secretion by platelets. A methodological report. Thromb Res. 1982 Jul 1;27(1):59–64. doi: 10.1016/0049-3848(82)90278-x. [DOI] [PubMed] [Google Scholar]
- Akkerman J. W., Gorter G. Relation between energy production and adenine nucleotide metabolism in human blood platelets. Biochim Biophys Acta. 1980 Mar 7;590(1):107–116. doi: 10.1016/0005-2728(80)90150-4. [DOI] [PubMed] [Google Scholar]
- Akkerman J. W., Gorter G., Sixma J. J. Regulation of glycolytic flux in human platelets: relation between energy production by glyco(geno)lysis and energy consumption. Biochim Biophys Acta. 1978 Jun 15;541(2):241–250. doi: 10.1016/0304-4165(78)90397-5. [DOI] [PubMed] [Google Scholar]
- Akkerman J. W., Holmsen H., Driver H. A. Platelet aggregation and Ca2+ secretion are independent of simultaneous ATP production. FEBS Lett. 1979 Apr 15;100(2):286–290. doi: 10.1016/0014-5793(79)80353-1. [DOI] [PubMed] [Google Scholar]
- Akkerman J. W., Holmsen H. Interrelationships among platelet responses: studies on the burst in proton liberation, lactate production, and oxygen uptake during platelet aggregation and Ca2+ secretion. Blood. 1981 May;57(5):956–966. [PubMed] [Google Scholar]
- Costa J. L., Murphy D. L. Platelet 5-HT uptake and release stopped rapidly by formaldehyde. Nature. 1975 May 29;255(5507):407–408. doi: 10.1038/255407a0. [DOI] [PubMed] [Google Scholar]
- Dangelmaier C. A., Holmsen H. Determination of acid hydrolases in human platelets. Anal Biochem. 1980 May 1;104(1):182–191. doi: 10.1016/0003-2697(80)90296-1. [DOI] [PubMed] [Google Scholar]
- Daniel J. L., Molish I. R., Holmsen H. Radioactive labeling of the adenine nucleotide pool of cells as a method to distinguish among intracellular compartments. Studies on human platelets. Biochim Biophys Acta. 1980 Oct 15;632(3):444–453. doi: 10.1016/0304-4165(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Daniel J. L., Robkin L., Molish I. R., Holmsen H. Determination of the ADP concentration available to participate in energy metabolism in an actin-rich cell, the platelet. J Biol Chem. 1979 Aug 25;254(16):7870–7873. [PubMed] [Google Scholar]
- Gold A. M., Legrand E., Sánchez G. R. Inhibition of muscle phosphorylase a by 5-gluconolactone. J Biol Chem. 1971 Sep 25;246(18):5700–5706. [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- HOHORST H. J. Enzymatische Bestimmung von L(+)-Milchsäure. Biochem Z. 1957;328(7):509–521. [PubMed] [Google Scholar]
- Herman I. M., Pollard T. D. Comparison of purified anti-actin and fluorescent-heavy meromyosin staining patterns in dividing cells. J Cell Biol. 1979 Mar;80(3):509–520. doi: 10.1083/jcb.80.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Storm E. Adenine nucleotide metabolism of blood platelets. VI. Subcellular localization of nucleotide pools with different functions in the platelet release reaction. Biochim Biophys Acta. 1969 Aug 20;186(2):254–266. doi: 10.1016/0005-2787(69)90003-3. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Kaplan K. L., Dangelmaier C. A. Differential energy requirements for platelet responses. A simultaneous study of aggregation, three secretory processes, arachidonate liberation, phosphatidylinositol breakdown and phosphatidate production. Biochem J. 1982 Oct 15;208(1):9–18. doi: 10.1042/bj2080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Robkin L., Day H. J. Effects of antimycin A and 2-deoxyglucose on secretion in human platelets. Differential inhibition of the secretion of acid hydrolases and adenine nucleotides. Biochem J. 1979 Aug 15;182(2):413–419. doi: 10.1042/bj1820413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Robkin L. Effects of antimycin A and 2-deoxyglucose on energy metabolism in washed human platelets. Thromb Haemost. 1980 Feb 29;42(5):1460–1472. [PubMed] [Google Scholar]
- Holmsen H., Robkin L. Hydrogen peroxide lowers ATP levels in platelets without altering adenyalte energy charge and platelet function. J Biol Chem. 1977 Mar 10;252(5):1752–1757. [PubMed] [Google Scholar]
