Abstract
In military populations, gene-environment interactions can influence performance and health outcomes. Brain-derived neurotrophic factor (BDNF) is a central nervous system protein that is important for neuronal function and synaptic plasticity. A BDNF single nucleotide polymorphism, rs6265, leads to an amino acid substitution of valine (Val) with methionine (Met) at codon 66 (Val66Met), which may influence an individual’s response to occupational stress, and predispose military members to psychological disorders. Telomere length (TL), a novel measure of biological aging, can be used as a biomarker of stress. Accordingly, telomere shortening may be a surrogate indicator of physiological weathering due to chronic disease and stressful life events. To increase our understanding about the potential effect of the Val66Met mutation on the human stress response, we evaluated the relationships between Val66Met, TL, and mental health symptoms in a military population. In this pilot study (N = 164), we observed an association between Val66Met and reduced TL (p = 0.048). There was no relationship between Val66Met and mental health symptoms. These results support the investigation of gene-environment interactions, and their potential influence on TL due to occupational stress such as military service.
Subject terms: Genetics, Biomarkers
Introduction
Monitoring psychological and physiological effects of chronic stress in military members is key to ensuring readiness. Gene-environment interactions can influence health, performance, and quality of life, during and after service. Stress is an inherent part of the military experience. There has been a considerable research effort to explore factors related to disease risk in military personnel and veterans in order to improve short- and long-term health outcomes.
Brain-derived neurotrophic factor (BDNF) is a prominent, central nervous system growth factor. It supports neural plasticity1and is involved in stress response regulation2. A BDNF single nucleotide polymorphism, rs6265, leads to an amino acid substitution of valine (Val) with methionine (Met) at codon 66 (Val66Met). This Val66Met point mutation is a relatively common variant that reduces BDNF secretion and expression levels, but not the coding of mature BDNF3. More specifically, the Met allele has been linked to deficits in normal BDNF expression4, reduced synaptic plasticity of hippocampal neurons, and poorer episodic memory3, as compared to the Val allele. Furthermore, altered BDNF levels may lead to maladaptive stress responses and Met allele carriage has been linked to depression5. and posttraumatic stress disorder (PTSD6). Also, results from a systematic review and meta-analysis revealed that the Met allele is a moderator of the life stress and depression relationship7. There is ample evidence that BDNF expression is modulated by both genetic and environmental factors8. To date, there have been very few studies on the relationship between Val66Met and mental health in active duty military members.
Telomere length (TL) is a marker of biological aging, and stress can induce TL shortening9. While telomere attrition is a normal part of the aging process, short telomeres can predispose individuals to disease9and psychological disorders10. However, excessively long telomeres are linked to disease risk11. The data on TL in military members are limited, but TL shortening has been associated with higher hostility in special operations personnel12. A similar finding was also reported in military veterans13. A recent study found that TL was significantly reduced in men with prior military service suggesting that military experience may expedite the aging process14. In veterans, TL attrition has been associated with perceived stress15. and PTSD16. Conversely, others have reported TL lengthening in active duty personnel with PTSD symptoms17. TL has yet to be characterized in younger military personnel and the influence of Val66Met on TL in this, or any other population is currently unknown.
The goal of this pilot study was to assess the effect of Val66Met on TL, and mental health in a relatively young military population. We hypothesized that the presence of the Val66Met Met allele would be linked to shorter TL and to greater mental health symptoms.
Methods
Participants (N = 164) were recruited from the Center for Explosive Ordnance Disposal and Diving (CEODD; Great Lakes, IL) and from units within U.S. Navy EOD Group One (San Diego, CA). This sample was comprised of EOD accessions (new recruits) from CEODD (n = 75), EOD trainees enrolled in the U.S. Navy Tactical Training Course (TTC; n = 24), and EOD technicians (n = 63). Accessions were either military recruits who were attempting to join U.S. Navy EOD or active duty personnel. Trainees had completed EOD A-School and were in the final training phase (TTC) before they could be assigned to their first duty station as an EOD technician. U.S. Navy EOD technicians are the world’s premier combat force for countering explosive hazards and all other types of ordnance. This study protocol was approved by Naval Health Research Center (NHRC) Institutional Review Board (NHRC.2018.0019). All study procedures were performed in accordance with U.S. Federal regulations, U.S. Department of Defense instructions, and U.S. Navy instructions.
