Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematological malignancy that expresses high levels of the enzyme aldo-keto reductase family 1 member C3 (AKR1C3). To exploit this finding, we developed a novel prodrug, ACHM-025, which is selectively activated by AKR1C3 to a nitrogen mustard DNA alkylating agent. We show that ACHM-025 has potent in vivo efficacy against T-ALL patient-derived xenografts (PDXs) and eradicated the disease in 7 PDXs. ACHM-025 was significantly more effective than cyclophosphamide both as a single agent and when used in combination with cytarabine/6-mercaptopurine. Notably, ACHM-025 in combination with nelarabine was curative when used to treat a chemoresistant T-ALL PDX in vivo. The in vivo efficacy of ACHM-025 directly correlated with AKR1C3 expression levels, providing a predictive biomarker for response. Together, our work provides strong preclinical evidence highlighting the potential of ACHM-025 as a targeted and effective therapy for aggressive forms of T-ALL.
Subject terms: Targeted therapies, Preclinical research, Drug development, Cancer genetics
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy and remains one of the most common causes of death from disease in children despite improvements in treatment that have seen cure rates increase to 90% [1, 2]. Pediatric ALL can be divided into two major subtypes, B-cell ALL (B-ALL) and T-cell ALL (T-ALL), with T-ALL accounting for approximately 15% of cases [3]. T-ALL is an aggressive malignancy with historically inferior outcomes compared with B-ALL, but with intensified risk-adapted chemotherapy protocols outcomes for T-ALL and B-ALL are now comparable [3, 4]. However, relapsed T-ALL has a poor survival rate of approximately 50% in children, with considerably worse outcomes for patients commensurate with short duration of remission [5, 6]. Therefore, a significant unmet need remains for new targeted therapies that can effectively treat relapsed/refractory T-ALL.
AKR1C3 is a member of the aldo-keto reductase (AKR) superfamily of NADPH-dependent oxidoreductases, that reduce aldehydes and ketones to their corresponding alcohols [7, 8]. A wide variety of substrates are potential targets of AKR enzymes, including simple carbohydrates, steroid hormones, endogenous prostaglandins and chemotherapeutics [9, 10]. The AKR1C3 enzyme is a 17β-hydroxysteroid dehydrogenase involved in the regulation of steroid hormones, and also functions as a prostaglandin synthase [8]. AKR1C3 is overexpressed in T-ALL compared to both B-ALL and healthy human tissues and is considered a biomarker for disease burden where patients with refractory/recurrent disease show increased AKR1C3 expression [9, 11, 12]. Similarly, AKR1C3 expression was shown to be higher in treatment-resistant T-ALL patients, compared to treatment-sensitive T-ALL patients [10]. However, AKR1C3 inhibitors under preclinical development have only had limited efficacy in phase I/II clinical trials [8]. An alternative approach is to exploit pathologically high AKR1C3 expression using AKR1C3-activated prodrugs.
Previous generation AKR1C3-activated prodrugs include PR-104 and OBI-3424. PR-104 is a water-soluble phosphate ester ‘pre-prodrug’ that is hydrolyzed in vivo to form the prodrug, PR-104A, a primary metabolite which is activated by AKR1C3 to predominantly produce the nitrogen mustard product PR-104H [12]. However, PR-104A is not selectively activated by AKR1C3, since it was originally designed to also undergo activation under hypoxic conditions [13]. PR-104 displayed significant efficacy in phase I/II clinical trials in adult leukemia, although there was insufficient activity for advancement to registration [14]. Alternately, OBI-3424 activation is a multi-step process that requires AKR1C3 reduction of a nitro group to a hydroxylamine followed by fragmentation of a ‘trigger’ moiety to release the cytotoxin, bis-aziridine phosphoramidate, a cytotoxin class which is not widely used in humans (compared to nitrogen mustards) and has an unknown mechanism of DNA crosslink formation [11]. OBI-3424 is currently being evaluated in a phase II clinical trial in relapsed/refractory T-ALL (ClinicalTrials.gov Identifier: NCT04315324). Significant justification exists to expand the class of AKR1C3-activated prodrugs to additional small molecules with improved physicochemical properties.
In the present study we have developed and evaluated the in vitro/in vivo activity of a new prodrug, ACHM-025, designed to address the short-comings of PR-104 and OBI-3424. ACHM-025 does not require the phosphate ester pre-prodrug strategy of PR-104 to achieve acceptable solubility and avoids all ‘mixed-mechanism’ hypoxic activation, being specifically activated by AKR1C3-mediated reduction, that results in the direct formation of a potent nitrogen mustard, thus negating the need for a second trigger fragmentation event. We show that ACHM-025 is highly effective as a single agent across a panel of T-ALL patient-derived xenografts (PDXs) in vivo including those derived from relapsed/refractory patients. ACHM-025 was more effective than cyclophosphamide (CP) when used in combination with cytarabine/6-mercaptopurine in a consolidation-type regimen. Moreover, ACHM-025 was also remarkably effective in combination with nelarabine where it was able to eradicate the disease in vivo.
Materials and methods
PDXs and cell lines
The generation and validation of HCT116 cells (American Type Culture Collection, ATCC) overexpressing the human AKR1C family members (AKR1C1-AKR1C4) was previously described [12]. ALL PDXs were previously established in nonobese diabetic/SCID (NOD.Cg-Prkdcscid/J, NOD/SCID) or NOD/SCID/IL2 receptor g–negative (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, NSG) female mice as previously described [15]. The generation and validation of lentivirally transduced ALL-11 PDXs overexpressing AKR1C3 (ALL-11/AKR1C3) or empty vector (ALL-11/EV) was previously described [16]. The generation and STR validation of acute myeloid leukemia (AML), neuroblastoma (NB), Ewing sarcoma (ES), diffuse midline glioma (DMG), hepatoblastoma (HB), and ependymoma (EPD) PDXs was previously described [17, 18].
In vitro and ex vivo cytotoxicity assays
HCT116 cells were treated with ACHM-025 for 96 h, then cell viability was assessed by sulforhodamine B staining as previously described [13]. ALL PDXs were cultured in AIM V medium (Thermo Fisher Scientific) at a cell concentration previously optimized for each PDX (1–5 × 106 cells per mL). Cytotoxicity was assessed by Resazurin assay (made in house) or Cell Titer-Glo (Promega). Assays were performed in three biological experiments and half-maximal inhibitory concentration (IC50) was calculated by interpolation of nonlinear regression curves calculated by GraphPad Prism 10 software.
RNA-sequencing analysis
RNA-sequencing data for AKR1C1-AKR1C4 gene expression of 90 ALL PDXs can be accessed at https://pedcbioportal.org. AKR1C3 gene expression of 90 ALL PDXs was previously published [11]. RNA-sequencing data analysis and expression profiling for 759 patients from the ZERO childhood cancer precision medicine program was performed as previously reported in Wong et al. [19] RNA-sequencing data and processing for the 90 ALL PDX models was performed as reported in Rokita et al. [20] Expression data of the patient and PDX data was integrated using R (v1.4.2).
Quantitative real-time reverse transcription-PCR (qRT-PCR)
RNA from ALL PDX cells was isolated and purified using the RNeasy RNA isolation kit (Qiagen) according to the manufacturer’s instructions. First-strand complementary DNA was synthesized using MMLV reverse transcriptase (Thermo Fisher Scientific). TaqMan primers and probes for AKR1C3 (Hs00366267_m1) and EF1α (elongation factor 1α; Hs00265885_g1) were purchased from Thermo Fisher Scientific and qRT-PCR was carried out in duplicate under cycling conditions according to the manufacturer’s instructions. AKR1C3 expression was normalized to EF1α in each sample using the ΔCt method.
Immunoblotting
PDX cells were lysed in RIPA lysis buffer. The membranes were probed with primary monoclonal antibodies; mouse anti-AKR1C3 (#A6229, Sigma-Aldrich) or rabbit anti-actin (#A2066, Sigma). AKR1C3 protein expression of a panel of 6 T-ALL and 6 B-ALL PDXs was previously published (shown in Supplementary Fig. S3) [11].
AKR1C3 intracellular flow cytometry
Cells were incubated with eBiosience Fixable Viability Dye eFluor 450 (Thermo Fisher Scientific), incubated with eBiosience Intracellular Fixation buffer (Thermo Fisher Scientific) and then Permeabilization buffer (Thermo Fisher Scientific). The following day, cells were incubated in BD Pharmingen Human BD Fc Block (BD Biosciences) and stained with anti-AKR1C3 antibody (#A6229, Sigma-Aldrich). The median fluorescence intensity (MFI) for each sample was normalized to MFI of the secondary antibody only controls of each sample to obtain the Ratio of MFI (RFI) values.
In vivo efficacy studies
All studies using mice received prior approval from the UNSW Sydney Animal Care and Ethics Committee. Drug efficacy was assessed by mouse event-free survival (EFS), where the difference between EFS of vehicle control (C) and drug treated (T) cohorts (T-C and T/C) was evaluated. Drug efficacy was also assessed by stringent objective response measures (ORMs) as defined in Supplementary Table S1 [21]. Efficacy studies were performed as either conventional studies (n = 6 mice per treatment) or Single Mouse Testing (SMT) studies (n = 1 mouse per treatment) [22]. In conventional studies, additional mice were used to assess leukemic infiltration in the femoral bone marrow, spleen, kidney and liver at Day 0 (n = 3 mice) and Day 28 or event (n = 3 mice), whichever occurred first.
Statistical analyses
Statistical differences between data were determined using unpaired t-tests with Welch’s correction, or if data were not normally distributed, using Mann–Whitney U-tests. EFS curves were compared using Log-rank (Mantel–Cox) test (conservative) and data were analyzed using GraphPad Prism 10 software. Significance was inferred from tests with p values < 0.05. For in vivo combination efficacy studies, therapeutic enhancement was considered if the EFS of mice treated with the combination was significantly greater than those induced by the single agent treatment cohorts [23].
