Abstract
This study assessed plasma levels of essential amino acids (EAA) in drug-naïve first episode psychosis (FEP) patients at diagnosis and after 10 weeks of antipsychotic treatment. Forty FEP patients were enrolled at baseline, with blood samples collected before and after a 10-week antipsychotic treatment period. Plasma EAA levels were measured using an LC/MS/MS method. Psychotic symptoms were evaluated using standardized inventories before and after treatment. A decrease in BPRS score of more than 40% was used to indicate treatment response. Thirty-five healthy volunteers served as the control group. Baseline plasma levels of Thr, Met, Leu, Lys, His, and Tyr were higher in FEP patients than in healthy controls. After 10 weeks of treatment, Leu, His, and Tyr increased further, primarily in treatment-responsive patients. Conversely, Val level was lower than controls in patients at baseline and remained unchanged after treatment. Increased EAA levels were correlated with lower (less severe) scores in positive symptom scales. Treatment non-responders had persistently low Tyr/large neutral amino acid (LNAA) ratio. Tyr/LNAA ratio increased after treatment, specifically in treatment-responders. Phe/Tyr ratio decreased post-treatment in both responder and non-responder groups. Elevated EAA levels in FEP patients may signify compensatory responses to increased physiological demand for neurotransmitters or energy. Combining specific EAA supplementation with antipsychotic treatment may enhance treatment response in these patients.
Subject terms: Biomarkers, Psychosis
Introduction
Schizophrenia is a chronic deteriorating disorder characterized by positive, negative, and cognitive symptoms. The overall prevalence of the disease ranges from 0.85 to 1%, according to epidemiological studies1. There are a growing number of studies addressing the possible mechanisms underlying the disorder, but are still far from explaining the main pathophysiology.
In general, the patients initially contact a health institution with a psychotic episode. The typical age of onset is 18–25 in men, and 25–35 in women2. These patients are diagnosed with schizophrenia at a rate of 12.6% in 1.7 years, and this diagnostic shift increases to 80% in a period of 2.8 years. Younger age of onset and male gender are associated with increased risk and earlier development of schizophrenia3. Usually, the transition to a chronic disorder is exacerbated by several factors, such as stress, substance use, medication incompliance, social problems or negative life events, and medical factors.
Drug-naïve first episode psychotic (FEP) patients provide a unique opportunity to study the underlying mechanisms of psychotic disorders because they have not been exposed to the confounding effects of antipsychotic medications, allowing researchers to more accurately assess the natural progression of the illness and its neurobiological basis. These studies offer a critical window into understanding the early stages of psychosis, potentially shedding light on the neurochemical, genetic, and environmental factors that contribute to the development of the disorder.
Amino acids are fundamental components of our bodies, they are vital for physiological functions and play a crucial role in brain neurotransmitter function. The biosynthesis of dopamine, noradrenaline, and serotonin is related to the availability of their amino acid precursors, tyrosine (Tyr) and tryptophan (Trp)4. Moreover, glutamate is a derivative of the amino acid glutamine, and glycine is a co-agonist of the N‐methyl‐d‐aspartate receptor (NMDAR). Previously, we showed that the plasma l-arginine and its metabolites l-citrulline and agmatine, but not l-ornithine levels were higher during FEP; antipsychotic treatment for 10 weeks decreased the agmatine levels5. Because agmatine can act as an endogenous NMDA receptor antagonist, we further investigated the amino acids that are related to NMDAR functioning, such as serine, asparagine, glutamine, glutamic acid, proline, and hydroxyproline. These amino acids were higher in FEP patients, and they remained high or increased further after 10 weeks of antipsychotic treatment6. Among the 20 amino acids, the essential amino acids (EAA), namely phenylalanine (Phe), Trp, valine (Val), threonine (Thr), methionine (Met), leucine (Leu), isoleucine (Ile), lysine (Lys), and histidine (His) cannot be synthesized by the human metabolism therefore must be supplied in the diet (Table 1).
Table 1.