This was a cross-sectional study and participants provided informed consent before study participation. During a single, in-person session, participants completed a computer tablet-based survey and provided a saliva sample. Participants self-reported their background information (e.g., age, education) and responded to mental health measures. All data were deidentified using a non-traceable, unique identifier for each participant, which was then linked to their survey and saliva data.
A single saliva sample was collected from each participant using an oral swab (SalivaBio Oral Swab, part no. 5001.02, Salimetrics, LLC, Carlsbad, CA) housed in a storage tube (Swab Storage Tube, part no. 5001.05, Salimetrics, LLC, Carlsbad, CA). Ten minutes prior to sample collection, participants rinsed their mouths with water. Participants uncapped the tube and deposited the swab under their tongue. After 2 min, the saturated swab was placed back into the storage tube and the cap was replaced. Samples were immediately placed on ice or dry ice and then stored at the NHRC laboratory in a -80oC freezer until ready for shipment. Genotyping was performed by Salimetrics, LLC and TL measurement was conducted by the Telomere Core, Elizabeth Blackburn Lab at University of California, San Francisco.
The Genebank accession number for the human BDNF gene DNA region containing the rs6265 polymorphism is AB038670. A Taqman™ single nucleotide polymorphism (SNP) genotyping assay was used to amplify and detect the two alleles for BDNF rs6265 SNP (catalog no.: 4351379, assay ID: C_11562758_10, Life Technologies™ [LifeTech]/Applied Biosystems [ABI]). Polymerase chain reaction (PCR) amplification was performed by an ABI 7500 Real-Time PCR machine using sequence specific DNA primers and Taqman™ PCR universal Master Mix (catalog no.: 4303337, LifeTech/ABI). To detect each allele, Taqman™ DNA probes that contained fluorescent reporter dyes at their 5’ ends, and non-fluorescent quenchers at their 3’ ends, were used.
The measurement assay for telomere length was adapted18from the original published method19. The telomere thermal cycling profile was as follows: Cycling for T (telomere copy) PCR: Denature at 96 °C for 1 min, one cycle; denature at 96 °C for 1 s then, anneal/extend at 54 °C for 60 s for 30 cycles. Cycling for S (single copy gene) PCR: Denature at 96 °C for 1 min, one cycle; denature at 95 °C for 15 s, anneal at 58 °C for 1 s, extend at 72 °C for 20 s, hold at 83 °C for 5 s for 35 cycles.
The primers for the telomere PCR were tel1b [5’-CGGTTT(GTTTGG)5GTT-3’], final concentration 100 nM, and tel2b [5’-GGCTTG(CCTTAC)5CCT-3’], final concentration of 900 nM. The primers for the single-copy gene (human beta-globin) PCR were hbg1 [5’ GCTTCTGACACAACTGTGTTCACTAGC-3’], final concentration of 300 nM, and hbg2 [5’-CACCAACTTCATCCACGTTCACC-3’], final concentration of 700 nM. The final reaction mix contained 20 mM Tris-HCl, pH 8.4; 50 mM KCl; 200 µM each dNTP; 1% DMSO; 0.4x Syber Green I; 22 ng E. coli DNA; 0.4 Units of Platinum Taq DNA polymerase (Invitrogen Inc.); approximately 6.6 ng of genomic DNA per 11 µL reaction. Serial dilution (26 ng to 0.108 ng) of a reference DNA (Human genomic DNA from buffy coat, Sigma cat# 11691112001) was included in each PCR run to establish a standard curve for quantification of targeted templates in each research sample. Assays were run in triplicate wells on 384-well assay plates in a Roche LightCycler 480. The average concentrations of T and S from the triplicate wells were used to calculate the T/S ratios after a Dixon’s Q test to remove outlier wells. The T/S ratio for each sample was measured twice. When the duplicate T/S value and the initial value varied by more than 7%, the sample was run a third time, and the two closest values were reported. The average coefficient of variation was 2.4% ± 2.2%.