Results
ACHM-025 is a new AKR1C3-activated prodrug with enhanced ex vivo selectivity
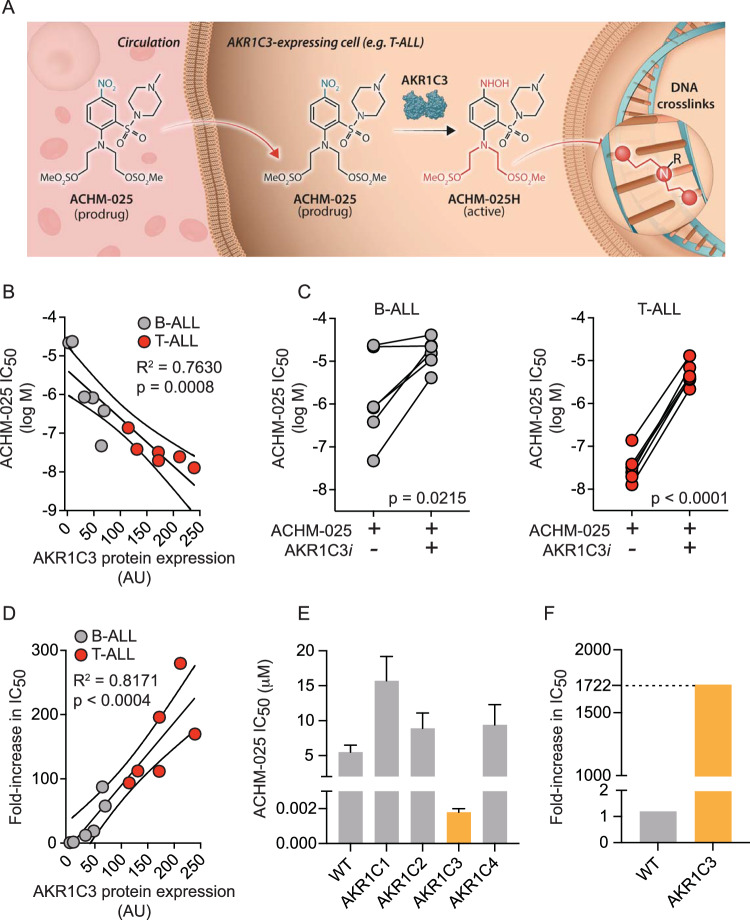
ACHM-025 (((2-((4-methylpiperazin-1-yl)sulfonyl)-4-nitrophenyl)azanediyl)bis(ethane-2,1-diyl) dimethanesulfonate) is a novel prodrug selectively activated by AKR1C3 in the presence of NADPH to form the nitrogen mustard DNA alkylating agent, ACHM-025H (Fig. 1A). The nitrogen mustard, ACHM-025H, is able to bis-alkylate DNA via the amino group of two guanine bases, forming DNA intra- or inter-strand crosslinks (Fig. 1A). CP is a DNA alkylating prodrug, currently used in the treatment of pediatric ALL (Supplementary Fig. S1). Unlike ACHM-025, CP is activated by liver enzymes in a multi-step process that culminates in fragmentation to form the nitrogen mustard DNA alkylating agent, phosphoramide mustard (Supplementary Fig. S1) [24]. Importantly, and unlike earlier AKR1C3-activated prodrugs such as OBI-3424 [11] and PR-104 [12, 13, 25], ACHM-025 forms the active agent ACHM-025H in a single step (Supplementary Figs. S1 and S2).
Fig. 1. Ex vivo activity and selectivity of ACHM-025.
A Mechanism of activation of prodrug ACHM-025: the nitro group of ACHM-025 is reduced by AKR1C3 in the presence of NADPH to a hydroxylamine group, forming the active agent ACHM-025H in a single step (nitro group highlighted blue and hydroxylamine group highlighted red). ACHM-025H is a nitrogen mustard, which is able to bis-alkylate DNA (nitrogen mustard highlighted red). B–D A panel of 6 T-ALL PDXs (red) and 6 B-ALL PDXs (gray) were treated ex vivo with ACHM-025± an AKR1C3 specific inhibitor (AKR1C3i). B ACHM-025 IC50 values plotted against AKR1C3 protein expression as measured by immunoblotting. C ACHM-025 IC50 values ± AKR1C3i against B-ALL (gray) and T-ALL (red) PDXs. D Fold-increase in IC50 (ACHM-025 + AKR1C3i/ACHM-025 alone) versus AKR1C3 protein expression. E, F HCT116 parental (WT) and HCT116 cells overexpressing the AKR1C family members, AKR1C1-AKR1C4, were treated in vitro with ACHM-025 ± an AKR1C3 specific inhibitor (AKR1C3i). E ACHM-025 IC50 values. F Fold-increase in IC50 (ACHM-025 + AKR1C3i/ACHM-025 alone) of HCT116 WT and HCT116 overexpressing AKR1C3. B–F Each data point represents the mean of at least three independent experiments. p-values were corrected for multiple comparisons. B, D Centre and curved lines represent linear regression and 95% confidence interval, respectively.
To evaluate the activity of ACHM-025 compared with OBI-3424 and PR-104A, ex vivo cytotoxicity assays were carried out on 12 PDXs (6 T-ALL PDXs and 6 B-ALL PDXs) with varying levels of AKR1C3 protein expression. The IC50 values for ACHM-025, OBI-3424 and PR-104A all displayed a significant inverse correlation with basal AKR1C3 protein expression (Fig. 1B, Supplementary Fig. S3). The strongest inverse correlation was observed with PR-104A (R2 = 0.8166; p < 0.0004; Supplementary Fig. S3A), followed by ACHM-025 (R2 = 0.7630; p = 0.0008; Fig. 1B), then OBI-3424 (R2 = 0.4834; p = 0.048; Supplementary Fig. S3B). However, PR-104A showed a modest interdependence (slope) and reduced overall dose potency, where the median IC50 values were 26.6 µM for B-ALL and 1.8 µM for T-ALL PDXs (Supplementary Table S2). Comparatively, the median IC50 values for ACHM-025 were 840 nM for B-ALL and 29 nM for T-ALL. The median IC50 values for OBI-3424 were 60 nM for B-ALL and 8 nM for T-ALL, although the overall correlation with AKR1C3 expression was weak (Supplementary Table S2).
To further assess the selectivity of ACHM-025 for AKR1C3, all 12 PDXs were pre-treated ex vivo with the isoform-specific AKR1C3 inhibitor, SN34037, prior to treatment with ACHM-025 (Supplementary Fig. S4) [16]. Inhibition of AKR1C3 enzymatic activity prevented ACHM-025 cytotoxicity with a significant increase in IC50 in both B-ALL and T-ALL PDXs (Fig. 1C, Supplementary Table S2). Furthermore, the fold-increase in IC50 (ACHM-025 + SN34037/ACHM-025 alone) showed a strong and significant correlation with basal AKR1C3 protein expression (R2 = 0.8171; p < 0.0004; Fig. 1D). Further, ACHM-025 showed selectivity for activation by the AKR1C3 isoform, compared to AKR1C1, AKR1C2 and AKR1C4 in HCT116 cells (Fig. 1E, Supplementary Table S3). Incubation of HCT116/AKR1C3 overexpressing cells with SN34037 caused a 1,700-fold increase in IC50 (Fig. 1F, Supplementary Table S3). Moreover, ACHM-025 ex vivo cytotoxicity showed a significant inverse correlation with AKR1C3 mRNA expression (R2 = 0.8720; p < 0.0004; Supplementary Fig. S5A), compared to isoforms AKR1C1, AKR1C2 and AKR1C4 (Supplementary Fig. S5B–D). Taken together, these data show that ACHM-025 has improved specificity for AKR1C3 and dose potency compared to OBI-3424 and PR-104A, respectively.
ACHM-025 displays profound single agent in vivo efficacy against chemoresistant T-ALL PDXs
We next sought to determine the in vivo efficacy of ACHM-025 as a single agent. Initial in vivo pharmacokinetic analysis of ACHM-025 in murine plasma and brain tissue was evaluated at 3 dose levels (5, 10, 25 mg/kg) and 6 time points (0.25–2 h) via intraperitoneal injection (IP). ACHM-025 was cleared rapidly from the plasma, with Cmax ranging 1.6–9.3 µM across the dose levels tested, with a total plasma exposure (AUC0-inf) ranging 0.5–3.3 µmol-h/L (Supplementary Fig. S6A, Supplementary Table S4). ACHM-025 concentration in brain tissue was low, although the peak concentration (0.14 µM, 0.25 h, 25 mg/kg ACHM-025) exceeded the IC50 values across all T-ALLs (median 0.029 µM; Supplementary Tables S2 and S4). ACHM-025 displayed limited protein binding, with 43.1% and 35.4% bound to mouse and human plasma proteins, respectively. The maximum dose of ACHM-025 evaluated (25 mg/kg via IP weekly ×3) was well tolerated in naïve immunodeficient mice including no major hematology effects (Supplementary Fig. S6B, Supplementary Tables S5, S6) [26]. Therefore, the IP dose of 25 mg/kg was selected as the maximum dose to assess the in vivo efficacy of ACHM-025 in murine PDX models.
