Abstract
Viral nervous necrosis (VNN) poses a significant threat to the aquaculture industry, causing substantial losses and economic burdens. The disease, attributed to nervous necrosis viruses within the Betanodavirus genus, is particularly pervasive in the Mediterranean region, affecting various fish species across all production stages with mortality rates reaching 100%. Developing effective preventive measures against VNN is imperative. In this study, we employed rigorous immunoinformatics techniques to design a novel multi-epitope vaccine targeting VNN. Five RNA-directed RNA polymerases, crucial to the lifecycle of Betanodavirus, were selected as vaccine targets. The antigenicity and favorable physicochemical properties of these proteins were confirmed, and epitope mapping identified cytotoxic T lymphocyte, helper T lymphocyte, and linear B lymphocyte epitopes essential for eliciting a robust immune response. The selected epitopes, characterized by high antigenicity, non-allergenicity, and non-toxicity, were further enhanced by adding PADRE sequences and hBD adjuvants to increase immunogenicity. Two vaccine constructs were developed by linking epitopes using appropriate linkers, demonstrating high antigenicity, solubility, and stability. Molecular dynamics simulations revealed stable interactions between the vaccine constructs and Toll-like receptors (TLRs), essential for pathogen recognition and immune response activation in fish. Notably, vaccine construct V2 exhibited superior stability and binding affinity with TLR8, suggesting its potential as a promising candidate for VNN prevention. Overall, our study presents a comprehensive approach to VNN vaccine design utilizing immunoinformatics, offering safe, immunogenic, and effective solutions across multiple Betanodavirus species. Further experimental validation in model animals is recommended to fully assess the vaccine’s efficacy. This research contributes to improved vaccine development against diverse fish pathogens by addressing emerging challenges and individualized immunization requirements in aquaculture.
Keywords: Viral nervous necrosis (VNN), Fish vaccine, Betanodavirus, Multi-epitope vaccine, Immunoinformatics, Molecular dynamics simulation, Aquaculture
Subject terms: Computational biology and bioinformatics, Drug discovery, Microbiology, Environmental sciences, Ocean sciences
Introduction
Aquaculture is recognized as one of the fastest-growing food-producing sectors. The production of marine aquaculture fish has expanded considerably in recent years. However, this rapid growth and increased intensity have led to disease outbreaks1,2. Among these diseases, viruses pose the most significant threat, causing substantial losses in fish aquaculture productivity. Betanodavirus, also known as nervous necrosis virus (NNV), is particularly concerning as it has emerged as a major impediment to fish culture worldwide, causing devastating losses with mortality rates reaching up to 100% in some regions3.
Viral Nervous Necrosis (VNN), caused by NNV, was initially described in Australia and the Caribbean around the end of the 1980s. Since then, VNN has caused numerous deaths and significant economic losses in various marine and freshwater fish species globally. Currently, around 40 different fish species are known to be infected with Betanodavirus, including freshwater guppy and aquarium fish like rainbow shark and goldfish. Originally referred to as a "picorna-like virus," Betanodavirus was later classified as a member of the Nodaviridae family. The International Committee on Virus Taxonomy (ICTV) placed piscine nodaviruses in the genus Betanodavirus within the Nodaviridae family4–6.
There are currently four recognized species of Betanodavirus: red-spotted grouper nervous necrosis virus (RGNNV), barfin flounder nervous necrosis virus (BFNNV), tiger puffer nervous necrosis virus (TPNNV), and striped jack nervous necrosis virus (SJNNV), along with the less prevalent sevenband grouper nervous necrosis virus (SBGNNV)7. NNV is a small non-enveloped virus, about 25–30 nm in diameter, with a viral genome consisting of two single-stranded positive-sense RNA molecules, RNA1 (1.01 × 10^6 Da) and RNA2 (0.49 × 10^6 Da), co-packaged to form a single virion4,8. Disease outbreaks have been documented mostly in larvae and juveniles, though significant fatalities have also been reported in older fishes. Histopathological investigations indicate severe necrosis of the central nervous system (CNS), with significant vacuolation, neural deterioration, and retinal vacuolation. Several viral entry points have been identified, including cells of the epithelial lining of the body, fins, gills, nasal, and oral cavities9–12.
For the past decade, Betanodavirus has been a key limiting factor in fish culture globally and continues to be so today. Current treatment approaches are limited and generally lack significant impact. Plant-derived natural compounds have shown efficacy against viruses and can be used as antiviral medicines13,14. Several antiviral agents, such as tilapia hepcidin 1–5 (TH1-5) and cyclic prawn anti-lipopolysaccharide factor, have been found effective against Betanodavirus3. However, vaccines are believed to be more efficient for preventing viral diseases in fish. Vaccinating fish and boosting their immune system is a valuable and practical strategy to ensure they remain as disease-free as possible. Various vaccines, including the nodavirus-like particle vaccine, the subunit vaccine containing recombinant Betanodavirus coat protein, and the formalin-inactivated vaccine, have been developed and shown efficacy in preventing Betanodavirus infection3,15–17. However, these vaccines require high concentrations and multiple doses to provide effective and long-term immunity. Moreover, all these vaccines are monovalent, providing immune protection only against a single antigen or strain of the virus, limiting their effectiveness18,19. Currently, no epitope-based polyvalent vaccine has been proposed to provide broad-range protection against multiple strains of Betanodavirus simultaneously. In this study, we developed two multi-epitope polyvalent vaccines targeting the RNA-directed RNA polymerase (RdRp) protein of five virulent species: RGNNV, BFNNV, TPNNV, SJNNV, and SBGNNV. This approach aims to provide protection against all these strains. RdRp plays a crucial role in the lifecycle of Betanodavirus and the translation process in the host20.
From an immunological perspective, the designed vaccines have the potential to elicit strong and specific immune responses. By incorporating multiple epitopes known to be immunogenic, the vaccine is expected to activate both the humoral and cellular arms of the immune system, leading to robust and long-lasting immunity. The inclusion of epitopes that stimulate T-cell responses is particularly important for generating memory cells that can provide long-term protection21. Additionally, the designed vaccines aim to overcome the limitations of monovalent vaccines by targeting multiple strains of the virus, thus offering broader protection and reducing the risk of vaccine escape mutants.
The use of immunoinformatics tools ensures that the selected epitopes are highly conserved and likely to be effective across different viral strains, enhancing the vaccine’s potential to provide comprehensive protection against Betanodavirus infections22–24. This work paves the way for the aquaculture sector to use epitope-based immunizations to combat infections, enabling researchers to create quick and effective vaccines for emerging diseases.
Methods
The stepwise methods of the entire study are depicted in Supplementary Figure S1 as a flowchart.
Strain and protein selection with biophysical property analyses
In this study, we selected five RdRp proteins that were retrieved in FASTA format from the UniProt (https://www.uniprot.org) and NCBI (https://www.ncbi.nlm.nih.gov/) databases. To evaluate the antigenic properties, the selected protein sequences were submitted to the VaxiJen v2.0 online antigenicity program (https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html) using a threshold of 0.425. The TMHMM-2.0 server (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0) was used to evaluate the transmembrane topology of the selected proteins26. Various physicochemical characteristics of the proteins were examined using the ExPASy ProtParam server (https://web.expasy.org/protparam/)27. To identify conservancy patterns, homologous sequence sets of the selected antigenic proteins were obtained from the NCBI database using the BLASTp program.