Symptoms of anxiety, depression, posttraumatic stress, and perceived stress were measured with widely used, validated, and reliable scales. For each scale, items were summed with higher total scores indicating greater symptoms or stress.
The Generalized Anxiety Disorder-7 Scale (GAD-7; Cronbach’s α = 0.9220) was used to assess generalized anxiety symptoms including “feel nervous, anxious, or on edge” and “being so restless that it is hard to sit still.” Participants reported how often they were bothered by each problem in the past 2 weeks on a scale ranging from 0 (not at all) to 3 (nearly every day) with a total possible score = 21. A GAD-7 total score > 10 is qualified as moderate to severe anxiety.
The Patient Health Questionnaire-8 (PHQ-8; Cronbach’s α = 0.8521) was used to assess Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria for major depression including “little interest or pleasure in doing things” and “feeling down, depressed, or hopeless.” Participants reported how often they have been bothered by each symptom in the past 2 weeks on a scale ranging from 0 (not at all) to 3 (nearly every day) with a total possible score = 24. A PHQ-8 total score > 10 is qualified as moderate to severe depression.
The Posttraumatic Stress Disorder (PTSD) Checklist (PCL-5; Cronbach’s α = 0.9522) is comprised of 20 Likert-type items assessing criteria for PTSD. PCL-5 items are consistent with the DSM, Fifth Edition (DSM-V) PTSD criteria. Participants reported how much they felt bothered by symptoms over the previous month on a scale from 1 (not at all) to 5 (extremely) with a total possible score = 80. Sample items include, “feeling very upset when something reminded you of a stressful experience,” and “being ‘super alert’ or watchful or on guard.” Total scores from 31 to 33 are commonly used as the threshold for probable PTSD.
The Perceived Stress Scale-10 (PSS-10; Cronbach’s α = 0.8623) was used to assess stress perceptions during the last month, such as “how often have you felt nervous and stressed” and “how often have you felt that you were on top of things” on a scale from 1 (never) to 5 (very often) with a total possible score = 40. There are no cut-off scores or criteria for the PSS-10.
Missing data were noted and accounted for in the dataset. Missing survey data resulted from a participant intentionally, or accidentally, skipping a question. Missing TL values resulted from saliva sample degradation or insufficient volume. No data were imputed for missing values. Data were analyzed using SPSS statistical software, version 25.0 (IBM, Armonk, NY, USA). Descriptive analyses were used to summarize participant characteristics and demographic information. Frequencies were run for all continuous data to determine normality. Anxiety, depression, and posttraumatic stress scores were log transformed to render skewness and kurtosis within predefined limits. Log transformed data were used for analyses, but untransformed means are reported for ease of interpretation. Univariate analyses of variance were used to evaluate the relationships between Val66Met genotype and TL or mental health symptoms. Bivariate correlations were performed to evaluate the relationship between TL, age, deployments, mental health symptoms, and perceived stress. One-way analyses of variance were used to evaluate differences in mental health symptoms between subgroups. Alpha level was set at 0.05. For all models, theoretically relevant variables were screened as candidate covariates (i.e., age, deployments) following standardized criteria. Specifically, a variable was selected as a covariate if it related theoretically, and statistically, (p< 0.05) to the independent and dependent variable, thus qualifying it as a potential confounder or mediator24.
Results
Relevant participant characteristics are provided in Table 1. Participants (N = 164) were predominantly White and male. Most were 30 years old or younger (n = 136; 85. 5%). TL did not associate with age, number of deployments, mental health symptoms, or perceived stress (p > 0.05). Overall, symptoms of anxiety, depression, and posttraumatic stress, were low to mild. Sixteen participants (10%) endorsed moderate to severe anxiety. Ten individuals (6.2%) met criteria for moderate to severe depression and 8 participants (5.1%) met criteria for probable PTSD. Approximately 93% (n = 147) rated their perceived stress as less than 20 out of 40 total possible points. None of the candidate covariates met criteria for inclusion in any of the models.
Table 1.