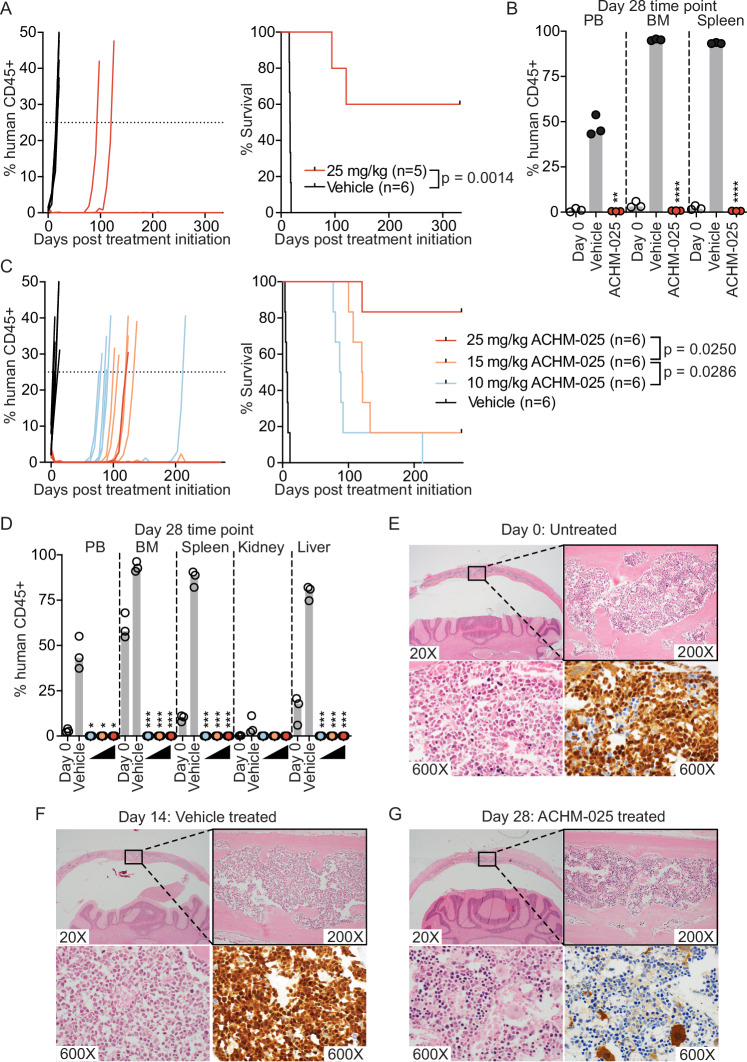
The in vivo efficacy of ACHM-025 (25 mg/kg, IP Days 0, 7, 14) was first evaluated against the aggressive and chemoresistant T-ALL PDX, ALL-31, which has high AKR1C3 expression (Supplementary Table S7). ACHM-025 caused a rapid reduction of human leukemia cells in the murine peripheral blood (PB) to <1%, which was maintained for 10 consecutive weeks with a concomitant significant increase in event-free survival (EFS) compared to vehicle control treated mice (vehicle median EFS = 16.7 days vs. ACHM-025 median EFS > 332 days, p = 0.0014) (Fig. 2A, Supplementary Table S8). Remarkably, 3 out of 5 mice treated with ACHM-025 did not relapse over the >300-day observation period following the last treatment. When these mice were humanely killed on day 332, flow cytometry analysis revealed no detectable human leukemia cells in the PB, bone marrow (BM), spleen, kidney or liver (data not shown). Three sentinel mice included in the study for Day 28 time point analyses also revealed no detectable human leukemia cells in 3 out of 3 mice in the PB, BM or spleen, which was 14 days after the last ACHM-025 treatment when analyzed as either the % human CD45+ cells versus total mouse cells (Fig. 2B) or the % human CD45+ cells versus mouse CD45+ cells (Supplementary Fig. S7A).
Fig. 2. ACHM-025 displays profound single agent efficacy against chemoresistant T-ALL PDXs in vivo.
A Mice engrafted with a diagnosis/refractory T-ALL PDX (ALL-31) were treated in vivo with ACHM-025 (red; 25 mg/kg, IP Days 0, 7, 14) or vehicle (black). Engraftment of individual mice for each treatment, showing the % human CD45+ over time (left). Mouse EFS, where dashes denote censored mice that did not reach event (right). B Engraftment (% human CD45+ cells versus all mouse cells) of ALL-31 in PB, BM and spleen at Day 0 (open circles), vehicle treated at event (black circles), 25 mg/kg ACHM-025 treated at Day 28 (red circles). C Mice engrafted with a relapse/refractory T-ALL PDX (ALL-8) were treated in vivo with 25 mg/kg ACHM-025 (red; IP Days 0, 7, 14), 15 mg/kg ACHM-025 (orange; IP Days 0, 7, 14), 10 mg/kg ACHM-025 (blue; IP Days 0, 7, 14) or vehicle (black). Engraftment of individual mice for each treatment, showing the % human CD45+ over time (left). Mouse EFS, where dashes denote censored mice that did not reach event (right). D Engraftment (% human CD45+ cells versus all mouse cells) of ALL-8 in PB, BM and organs at Day 0 (open circles), vehicle treated at event (black circles), 10 mg/kg ACHM-025 treated at Day 28 (blue circles), 15 mg/kg ACHM-025 treated at Day 28 (orange circles), and 25 mg/kg ACHM-025 treated at Day 28 (red circles). E, F Engraftment of ALL-8 in the skull demonstrated with routine H&E staining (upper panels and lower left panels) and nuclear TdT immunohistochemical staining (lower right panels). E Untreated mouse at Day 0. F Vehicle treated mouse at event (Day 14). G Mouse treated with 10 mg/kg ACHM-025 (IP Days 0, 7, 14) at Day 28. Widespread leukemic infiltration of the calvarial bone marrow is present in the untreated (E) and vehicle treated (F) mice; in contrast, leukemic infiltration is absent in the 10 mg/kg ACHM-025 treated mouse at Day 28 (G). B, D Gray bars represent the median of 3 mice. Statistical differences between vehicle treated mice at event and ACHM-025 treated mice at Day 28 were determined using unpaired t-tests with Welch’s correction (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Given the remarkable single agent efficacy of ACHM-025, we next undertook an in vivo dose-response study against an additional aggressive T-ALL PDX (ALL-8) (Supplementary Table S7). All three doses of ACHM-025 (10, 15 or 25 mg/kg, IP Days 0, 7, 14) induced significantly prolonged leukemia regressions (maintained complete responses, MCRs) (Fig. 2C, Supplementary Tables S8, S9) and a clear dose response (Fig. 2C, Supplementary Table S8). Similar to ALL-31, 5 out of 6 mice treated with the highest dose of 25 mg/kg had no detectable human leukemia cells in the PB at 250 days after the last treatment (Fig. 2C). Analysis of mice at Day 28, 14 days after the last treatment there was no detectable human leukemia cells in the PB, BM, spleen, kidney or liver at any of the three doses of ACHM-025 when expressed relative to total (Fig. 2D) or CD45+ (Supplementary Fig. 7B) murine cells.
Since ALL can cause relapse via the central nervous system (CNS), we histopathologically analyzed murine skull and brain samples at Day 0 (pre-treatment), vehicle treated at Day 14 (event), and 10 mg/kg ACHM-025 treated and harvested at Day 28. Hematoxylin and eosin (H&E) and terminal deoxynucleotidyl transferase (TdT) staining showed that, while leukemic infiltration was observed in the calvarial bone marrow at Day 0 (Fig. 2E) and more extensively at Day 14 of the vehicle treated mice (Fig. 2F), there was no observable leukemic infiltration at Day 28 for ACHM-025 treated mice (Fig. 2G). Therefore, we reasoned that relapse is likely to occur through measurable/minimal residual disease (MRD) cells residing in the BM or elsewhere that were not detected at the level of sensitivity of flow cytometry.
To determine whether disease relapse could be due to acquired drug resistance via down-regulation of AKR1C3, we analyzed AKRIC3 mRNA expression in leukemia cells isolated from the spleens of vehicle treated mice and mice treated with ACHM-025 that subsequently relapsed. There was no significant difference in AKR1C3 expression at the mRNA level suggesting that relapse was not due to outgrowth of low AKR1C3 expressing cells (Supplementary Fig. S7C). Taken together, these data show impressive single agent efficacy of ACHM-025 against aggressive and chemoresistant T-ALL PDXs.