Epitope mapping and vaccine construction
The T-cell and B-cell epitopes of the selected protein sequences were predicted using the Immune Epitope Database (IEDB; https://www.iedb.org/), maintaining default parameters. The NetMHCpan EL 4.0 prediction method (http://tools.iedb.org/mhci/) was utilized for predicting MHC class I-restricted CD8+ cytotoxic T-lymphocyte (CTL) epitopes, using the reference set of human leukocyte antigen (HLA) alleles and setting the epitope length to 9 (9-mer epitopes). Similarly, the IEDB recommended 2.22 prediction method (http://tools.iedb.org/mhcii/) was used to determine MHC class II-restricted CD4+ helper T-lymphocyte (HTL) epitopes with a length of 15 (15-mer epitopes). The BepiPred 2.0 server (https://services.healthtech.dtu.dk/services/BepiPred-2.0/) was used to predict potential Linear B Lymphocytes (LBL) epitopes of our selected proteins, retaining all default parameters. The top-scored LBL epitopes were selected as potential candidates for further analysis28,29.
Following the initial epitope predictions, the antigenicity of those epitopes was determined using the VaxiJen v2.0 server (https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html). The transmembrane topology was predicted using the TMHMM 2.0 server (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0), and allergenicity was assessed using the AllergenFP (https://ddg-pharmfac.net/AllergenFP/), AllerTOP (https://www.ddg-pharmfac.net/AllerTOP/), and AlgPred2.0 (https://webs.iiitd.edu.in/raghava/algpred2/ige.html) servers30. The toxicity of the epitopes was evaluated using the ToxinPred server (http://crdd.osdd.net/raghava/toxinpred/). The capacity of HTL epitopes to induce IFN-γ, IL-4, and IL-10 was predicted using the IFNepitope (http://crdd.osdd.net/raghava/ifnepitope/), IL4pred (http://crdd.osdd.net/raghava/il4pred/), and IL10pred (http://crdd.osdd.net/raghava/IL-10pred/) servers, respectively31–33. Additionally, epitope conservancy was evaluated using the IEDB epitope conservancy tool (http://tools.iedb.org/conservancy/). Epitopes with high antigenicity, non-toxicity, non-allergenicity, and conservancy were chosen for the final potential vaccine constructs. The selected epitopes were linked using different linkers: AAY linkers for CTL epitopes, GPGPG linkers for HTL epitopes, KK linkers for LBL epitopes, and EAAAK linkers for adjuvants like PADRE and human beta-defensin at the N-terminal end of the vaccine constructs29.
Analyses of the biophysical and structural properties of the vaccine
To ensure the safety and efficacy of the vaccine, antigenicity and allergenicity analyses were conducted. The solubility of the vaccine construct upon expression in Escherichia coli was evaluated using the Protein-Sol server (https://protein-sol.manchester.ac.uk/)34. Biophysical characteristics of the vaccine constructs, such as isoelectric pH, aliphatic and instability index, GRAVY values, hydropathicity, and anticipated half-life, were assessed using the ProtParam tool of the ExPASy server (https://web.expasy.org/protparam/)27. The secondary structure of the final multi-epitope vaccines was predicted using the SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html) and PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/) servers35,36. The trRosetta server (https://yanglab.nankai.edu.cn/trRosetta/) was used to predict the tertiary structure of the vaccine constructs, which were later refined using the GalaxyRefine module of the GalaxyWEB server (http://galaxy.seoklab.org/)37,38. The improved models were validated through the PROCHECK server (https://saves.mbi.ucla.edu/) for Ramachandran plots, ERRAT score plots, and the ProSA-web server (https://prosa.services.came.sbg.ac.at/prosa.php) for Z score plots39,40.
Disulfide engineering of the vaccine construct
Disulfide engineering of the vaccine constructs was performed using the Disulfide by Design 2 server (http://cptweb.cpt.wayne.edu/DbD2/) to investigate the conformational stability of the folded proteins. During the analysis, the Cα-Cβ-Sγ angle was kept at its default value of 114.6° ± 10, and the χ3 angle was set at − 87° or + 97°. Residue pairs with energies lower than 2.5 kcal/mol were chosen and converted to cysteine residues to form disulfide bridges41.
Molecular docking analyses
Initially, the binding affinity of the best CTL epitopes was investigated using a molecular docking approach. The CTL epitopes were docked against three different HLA alleles: HLA-A*0201 (PDB ID: 3mgt), HLA-B*3501 (PDB ID: 3lks), and HLA-B*3508 (PDB ID: 3vfm) using the HDOCK server (http://hdock.phys.hust.edu.cn/). The server reliably asserts receptor and ligand interactions using a hybrid method of template-based and template-free docking. These alleles are prevalent in different fish species42.
Molecular docking analysis was carried out between the vaccine constructs and TLR2 (PDB ID: 3a7c), TLR7 (PDB ID: 5gmf), and TLR8 (PDB ID: 5wyx) receptors to predict their binding affinities and interaction patterns. The TLR2, TLR7, and TLR8 receptor structures were obtained from the RCSB PDB database and prepared using BIOVIA Discovery Studio. The mutant 3D structure of the multi-epitope constructs served as the ligand. The binding affinity between the vaccine constructs and TLRs was calculated using the ClusPro 2.0 server, where docking algorithms identify complexes with favorable surface complementarity, then screen and select those with high electrostatic and desolvation free energies for clustering43. The best-docked complexes were identified based on the lowest energy-weighted score and docking efficiency.
Molecular dynamics simulation studies
From the docking studies, the complexes of vaccine constructs V1 and V2 with TLR7 and TLR8 were taken up for molecular dynamics simulations to gain deeper insights into the stability of the respective TLR-vaccine construct complexes. We conducted 100 ns MD simulations of TLR7-V1, TLR7-V2, TLR8-V1, and TLR8-V2 complexes using the Groningen Machine for Chemical Simulations (Gromacs-2020.4)44,45 program, performed on the HPC cluster at Bioinformatics Resources and Applications Facility (BRAF), C-DAC, Pune. The topologies of the respective TLRs and vaccine constructs in each complex were built using the CHARMM-36 force field46,47 parameters. Each complex was initially solvated with TIP3P water models48 in a dodecahedron simulation box, ensuring the edges of the system and box were 1 nm apart. The systems were neutralized by the addition of appropriate sodium and chloride counter ions to achieve a concentration of 0.15 M.
Subsequently, the systems were energy minimized using the steepest descent energy minimization criteria until the force-constant threshold of 100 kJ/mol/nm was reached. The systems were equilibrated at constant volume, temperature, and pressure conditions: the initial constant volume and temperature of 300 K conditions (NVT) were achieved using a modified Berendsen thermostat49, and then a constant volume and pressure of 1 atm conditions (NPT) were achieved through a Berendsen barostat50. Both NVT and NPT equilibrations were performed for 1 ns each. The final production phase MDS of 100 ns was performed using the same thermostat and the Parrinello-Rahman barostat51, employing a time step of 2 fs and storing the trajectories every 10 ps. The restraint on covalent bonds was obtained using the Linear Constraint Solver (LINCS) algorithm52. Long-range electrostatic energies were obtained using the Particle Mesh Ewald (PME) method53 with a cut-off distance of 1.2 nm. Post-MD simulation, the output trajectories were refined for periodic boundary conditions.
The stability of the respective TLR-vaccine construct complexes was assessed through root mean square deviations (RMSD) in the C-α atoms of TLRs and the vaccine constructs, root mean square fluctuations (RMSF) in the side chain atoms of TLRs and vaccine constructs, and radius of gyration (Rg) analyses. Further, hydrogen bonds between TLRs and vaccine constructs, a crucial non-bonded interaction, were analyzed. The major paths of motion for each TLR-vaccine construct complex were investigated through principal component analysis (PCA)54. In PCA, the covariance matrix for the C-α atoms was constructed and diagonalized to obtain eigenvectors and eigenvalues, where eigenvectors (principal components) signify the motion path, and eigenvalues signify the mean square fluctuation in the C-α atoms of the complex. The first two principal components (PC1 and PC2) were used as reaction coordinates in the Gibbs free energy landscape55 analysis. Residue-wise contacts between TLRs and vaccine constructs were analyzed through the dynamic cross-correlation matrix (DCCM) analysis using the Bio3D package56. In DCCM plots, the blue color gradient indicates negative correlation (less likely) with correlation coefficients of − 1, and the red color gradient indicates positive correlation (more evident) with correlation coefficients of + 1. Lighter shades indicate weaker correlations, and white indicates no correlation. The binding energy between the TLRs and vaccine constructs was approximated using the Molecular Mechanics Poisson–Boltzmann Surface Area continuum solvation (MM-PBSA) method with the gmx_MMPBSA version 1.52 tool57, performed on trajectories from 75 to 100 ns at each 100 ps time step.