Participant characteristics. *Two participants identified as American Indian, but both also selected another race or ethnicity. ^Indicates self-report of more than one race and ethnicity. M = mean. SD = standard deviation. EOD = Explosive Ordnance Disposal.
| Characteristic | n (%) | M ± SD | Range |
|---|---|---|---|
| EOD Group | |||
| Accession | 75 (45.7%) | ||
| Trainee | 24 (14.6%) | ||
| Technician | 63 (38.4%) | ||
| Missing | 2 (1.2%) | ||
| Sex | |||
| Male | 157 (95.7%) | ||
| Female | 3 (1.8%) | ||
| Missing | 4 (2.4%) | ||
| Race and Ethnicity | |||
| American Indian* | 0 (0%) | ||
| Asian/Pacific Islander | 12 (7.3%) | ||
| Black | 2 (1.2%) | ||
| Hispanic | 2 (1.2%) | ||
| White | 130 (79.3%) | ||
| More than 1 race and ethnicity^ | 15 (9.1%) | ||
| Missing | 3 (1.8%) | ||
| Education | |||
| Less than high school | 1 (0.6%) | ||
| High school graduate | 42 (25.6%) | ||
| Some college | 46 (28.0%) | ||
| Associate degree | 10 (6.1%) | ||
| Bachelor’s degree or higher | 62 (37.8%) | ||
| Missing | 3 (1.8%) | ||
| Rank | |||
| Officer | 15 (9.1%) | ||
| Enlisted | 96 (58.5%) | ||
| Missing / N/A | 53 (32.3%) | ||
| Age (years) | 159 (97.0%) | 25.9 ± 4.7 | 18–43 |
| Missing | 5 (3.0%) | ||
| Military Experience (years) | 159 (97.0%) | 3.5 ± 3.9 | 0–18 |
| Missing | 5 (3.0%) | ||
| Telomere Length (T/S ratio) | 148 (90.2%) | 1.28 ± 0.3 | 0.6–2.1 |
| Missing | 16 (9.8%) | ||
| Anxiety Symptom Score | 160 (97.6%) | 3.9 ± 4.2 | 0–20 |
| Missing | 4 (2.4%) | ||
| Depressive Symptom Score | 161 (98.2%) | 3.3 ± 3.9 | 0–23 |
| Missing | 3 (1.8%) | ||
| Posttraumatic Stress Symptom Score | 157 (95.7%) | 7.7 ± 12.0 | 0–66 |
| Missing | 7 (1.3%) | ||
| Perceived Stress Score | 159 (97.0%) | 9.8 ± 6.8 | 0–31 |
| Missing | 5 (3.0%) | ||
Val66Met and telomere length
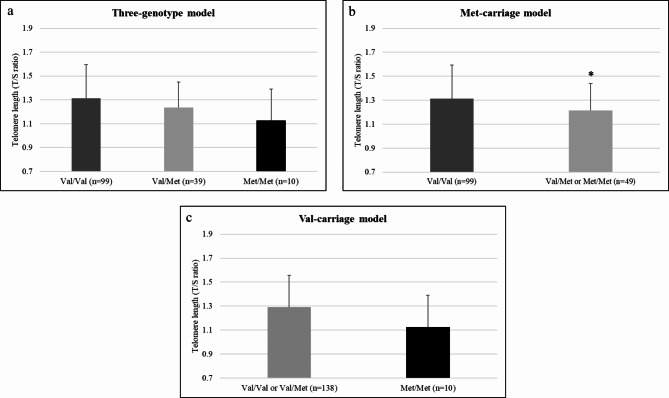
TL means ± standard deviations for Val/Val, Val/Met, and Met/Met were 1.31 ± 0.28, 1.24 ± 0.21, and 1.13 ± 0.26, respectively. In a three-genotype model, overall differences were observed between Val66Met genotypes TL (F(2,145) = 3.09, p = 0.048, ηp2 = 0.04, 1-β = 0.59). As shown in Fig. 1a, homozygous Val carriers exhibited the highest TL, followed by heterozygous carriers (Val/Met), and then homozygous Met carriers. Post hoc comparisons revealed the most pronounced difference between homozygous Val carriers and homozygous Met carriers (T(107) = 2.0, p = 0.02, Cohen’s D = 0.67). In addition, we conducted exploratory analyses using the three-genotype model within each subgroup of EOD accessions, trainees, and technicians, which revealed similar, non-significant trends.