A mouse “clinical” trial to evaluate AKR1C3 expression as a biomarker for in vivo ACHM-025 efficacy
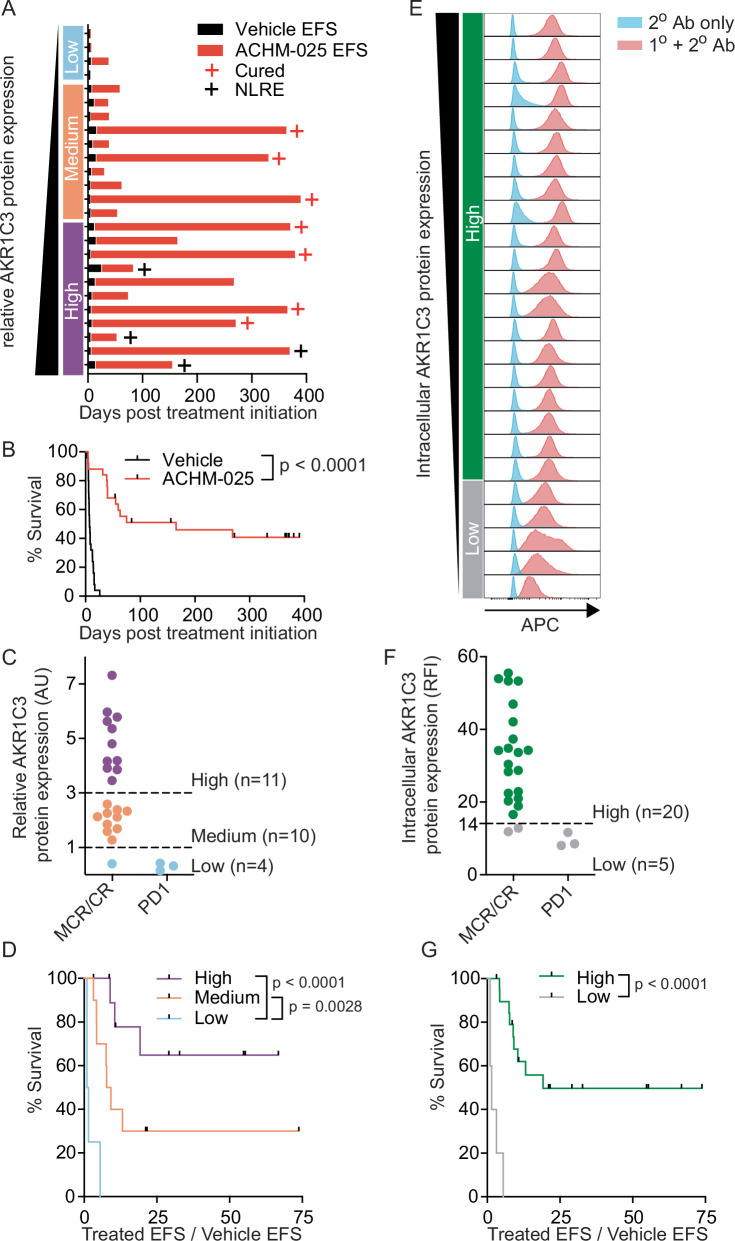
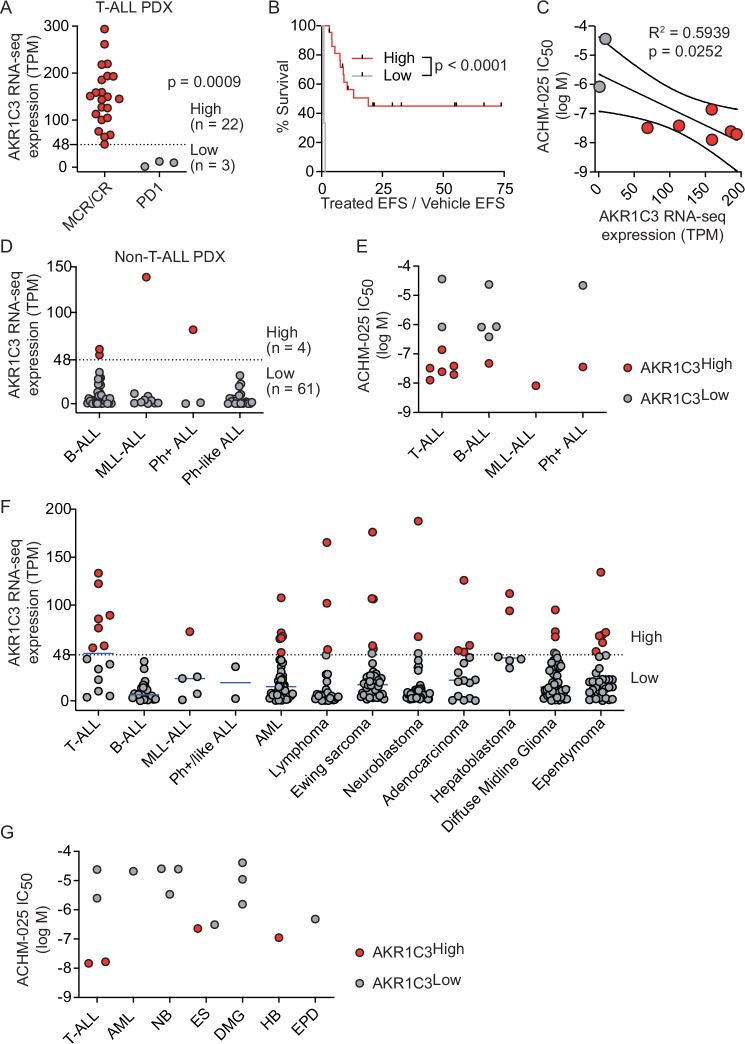
We next evaluated the relationship between AKR1C3 expression and in vivo ACHM-025 activity using a single mouse trial (SMT) format (one vehicle control treated mouse, one drug treated mouse) [22] across an extended panel of 25 T-ALL PDXs that better addresses the impact of genetic heterogeneity in pediatric ALL on drug response (Supplementary Fig. S8A). All 25 PDXs treated with vehicle control reached event (huCD45+ >25% in the PB) between 3.1 and 26 days following treatment initiation (Fig. 3A, Supplementary Table S9, Supplementary Fig. S8B). Of the ACHM-025 treated mice, 7/25 PDXs did not reach event during the 250 day monitoring period following the final treatment (Fig. 3A, Supplementary Table S9, Supplementary Fig. S8B). In 22/25 PDXs there were no human leukemia cells detected in the murine PB for at least two weeks after treatment initiation, and all of these mice achieved CRs or MCRs (Supplementary Fig. S9A, Supplementary Table S9). When all vehicle vs. all treated mice were evaluated, ACHM-025 significantly increased EFS compared to vehicle control mice (p < 0.0001; Fig. 3B). Moreover, the 3 PDXs in which only Progressive Disease 1 (PD1) was achieved all expressed minimal levels of AKR1C3 mRNA and protein (Supplementary Fig. S9B–D, Supplementary Table S9).
Fig. 3. A single mouse trial across a panel of T-ALL PDXs identified the levels of AKR1C3 expression as a biomarker for in vivo ACHM-025 efficacy.
A Event free survival (EFS) for 25 T-ALL PDXs treated in vivo with vehicle (black bars) or ACHM-025 (red bars; 25 mg/kg, IP Days 0, 7, 14). Red crosses denote PDXs that did not reach event, black crosses denote non-leukemia related events (NLRE). PDXs are listed in order of increasing AKR1C3 protein expression; low (blue, n = 4), medium (orange, n = 10) or high (purple, n = 11). B Survival of 25 T-ALL PDXs treated in vivo with vehicle (black) or ACHM-025 (red; 25 mg/kg, IP Days 0, 7, 14). C Objective response measures versus AKR1C3 protein expression; high (purple, n = 11), medium (orange, n = 10) or low (blue, n = 4). D Leukemia growth delay (Treated EFS/Vehicle EFS) of PDXs with high (purple, n = 11), medium (orange, n = 10) or low (blue, n = 4) AKR1C3 protein expression. E Basal AKR1C3 intracellular expression as measured by flow cytometry for 25 T-ALL PDXs. Histograms for AKR1C3 unconjugated primary antibody (blue) and APC-conjugated secondary antibody (red). PDXs are listed in order of decreasing AKR1C3 intracellular expression; high (green, n = 20) or low (gray, n = 5). F Objective response measures versus AKR1C3 intracellular expression according to relative fluorescent intensity (RFI) cut-off at 14 as measured in E; high (green, n = 20) or low (gray, n = 5). G Leukemia growth delay (Treated EFS / Vehicle EFS) of PDXs with high (green, n = 20) or low (gray, n = 5) AKR1C3 intracellular expression. B, D, G Significance calculated by Log-rank (Mantel-Cox). Dashes denote censored PDXs that did not reach event or are NLREs.
Comparing the panel of 25 T-ALL PDXs that achieved an objective response (CR or MCR) and those that did not (PD1) with AKR1C3 protein expression, ACHM-025 was significantly less effective against T-ALL PDXs with low AKR1C3 expression compared to those with medium (p = 0.0028) or high (p < 0.0001) AKR1C3 expression (Fig. 3C, D). This was also found using intracellular flow cytometry for measuring AKR1C3 expression with ACHM-025 significantly less effective against T-ALL PDXs below minimum AKR1C3 intracellular expression values required for an objective response (CR or MCR) (p < 0.0001; Fig. 3E–G).
Furthermore, a B-ALL PDX (ALL-11) that had been lentivirally transduced to overexpress AKR1C3 (ALL-11/AKR1C3; AKR1C3high) [27] exhibited an ex vivo ACHM-025 IC50 (13.1 nM) that was more reflective of AKR1C3high T-ALL PDXs (median IC50 29 nM) compared with empty vector control cells (ALL-11/EV; AKR1C3low), in which the IC50 (1261 nM) was more consistent with AKR1C3low B-ALL PDXs (median IC50 840 nM) (Supplementary Fig. S10A, B, Supplementary Table S2). Similarly, the AKR1C3 inhibitor, SN34037, caused >20-fold shift in the ACHM-025 IC50 value in ALL-11/AKR1C3 cells relative to ALL-11/EV cells (Supplementary Fig. S10B, Supplementary Table S2), while ACHM-025 was profoundly more effective in vivo against ALL-11/AKR1C3 than ALL-11/EV (EFS 104 and 49.1 days, respectively; Supplementary Fig. S10C, Supplementary Table S9). Taken together, these findings support AKR1C3 expression as a biomarker for ACHM-025 activity both in vitro and in vivo, and suggest that the potency of ACHM-025 is dependent on AKR1C3 expression levels.