Results
Strain and protein selection with biophysical property analyses
The protein sequences of the target proteins were retrieved in FASTA format from the UniProt and NCBI databases. Table 1 provides the accession numbers of the selected protein sequences. All the selected proteins exhibited high antigenicity, stability, and desirable physicochemical properties, as detailed in Supplementary Table S1.
Table 1.
List of the proteins used in this study with their UniProt accession numbers.
| No | Species | Name of the protein | UniProt and NCBI accession no |
|---|---|---|---|
| 01 | Striped Jack nervous necrosis virus | RNA-directed RNA polymerase | Q9QAZ8 (UniProt) |
| 02 | Tiger puffer nervous necrosis virus | D0PQF3 (UniProt) | |
| 03 | Red-spotted grouper nervous necrosis virus | A9Q622 (UniProt) | |
| 04 | Barfin flounder nervous necrosis virus | D0PQF0 (UniProt) | |
| 05 | Seven banded grouper nervous necrosis virus | AIK23437.1 (NCBI) |
Epitope mapping for vaccine construction
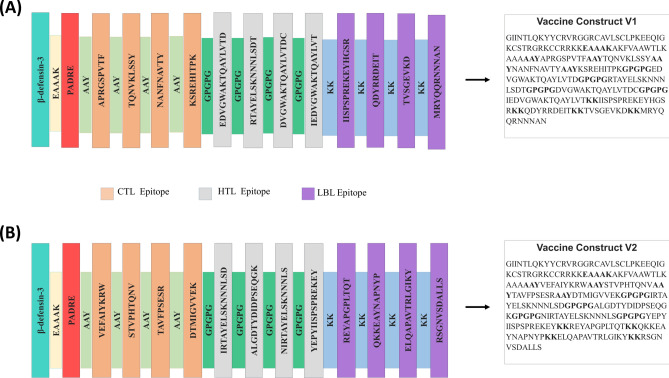
The vaccine development process included predicting T-cell and B-cell epitopes and evaluating their biophysical properties. The epitopes were assessed for high antigenicity, non-allergenicity, non-toxicity, high conservancy, and dissimilarity to the fish proteome. Epitopes meeting these criteria were selected. Additionally, HTL epitopes were further evaluated for their cytokine-inducing potential, and those capable of inducing at least one cytokine were included in the vaccine constructs. Supplementary Table S2 lists the potential CTL, HTL, and LBL epitopes. Based on stringent criteria, 8 CTL, 8 HTL, and 8 LBL epitopes were chosen for vaccine construction, as detailed in Supplementary Table S3. Supplementary Table S4 provides a list of all tools used for epitope prediction, including their specific applications, inputs, and outputs. Specific linkers and adjuvants were used to conjugate the epitopes, and the vaccine designs underwent rigorous testing to confirm their high antigenicity, non-allergenicity, and non-toxicity. Figure 1A,B illustrate schematic and constructive representations of vaccine constructs V1 and V2.
Fig. 1.
Schematic and constructive representation of (A) vaccine construct V1 and (B) vaccine construct V2. Schematic representation illustrates CTL, HTL, LBL epitopes, and all linkers of the vaccines in bar format. Constructive representation shows the sequence of the vaccines.
Analyses of the biophysical and structural properties of the vaccine
Biophysical analyses revealed that vaccine constructs V1 and V2 exhibited favorable qualities such as solubility, stability, and suitability for further examination, as shown in Supplementary Table S5. Secondary structure analysis indicated that random coil was the most prevalent structure. Subsequently, 3D structures of the vaccine constructs were generated, refined, and validated. The ERRAT values for the best models of vaccine constructs V1 and V2 were 94.348 and 96.121, respectively, while the Z scores were − 6.12 and − 5.24, respectively. The Ramachandran plot showed that most residues for both vaccine designs (89.2% for V1 and 90.1% for V2) were in the favored region, with no segments in the disallowed region for either vaccine.
Physicochemical property analysis revealed that vaccine construct V1 had a better theoretical isoelectric point (9.76 vs. 9.68). The aliphatic index was 57.77 for V1 and 64.13 for V2, with both vaccines showing negative GRAVY values. Overall, the biophysical and structural characteristics indicated that both vaccine constructs possessed desirable qualities. Figures 2A–J present the 3D model and validation of both vaccine constructs V1 and V2, while Supplementary Figure S2 provides additional information on the secondary structure of the vaccine constructs.
Fig. 2.
Structure prediction and validation of vaccine construct V1: (A) 3D model, (B) ERRAT quality value, (C) Ramachandran plot, (D) Z-score graph (overall quality), and (E) Z-score graph (sequence position); and of vaccine construct V2: (F) 3D model, (G) ERRAT quality value, (H) Ramachandran plot, (I) Z-score graph (overall quality), and (J) Z-score graph (sequence position).
Disulfide engineering of vaccine constructs
Using the DbD2 server, 17 pairs of amino acid residues for vaccine construct V1 and 21 pairs for V2 were identified as potential candidates for disulfide bond formation. Three pairs of amino acid residues for V1 (THR 5–LYS 8, GLU 27–CYS 41, and CYS 33–ARG 38) and three pairs for V2 (GLY 15–ALA 56, LYS 44–ALA 49, and ALA 244–SER 247) were carefully selected due to their compatibility with standard disulfide bond formation conditions, with energy levels lower than 2.5 kcal/mol. Supplementary Figures S3 and S4 provide further details.
Molecular docking analyses
Initially, molecular docking of the best CTL epitopes with HLA alleles revealed great binding affinity. The epitope VEFAIYKRW displayed the highest docking score among all the epitopes with each of these prevalent HLA alleles. Supplementary Table S6 lists the docking scores between the best CTL epitopes and HLA alleles.
Molecular docking analysis assessed the binding affinity of both vaccine constructs with TLR2, TLR7, and TLR8. Results indicated that both vaccine constructs had significantly higher free binding energy and demonstrated greater docking scores with TLR7. Both vaccine models also showed good binding affinity towards TLR2 and TLR8. Table 2 provides the docking scores obtained from the ClusPro server. Based on the assigned docking score, solubility, and other desired criteria, both vaccine constructs were selected for further Molecular Dynamics simulations using the GROMACS 2020.4 package to evaluate their interaction and binding affinity with TLR7 and TLR8.
Table 2.