Fig. 1.
Effect of the Val66Met variant of BDNF on telomere length (TL). TL means and standard deviations are shown for the total sample. There was a main effect of genotype on TL (p < 0.05) in three-genotype model (a), group differences (*p < 0.05) in the Met-carriage model (b), and no differences (p > 0.05) in the Val-carriage model (c).
To further parcel the unique functional roles of each allele, we also performed post-hoc comparisons of Met-carriage (versus Val/Val + Val/Met; Fig. 1b) and Val-carriage (versus Met/Met + Val/Met; Fig. 1c), respectively. In the Met-carriage model, there were differences between Val/Val and Met carriers (F(1,146) = 4.79, p = 0.030, ηp2 = 0.03, 1-β = 0.59). There were no significant differences between Val carriers or a Met/Met in the Val-carriage model.
Val66Met and mental health
In this sample, 111 participants (67.7%) were homozygous Val carriers, 43 (26.2%) were heterozygous, and 10 (6.1%) were homozygous Met carriers. Mental health differences between subgroups were observed, but only for depressive symptoms (F(2,158) = 4.35, p = 0.015, ηp2 = 0.05, 1-β = 0.75). Post-hoc test revealed that technicians had greater symptoms than trainees (p = 0.011). There were no clear associations between the Val66Met genotype and symptoms of anxiety, depression, posttraumatic stress, or perceived stress (all p < 0.05) in the total sample.
Discussion
Gene-environment research has the potential to identify those who are at increased risk for certain health outcomes, which ultimately supports the advancement of precision medicine. For instance, it is possible that Val66Met genotype may be incorporated into preventive and/or therapeutic modalities for traumatic brain injury, healthy aging/memory care, blast exposure, and other injuries that can affect neural (synaptic) function. This pilot study established a relationship between the Val66Met (rs6265) variant of BDNF and TL in an apparently healthy military population. To our knowledge, this is the first study of its kind, and the results coalesce with substantial literature that implicates Met-carriage in compromised BDNF expression and in reduced synaptic function of hippocampal neurons.
In a three-genotype model, the Val66Met variant associated with TL and Met/Met carriers had the shortest TL, on average. The Met-carriage model revealed that Met carriage had a unique influence on TL. Our analyses imply that Val carriage may also have an equally important contribution, but the Val-carriage model was underpowered. All the observed associations in this study were independent of age. This contrasts with the broader literature which describes telomere shortening with age9,10,25. TL values in this study were comparable to T/S ratios in healthy adults 0.3–1.526,27,28,29.), and less than 15% of participants had T/S ratios < 1, which are classified as short13,30. It is possible that age-dependent effects on TL cannot be captured in samples with a narrow age range or perhaps that TL shortening over the lifespan is non-linear. Altogether, the effect of the Val66Met genotype on TL in young, healthy participants is a novel finding amongst the accruing research on stress and biological aging in military populations. These findings suggest that there are genetically determined individual differences that may affect when TL shortening transpires, and that genetic influence on TL may begin earlier in life, which further supports the prognostic capabilities of genetic biomarkers. Confirmation of our results about the respective contribution of the Val and Met alleles on TL awaits replication studies.
In the present study of non-clinical participants, the Val66Met variant did not associate with measures of mental health symptoms. One possible explanation is that overall, participants self-reported no to low symptoms of anxiety and depression and very few met the clinical threshold for posttraumatic stress. Total scores for perceived stress in this study were similar to a recent study in elite military men31. These factors, coupled with a broad spectrum of military experience/exposure in this sample, may have diluted any effect of Val66Met on mental health. Val66Met has been explored as a potential genetic marker of PTSD, mostly in military veterans. Studies implicating Val66Met as a risk for PTSD have been equivocal32and a meta-analysis suggested that this link is tenuous33. The Met allele may both favorably, and unfavorably, modulateclinical outcomes as opposed to directly affecting mental health34, found that the Met allele moderated the effects of a cognitive enhancer during PTSD exposure therapy, resulting in greater symptom improvement for Met carriers, while Val/Val carriers improved more without the enhancer34. Such studies support the advancement of precision medicine by demonstrating that genetic predisposition can profoundly influence clinical effects. In older military veterans, the Val66Met genotype and physical exercise moderated the association between depression and cognitive dysfunction35. Others observed that U.S. military veterans who were Met carriers with probable PTSD had greater cognitive impairments as compared to those who did not have PTSD6. In summary, framing the Val66Met mutation as a risk, versus protective, factor for mental health is perhaps an oversimplified approach8. We suggest that future studies focus on the role of the Met allele as a moderator of mental health outcomes and interventions.