Essential amino acids, their chemical structures, and important metabolites.
| Amino acid | Abbreviation | Chemical structure/category | Important metabolites |
|---|---|---|---|
| Lysine | Lys | Basic amino acid | Carnitine |
| Histidine | His | Basic amino acid | Histamine |
| Methionine | Met | Sulfur-containing amino acid | S-adenosylmethionine |
| Phenylalanine | Phe | Aromatic amino acid | Tyrosine |
| Tyrosine | Tyr | Aromatic amino acid | Dopamine, noradrenaline, adrenaline |
| Tryptophan | Trp | Aromatic amino acid | Serotonin, melatonin |
| Threonine | Thr | Hydroxy amino acid | Nucleotides |
| Isoleucine | Ile | Branched-chain amino acid | Acetyl-Co-A |
| Leucine | Leu | Branched-chain amino acid | Acetyl-Co-A |
| Valine | Val | Branched-chain amino acid | Acetyl-Co-A |
Trp, besides serotonin, is a precursor for kynurenine and kynurenic acid, that are known to play a significant role in psychotic disorders7. Tyr and Trp compete with Ile, Leu, Val, Met, His, and Phe for the same transporter, Large Neutral Amino Acid-Transporter 1 (LAT1) in the blood-brain barrier, thus the brain concentrations of Tyr and Trp are influenced by blood concentrations of these amino acids8. Moreover, brain glutamate synthesis is highly dependent on branched-chain amino acids and Leu availability9, which has been previously implicated in schizophrenia and other neuropsychiatric conditions10.
Two of the important neurotransmitters in the etiology of schizophrenia, namely dopamine and serotonin, are synthetized from Tyr and Trp, respectively. Tyr is not an EAA, but one of the sources of Tyr is Phe, and it is usually defined as a conditional EAA. Previous studies investigating the EAA in psychotic disorders were mostly focused on schizophrenia rather than FEP. Also, several other clinically useful indicatives can be assessed using some of the EAA measured in the present study. For instance, the Tyr and Trp to large neutral amino acids (LNAA; Val, Met, Ile, Leu, His, Thr, Phe) ratios are indicative of brain Tyr and Trp availability; the Phe to Tyr ratio shows the activity of the enzyme phenylalanine-hydroxylase (PAH), the rate-limiting step in dopamine biosynthesis. As a general indicative of metabolic changes, the Fisher’s ratio can be calculated by dividing branched-chain amino acid (BCAA-Val, Leu, Ile) levels by aromatic amino acids (AAA-Phe, Tyr, Trp) levels. These measures were rarely studied for any psychiatric conditions.
Building on our two prior studies, which documented changes in specific amino acids in FEP patients both pre- and post-treatment, and considering the crucial role of EAAs in overall metabolism, including neurotransmission and energy metabolism, we hypothesized that plasma EAA profiles would differ between drug-naïve FEP patients and healthy controls. Additionally, we anticipated that 10 weeks of antipsychotic treatment would modulate these EAA levels. We also sought to compare these biochemical markers between treatment-responsive and non-responsive patients, as defined by changes in the Brief Psychiatric Rating Scale (BPRS). Furthermore, asides these main purposes, our study examined the impact of antipsychotic type, the presence of an affective component, psychosis subtype, and smoking status on EAA levels, aiming to elucidate the interactions between these clinical factors and metabolic processes.
Results
According to age, sex, and marital status, there were no significant differences between patients and healthy control groups. The average age in patients and control groups was 21.97 ± 0.49 and 22.51 ± 0.56 years, respectively. After 10 weeks of antipsychotic treatment, positive psychotic symptoms (SAPS) and general psychiatric symptoms (BPRS) had shown significant improvement while the negative symptoms scale (SANS) worsened, and CDSS score remained unchanged (please see Supplementary Table 1).