ACHM-025 significantly improves standard-of-care consolidation therapy compared to cyclophosphamide
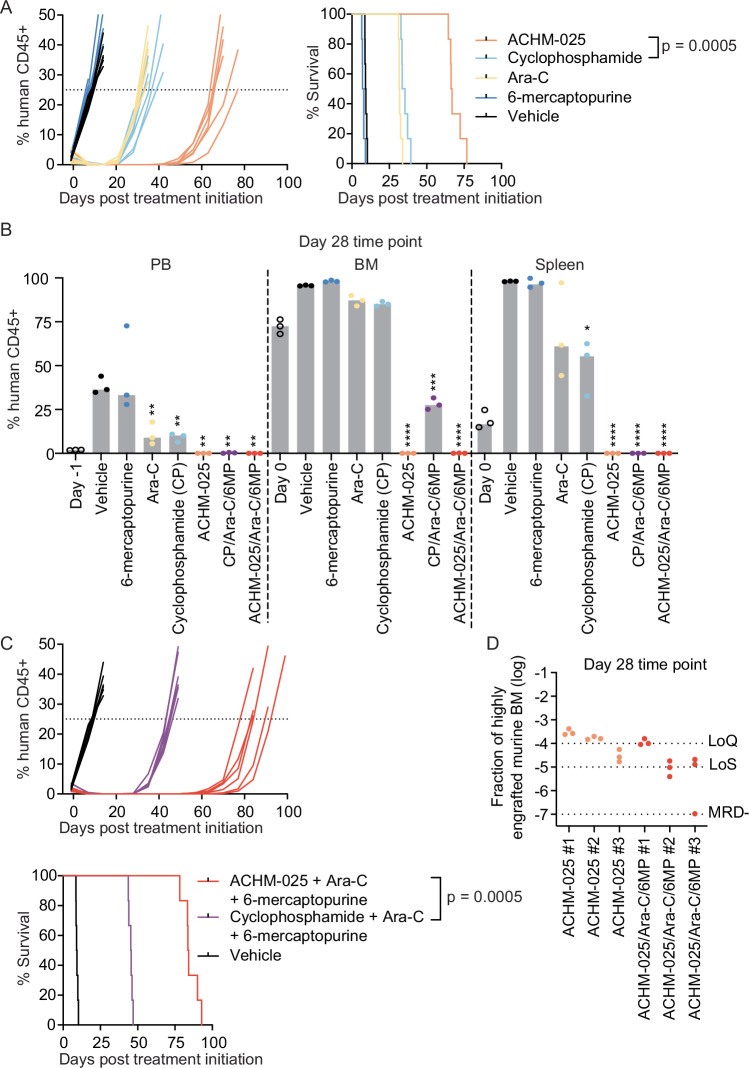
Both ACHM-025 and CP are DNA bis-alkylating prodrugs, with CP included in standard-of-care consolidation therapy for ALL in combination with, amongst other drugs, cytarabine (Ara-C) and 6-mercaptopurine (6MP). Therefore, we compared the combination in vivo efficacy of ACHM-025/Ara-C/6MP with CP/Ara-C/6MP against ALL-8. ACHM-025 (5 mg/kg, IP Days 0, 7) or CP (75 mg/kg, IP Days 0, 7) combined with Ara-C (12.5 mg/kg, IP Days 0–4, 7–11) and 6MP (12.5 mg/kg, IP Days 0–4, 7–11). Combination dosing and single agents used as twice the dose were well tolerated in naïve immunodeficient mice (Supplementary Fig. S11A, Supplementary Tables S10, S11).
With the exception of 6MP, all single agents caused significant delays in the progression of ALL-8, with treated minus control (T-C) median EFS values of 22.5 days for Ara-C (p = 0.0005), 25.3 days for CP (p = 0.0005) and 57.4 days for ACHM-025 (p = 0.0005) (Table 1, Fig. 4A). While ACHM-025 decreased the levels of human leukemia cells in the murine PB to <1% for six consecutive weeks and elicited an MCR, CP was only able to do so for two weeks (CR; Table 1, Fig. 4A, Supplementary Fig. S11B). Flow cytometric analysis of a separate cohort of mice at Day 28 post treatment initiation showed that only ACHM-025 reduced human leukemia levels in the PB, BM and spleen to undetectable levels at Day 28 (Fig. 4B).
Table 1.
In vivo activity of ACHM-025 in combination against a relapsed/refractory T-ALL PDX (ALL-8).
| Treatment | Dose (mg/kg) | EFS (days) | T-C (days) | T/C (days) | p-value (vs vehicle) | p-value (vs treatment) | ORM |
|---|---|---|---|---|---|---|---|
| Vehicle | 0 | 9.2 | – | – | – | – | – |
| Ara-C | 12.5 | 31.7 | 22.5 | 3.4 | 0.0005 | – | CR |
| 6MP | 12.5 | 7.4 | −1.8 | 0.8 | 0.0053 | – | PD1 |
| CP | 75 | 34.5 | 25.3 | 3.7 | 0.0005 | 0.0005 | CR |
| ACHM-025 | 5 | 66.6 | 57.4 | 7.2 | 0.0005 | MCR | |
| CP + Ara-C + 6MP | – | 45.5 | 36.3 | 4.9 | 0.0005 | 0.0005 | MCR |
| ACHM-025 + Ara-C + 6MP | – | 83.9 | 74.7 | 9.1 | 0.0005 | MCR | |
| ACMH-025 | 10 | 207 | 198 | 22.5 | 0.0005 | 0.0005 | MCR |
| Nelarabine | 125 | 28.4 | 19.1 | 3.1 | 0.0005 | SD | |
| ACHM-025 + Nelarabine | – | >293 | >284 | >31.8 | 0.0005 | – | MCR |
6MP 6-mercaptopurine, CP cyclophosphamide, CR complete response, EFS median event free survival, MCR maintained complete response, ORM median objective response measure, PD1 progressive disease 1, p-value log-rank (Mantel–Cox) test (conservative), SD stable disease, T-C treated EFS – control EFS, T/C treated EFS/control EFS.
Fig. 4. ACHM-025 significantly improves standard-of-care consolidation therapy compared to CP.
A Mice engrafted with a relapse/refractory T-ALL PDX (ALL-8) were treated in vivo with vehicle (black), 6-mercaptopurine (dark blue; 12.5 mg/kg, IP Days 0–4, 7–11), Ara-C (yellow; 12.5 mg/kg, IP Days 0–4, 7–11), CP (light blue; 75 mg/kg, IP Days 0, 7), or ACHM-025 (orange; 5 mg/kg, IP Days 0, 7). Engraftment of individual mice for each treatment, showing the % human CD45+ over time (left) and mouse EFS (right). B Engraftment (% human CD45+ cells versus mouse CD45+ cells) of ALL-8 in PB, BM and spleen at Day 0 (open circles), vehicle treated (black circles; event), 6-mercaptopurine (dark blue; event), Ara-C (yellow; Day 28), CP (light blue; Day 28), ACHM-025 (orange; Day 28), the combination of Ara-C, 6-mercaptopurine and CP (purple; Day 28), or the combination of Ara-C, 6-mercaptopurine and ACHM-025 (red; Day 28). Gray bars represent the median of 3 mice. Statistical differences between vehicle treated mice at event and drug treated mice at Day 28 were determined using unpaired t-tests with Welch’s correction (**p < 0.01; ***p < 0.001; ****p < 0.0001). C Mice engrafted with ALL-8 were treated in vivo with vehicle (black), the combination of Ara-C, 6-mercaptopurine and CP (purple) or the combination of Ara-C, 6-mercaptopurine and ACHM-025 (red). Engraftment of individual mice for each treatment, showing the % human CD45+ over time (upper) and mouse EFS (lower). D Minimal residual disease in the bone marrow at Day 28 for ACHM-025 treated mice (orange circles; n = 3) and ACHM-025/Ara-C/6-mercaptopurine treated mice (red circles; n = 3). Samples displayed as the fraction of a highly engrafted (96% huCD45+) murine bone marrow (BM). Technical triplicates of n = 3 mice per treatment, where dotted lines represent the limit of quantification (LoQ), limit of sensitivity (LoS) and MRD-. A, C Significance calculated by Log-rank (Mantel–Cox).
We next compared the 3-drug combinations and found that ACHM-025/Ara-C/6MP induced sustained remissions in the mice (7 consecutive weeks) compared with only two weeks for CP/Ara-C/6MP (Fig. 4C, Supplementary Fig. S11C), resulting in a median EFS that was 38 days longer (p = 0.0005; Table 1, Fig. 4C). Furthermore, ACHM-025/Ara-C/6MP significantly improved the clearance of leukemia cells from the BM compared with CP/Ara-C/6MP, which still had 28% huCD45+ cells in the BM at Day 28 (Fig. 4B). Using clinically validated and patient-specific quantitative-PCR based minimal residual disease (MRD) testing [28, 29], there was a trend for lower MRD levels in the ACHM-025/Ara-C/6MP combination compared to ACHM-025 alone (Fig. 4D), albeit with most values at borderline limits of quantification.
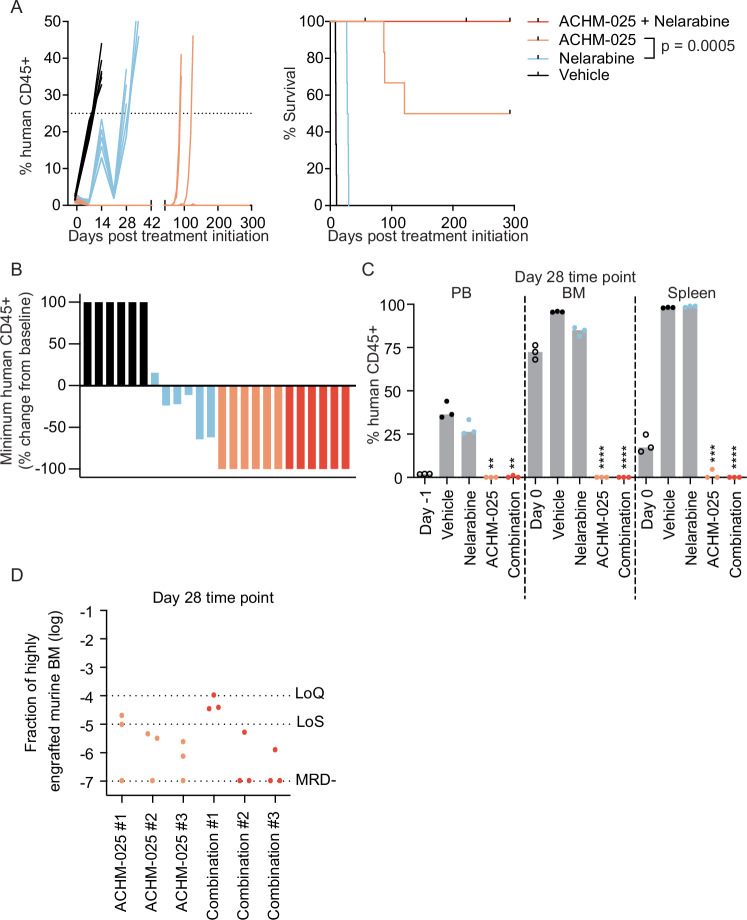
ACHM-025 in combination with nelarabine is highly effective against a chemoresistant T-ALL PDX
Currently, the only chemotherapy FDA and EMA approved for the treatment of relapsed/refractory T-ALL is the nucleoside analog, nelarabine [30]. Therefore we next compared the in vivo efficacy of nelarabine and ACHM-025 as single agents and in combination against ALL-8. Due to the exceptional efficacy of ACHM-025 as a single agent, its dose was lowered to better assess any ACHM-025/nelarabine combinatorial effect. The combination of ACHM-025 (10 mg/kg, IP Days 0, 7, 14) and nelarabine (125 mg/kg, IP Days 0–4, 14–18) was well tolerated in naïve immunodeficient mice (Supplementary Fig. S12A).