Binding affinity between vaccine models and TLRs by ClusPro server.
| TLR | Vaccine | Cluspro docking score (Kcal/mol) |
|---|---|---|
| TLR2 (PDB ID: 3a7c) | V1 | − 806.3 |
| V2 | − 809.3 | |
| TLR7 (PDB ID: 5gmf) | V1 | − 1131.1 |
| V2 | − 946.5 | |
| TLR8 (PDB ID: 5wyx) | V1 | − 902.5 |
| V2 | − 898.9 |
Molecular dynamics studies
Root mean square deviation analysis
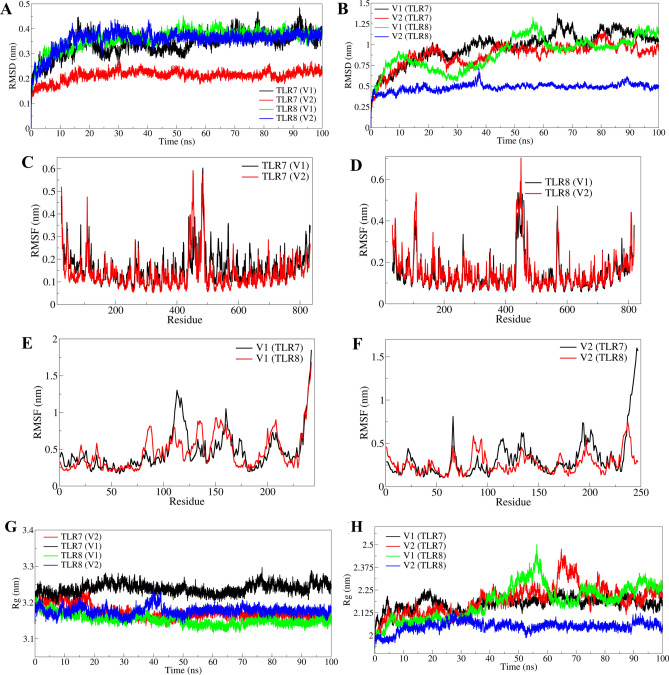
The RMSD in C-α atoms of TLR7 complexed with V2 was notably lower, averaging 0.2120 nm, compared to TLR7 complexed with V1, which averaged 0.3403 nm (Fig. 3A, Table 3). Similarly, TLR8 in complex with V2 exhibited lower RMSD at 0.3566 nm on average, whereas TLR8 in complex with V1 showed higher fluctuations with an average RMSD of 0.8969 nm. Initial fluctuations were observed in TLR7-V1 and TLR8-V1 complexes until around 20 ns, after which TLR7-V1 continued to fluctuate significantly throughout the simulation. In contrast, TLR7-V2 and TLR8-V1 complexes stabilized reasonably after approximately 30 ns.
Fig. 3.
RMSD, RMSF and Rg analyses: (A) RMSD in C-α atoms of TLR7 and TLR8 bound to respective vaccine constructs, (B) RMSD in C-α atoms of vaccine constructs V1 and V2 bound to TLRs, (C) RMSF in side chain atoms of TLR7 bound to respective vaccine constructs, (D) RMSF in side chain atoms of TLR8 bound to respective vaccine constructs, (E) RMSF in side chain atoms of vaccine construct V1 bound to respective TLRs, and (F) RMSF in side chain atoms of vaccine construct V2 bound to respective TLRs. Radius of gyration analysis: (G) Rg in TLRs bound to respective vaccine constructs and (H) Rg in vaccine constructs bound to respective TLRs.
Table 3.
Estimates of averages for different MDS analysis parameters.
| Average (nm) | ||||
|---|---|---|---|---|
| RMSD in Cα atoms | RMSF in side chain atoms | Radius of gyration | Number of hydrogen bonds | |
| TLR7-vaccine construct V1 complex | ||||
| TLR7 | 0.3403 (0.0460) | 0.1606 (0.0702) | 3.2385 (0.0138) | 5.82 |
| V1 | 0.9728 (0.1826) | 0.4667 (0.2623) | 2.1939 (0.0791) | |
| TLR7-vaccine construct V2 complex | ||||
| TLR7 | 0.2120 (0.02129) | 0.1372 (0.0750) | 3.1732 (0.0175) | 9.73 |
| V2 | 0.8800 (0.1431) | 0.3234 (0.2371) | 2.1752 (0.0364) | |
| TLR8-vaccine construct V1 complex | ||||
| TLR8 | 0.3613 (0.0409) | 0.1439 (0.0779) | 3.1526 (0.0145) | 10.29 |
| V1 | 0.8969 (0.1908) | 0.4682 (0.2479) | 2.1853 (0.0940) | |
| TLR8-vaccine construct V2 complex | ||||
| TLR8 | 0.3566 (0.0387) | 0.1518 (0.0910) | 3.1772 (0.0118) | 10.51 |
| V2 | 0.4861 (0.0435) | 0.2659 (0.1167) | 2.0492 (0.0273) | |
Standard deviations in average values are given in parentheses.
The RMSD analysis of vaccine constructs showed that V2 in complex with TLR8 had the lowest average RMSD at 0.4861 nm with minimal deviations (Fig. 3B). V2 in complex with TLR7 also exhibited lower RMSD, averaging 0.3234 nm, with significant stabilization observed after 40 ns. In contrast, V1 showed significant fluctuations throughout the simulation period in complexes with both TLR7 and TLR8, with average RMSD values of 0.4667 nm and 0.4682 nm, respectively.
Root mean square fluctuation analysis
The RMSF of side chain atoms in TLRs and vaccine constructs was analyzed separately. TLR7 in complex with V2 showed the lowest RMSF of side chain atoms, averaging 0.1372 nm, compared to 0.1606 nm in complex with V1 (Fig. 3C). TLR8 in complex with V1 exhibited lower RMSF at 0.1439 nm, whereas in complex with V2 it averaged 0.1518 nm (Fig. 3D). Both TLRs displayed major fluctuations in the loop region spanning residues 425–475, while structured α-helices and β-sheets showed significantly lower RMSF, particularly in complexes with V2.
In vaccine constructs, V1 in complex with TLR8 showed lower RMSF compared to TLR7 (Fig. 3E). Similarly, V2 exhibited lower RMSF in complex with TLR8 compared to TLR7 (Fig. 3F). Notably, V2 in complex with TLR8 had significantly lower RMSF, averaging 0.2659 nm, with minor fluctuations observed except in residues 75–100. Conversely, V2 in complex with TLR7 showed higher RMSF, averaging 0.3234 nm, with significant fluctuations in most residues except in the range 75–100. V1 exhibited similar RMSF values in complexes with TLR7 and TLR8, averaging 0.4667 nm and 0.4682 nm, respectively, with larger fluctuations observed in residues 100–125. These analyses provide insights into the stability and dynamic behavior of the TLR and vaccine construct complexes, highlighting differences between V1 and V2 in their interactions with TLR7 and TLR8.
Radius of gyration analysis
The radius of gyration (Rg) analysis revealed distinct characteristics for the complexes studied. In TLR8 bound to V1, Rg averaged at 3.1526 nm, marking the lowest value observed, whereas TLR7 bound to V1 exhibited the largest Rg at 3.2385 nm (Fig. 3G). Both complexes maintained stable Rg values throughout the simulation. Conversely, TLR7 and TLR8 bound to V2 displayed intermediate Rg values, averaging 3.1732 nm and 3.1526 nm, respectively. Initially, TLR8 bound to V2 showed deviations until approximately 45 ns, stabilizing thereafter, similar to TLR7 bound to V2, which stabilized after the first 20 ns of simulation (Fig. 3G). Analysis of vaccine constructs showed that V2 bound to TLR8 had the lowest Rg with an average of 2.0492 nm, whereas V1 bound to TLR7 had the highest Rg with an average of 2.1939 nm (Fig. 3H). Notably, V2 bound to TLR8 exhibited significantly lower deviations compared to its counterpart V2 bound to TLR7 and V1, which showed more pronounced fluctuations in Rg (Fig. 3H). Specifically, V2 bound to TLR7 experienced notable deviations, particularly during 60–80 ns, averaging 2.1752 nm, while V1 bound to TLR8 also showed significant deviations from 40 to 65 ns, averaging 2.1853 nm.