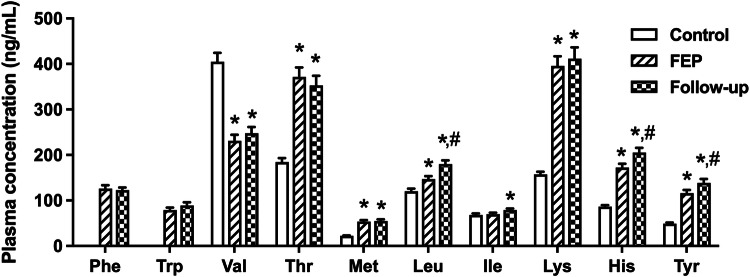
The plasma levels of Thr, Met, Leu, Lys, His, and Tyr were significantly higher; although Val plasma level was lower in FEP patients compared to healthy controls (p values <0.05, Fig. 1). No difference was observed in plasma Ile level between FEP and healthy control groups (p > 0.05, Fig. 1). The levels of Phe and Trp were not evaluated because of a data loss. After 10 weeks of antipsychotic treatment, the plasma Leu, His, and Tyr levels were significantly increased (p values <0.05, Fig. 1), whereas the other amino acids remained unchanged compared to their levels at diagnosis (p values >0.05, Fig. 1). Detailed statistical results of these comparisons are presented in Supplementary Tables 2 and 3.
Fig. 1.
Plasma concentrations of the essential amino acids, and tyrosine (Tyr) in healthy controls, and the patients at the diagnosis of first episode psychosis (FEP), and during the follow-up after 10 weeks of treatment (Phe phenylalanine, Trp tryptophan, Val valine, Thr threonine, Met methionine, Leu leucine, Ile isoleucine, Lys lysine, His histidine, *p < 0.05, compared to the control group; #p < 0.05, compared to initial measurements).
We observed significant correlations between the clinical assessment scores and the plasma levels of EAA. The correlation coefficients, statistical results, and the confidence intervals (95%) regarding these results are presented in Supplementary Table 4, and a heatmap showing the correlation coefficients for all the comparisons is provided in Supplementary Fig. 1. Shortly, the initial SAPS scores were negatively correlated with Met, Lys, His, and Thr levels measured before treatment, and with His and Thr levels during follow-up. Follow-up SAPS scores were negatively correlated with follow-up Phe, Ile, Leu levels, and initial His levels. The increase rate of Leu after treatment was also negatively correlated with follow-up SAPS score. The decrease rate of SAPS score during follow-up was correlated with follow-up Ile levels. The initial SANS scores and the increased rate of SANS scores during treatment were correlated with both initial and follow-up Tyr/LNAA ratios. Initial SANS scores were also correlated with follow-up Tyr and Lys levels while negatively correlated with the Fisher’s ratio. Finally, BPRS score during follow-up was negatively correlated with initial Lys and follow-up His levels, and negatively correlated with the change in Val levels (BPRS decreased by the percent increase in individual Val levels).
The amino acid levels were differentially impacted in treatment responder and non-responder patients. Lys level was significantly higher in treatment responder patients at diagnosis and during the follow-up after antipsychotic treatment (p values <0.05, Fig. 2). Although the levels of Leu, His, and Tyr were not different between treatment responder and non-responders at diagnosis (p values >0.05, Fig. 2), their levels were significantly increased compared to the initial levels only in treatment responder patients after 10 weeks of antipsychotic treatment (p values <0.05, Fig. 2). His and Tyr levels were higher in treatment responder patients compared to non-responders during follow up (p < 0.05, Fig. 2).
Fig. 2.
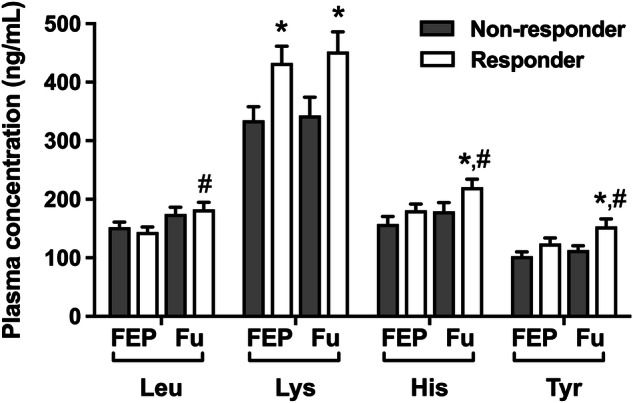
Plasma concentrations of the essential amino acids according to the treatment response during follow-up (Fu) (FEP first episode psychosis, Leu leucine, Lys lysine, His histidine, Tyr tyrosine, *p < 0.05, compared to treatment non-responder group; #p < 0.05, compared to initial measurements).