There were some limitations in this pilot study. The primary limitation of this pilot study is its relatively small sample size. As a result, some key results were not optimally powered and there was a lack of gender and ethnic diversity. Thus, our findings are not generalizable. These are important considerations for our upcoming research. Additionally, although we accounted for several potential confounders (e.g., posttraumatic stress symptoms), we did not quantify exposures to traumatic events per se. Trauma exposure is known to influence hippocampal structure36and function37, and the clinical symptoms associated with traumatic memory, fear avoidance, and stress regulation41. Moreover, the impact of trauma on the hippocampus can be independent of the effects asserted by posttraumatic stress disorder36. It is plausible that the effects of trauma exposure may mediate, or extend directly to, TL. This may, in turn, moderate, or confound, the observed associations between Val66Met and TL. Bias in self-report data is well known, but this is offset by our use of valid measures of mental health symptoms and objective biological markers. While allele frequencies of the Val66Met variant in this study were consistent with other European American samples38, there is notable variation in Val66Met across gender38and ethnicity39. Larger studies with more diverse participant characteristics are needed to adequately evaluate the impact of Val66Met on telomere length and mental health in military personnel. These efforts are currently underway in our lab and will include replicating this study in a large sample of active-duty military personnel, allowing us to increase the complexity of our hypothesis tests (e.g., moderating role of Val66Met).
BDNF is an important protein for adaptive neuronal function and supports improved health outcomes in military personnel who are at greater risk for accelerated biological aging due to stress and trauma. The effect of the Val66Met variant on TL in the present study warrants further investigation of not only Val66Met, but of other genetic variants that are implicated in mental health and secondary cognitive deficits. Both Val66Met and TL show great promise as clinical tools that can be used to refine health care treatments for military members and civilians alike. In one practical illustration, aerobic exercise is known to influence neurogenesis and TL26, and preliminary evidence links Met carriage of Val66Met to more positive BDNF responses to acute aerobic exercise in older adults25. This latter finding might be explained by a “law of initial values,” whereby lower inherent function of BDNF in Met carriers might facilitate greater improvements. Of course, it is not known is such effects would prevail in relatively younger cohorts. Clearly, further advancements in this area of research are warranted to strengthen these theoretical linkages, and, ultimately, to affect precision medicine.
Author contributions
R.C.A. provided conceptualization and writing (original draft and review). L.M.H. provided data curation, formal analysis, funding acquisition, project administration, visualization, and writing (original draft, review, and editing). M.K.T. provided conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, resources, supervision, and writing (review and editing). All authors reviewed the manuscript.
Funding
This work was supported by the Office of Naval Research, Summer Faculty Research Program and the Defense Health Agency under work unit number N1522.