As a single agent, nelarabine was able to significantly delay the progression of leukemia in mice engrafted with ALL-8 but only achieved stable disease (SD) (Table 1, Fig. 5A, B). However, nelarabine did not significantly decrease human leukemia levels in the PB, BM or spleen at Day 28 (Fig. 5C, Supplementary Fig. S12B). In contrast, both ACHM-025 and the ACHM-025/nelarabine combination profoundly delayed leukemia progression and induced MCRs, with no mice relapsing in the combination cohort of ACHM-025/nelarabine over the observable period of 300 days following the final day of treatment (Table 1, Fig. 5A, B, Supplementary Fig. S12B). At Day 28 there was no detectable human leukemia cells in the BM of ACHM-025 and ACHM-025/nelarabine treated mice by flow cytometry (Fig. 5C) and although BM samples were MRD positive, they were not within the quantitative range of the PCR-based assay (Fig. 5D). Finally, once all surviving mice treated with the ACHM-025/nelarabine combination were euthanized we found no detectable human leukemia cells in the PB, BM or other organs (spleen, kidney and liver) as measured by flow cytometry (>275 days after the last treatment, data not shown). Taken together, the combination of ACHM-25 and nelarabine appeared to eradicate this chemoresistant T-ALL PDX compared to each single agent alone.
Fig. 5. ACHM-025 in combination with nelarabine is highly effective against chemoresistant T-ALL.
A Mice engrafted with a relapse/refractory T-ALL PDX (ALL-8) were treated in vivo with vehicle (black), nelarabine (blue; 125 mg/kg, IP Days 0–4, 14–18), ACHM-025 (orange; 10 mg/kg, IP Days 0, 7, 14), or the combination ACHM-025/nelarabine (red). Engraftment of individual mice for each treatment, showing the % human CD45+ over time (left). Mouse EFS, where dashes denote censored mice that did not reach event or are NLREs (right). Significance calculated by Log-rank (Mantel–Cox). B Percentage change from baseline of the minimum human CD45+ values in ALL-8 PB engraftment from treatment initiation of individual mice (n = 6 per treatment group). C Engraftment (% human CD45+ cells versus mouse CD45+ cells) of ALL-8 in the PB, BM and spleen at Day 0 (open circles), vehicle treated at event (black circles), nelarabine treated at Day 28 (blue circles), ACHM-025 treated at Day 28 (orange circles), and ACHM-025/nelarabine treated at Day 28 (red circles). Gray bars represent the median of 3 mice. Statistical differences between vehicle treated mice at event and drug treated mice at Day 28 were determined using unpaired t-tests with Welch’s correction (**p < 0.01; ***p < 0.001; ****p < 0.0001). D Minimal residual disease in the bone marrow at Day 28 for ACHM-025 treated (orange circles; n = 3) and ACHM-025/nelarabine treated (red circles; n = 3). Samples displayed as the fraction of a highly engrafted (96% huCD45+) murine bone marrow (BM). Technical triplicates of n = 3 mice per treatment, where dotted lines represent the limit of quantification (LoQ), limit of sensitivity (LoS) and MRD-.
Evaluation of AKR1C3 expression as a biomarker for ACHM-025 efficacy in other pediatric cancers
Based on the promising results of the in vivo ACHM-025 SMT study against 25 T-ALL PDXs, we interrogated a recently published large cohort of 1,335 pediatric T-ALL patients to reveal potential subtype-specific AKR1C3 mRNA expression [31]. AKR1C3 expression appeared consistent across most T-ALL subtypes, with notably lower expression in the SPI1, TLX1 and TLX3 subtypes (Supplementary Fig. S13). We next evaluated whether AKR1C3 expression could be used as a biomarker for ACHM-025 efficacy in other pediatric cancers. Similar to Fig. 3, we separated the panel of 25 T-ALL PDXs into those that achieved an objective response (CR or MCR) and those that did not (PD1) following ACHM-025 in vivo treatment and assessed AKR1C3 RNA-seq expression (Fig. 6A). The minimum AKR1C3 RNA-seq expression value required for an objective response was determined as transcripts per million (TPM) > 48, where T-ALL PDXs above this threshold were designated AKR1C3high (Fig. 6A). As we have shown with AKR1C3 protein and intracellular expression, ACHM-025 was significantly more effective in vivo against T-ALL PDXs with high AKR1C3 expression compared to those with low AKR1C3 expression (p < 0.0001; Fig. 6B). Importantly, AKR1C3 RNA-seq expression significantly correlated with ex vivo ACHM-025 efficacy against T-ALL PDXs (Fig. 6C).
Fig. 6. ACHM-025 displays ex vivo activity against multiple pediatric cancers with elevated AKR1C3 expression.
A Objective response measures of 25 T-ALL PDXs treated in vivo with ACHM-025 (25 mg/kg, IP Days 0, 7, 14) versus AKR1C3 mRNA expression as measured by RNA-seq; high (red, n = 22) or low (gray, n = 3). B Leukemia growth delay (Treated EFS / Vehicle EFS) of PDXs with high (red, n = 22) or low (gray, n = 3) AKR1C3 mRNA expression (RNA-seq). Significance calculated by Log-rank (mantel-Cox). Dashes denote censored PDXs that did not reach event or are NLREs. C A panel of 8 T-ALL PDXs with high (red, n = 6) or low (gray, n = 2) AKR1C3 mRNA expression were treated ex vivo with ACHM-025. ACHM-025 IC50 values plotted against AKR1C3 mRNA expression (RNA-seq). Centre and curved lines represent linear regression and 95% confidence interval, respectively. D AKR1C3 mRNA expression of 65 ALL PDXs as measured by RNA-seq; high (red, n = 4) or low (gray, n = 61). The dashed line represents the minimum AKR1C3 expression level to be classified as AKR1C3high (TPM > 48). E ACHM-025 IC50 values of ALL PDXs with high (red, n = 9) or low (gray, n = 7) AKR1C3 mRNA expression (RNA-seq). F AKR1C3 mRNA expression of 371 pediatric patient samples as measured by RNA-seq. Each data point represents AKR1C3 expression of an individual patient sample, where the solid blue line represents the median AKR1C3 expression of each type of cancer. The dashed line represents the minimum AKR1C3 expression level to be classified as AKR1C3high (TPM > 48). G ACHM-025 IC50 values of T-ALL, acute myeloid leukemia (AML), neuroblastoma (NB), Ewing sarcoma (ES), diffuse midline glioma (DMG), hepatoblastoma (HB), and ependymoma (EPD) PDXs. PDXs denoted AKR1C3 high (red, n = 4) or low (gray, n = 11) as measured by qRT-PCR (CNRQ). Each data point represents the mean of three technical repeats. (C, E) Each data point represents the mean of at least three independent experiments.
To evaluate whether AKR1C3 expression could be used as a biomarker for ACHM-025 ex vivo efficacy against non-T-ALL PDXs, we applied the AKR1C3 RNA-seq threshold identified in Fig. 6A (TPM > 48) to a panel of 65 non-T-ALL PDXs (Fig. 6D). Although only 6% of non-T-ALL PDXs (4/65) were assigned AKR1C3high, these PDXs showed similar levels of ACHM-025 ex vivo sensitivity as AKR1C3high T-ALL PDXs (Fig. 6E, Supplementary Figs. S4 and S14, Supplementary Table S12). We next evaluated AKR1C3 RNA-seq expression in 759 patients enrolled in the ZERO Childhood Cancer Program [32], which is a precision medicine program for children with poor-outcome, rare, relapsed or refractory cancer (subset shown in Fig. 6F, whole cohort shown in Supplementary Fig. S15). We applied the AKR1C3 RNA-seq threshold identified in Fig. 6A (TPM > 48) to the 759 patient samples and 11% (87/759) were assigned AKR1C3high (Fig. 6F, Supplementary Fig. S15), with hepatocellular cancers displaying the highest AKR1C3 expression with all patients (8/8) assigned AKR1C3high (Supplementary Fig. S15) [33].
We evaluated ex vivo ACHM-025 activity against a subset of PDX samples that were established from patients with acute myeloid leukemia (AML), neuroblastoma (NB), Ewing sarcoma (ES), diffuse midline glioma (DMG), hepatoblastoma (HB), and ependymoma (EPD) (Supplementary Fig. S16). Interestingly, AKR1C3 expression of PDX samples (as measured by qRT-PCR) did not reflect AKR1C3 expression of matched patient samples (as measured by RNA-seq) for NB or DMG (Supplementary Fig. S17A–C, Supplementary Table S13). For this reason, PDXs were assigned AKR1C3high based on qRT-PCR data of the PDX samples (CNRQ > 7; Fig. 6G, Supplementary Table S13). ACHM-025 displayed ex vivo activity in AKR1C3high ES and HB PDXs, however ACHM-025 was more dose potent against AKR1C3high T-ALL PDXs (Fig. 6G). Taken together, AKR1C3 expression is elevated in multiple high-risk pediatric cancers and can be used as a biomarker for ex vivo ACHM-025 activity.
Discussion
AKR1C3 is highly expressed in several cancers including liver, prostate, pediatric and adult T-ALL [11, 12, 33, 34] and this observation has been therapeutically exploited through the development of AKR1C3-activated prodrugs. In this study we have shown that ACHM-025 has both excellent dose potency and AKR1C3 selectivity, as measured ex vivo across a panel of both B- and T-ALL PDXs. ACHM-025, when administered at a conservative dose (25 mg/kg) and schedule (once weekly × 3), exerted potent in vivo efficacy as a single agent against a diagnosis/refractory T-ALL PDX (ALL-31) and a relapse/refractory T-ALL PDX (ALL-8) delaying leukemia progression in both PDXs by greater than 265 days. ACHM-025 showed notably superior efficacy compared to other AKR1C3-activated prodrugs, OBI-3424 (2.5 mg/kg) and PR-104 (200 mg/kg) which delayed the progression of ALL-8 by 67.3 and 54.3 days respectively, and delayed the progression of ALL-31 by 77.8 and 44.4 days respectively [11, 27].