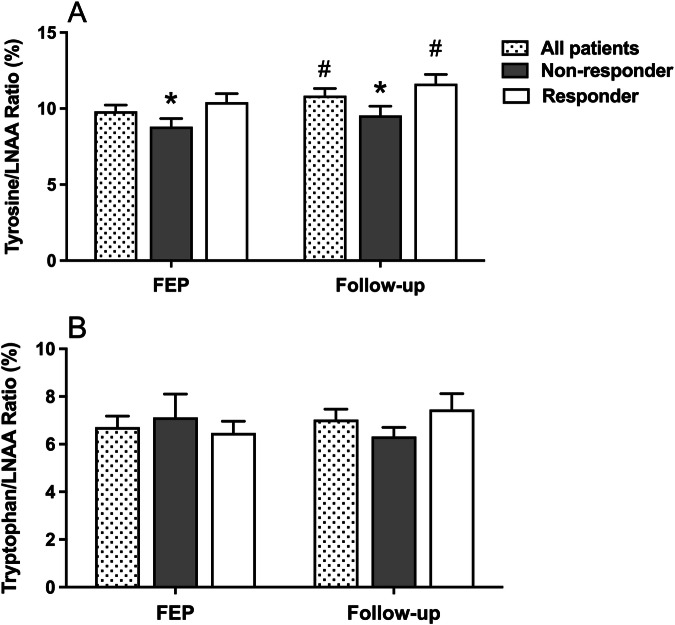
Tyr/LNAA ratio was significantly lower in treatment non-responders compared to responders at diagnosis, and after 10-week antipsychotic treatment, it increased only in treatment responder patients compared to initial values (p values <0.05, Fig. 3A). No significant change was observed in Trp/LNAA ratio in treatment responder or non-responders during FEP or follow-up (p values >0.05, Fig. 3B).
Fig. 3.
Ratio of tyrosine (A) and tryptophan (B) to large neutral amino acids (LNAA) according to the treatment response at the diagnosis of first episode psychosis (FEP), and during follow up (LNAA are isoleucine, leucine, valine, methionine, histidine, threonine, and phenylalanine; *p < 0.05, compared to treatment responder group; #p < 0.05, compared to initial measurements).
The Phe/Tyr ratio and Fisher’s Ratio were not compared with healthy controls because of the missing Phe level. In the patients, Phe/Tyr was 1.17 ± 0.08 at diagnosis, and it significantly decreased to 0.94 ± 0.04 after treatment during follow-up (p = 0.011). The Fisher’s ratio in patients was 1.55 ± 0.12 at diagnosis, and it remained at similar levels during follow-up (1.66 ± 0.14, p > 0.05). Treatment-responder and -non-responder patients did not show any difference in Phe/Tyr or Fisher’s ratio both at diagnosis and during follow-up (p values >0.05).
Multifactorial ANOVA revealed that there were significant interactions with SAPS score change and subtype of psychosis [F(1,22) = 7.890; p = 0.01, partial η2 = 0.264], and antipsychotic group [F(2.22) = 4.333; p = 0.026, partial η2 = 0.283]. Post hoc comparisons indicated that disorganized patients had a higher score of SAPS before treatment (p = 0.021), and both disorganized and paranoid patients had lower SAPS scores after treatment. On the other hand, the antipsychotic group effect did not survive after the post hoc comparisons. The SANS score was only changed according to the presence of the affective component, as expected [F(1,22) = 5.981; p = 0.023, partial η2 = 0.214]. BPRS scores before and after the treatment were interacted only with the subtype of the psychosis [F(1,23) = 4.393; p = 0.047, partial η2 = 0.16], whereas the disorganized patients had a higher BPRS score than paranoid patients during the diagnosis (p = 0.011, partial η2 = 0.25), and they were significantly benefitted from the treatment (p < 0.001, partial η2 = 0.714) but not the paranoid patients (p = 0.124, partial η2 = 0.1). The antipsychotic group and smoking status had no significant effect on any of the variables.