- Holmsen H., Setkowsky C. A., Day H. J. Effects of antimycin and 2-deoxyglucose on adenine nucleotides in human platelets. Role of metabolic adenosine triphosphate in primary aggregation, secondary aggregation and shape change of platetets. Biochem J. 1974 Nov;144(2):385–396. doi: 10.1042/bj1440385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K. L., Nossel H. L., Drillings M., Lesznik G. Radioimmunoassay of platelet factor 4 and beta-thromboglobulin: development and application to studies of platelet release in relation to fibrinopeptide A generation. Br J Haematol. 1978 May;39(1):129–146. doi: 10.1111/j.1365-2141.1978.tb07135.x. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. Phosphatidylinositol metabolism in cells receiving extracellular stimulation. FEBS Lett. 1973 Apr 1;31(1):1–10. doi: 10.1016/0014-5793(73)80061-4. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Koser M., Ravazzola M., Malaisse-Lagae F. The stimulus-secretion coupling of glucose-induced insulin release. Insulin release due to glycogenolysis in glucose-deprived islets. Biochem J. 1977 May 15;164(2):447–454. doi: 10.1042/bj1640447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson M., Schofield J. G. Requirement for adenosine triphosphate for stimulation in vitro of ox growth-hormone release. Biochem J. 1974 Jun;140(3):479–485. doi: 10.1042/bj1400479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. Receptor occupancy dose--response curve suggests that phosphatidyl-inositol breakdown may be intrinsic to the mechanism of the muscarinic cholinergic receptor. FEBS Lett. 1976 Oct 15;69(1):1–5. doi: 10.1016/0014-5793(76)80640-0. [DOI] [PubMed] [Google Scholar]
- Mills D. C. Changes in the adenylate energy charge in human blood platelets induced by adenosine diphosphate. Nat New Biol. 1973 Jun 13;243(128):220–222. doi: 10.1038/newbio243220a0. [DOI] [PubMed] [Google Scholar]
- Mürer E. H. Release reaction and energy metabolism in blood platelets with special reference to the burst in oxygen uptake. Biochim Biophys Acta. 1968 Oct 1;162(3):320–326. doi: 10.1016/0005-2728(68)90118-7. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Stimulus-permeability coupling: role of calcium in the receptor regulation of membrane permeability. Pharmacol Rev. 1978 Jun;30(2):209–245. [PubMed] [Google Scholar]
- Reimers H. J., Packham M. A., Mustard J. F. Labeling of the releasable adenine nucleotides of washed human platelets. Blood. 1977 Jan;49(1):89–99. [PubMed] [Google Scholar]
- Rivard G. E., McLaren J. D., Brunst R. F. Incorporation of hypoxanthine into adenine and guanine nucleotides by human platelets. Biochim Biophys Acta. 1975 Jan 13;381(1):144–156. doi: 10.1016/0304-4165(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Rossignol B., Herman G., Chambaut A. M., Keryer G. The calcium ionophore A 23 187 as a probe for studying the role of Ca2+ ions in the mediation of carbachol effects on rat salivary glands: protein secretion and metabolism of phospholipids and glycogen. FEBS Lett. 1974 Jul 15;43(2):241–246. doi: 10.1016/0014-5793(74)81010-0. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The metabolic requirements from catecholamine release from the adrenal medulla. J Physiol. 1969 May;202(1):197–209. doi: 10.1113/jphysiol.1969.sp008804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Troost J., van der Heijden M. C., Staal G. E. Characterization of alpha-L-fucosidase from two different families with fucosidosis. Clin Chim Acta. 1976 Dec 1;73(2):329–346. doi: 10.1016/0009-8981(76)90180-7. [DOI] [PubMed] [Google Scholar]
- Tu J. I., Jacobson G. R., Graves D. J. Isotopic effects and inhibition of polysaccharide phosphorylase by 1,5-gluconolactone. Relationship to the catalytic mechanism. Biochemistry. 1971 Mar 30;10(7):1229–1236. doi: 10.1021/bi00783a020. [DOI] [PubMed] [Google Scholar]
- Walsh P. N. Albumin density gradient separation and washing of platelets and the study of platelet coagulant activities. Br J Haematol. 1972 Feb;22(2):205–217. doi: 10.1111/j.1365-2141.1972.tb08801.x. [DOI] [PubMed] [Google Scholar]
- Walsh P. N., Gagnatelli G. Platelet antiheparin activity: storage site and release mechanism. Blood. 1974 Aug;44(2):157–168. [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Tubulin and calmodulin. Effects of microtubule and microfilament inhibitors on localization in the mitotic apparatus. J Cell Biol. 1979 Jun;81(3):624–634. doi: 10.1083/jcb.81.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]