Data availability
All data are property of the U.S. Government and can be shared after a data sharing agreement has been established between the interested party and the Naval Health Research Center. Interested parties may send inquiries to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Disclaimer
MKT is an employee of the U.S. Government. This work was prepared as part of his official duties. Title 17, U.S.C. § 105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C. § 101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. Report No. 23 − 05 was supported by the Office of Naval Research, Summer Faculty Research Program and the Defense Health Agency under work unit number N1522. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. The study protocol was approved by the Naval Health Research Center Institutional Review Board in compliance with all applicable Federal regulations governing the protection of human subjects. Research data were derived from an approved Naval Health Research Center Institutional Review Board protocol, number NHRC.2018.0019.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bramham, C. R. & Messaoudi, E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol.76 (2), 99–125. 10.1016/j.pneurobio.2005.06.003 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Miller, J. K., McDougall, S., Thomas, S. & Wiener, J. The impact of the brain-derived neurotrophic factor gene on trauma and spatial processing. J. Clin. Med.6 (12), 108. 10.3390/jcm6120108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egan, M. et al. The BDNF Val66Met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 112 (2), 257–269. 10.1016/S0092-8674(03)00035-7 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Brown, D. T., Vickers, J. C., Stuart, K. E., Cechova, K. & Ward, D. D. The BDNF Val66Met polymorphism modulates resilience of neurological function to brain ageing and dementia: a narrative review. Brain Sci. 202025;10(4):195. 10.3390/brainsci10040195. PMID: 32218234; PMCID: PMC7226504. [DOI] [PMC free article] [PubMed]
- 5.Youssef, M. M. et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int. J. Neuropsychopharmacol.21 (6), 528–538. 10.1093/ijnp/pyy008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rippey, C. S., Pietrzak, R. H., Maruff, P. & Adams, T. G. Interactive effects of the BDNF Val66Met polymorphism and posttraumatic stress disorder on cognition in U.S. military veterans. Psychoneuroendocrinology. 142, 105820. 10.1016/j.psyneuen.2022.105820 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Hosang, G. M., Shiles, C., Tansey, K. E., McGuffin, P. & Uher, R. Interaction between stress and the BDNFVal66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med.12 (1), 1–1. 10.1186/1741-7015-12-7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda, M., Morici, J. F., Zanoni, M. B. & Bekinschtein, P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 363. 10.3389/fncel.2019.00363 (2019). [DOI] [PMC free article] [PubMed]
- 9.Lin, J. & Epel, E. Stress and telomere shortening: insights from cellular mechanisms. Ageing Res. Rev.73, 101507. 10.1016/j.arr.2021.101507 (2022). [DOI] [PMC free article] [PubMed]
- 10.Epel, E. S. & Prather, A. A. Stress, telomeres, and psychopathology: toward a deeper understanding of a triad of early aging. Ann. Rev. Clin. Psychol.14, 371. 10.1146/annurev-clinpsy-032816-045054 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Protsenko, E., Rehkopf, D., Prather, A. A., Epel, E. & Lin, J. Are long telomeres better than short? Relative contributions of genetically predicted telomere length to neoplastic and non-neoplastic disease risk and population health burden. PloS One. 15 (10), e0240185. 10.1371/journal.pone.0240185 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang, L. et al. Association between leukocyte telomere length and hostility in US army service members. Neurosci. Lett.706, 24–29 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Watkins, L. E. et al. Hostility and telomere shortening among US military veterans: results from the National Health and Resilience in Veterans Study. Psychoneuroendocrinology. 74, 251–257. 10.1016/j.psyneuen.2016.09.006 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Howard, J. T. et al. Telomere shortening and accelerated aging in US military veterans. Int. J. Environ. Res. Public Health. 18 (4), 1743. 10.3390/ijerph18041743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bersani, F. S. et al. Association of dimensional psychological health measures with telomere length in male war veterans. J. Affect. Disord.190, 537–542. 10.1016/j.jad.2015.10.037 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Kang, J. I. et al. Effect of combat exposure and posttraumatic stress disorder on telomere length and amygdala volume. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 5 (7), 678–687. 10.1016/j.bpsc.2020.03.007 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Boks, M. P. et al. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 51, 506–512. 10.1016/j.psyneuen.2014.07.011 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Lin, J. et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J. Immunol. Methods. 352 (1–2), 71–80. 