Hydrogen bond analysis
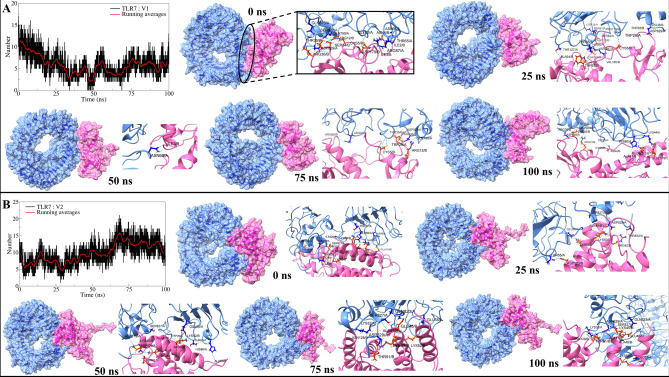
The analysis of interchain hydrogen bonds between TLRs and vaccine constructs provided insights into their binding affinities. Vaccine constructs V1 and V2 formed an average of approximately 5 and 9 hydrogen bonds with TLR7, respectively, with a higher frequency observed during the initial 25 ns simulation period (Fig. 4A). Detailed examination of TLR7-V1 interactions revealed the formation of nine hydrogen bonds initially, including notable pairs such as Thr65-Ile3 and Asp829-Ser34, among others (Supplementary Figure S5). While most bonds were transient, bonds like Asp829-Arg36 and Asp829-Thr35 remained stable throughout the simulation. New bonds emerged at 25 ns, with continued dynamics observed across subsequent trajectories up to 100 ns, indicating evolving interactions involving residues like Thr65-Trp57 and His86-Thr169. Similarly, TLR7 bound to V2 initially showed around 7 hydrogen bonds in the first 60 ns, increasing to approximately 10 bonds thereafter (Fig. 4B). Initial interactions included bonds between residues such as His90-Glu110 and Asp829-Ala88 (Supplementary Figure S6), with stable bonds like Asp829-Lys50 and Ser827-Ala47 persisting throughout the simulation. Additional bonds formed over time, highlighting dynamic interactions within the complex.
Fig. 4.
Hydrogen bond analysis: (A) interchain hydrogen bonds between TLR7 and V1, and (B) Interchain hydrogen bonds between TLR7 and V2. (Surface view and cartoon representation showing interchain hydrogen bonds at different time intervals. TLR surface and cartoon in light blue, vaccine constructs in pink).
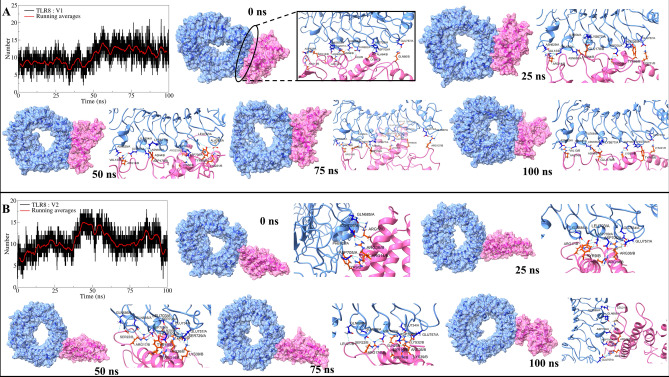
More interchain hydrogen bonds were observed in complexes involving TLR8, with both V1 and V2 forming around 10 hydrogen bonds on average. TLR8 bound to V1 initially exhibited fewer than 10 bonds in the first 50 ns, increasing to occasionally exceed 10 bonds in subsequent trajectories (Fig. 5A). Stable bonds such as Asp705-Lys60 and Glu657-Arg14 persisted throughout the simulation (Supplementary Figure S7), with additional interactions involving residues like Asn656-Asn4 and Glu757-Lys221 evident across all trajectories. Transient bonds were observed initially and intermittently during the simulation, illustrating dynamic binding patterns. TLR8 bound to V2 showed approximately 10 bonds initially, increasing to around 15 bonds from 30 to 60 ns before stabilizing again (Fig. 5B). Stable bonds included interactions like Gln685-Arg17 and Asp705-Arg14 (Supplementary Figure S8), with additional transient bonds appearing in early trajectories and intermittently thereafter, reflecting complex dynamics in hydrogen bond formation.
Fig. 5.
Hydrogen bond analysis: (A) interchain hydrogen bonds between TLR8 and V1, and (B) Interchain hydrogen bonds between TLR8 and V2. (Same color schemes as Fig. 4 for surface view and cartoon representations).
The 25 to 75 ns trajectories showed hydrogen bonds between Ser729-Lys39 and Glu757-Lys32. In addition to these stable hydrogen bonds, some transient hydrogen bonds were seen in the initial trajectory between Asp709-Arg36 and Phe679-Tyr9. The 25 ns trajectory showed the transient hydrogen bonds between Gln757-Lys32, Leu703-Tyr9, Gln685-Cys23, and Gln734-Lys32. The 50 ns trajectory showed transient hydrogen bonds between Glu757-Lys39, Glu734-Arg36, Gln686-Ser22, Ser708-Gln29, and Leu703-Tyr10. While, the 75 ns trajectory showed the transient hydrogen bonds between Glu734-Gln29, Gln685-Ser22, and Glu734-Lys32. The 100 ns trajectory showed a transient hydrogen bond between Gln686-Leu21. These analyses underscore the dynamic nature of interchain interactions between TLRs and vaccine constructs, influencing their stability and binding affinity over simulation time.
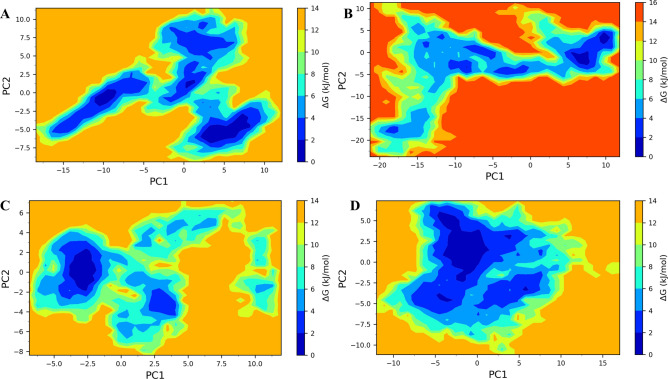
Gibb’s free energy analysis showed that the lowest energy metastable conformations of TLR7 bound to V1 occupy three small energy basins (Fig. 6A). The largest of these energy basins occupy between 2 to 8 on PC1 and -7.5 to -5 on PC2. On the other hand, the TLR8 bound to V1 showed two smaller energy basins, occupying the region between 5 to 10 on PC1 and -5 to 2 on PC2 (Fig. 6B). Both the energy basins were significantly smaller compared to the energy basins of TLR7 bound to V1. TLR7 bound to V2 showed a single energy basin occupied between -3 to -2.5 on PC1 and -2 to 2 on PC2 (Fig. 6C). While TLR8 bound to V2 showed the largest energy basin among all studied systems which occupied between -5 to 0 on PC1 and -2.5 to 5 on PC2 (Fig. 6D).
Fig. 6.
Gibbs free energy landscapes: (A) TLR7-V1 complex, (B) TLR8-V1 complex, (C) TLR7-V2 complex, and (D) TLR8-V2 complex.