Similarly, the effects of these fixed variables on plasma amino acid levels and the calculated ratios were investigated in patients. Multifactorial ANOVA revealed that Met levels were interacted with the presence of affective component and smoking status [F(1,23) = 7.079; p = 0.014, partial η2 = 0.235] and interacted with the antipsychotic group and smoking status [F(2,23) = 3.508; p = 0.047, partial η2 = 0.234]. However, these effects did not survive after post hoc analyses. The presence of affective component and smoking status [F(1,23) = 5.087; p = 0.034, partial η2 = 0.181], and subtype of psychosis and antipsychotic group [F(2,23) = 4.740; p = 0.019, partial η2 = 0.292] had significant effects on Val levels. Post hoc Bonferroni test revealed that olanzapine-treated disorganized patients had a higher level of Val compared to risperidone or other antipsychotic-treated patients (p = 0.006 and p = 0.026, respectively). The effect of the affective component and smoking status on Val level did not survive after post hoc analyses. Finally, the Fisher’s ratio was influenced by the subtype of psychosis and antipsychotic group [F(2,23) = 5.287; p = 0.013, partial η2 = 0.315], and antipsychotic group and smoking status [F(2,23) = 3.912; p = 0.034, partial η2 = 0.254]. Similar to Val levels, olanzapine-treated disorganized patients had a higher Fisher’s ratio compared to risperidone or other antipsychotic-treated patients (p = 0.041 and p = 0.002, respectively).
Methods
Participants
The present case-control study was conducted in antipsychotic naïve FEP patients. All male patients were enrolled in the study after being admitted by the Gulhane School of Medicine Department of Psychiatry. FEP was defined as the presence of a moderate level of positive symptoms that disrupt functioning for a minimum of one week, but not more than one month, in the absence of predominant mood symptoms. Initially, 81 patients were recruited for the study. Patients were diagnosed using a semi-structured clinical interview (SCID) form for Axis 1 disorders from the Diagnostic and Statistical Manual of Mental Disorders (DSM) based on DSM-IV-TR criteria. Scale for the assessment of positive symptoms (SAPS), BPRS, scale for the assessment of negative symptoms (SANS), and the Calgary depression scale for schizophrenia (CDSS) were used to evaluate the positive, negative, and depressive symptoms.
Forty of the patients were excluded from the study during the 10-week follow up period because of meeting at least one exclusion criterion. The exclusion criteria were the presence of history of previous antipsychotic exposure, any substance use disorder, organic brain disorders (confounding factors such as infection, inflammation, cancer, trauma, bleeding, etc.), mental deficiency, previous psychotic episodes or mood disorders. During the follow-up period, only one patient dropped out due to poor medication compliance and not coming to scheduled assessments, and 40 patients completed the study. The healthy volunteers (n = 35) matched for age, gender, and education level were selected as a control group with no family history of schizophrenia. The antipsychotic medication and clinical management of the patients during the follow-up period were performed by their psychiatrist, who were not involved with the present study. The patients were followed up at an inpatient unit for 3 weeks, then they were discharged. All the patients were treated with a single antipsychotic drug, and the treatment regime was not changed during the 10 weeks of follow-up. The ethical committee approved this study (Date: 15 October 2014/No: B.10.4.ISM.4.06.68.49) at Kecioren Research and Training Hospital. Written informed consent was obtained from all participants.
Amino acid measurement procedure
The blood samples were obtained before initiating the treatment and after 10 weeks of antipsychotic treatment. Lithium heparin-containing tubes were used and blood samples were incubated at +4 °C for 30 min and then centrifuged at +4 °C for 5 min at 5000 rpm to obtain plasma preserved at −80 °C until the analysis time. Those procedures were repeated before and after 10 weeks of antipsychotic treatment. The EAAs, Phe, Trp, Val, Thr, Met, Leu, Ile, Lys, His, and conditionally essential amino acid Tyr were measured by using a modified liquid chromatography/mass spectrometric (LC-MS/MS) method11,12. The separation was performed using an LC20AC HPLC system (Shimadzu) equipped with an autosampler, two solvent pumps, and a column oven. The chromatography column was a Zorbax HILIC column (3.5 μm, 3 × 15 mm, Agilent). The column temperature was set at 30 °C. The mobile phase A consisted of ammonium formate (40 mM) and formic acid (0.2%); the mobile phase B consisted of acetonitrile. The mobile phase B concentration was gradually decreased from 75 to 35% between 2–4.5 min. The samples (3 μL) were delivered at a flow rate of 0.7 mL/min. The ionized analytes by electrospray ionization (ESI) in positive ion mode were analyzed by using a triple quadrupole MS (Shimadzu 8030) with a source temperature of 400 °C. Because of a technical problem during data collection, we lost the data regarding Phe and Trp levels in control group, thus the comparisons were only performed between before and after treatment in the patient group, and between treatment-responder and -non-responder subgroups. The samples were studied by a technician that blinds to the study groups.