ACHM-025 has shown remarkable single agent efficacy compared to the vast majority of single agents tested against pediatric ALL PDXs by the NCI-funded pediatric preclinical testing consortium [35]. To put the single-agent in vivo efficacy of ACHM-025 in perspective, standard-of-care induction therapy consisting of a combination of vincristine, dexamethasone and L-asparaginase only delayed progression of ALL-8 and ALL-31 by 53.7 and 17.5 days, respectively [36]. In the present study, the dose range of ACHM-025 evaluated was conservative, ranging from 2.5% to 12.5% of the murine MTD (≥200 mg/kg). Murine orthologs of AKR1C3 are not capable of activating AKR1C3-activated prodrugs, however cynomolgus monkey and human liver cytosolic fractions have been shown to comparably activate the AKR1C3-activated prodrug OBI-3424 [11, 26, 37]. Preliminary studies have shown that ACHM-025 was well tolerated in cynomolgus monkeys at doses ≤0.5 mg/kg, and neutropenia was the single dose-limiting toxicity at 0.6 mg/kg. At 2.0 mg/kg, the highest dose tested, all other hematological, clinical chemistry, urinalysis and histopathology parameters were observed to be within normal range.
From a clinical perspective, understanding which patients would benefit from ACHM-025 treatment relies on the identification of an effective biomarker. Our in vivo SMT study of 25 T-ALL PDXs showed a clear distinction between ACHM-025 responders (CR or MCR) and non-responders (PD1), with no PDXs displaying stable disease (SD) or partial response (PR). ACHM-025 response was directly related to AKR1C3 protein expression by immunoblot, AKR1C3 intracellular expression by flow cytometry, and AKR1C3 expression by RNA-seq. Therefore, in a clinical setting, AKR1C3 expression could be determined by immunoblot, flow cytometry or RNA-seq to effectively identify ACHM-025 responders. Determining AKR1C3 expression by RNA-seq was the most effective biomarker, providing greatest separation between ACHM-025 responders and non-responders, with no ACHM-025 responders falling below the minimum AKR1C3 expression threshold. Regardless of the method used to determine AKR1C3 expression, every PDX above the minimum AKR1C3 threshold responded to ACHM-025 (CR or MCR).
Precision medicine based clinical trials, where real-time molecular profiling of patients is used to identify tailored treatment options, are becoming increasingly common in high-risk pediatric cancer [32, 38, 39]. Our pilot study suggests that ACHM-025 efficacy relates to AKR1C3 expression in multiple pediatric cancers and, pending first-in-human clinical trials, could be incorporated into future precision medicine-based clinical trials.
Consolidation therapy for T-ALL includes Ara-C, 6MP, and the DNA bis-alkylating prodrug, CP. When activated, ACHM-025 forms a potent nitrogen mustard-based DNA alkylating agent, however the activation mechanisms of CP and ACHM-025 vary considerably. CP is activated by mixed-function oxidases in the liver and while highly effective, can cause significant side effects including, myelosuppression, infertility and secondary neoplasms [40]. While pulmonary injury and fibrosis are common side effects associated with nitrogen mustard therapy [41], the ACHM-025 dose-limiting side effect of neutropenia in cynomolgus monkeys noted above suggests that the intracellular ACHM-025 activation mechanism may result in a more favorable side effect profile compared with both nitrogen mustard and CP therapy, as well as improved efficacy compared to CP. Here we showed that ACHM-025 was significantly more effective than CP, delaying leukemia progression in a relapse/refractory T-ALL PDX (ALL-8) by 57 versus 25 days. Similarly, replacing CP with ACHM-025 as part of a consolidation-based therapy with Ara-C and 6MP was significantly more effective, delaying leukemia progression in ALL-8 by 75 days, compared to 36 days for CP based consolidation therapy.
The treatment of relapsed/refractory T-ALL represents an unmet need in cancer. Relapsed/refractory T-ALL is highly aggressive, often chemoresistant, and has poor survival rates [5]. Treatment options for relapsed/refractory T-ALL remain limited, with current clinical and translational research investigating NOTCH inhibitors, PI3K inhibitors, BCL2 inhibitors, tyrosine kinase inhibitors, BET inhibitors, JAK inhibitors, and immunotherapy, with a bridge to hematopoietic stem cell transplantation (HSCT) considered the best outcome [5, 42]. Currently, nelarabine is the only FDA approved chemotherapy for the treatment of relapsed/refractory T-ALL, in patients who have failed at least two prior regimens [30]. Nelarabine is a purine nucleoside analog that inhibits DNA synthesis, and displays selectivity towards T-lymphoblasts compared to other hematopoietic cells [42]. However, nelarabine treatment can cause neurotoxicity, particularly when combined with other CNS-directed therapy [30, 43, 44]. Here we showed that ACHM-025 was significantly more effective than nelarabine, delaying leukemia progression in a relapse/refractory T-ALL PDX (ALL-8) by 198 versus 19 days. Remarkably, none of the mice treated with the combination of ACHM-025 and nelarabine relapsed during the remaining 275 days post treatment. Importantly, in both the combinatorial drug analyses, combining ACHM-025 with nelarabine or Ara-C/6MP showed no antagonism in vivo. One of the current challenges in using CAR T-cells to treat T-ALL is fratricide [45]. While the role of AKR1C3 in T-cell development and function is currently unknown, AKR1C3 expression appears to be relatively low in healthy mature T-cells [46, 47]. Therefore, ACHM-025 may not adversely affect CAR T-cell therapy if used in future combination protocols. Overall, these promising results highlight the potential of ACHM-025 to be incorporated into current therapy for relapsed/refractory T-ALL to achieve prolonged remission prior to HSCT.
In conclusion, we have demonstrated the in vivo efficacy and utility of the new AKR1C3-activated prodrug, ACHM-025, which overcomes many of the limitations observed for both PR-104 and OB1-3424. This study provides strong preclinical evidence highlighting the potential of ACHM-025 as a targeted and effective therapy for aggressive forms of T-ALL and potentially other cancers, with AKR1C3 expression as a predictive biomarker of its activity.
Supplementary information
Toscan et al Supplementary Materials and Methods
Acknowledgements
The authors thank Professor William R Wilson for kindly providing PR-104A and SN34037, and Dr Adrian Blaser and Mr Sisira Kumara from the University of Auckland for technical support. The authors thank staff at the Katharina Gaus Light Microscopy Facility, UNSW Sydney, for histology preparation; and Anatomical Pathology, Prince of Wales Hospital, Randwick, for performing CD3 and TdT immunostaining. The authors thank Shiloh Middlemiss, Simon Sleep and Claudia Flemming for their valuable contributions to grant applications. The authors thank Libby Huang and Jodie Giles for technical assistance with the MRD assays, Jayne Murray for assistance with the histology, Caitlin Ung and Ben Watts for assistance with the pharmacokinetics assays. The results published as part of Fig. 6 (panels F and G), Supplementary Figs. 15–17, and Supplementary Table 13 are whole or part based upon the data generated by the Zero Childhood Cancer Program. The authors thank the Sydney Children’s Tumour Bank Network for providing samples and related clinical information for this study. The authors thank the Children’s Cancer Institute Animal Facility for providing support to this study. Children’s Cancer Institute Australia is affiliated with UNSW Sydney and the Sydney Children’s Hospitals Network. This research was supported by the Kid’s Cancer Alliance Translational Cancer Research Project Grant, the Anthony Rothe Memorial Trust Project Grant, and the Leukemia & Lymphoma Society Translational Research Program Grant (jointly funded by the Leukemia & Lymphoma Society, Snowdome Foundation, and the Leukaemia Foundation). RBL was supported by a Fellowship from the Australian National Health and Medical Research Council (1157871). AVP and JBS were supported by Senior Research Fellowships from Cancer Society Auckland Northland.
Author contributions
CT was responsible for conceptualizing the study, acquiring and analyzing the data, supervision, funding acquisition, and writing the original draft. HM, AA, XL, ZF, LD, HJK, RC, AZ, SW, KE, FK, RC, KLY, AJG, RP were responsible for acquiring and analyzing the data and reviewing the final draft. CM was responsible for analyzing the data and reviewing the final draft. JX was responsible for conceptualizing the study and reviewing the final draft. MJH was responsible for providing the resources and reviewing the final draft. TNT was responsible for conceptualizing the study, funding acquisition and reviewing the final draft. AVP and JBS were responsible for conceptualizing the study, supervision, funding acquisition, providing resources, and reviewing the final draft. CEdB and RBL were responsible for conceptualizing the study, supervision, funding acquisition, providing resources, writing the original draft and reviewing the final draft.
Data availability
All data generated or analyzed during this study are included in this manuscript. Supplementary information is available.
Competing interests
AVP and JBS are inventors on patents related to ACHM-025 (US11661404B2, EP3774743B1, CN111918864B) assigned to Achilles Medical Ltd., a privately-held company. AVP and JBS are part-owners of Achilles Medical Ltd. and stand to benefit financially from commercial development of ACHM-025. All other authors declare no competing interests.
Ethics approval and consent to participate
All methods used in this study were performed in accordance with the relevant guidelines and regulations. The PDXs used in this study were previously established under approval from the Sydney Children’s Hospitals Network and the UNSW Sydney Human Research Ethics Committees (HREC10/114, HC10442 and 2019/ETH10580) and the UNSW Sydney Animal Care and Ethics Committee (ACECs 16/168B, 17/101B, 19/82B, 19/136B, 20/119B, 22/58B, 22/152B and 23/82B). The RNA-sequencing data derived from primary patient samples used in this study were under approval from the Sydney Children’s Hospitals Network and the UNSW Sydney Human Research Ethics Committees (LNR/14/SCH/497, 2109/ETH10480 and 2022/ETH01232). Informed consent was obtained from all participants. No identifiable images from human research participants were used in this study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Charles E. de Bock, Richard B. Lock.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-024-01180-x.