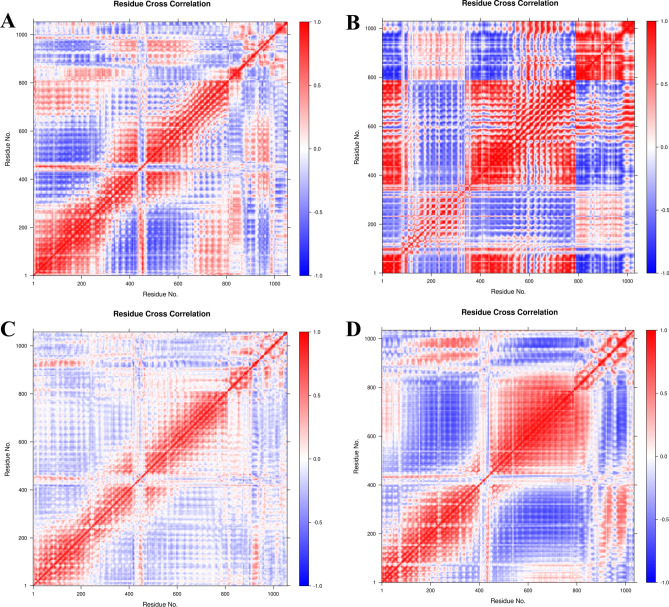
The time-correlated inter-chain and intra-chain residue-to-residue contacts and motions were analyzed from the DCC matrix. It is evident that vaccine construct V1 had a slightly weaker correlation in terms of interchain residue contacts between TLR7 and V1 (Fig. 7A) compared to the more strongly correlated interchain residue contacts between TLR8 and V1 (Fig. 7B). Particularly, almost all the residues of V1 showed a strong positive correlation with the residues of TLR8 in the ranges 150-380 and 600-800. The vaccine construct V2 showed a further weaker correlation between the interchain residues of TLR7 and V2 (Fig. 7C), compared to that of correlations between TLR7 and V1. While, in the case of TLR8 bound to V2, the interchain residue correlation was significantly stronger compared to that of TLR7 bound to V2 (Fig. 7D).
Fig. 7.
DCCM analysis: DCCM plots for (A) TLR7-V1 complex, (B) TLR8-V1 complex, (C) TLR7-V2 complex, and (D) TLR8-V2 complex.
MM-PBSA calculations
The MM-PBSA calculations provided insights into the binding free energies (ΔTOTAL) of each TLR and vaccine construct complex. V2 in complex with TLR8 exhibited the most favorable binding free energy with ΔTOTAL of − 60.68 kcal/mol, while V2 with TLR7 showed a slightly higher ΔTOTAL of − 50.75 kcal/mol (Table 4). V1 bound to TLR7 displayed a higher ΔTOTAL of − 29.59 kcal/mol, whereas V1 with TLR8 showed a lower ΔTOTAL of − 35.67 kcal/mol. Notably, V2 complexes consistently showed lower electrostatic energies (ΔEEL), while nonpolar solvation energies (ΔEPB) varied significantly between TLR8 and TLR7 complexes, indicating distinct energetic contributions to complex stability.
Table 4.
MM-PBSA calculations.
| Energy component (Kcal/mol) | Vaccine construct V1 | Vaccine construct V2 | ||
|---|---|---|---|---|
| TLR7 | TLR8 | TLR7 | TLR8 | |
| ΔVDWAALS | − 99.59 | − 111.68 | − 98.44 | − 55.55 |
| ΔEEL | 776.46 | − 1602.93 | 162.38 | − 1696.92 |
| ΔEPB | − 691.53 | 1681.39 | − 83.49 | 1702.20 |
| ΔENPOLAR | − 14.93 | − 17.53 | − 16.13 | − 10.40 |
| ΔGGAS | 676.87 | − 1714.61 | 63.94 | − 1752.47 |
| ΔGSOLV | − 706.46 | 1663.86 | − 99.62 | 1691.79 |
| ΔTOTAL | − 29.59 | − 50.75 | − 35.67 | − 60.68 |
ΔVDWAALS: van der Waals energy; ΔEEL: electrostatic energies; ΔEPB: polar solvation energy; ΔENPOLAR: nonpolar solvation energy; ΔGGAS = ΔVDWAALS + ΔEEL; ΔGSOLV = ΔEPB + ΔENPOLAR; ΔTOTAL = ΔGSOLV + ΔGGAS.
Discussion
VNN poses a severe threat to aquaculture, particularly in the Mediterranean region, where its devastating impact on fish populations has spurred urgent efforts towards effective prevention and control. The disease, caused by NNVs of the Betanodavirus group within the Nodaviridae family, can lead to mortality rates as high as 100% across various stages of fish production3,4. This study utilized advanced immunoinformatics techniques to explore the feasibility of an in-silico vaccination strategy targeting key proteins crucial to the lifecycle of Betanodaviruses, specifically RNA-directed RNA polymerase (RdRp) proteins, known for their critical roles in viral replication and host cell interaction20. Amino acid sequences of these proteins were carefully sourced from databases like UniProt and NCBI to facilitate epitope mapping, crucial for identifying CTL, HTL, and LBL epitopes capable of eliciting robust immune responses against viral infections. The epitope selection process prioritized criteria such as high antigenicity, non-allergenicity, and non-toxicity to ensure the safety and efficacy of vaccine design. From a comprehensive pool of candidates, eight CTL, eight HTL, and eight LBL epitopes were selected based on their ability to induce cytokine responses and enhance immune system activation, critical for combating viral infections. To boost immunogenicity, selected epitopes were fused with PADRE sequences and human β-defensin (hBD) adjuvants, strategically linked using EAAAK, AAY, GPGPG, and KK linkers to enhance stability and ensure proper folding of the vaccine constructs. Solubility assessments revealed high scores for both vaccine designs (V1 and V2), essential attributes for their effective administration and immune response triggering34,58. Physicochemical analyses further bolstered confidence in the stability and functionality of the vaccine constructs. Both designs demonstrated thermostability at human body temperature, supported by moderate aliphatic indices indicating robust structural integrity (57.77 for V1 and 64.13 for V2)59. Their hydrophilicity, reflected in negative GRAVY values (− 0.771 for V1 and − 0.696 for V2), ensured solubility and bioavailability in aqueous environments, critical for efficient vaccine delivery60. Structural predictions confirmed predominant random coil formations in both V1 and V2, validated by high ERRAT scores (94.348 and 96.121, respectively), surpassing benchmarks set by previous vaccine designs for related pathogens, highlighting superior quality and stability61. Moreover, the Ramachandran plot showed 89.2% of V1 and 90.1% of V2 residues in the favored region, with no residues in the disallowed area, indicating both vaccine designs are stable and well-structured62. Molecular docking studies provided critical insights into the interaction dynamics between CTL epitopes and common fish HLA alleles (HLA-A0201, HLA-B3501, and HLA-B*3508), revealing strong binding affinities crucial for efficient antigen presentation and immune activation42. Docking simulations with TLR2, TLR7, and TLR8 elucidated robust interactions, particularly notable for TLR7 and TLR8 in recognizing viral single-stranded RNA, a hallmark feature of Betanodaviruses63–66. Molecular dynamics simulations offered mechanistic insights into the stability of vaccine-TLR complexes, showing minimal RMSD deviations over time67–69. RMSF analyses underscored consistent residue fluctuations in vaccine constructs bound to TLRs, indicating stable binding and conformational integrity70–74. Radius of gyration (Rg) analyses further supported compact and stable structures in most TLR-vaccine complexes, with TLR8-V2 interactions particularly noted for stability75. Interchain hydrogen bond analyses highlighted strong stabilizing interactions within TLR8-V2 complexes, enhancing their stability and affinity76,77. PCA-based Gibbs free energy analysis showed that the TLR8-V2 complex has more metastable conformations and a larger energy basin, indicating better stability36. DCCM analysis also revealed stronger and more positively correlated contacts in the TLR8-V2 complex compared to V1, suggesting greater stability for V229. MM-PBSA analyses also quantified V2's superior binding affinity to TLR7 and TLR8, characterized by favorable ΔTotal energies driven by low van der Waals and electrostatic energies22. Overall, both designed vaccines, V1 and V2, demonstrated high solubility, stability, and immunogenicity, with strong binding affinities to fish HLA alleles and TLRs. However, V2 demonstrated a stronger stabilization propensity and superior binding affinity to TLR8. These findings suggest their potential to provide broad-range protection against multiple Betanodavirus strains, offering a promising approach for controlling VNN in aquaculture.