Statistical analysis
Statistical analyses were performed using SPSS v.29.0.1.0 (IBM Corp., Armonk, NY) and GraphPad Prism v.10.3.1 (GraphPad Software Inc., San Diego, CA) software. The data were represented as the mean ± standard error of mean (S.E.M.). Shapiro–Wilk test was performed, and the histograms were visually inspected to analyze the normal distribution of the variables. Student’s t-test was used to compare plasma amino acid levels of the healthy controls and of the patients with schizophrenia. To maintain the family-wise error rate while preserving an overall alpha level of 0.05, a Bonferroni correction was applied by adjusting the p values. This method ensures that the likelihood of Type I errors across multiple comparisons remains controlled, reducing the risk of false positive findings due to multiple testing. Paired samples t-test or Wilcoxon signed-ranks test was used for comparing the plasma amino acid levels and Tyr/LNAA, Trp/LNAA, Fisher’s ratio, and Phe/Try ratios before and after antipsychotic treatment. Pearson’s correlations were performed between amino acid levels, changes in these levels after treatment, and other calculated variables with clinical scales. A decrease in the BPRS score of more than 40% was used to indicate a treatment response, and the analyses and comparisons were repeated for treatment responder and non-responder patients5,13. Repeated measures multifactorial ANOVA was used to evaluate the effect of fixed factors such as antipsychotic group, presence of an affective component, a subtype of psychosis (disorganized or paranoid), and smoking status on the measured or calculated variables, and the clinical assessment scores (pre- and post-treatment values assigned as within-subject factors). The post hoc Bonferroni test was used to compare variables when a significant group difference was established. Olanzapine (n = 12) and risperidone (n = 14) were found to be the most prevalent medications applied to the patients. Any other atypical antipsychotic (i.e., aripiprazole, paliperidone, quetiapine, or amisulpride) or a typical antipsychotic drug (i.e., haloperidol, chlorpromazine, or trifluoperazine) was prescribed to the rest of the patients (n = 14). Thus, olanzapine and risperidone formed two independent groups, and the remaining patients were categorized into a third “others” treatment group. The statistical significance was set at p < 0.05.
Discussion
The present study showed that plasma EAA levels showed significant changes during FEP and after 10 weeks of antipsychotic treatment compared to healthy controls. According to our results, the plasma levels of several EAA (namely Thr, Met, Leu, Lys, His, and Tyr) were significantly higher, whereas plasma Val levels were lower during FEP compared to healthy controls. Moreover, positive symptom severity was associated with the decrease of Met, Lys, His, and Thr. The antipsychotic treatment showed differential effects on these amino acids; for example, Leu, Ile, His, and Tyr levels significantly increased compared to initial levels, and the increase in Phe, Ile, and Leu levels was associated with the alleviation of the positive symptoms during follow-up. Positive symptom alleviation rate compared to the initial assessment was also linked with Ile level during follow-up. When the patients were evaluated according to their treatment response, it was revealed that Leu, His, and Tyr levels increased only in treatment responders during follow-up, and Lys level was higher in responders both at diagnosis and follow-up. Moreover, the Tyr/LNAA ratio (indicative of brain Tyr availability), but not the Trp/LNAA ratio, increased after treatment. Interestingly, the increased Tyr/LNAA ratio was only present in the treatment responder group according to the BPRS, but it was also positively correlated with negative symptom severity. On the other hand, the treatment non-responder patients had a lower Tyr/LNAA ratio at diagnosis, and it remained low after treatment compared to treatment-responder patients. The Phe/Tyr ratio decreased after treatment both in treatment responders and non-responders, indicative of increased activity in PAH activity. Finally, in a subgroup of olanzapine-treated disorganized patients, Val level and Fisher’s ratio were higher compared to patients treated with risperidone or another antipsychotic drug.