References
- 1.Pui C-H, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33:2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105:2524–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teachey DT, O’Connor D. How I treat newly diagnosed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma in children. Blood. 2020;135:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teachey DT, Pui C-H. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol. 2019;20:e142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pocock R, Farah N, Richardson SE, Mansour MR. Current and emerging therapeutic approaches for T-cell acute lymphoblastic leukaemia. Br J Haematol. 2021;194:28–43. [DOI] [PubMed] [Google Scholar]
- 6.Hunger SP, Raetz EA. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. 2020;136:1803–12. [DOI] [PubMed] [Google Scholar]
- 7.Reddi D, Seaton BW, Woolston D, Aicher L, Monroe LD, Mao ZJ, et al. AKR1C3 expression in T acute lymphoblastic leukemia/lymphoma for clinical use as a biomarker. Sci Rep. 2022;12:5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penning TM. AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase): roles in malignancy and endocrine disorders. Mol Cell Endocrinol. 2019;489:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penning TM. The aldo-keto reductases (AKRs): overview. Chem Biol Interact. 2015;234:236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bortolozzi R, Bresolin S, Rampazzo E, Paganin M, Maule F, Mariotto E, et al. AKR1C enzymes sustain therapy resistance in paediatric T-ALL. Br J Cancer. 2018;118:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans K, Duan J, Pritchard T, Jones CD, McDermott L, Gu Z, et al. OBI-3424, a novel AKR1C3-activated prodrug, exhibits potent efficacy against preclinical models of T-ALL. Clin Cancer Res. 2019;25:4493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guise CP, Abbattista MR, Singleton RS, Holford SD, Connolly J, Dachs GU, et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010;70:1573–84. [DOI] [PubMed] [Google Scholar]
- 13.Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel K, et al. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin Cancer Res. 2007;13:3922–32. [DOI] [PubMed] [Google Scholar]
- 14.Konopleva M, Thall PF, Yi CA, Borthakur G, Coveler A, Bueso-Ramos C, et al. Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica. 2015;100:927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lock RB, Liem N, Farnsworth ML, Milross CG, Xue C, Tajbakhsh M, et al. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood. 2002;99:4100–8. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson SMF, Gu Y, Manesh DM, El-Hoss J, Jing D, Mackenzie KL, et al. A novel fluorometric assay for aldo-keto reductase 1C3 predicts metabolic activation of the nitrogen mustard prodrug PR-104A in human leukaemia cells. Biochemical Pharmacol. 2014;88:36–45. [DOI] [PubMed] [Google Scholar]
- 17.Mayoh C, Mao J, Xie J, Tax G, Chow S-O, Cadiz R, et al. High-throughput drug screening of primary tumor cells identifies therapeutic strategies for treating children with high-risk cancer. Cancer Res. 2023;83:2716–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau LMS, Mayoh C, Xie J, Barahona P, MacKenzie KL, Wong M, et al. In vitro and in vivo drug screens of tumor cells identify novel therapies for high-risk child cancer. EMBO Mol Med. 2022;14:e14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong M, Mayoh C, Lau LMS, Khuong-Quang D-A, Pinese M, Kumar A, et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat Med. 2020;26:1742–53. [DOI] [PubMed] [Google Scholar]
- 20.Rokita JL, Rathi KS, Cardenas MF, Upton KA, Jayaseelan J, Cross KL, et al. Genomic profiling of childhood tumor patient-derived xenograft models to enable rational clinical trial design. Cell Rep. 2019;29:1675–1689.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houghton PJ, Morton CL, Tucker C, Payne D, Favours E, Cole C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–40. [DOI] [PubMed] [Google Scholar]
- 22.Murphy B, Yin H, Maris JM, Kolb EA, Gorlick R, Reynolds CP, et al. Evaluation of alternative in vivo drug screening methodology: a single mouse analysis. Cancer Res. 2016;76:5798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houghton PJ, Morton CL, Gorlick R, Lock RB, Carol H, Reynolds CP, et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol Cancer Therapeutics. 2010;9:101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. 2016;78:661–71. [DOI] [PubMed] [Google Scholar]
- 25.Phillips RM. Targeting the hypoxic fraction of tumours using hypoxia-activated prodrugs. Cancer Chemother Pharmacol. 2016;77:441–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Bellemare V, Labrie F, Luu-The V. Molecular characterization of the cynomolgus monkey Macaca fascicularis steroidogenic enzymes belonging to the aldo-keto reductase family. J Steroid Biochem Mol Biol. 2007;104:75–80. [DOI] [PubMed] [Google Scholar]
- 27.Moradi Manesh D, El-Hoss J, Evans K, Richmond J, Toscan CE, Bracken LS, et al. AKR1C3 is a biomarker of sensitivity to PR-104 in preclinical models of T-cell acute lymphoblastic leukemia. Blood. 2015;126:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton R, Bahar AY, Kwan E, Giles JE, Venn NC, Tran S, et al. Improving minimal residual disease detection in precursor B-ALL based on immunoglobulin-kappa and heavy-chain gene rearrangements. Leukemia. 2008;22:2265–7. [DOI] [PubMed] [Google Scholar]
- 29.Flohr T, Schrauder A, Cazzaniga G, Panzer-Grümayer R, van der Velden V, Fischer S, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22:771–82. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MH, Johnson JR, Justice R, Pazdur R. FDA drug approval summary: nelarabine (Arranon) for the treatment of T-cell lymphoblastic leukemia/lymphoma. Oncologist. 2008;13:709–14. [DOI] [PubMed] [Google Scholar]
- 31.Pölönen P, Di Giacomo D, Seffernick AE, Elsayed A, Kimura S, Benini F, et al. The genomic basis of childhood T-lineage acute lymphoblastic leukaemia. Nature. 2024;632:1082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau LMS, Khuong-Quang D-A, Mayoh C, Wong M, Barahona P, Ajuyah P, et al. Precision-guided treatment in high-risk pediatric cancers. Nat Med. 2024;30:1913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao S-F, Wang S-G, Zhao Z-Y, Li W-L. AKR1C1-3, notably AKR1C3, are distinct biomarkers for liver cancer diagnosis and prognosis: database mining in malignancies. Oncol Lett. 2019;18:4515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J, Zhang M, Liu J, Liu Z, Shen P, Nie L, et al. AKR1C3 expression in primary lesion rebiopsy at the time of metastatic castration-resistant prostate cancer is strongly associated with poor efficacy of abiraterone as a first-line therapy. Prostate. 2019;79:1553–62. [DOI] [PubMed] [Google Scholar]
- 35.Jones L, Carol H, Evans K, Richmond J, Houghton PJ, Smith MA, et al. A review of new agents evaluated against pediatric acute lymphoblastic leukemia by the Pediatric Preclinical Testing Program. Leukemia. 2016;30:2133–41. [DOI] [PubMed] [Google Scholar]
- 36.Szymanska B, Wilczynska-Kalak U, Kang MH, Liem NLM, Carol H, Boehm I, et al. Pharmacokinetic modeling of an induction regimen for in vivo combined testing of novel drugs against pediatric acute lymphoblastic leukemia xenografts. PLoS One. 2012;7:e33894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veliça P, Davies NJ, Rocha PP, Schrewe H, Ride JP, Bunce CM. Lack of functional and expression homology between human and mouse aldo-keto reductase 1C enzymes: implications for modelling human cancers. Mol Cancer. 2009;8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCabe MG, Geoerger B, Chesler L, Hargrave D, Parsons DW, van Tilburg CM, et al. Precision medicine for childhood cancer: current limitations and future perspectives. JCO Precision Oncol. 2024;1:e2300117. [DOI] [PubMed]
- 39.Vo KT, Parsons DW, Seibel NL. Precision medicine in pediatric oncology. Surgical Oncol Clin North Am. 2020;29:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Jonge ME, Huitema ADR, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinetics. 2005;44:1135–64. [DOI] [PubMed] [Google Scholar]
- 41.Sunil VR, Vayas KN, Abramova EV, Rancourt R, Cervelli JA, Malaviya R, et al. Lung injury, oxidative stress and fibrosis in mice following exposure to nitrogen mustard. Toxicol Appl Pharmacol. 2020;387:114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordo’ V, van der Zwet JCG, Canté-Barrett K, Pieters R, Meijerink JPP. T-cell acute lymphoblastic leukemia: a roadmap to targeted therapies. Blood Cancer Discov. 2021;2:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhlen M, Bleckmann K, Möricke A, Schrappe M, Vieth S, Escherich G, et al. Neurotoxic side effects in children with refractory or relapsed T-cell malignancies treated with nelarabine based therapy. Br J Haematol. 2017;179:272–83. [DOI] [PubMed] [Google Scholar]
- 44.Commander LA, Seif AE, Insogna IG, Rheingold SR. Salvage therapy with nelarabine, etoposide, and cyclophosphamide in relapsed/refractory paediatric T-cell lymphoblastic leukaemia and lymphoma. Br J Haematol. 2010;150:345–51. [DOI] [PubMed] [Google Scholar]
- 45.Dourthe ME, Baruchel A. CAR T-cells for T-cell acute lymphoblastic leukemia. EJC Paediatr Oncol. 2024;3:100150. [Google Scholar]
- 46.Immune cell- AKR1C3. The Human Protein Atlas. 2024. Available from: https://www.proteinatlas.org/ENSG00000196139-AKR1C3/immune+cell.
- 47.Normal human hematopoiesis (DMAP)- AKR1C3. BloodSpot. 2024. Available from: https://www.fobinf.com/?gene=AKR1C3&dataset=DMAP.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Toscan et al Supplementary Materials and Methods
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript. Supplementary information is available.