Conclusion
The study addresses the pressing issue of VNN in aquaculture caused by the NNVs. Using immunoinformatics, we developed an in silico multi-epitope vaccination strategy targeting RdRp proteins critical for Betanodavirus lifecycle. This comprehensive approach, encompassing epitope prediction, vaccine design, and molecular dynamics simulations, lays a robust foundation for practical application. While promising in combatting VNN across diverse Betanodavirus strains, further validation in animal models is essential to confirm efficacy. This research advances vaccine design methodologies, offering a versatile platform in immunoinformatics to tackle emerging diseases, antigenic variability, and tailored immunization needs in aquaculture.
Supplementary Information
Acknowledgements
The authors wholeheartedly dedicate this study to the passionate student researchers of Bangladesh, whose tireless dedication and unwavering commitment inspire us all. Their relentless pursuit of knowledge and excellence, often in the face of challenges, continues to elevate the nation's name on the global stage. This work stands as a tribute to their selfless determination and the boundless potential they embody.
Author contributions
A.T.M. conceptualized the study. N.A.R., R.B.P., and A.T.M. worked in methodology. N.A.R., A.T.M., and R.B.P. wrote the main manuscript and prepared the figures. Y.A.S., S.Y., I.H.R., U.S.R., M.S.M., S.A.J., T.A., M.U.R., D.B., M.H.A., and S.M.M. reviewed the manuscript. M.H.U. and R.U.N supervised the study. All the authors approved the manuscript.
Data availability
Data is provided within the manuscript or supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Abu Tayab Moin, Nurul Amin Rani, Yasin Arafath Sharker and Tanbir Ahammed
Contributor Information
Abu Tayab Moin, Email: tayabmoin786@gmail.com.
S. M. Murshid Ul Alam, Email: murshid.geb@cu.ac.bd.
Rajesh B. Patil, Email: rajesh.patil@sinhgad.edu
Rashed Un Nabi, Email: mrnimscu@yahoo.com.
Mohammad Helal Uddin, Email: mhuddincu@yahoo.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72116-5.
References
- 1.Salin, K. R. & Arome, A. G. Aquaculture and the environment: Towards sustainability. In Sustainable Aquaculture 1–62 (Springer, Cham, 2018). [Google Scholar]
- 2.Barange M. Fishery and aquaculture statistics. FAO yearbook Fishery and Aquaculture Statistics= FAO Annuaire Statistiques des Peches et de l'Aquaculture= FAO Anuario Estadisticas de Pesca y Acuicultura. pp. I-82. (2018).
- 3.Shetty, M., Maiti, B., Shivakumar Santhosh, K., Venugopal, M. N. & Karunasagar, I. Betanodavirus of marine and freshwater fish: Distribution, genomic organization, diagnosis and control measures. Indian J.Virol.23, 114–123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandín, I. & Souto, S. Betanodavirus and VER disease: a 30-year research review. Pathogens9(2), 106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callinan, R. Diseases of Australian native fishes. Fish Diseases Refresher Course for Veterinarians Proceedings. (1988).
- 6.Bajpai, V., Pragyan, D., Suman, K., Mohanty, J. & Sahoo, P. K. Viral diseases in Indian freshwater and marine water pisciculture. Curr. Sci.122, 261–280 (2022). [Google Scholar]
- 7.Fauquet, C. M., Mayo, M. A., Maniloff, J., Desselberger, U. & Ball, L. A. Virus taxonomy: VIIIth report of the international committee on taxonomy of viruses (Academic Press, Cambridge, 2005). [Google Scholar]
- 8.Mori, K.-I. et al. Properties of a new virus belonging to Nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology.187(1), 368–371 (1992). [DOI] [PubMed] [Google Scholar]
- 9.Zorriehzahra, M. J., Adel, M., Dadar, M., Ullah, S. & Ghasemi, M. Viral nervous necrosis (VNN) an emerging disease caused by Nodaviridae in aquatic hosts: Diagnosis, control and prevention: A review. Iran. J. Fish. Sci.18(1), 30–47 (2019). [Google Scholar]
- 10.Zorriehzahra, M. J. Viral nervous necrosis disease 673–703 (Elsevier, 2020). [Google Scholar]
- 11.Narang, P. K. et al. Genome-based identification and comparative analysis of enzymes for carotenoid biosynthesis in microalgae. World J. Microbiol. Biotechnol.38, 1–22 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Narang, P. K. et al. Functional annotation and sequence-structure characterization of a hypothetical protein putatively involved in carotenoid biosynthesis in microalgae. South Afr. J. Bot.141, 219–226 (2021). [Google Scholar]
- 13.Dey, J., Mahapatra, S. R., Raj, T. K., Misra, N. & Suar, M. Identification of potential flavonoid compounds as antibacterial therapeutics against Klebsiella pneumoniae infection using structure-based virtual screening and molecular dynamics simulation. Mol. Divers.10.1007/s11030-023-10738-z (2023). [DOI] [PubMed] [Google Scholar]
- 14.Mahapatra, S. R. et al. The potential of plant-derived secondary metabolites as novel drug candidates against Klebsiella pneumoniae: Molecular docking and simulation investigation. South Afr. J. Bot.149, 789–797 (2022). [Google Scholar]
- 15.Ma, J., Bruce, T. J., Jones, E. M. & Cain, K. D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms.7(11), 569 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yong, C. Y., Yeap, S. K., Omar, A. R. & Tan, W. S. Advances in the study of nodavirus. PeerJ.5, e3841 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dey, J. et al. Designing of multi-epitope peptide vaccine against Acinetobacter baumannii through combined immunoinformatics and protein interaction–based approaches. Immunol. Res.71(4), 639–662 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valero, Y., Olveira, J. G., López-Vázquez, C., Dopazo, C. P. & Bandín, I. BEI inactivated vaccine induces innate and adaptive responses and elicits partial protection upon reassortant Betanodavirus infection in Senegalese sole. Vaccines9(5), 458 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, W., Hsu, C.-H., Chang, C.-Y., Chen, H.-H. & Lin, C.-S. Immune response against grouper nervous necrosis virus by vaccination of virus-like particles. Vaccine24(37–39), 6282–6287 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Toffolo, V. et al. Phylogeny of Betanodaviruses and molecular evolution of their RNA polymerase and coat proteins. Mol. Phylogenetics Evolut.43(1), 298–308 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Welsh, R. M. & Fujinami, R. S. Pathogenic epitopes, heterologous immunity and vaccine design. Nat. Rev. Microbiol.5(7), 555–563 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moin, A. T. et al. Immunoinformatics approach to design novel subunit vaccine against the Epstein-Barr virus. Microbiol. Spectr.10(5), e01151-e1222 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dey, J. et al. Designing a novel multi-epitope vaccine to evoke a robust immune response against pathogenic multidrug-resistant Enterococcus faecium bacterium. Gut Pathogens14(1), 21 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dey, J. et al. Exploring Klebsiella pneumoniae capsule polysaccharide proteins to design multiepitope subunit vaccine to fight against pneumonia. Expert Rev. Vaccines21(4), 569–587 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Doytchinova, I. A. & Flower, D. R. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform.8(1), 1–7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou, H. & Zhou, Y. Predicting the topology of transmembrane helical proteins using mean burial propensity and a hidden-Markov-model-based method. Protein Sci.12(7), 1547–1555 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasteiger, E. et al.Protein identification and analysis tools on the ExPASy server (Springer, Totowa, 2005). [DOI] [PubMed] [Google Scholar]