There are a limited number of studies investigating the blood levels of amino acids performed in drug naïve FEP patients. Leppik et al.14 measured 21 amino acids in serum samples of patients with FEP before and after 7 months antipsychotic treatment in comparison to control subjects. Among the overlapping amino acids measured in our study, they did not show any difference for His, Trp, Tyr, and Val levels but, the ratio between Tyr and Phe were significantly low in FEP patients14; after treatment, Tyr/Phe ratio and His level increased in the patients. For the baseline measurements, we found significant changes in these amino acids except Trp, which is missing in our patient group. However, the findings on the increased Tyr/Phe (or decreased Phe/Tyr) ratio and decreased His and Try levels after treatment were replicated in our study despite the shorter treatment duration (2.5 months vs 7 months). These changes as early treatment response markers deserve further investigation. Observation of steady levels of Trp and Trp/LNAA ratio after treatment were replicated in accordance with the Leppik et al. study. Our previous studies in a subject group converging with the current study also showed increased citrulline5, and proline6 levels, however Leppik et al. showed no change in citrulline and a significant decrease in proline levels. The most prominent difference between these studies appears to be the definition of FEP; our patient group consisted of newly diagnosed in the presence of moderate levels of positive symptoms that disrupt functioning for a minimum of one week, but not more than one month, and they had no treatment history; on the other hand, the study by Leppik et al. included the patients experienced FEP for up to 3 years without a treatment. Thus, disease duration could have a noteworthy effect on the variables. Also, the different demographics of the study group could have an impact on the results. Our study group consisted of younger, showing a relatively small variability in age, and all male patients; as a result, we believe we reached out a distinct separation from the healthy control group.
Recently, Avigdor et al. profiled 408 metabolites, including amino acids in 31 FEP patients that were within the 24-month period of FEP diagnosis and treated with a range of psychotropic drugs with further diagnoses15. Besides other findings, such as lower serotonin and higher sarcosine, prominent changes in amino acid levels were identified. Compared to healthy controls, proline, l-glutamic acid, and ornithine levels were higher, but arginine, serin, His, and Thr levels were lower in the patients. These results contradicted our results, but the study group was considerably different from ours because their patient group was within 24 months from the psychotic onset and under treatment for a long time as compared to our drug-naïve and newly diagnosed participants.
As a matter of fact, our drug-naïve patient group is the main strength of this study. Recruiting, treating, and following up 40 FEP patients provided a unique opportunity for us to explore the baseline changes in the metabolism involving the EAA associated with the onset of psychosis, without the influence of medications. Furthermore, we could compare the effects of the antipsychotic treatment on these variables.
In the present study, besides overall changes in EAA levels we also observed profound differences between treatment responder and non-responder patients. While the Phe/Tyr ratio decreased both in responders and non-responders, the Tyr/LNAA ratio increased only in responders after treatment. In other words, PAH activity (the rate-limiting step in dopamine synthesis) increased in all the patients but an increase in brain availability of Tyr was observed only in treatment-responders. The production of dopamine and noradrenaline is dependent on tyrosine hydroxylation, but substrate availability has an influence on neuronal activity, especially when the demand increases or with dopamine receptor blockage16. Thus, an increase in dopamine availability seems to contribute to treatment response. This supports the idea that adequate dopamine could be necessary for an acceptable antipsychotic response in FEP17. Amato et al. suggested that antipsychotics suppress dopamine re-uptake by direct blockade of dopamine transporter and increase synaptic dopamine, which in turn increases therapeutic efficacy by phasic activation of presynaptic D2 receptors resulting in autoinhibition. Increased brain availability of Tyr in our study may also increase response to treatment in a similar manner. On the other hand, an increased Tyr/LNAA ratio is correlated with the worsening of negative symptoms (SANS). Because treatment worsened the negative symptoms in our study, its possible that the relationship between other measures with treatment might not directly be related to treatment response, instead other factors such as side effects of the antipsychotics could be contributing factors. Previously, antipsychotic drugs are thought to produce secondary negative symptoms, which can also exacerbate primary negative symptoms18.