10.1016/j.jim.2009.09.012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthon, R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res.30 (10), e47–e47. 10.1093/nar/30.10.e47 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitzer, R. L., Kroenke, K., Williams, J. B. W. & Löwe, B. A brief measure for assessing generalized anxiety disorder. Arch. Intern. Med.166 (10), 1092–1092. 10.1001/archinte.166.10.1092 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Kroenke, K. et al. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord.114 (1–3), 163–173. 10.1016/j.jad.2008.06.026 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Blevins, C. A., Weathers, F. W., Davis, M. T., Witte, T. K. & Domino, J. L. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J. Trauma. Stress. 28 (6), 489–498. 10.1002/jts.22059 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Cohen, S., Kamarck, T. & Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav.24 (4), 385–396. 10.2307/2136404 (1983). [PubMed] [Google Scholar]
- 24.MacKinnon, D. P., Krull, J. L. & Lockwood, C. M. Equivalence of the mediation, confounding and suppression effect. Prev. Sci.1 (4), 173–181. 10.1023/A:1026595011371 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bugge Kambestad, O. et al. Physical Exercise and serum BDNF levels: accounting for the Val66Met polymorphism in older adults. Cogn. Behav. Neurol.36 (4), 219–227 (2023). PMID: 37404130; PMCID: PMC10683974. [DOI] [PMC free article] [PubMed]
- 26.Østhus, I. B. et al. Telomere length and long-term endurance exercise: does exercise training affect biological age? A pilot study. PloS One. 7 (12), e52769. 10.1371/journal.pone.0052769 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denham, J., Marques, F. Z. & Charchar, F. J. Leukocyte telomere length variation due to DNA extraction method. BMC Res. Notes. 7 (1), 1–6. 10.1186/1756-0500-7-877 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, K. S. et al. Oxidative stress-induced telomere length shortening of circulating leukocyte in patients with obstructive sleep apnea. Aging Disease. 7 (5), 604. 10.14336/AD.2016.0215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furtado, F. M. et al. Telomere length analysis in monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia Binet A. Braz. J. Med. Biol. Res.50 (5). 10.1590/1414-431X20176019 (2017). [DOI] [PMC free article] [PubMed]
- 30.Brydon, L. et al. Hostility and cellular aging in men from the Whitehall II cohort. Biol. Psychiatry. 71 (9), 767–773. 10.1016/j.biopsych.2011.08.020 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández, L. M., Markwald, R. R., Kviatkovsky, S. A., Perry, L. N. & Taylor, M. K. Morning cortisol is associated with stress and sleep in elite military men: a brief report. Mil. Med.183 (9–10), e255–e259. 10.1093/milmed/usy047 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Wang, T. & Does, B. D. N. F. Val66Met polymorphism confer risk for posttraumatic stress disorder? Neuropsychobiology. 71 (3), 149–153. 10.1159/000381352 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Bountress, K. E. et al. The effects of a BDNF Val66Met polymorphism on posttraumatic stress disorder: a meta-analysis. Neuropsychobiology. 76, 136–142. 10.1159/000489407 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Difede, J. et al. Enhancing exposure therapy for posttraumatic stress disorder (PTSD): a randomized clinical trial of virtual reality and imaginal exposure with a cognitive enhancer. Translational Psychiatry. 12 (1), 1–9. 10.1038/s41398-022-02066-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitts, B. L. et al. Depression and cognitive dysfunction in older US military veterans: moderating effects of BDNF Val66Met polymorphism and physical exercise. Am. J. Geriatric Psychiatry. 28 (9), 959–967. 10.1016/j.jagp.2020.02.001 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Woon, F. L., Sood, S. & Hedges, D. W. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 34 (7), 1181–1188. 10.1016/j.pnpbp.2010.06.016 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Postel, C. et al. Variations in response to trauma and hippocampal subfield changes. Neurobiol. Stress. 15, 100346. 10.1016/j.ynstr.2021.100346 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahill, S., Chandola, T. & Hager, R. Genetic variants associated with resilience in human and animal studies. Front. Psychiatry. 1310.3389/fpsyt.2022.840120 (2022). [DOI] [PMC free article] [PubMed]
- 39.Petryshen, T. L. et al. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol. Psychiatry. 15 (8), 810–815. 10.1038/mp.2009.24 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee, E. H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs. Res.6 (4), 121–127. 10.1016/j.anr.2012.08.004 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Weissman, D. G. et al. Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depress. Anxiety. 37 (9), 916–925. 10.1002/da.23062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are property of the U.S. Government and can be shared after a data sharing agreement has been established between the interested party and the Naval Health Research Center. Interested parties may send inquiries to the corresponding author.