- 28.Kim, Y. et al. Immune epitope database analysis resource. Nucleic Acids Res.40(W1), W525–W530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moin, A. T. et al. An immunoinformatics and extended molecular dynamics approach for designing a polyvalent vaccine against multiple strains of Human T-lymphotropic virus (HTLV). Plos one18(9), e0287416 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Dimitrov, I., Naneva, L., Doytchinova, I. & Bangov, I. AllergenFP: allergenicity prediction by descriptor fingerprints. Bioinformatics30(6), 846–851 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Dhanda, S. K., Gupta, S., Vir, P. & Raghava, G. Prediction of IL4 inducing peptides. Clin. Dev. Immunol.2013, 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagpal, G. et al. Computer-aided designing of immunosuppressive peptides based on IL-10 inducing potential. Sci. Rep.7(1), 42851 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhanda, S. K., Vir, P. & Raghava, G. P. Designing of interferon-gamma inducing MHC class-II binders. Biol. Direct8(1), 1–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebditch, M., Carballo-Amador, M. A., Charonis, S., Curtis, R. & Warwicker, J. Protein–Sol: a web tool for predicting protein solubility from sequence. Bioinformatics33(19), 3098–3100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geourjon, C. & Deleage, G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics11(6), 681–684 (1995). [DOI] [PubMed] [Google Scholar]
- 36.McGuffin, L. J., Bryson, K. & Jones, D. T. The PSIPRED protein structure prediction server. Bioinformatics16(4), 404–405 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Du, Z. et al. The trRosetta server for fast and accurate protein structure prediction. Nat. Protoc.16(12), 5634–5651 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Ko, J., Park, H., Heo, L. & Seok, C. GalaxyWEB server for protein structure prediction and refinement. Nucleic Acids Res.40(W1), W294–W297 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laskowski, R., MacArthur, M. & Thornton, J. PROCHECK: Validation of protein-structure coordinates. Int. Tables Crystallogr.25, 722–725 (2006). [Google Scholar]
- 40.Wiederstein, M. & Sippl, M. J. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res.35(suppl_2), W407–W10 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig, D. B. & Dombkowski, A. A. Disulfide by design 2.0: a web-based tool for disulfide engineering in proteins. BMC Bioinform.14(1), 1–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Islam, S. I. et al. Designing a novel mRNA vaccine against Vibrio harveyi infection in fish: an immunoinformatics approach. Genom. Inform.20(1), e11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kozakov, D. et al. The ClusPro web server for protein–protein docking. Nat. Protoc.12(2), 255–278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham, M. J. et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX1, 19–25 (2015). [Google Scholar]
- 45.Berendsen, H. J., van der Spoel, D. & van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun.91(1–3), 43–56 (1995). [Google Scholar]
- 46.Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput.8(9), 3257–3273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanommeslaeghe, K. et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem.31(4), 671–690 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorgensen, W. L. & Madura, J. D. Quantum and statistical mechanical studies of liquids. 25. Solvation and conformation of methanol in water. J. Am. Chem. Soc.105(6), 1407–13 (1983). [Google Scholar]
- 49.Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys.10.1063/1.2408420 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Berendsen, H. J., Postma, J., Van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys.81(8), 3684–90 (1984). [Google Scholar]
- 51.Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys.52(12), 7182–7190 (1981). [Google Scholar]
- 52.Hess, B., Bekker, H., Berendsen, H. J. & Fraaije, J. G. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem.18(12), 1463–1472 (1997). [Google Scholar]
- 53.Petersen, H. G. Accuracy and efficiency of the particle mesh Ewald method. J. Chem. Phys.103(9), 3668–3679 (1995). [Google Scholar]
- 54.Sittel, F., Jain, A. & Stock, G. Principal component analysis of molecular dynamics: On the use of Cartesian vs. internal coordinates. J. Chem. Phys.141(1), 014111 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Maisuradze, G. G., Liwo, A. & Scheraga, H. A. Relation between free energy landscapes of proteins and dynamics. J. Chem. Theory Comput.6(2), 583–595 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grant, B. J., Rodrigues, A. P., ElSawy, K. M., McCammon, J. A. & Caves, L. S. Bio3d: An R package for the comparative analysis of protein structures. Bioinformatics22(21), 2695–2696 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Valdés-Tresanco, M. S., Valdés-Tresanco, M. E., Valiente, P. A. & Moreno, E. gmx_MMPBSA: A new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Comput.17(10), 6281–6291 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Lee, S. & Nguyen, M. T. Recent advances of vaccine adjuvants for infectious diseases. Immune Netw.15(2), 51–57 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikai, A. Thermostability and aliphatic index of globular proteins. J. Biochem.88(6), 1895–1898 (1980). [PubMed] [Google Scholar]
- 60.Kyte, J. & Doolittle, R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol.157(1), 105–132 (1982). [DOI] [PubMed] [Google Scholar]
- 61.Messaoudi, A., Belguith, H. & Ben, H. J. Homology modeling and virtual screening approaches to identify potent inhibitors of VEB-1 β-lactamase. Theor. Biol. Med. Model.10, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hooft, R. W., Sander, C. & Vriend, G. Objectively judging the quality of a protein structure from a Ramachandran plot. Bioinformatics.13(4), 425–430 (1997). [DOI] [PubMed] [Google Scholar]
- 63.Rani, N. A. et al. Development of multi epitope subunit vaccines against emerging carp viruses Cyprinid herpesvirus 1 and 3 using immunoinformatics approach. Sci. Rep.14(1), 11783 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lama, R., Pereiro, P., Figueras, A. & Novoa, B. Zebrafish as a vertebrate model for studying nodavirus infections. Front. Immunol.13, 863096 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Costa, J. Z. & Thompson, K. D. Understanding the interaction between Betanodavirus and its host for the development of prophylactic measures for viral encephalopathy and retinopathy. Fish Shellfish Immunol.53, 35–49 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Martínez-Espinoza, I. & Guerrero-Plata, A. The relevance of TLR8 in viral infections. Pathogens11(2), 134 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitera, J. W. Expected distributions of root-mean-square positional deviations in proteins. J. Phys. Chem. B.118(24), 6526–6530 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Moin, A. T. et al. A computational approach to design a polyvalent vaccine against human respiratory syncytial virus. Sci. Rep.13(1), 1–20 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nada, H., Elkamhawy, A. & Lee, K. Identification of 1H-purine-2, 6-dione derivative as a potential SARS-CoV-2 main protease inhibitor: molecular docking, dynamic simulations, and energy calculations. PeerJ.10, e14120 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Botos, I., Segal, D. M. & Davies, D. R. The structural biology of Toll-like receptors. Structure19(4), 447–459 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sudeshna Panda, S. et al. Investigation on structural prediction of pectate lyase enzymes from different microbes and comparative docking studies with pectin: the economical waste from food industry. Geomicrobiol. J.39(3–5), 294–305 (2022). [Google Scholar]
- 72.Manavalan, B., Basith, S. & Choi, S. Similar structures but different roles–an updated perspective on TLR structures. Front. Physiol.2, 41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martínez, L. Automatic identification of mobile and rigid substructures in molecular dynamics simulations and fractional structural fluctuation analysis. PloS one10(3), e0119264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amin Rani, N. et al. Designing a polyvalent vaccine targeting multiple strains of varicella zoster virus using integrated bioinformatics approaches. Front. Microbiol.14, 1291868 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lobanov, M. Y., Bogatyreva, N. & Galzitskaya, O. Radius of gyration as an indicator of protein structure compactness. Mol. Biol.42, 623–628 (2008). [PubMed] [Google Scholar]
- 76.Yu, H., Wang, M.-j, Xuan, N.-x, Shang, Z.-c & Wu, J. Molecular dynamics simulation of the interactions between EHD1 EH domain and multiple peptides. J. Zhejiang Univ. Sci. B.16(10), 883 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pace, C. N. et al. Contribution of hydrogen bonds to protein stability. Protein Sci.23(5), 652–661 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.