There can be individual differences in the amino acid transporter mechanisms. For instance, single nucleotide polymorphisms (SNPs) in the LAT1 coding gene SLC7A5 were detected in a case-control study with 315 schizophrenia patients that led to changes in CSF catecholamine levels19. To our knowledge, there is no further studies that performed with a larger sample size or investigating the role of antipsychotic treatments on this transporter system.
Leu is the other EAA that increased during follow-up only in treatment responder patients. Increased Leu levels during follow-up and its increase rate compared to baseline were also correlated with less severe positive symptoms. Leu is one of the three BCAA and has an importance in brain glutamate synthesis. The amount of glutamate or glutamine transported from peripheral blood to the brain is limited; thus, BCAA, and particularly leucine, could serve as precursors of glutamate synthesis in the brain. According to the Leu–glutamate cycle model proposed by Yudkoff et al. Leu enters astrocytes via the LNAA transporter, where it would be converted to glutamate and α-ketoisocaproate via branched-chain ketoacid dehydrogenase, α-ketoisocaproate is released to the neurons to regenerate Leu via reverse transamination with glutamate and transferred back to astrocytes9. In rats, as much as 50% of all brain glutamate nitrogen is derived from leucine, according to a study performed using a radioisotope labeled Leu20. In our previous study on NMDA receptor-related amino acid levels in FEP showed that glutamine and glutamic acid levels were significantly higher at the diagnosis, and after 10-week treatment, glutamic acid increased further. However, we did not observe any difference between treatment responder and non-responder patients6. Collectively, both Leu and glutamate-related amino acids may be increased in patients to compensate for the hypoglutamatergic state in FEP patients, and they further increased after treatment, but peripheral Leu seems to be a better indicative of treatment response possibly because of its easier transport to the brain. The interplay between Leu and brain glutamate metabolism in health and disease, especially FEP and schizophrenia, deserves more focused studies.
The third EAA that increased after treatment was His. His is the precursor of histamine which is one of the most widespread neurotransmitters in the brain. Histamine regulates basic physiological processes such as waking-sleeping, food intake, learning, and memory, primarily acting through the H1 receptor. Postmortem studies showed that brain histamine metabolism is higher in patients with schizophrenia; and an increased expression of H3 receptors in the dorsolateral prefrontal cortex was observed in the patients, especially those treated with atypical antipsychotics21. Increased levels of the histamine precursor amino acid His in treatment responders, and its inverse correlation with BPRS score in our study are directly in line with those studies proposing the essential role of this neurotransmitter system in schizophrenia.
In our previous studies in the same patient group, we observed increased levels of L-arginine, agmatine, glutamic acid, glutamine, proline, serine, asparagine, and hydroxyproline during FEP5,6. Almost all the time, these amino acids inversely correlated with the symptom severity in the patients showing a protective effect for psychosis. Also, agmatine, l-arginine, and l-citrulline have high sensitivity and specificity for diagnosing FEP, as indicated by the receiver-operator characteristic (ROC) analysis. Among the others, initial levels of L-arginine, L-citrulline, glutamine, proline, His, and Lys levels could predict the outcome of the treatment, such as decreases in SAPS and BPRS scores.
The majority of the tested amino acids throughout these studies were higher in the patient group; some of them were further increased by the treatment compared to the initial measurements. Thus, a general alteration in metabolism, such as reduced utilization of the amino acids, reduced amino acid transport to the cells, increased absorption, and altered metabolism, could be responsible for the observed changes. Metabolic changes such as insulin resistance or dyslipidemia observed in schizophrenia have been usually attributed to the side effects of medications or unhealthy lifestyle22. However, studies in drug-naïve FEP patients and follow up measurements after a short treatment period can rule out the influence of medications. The majority of the publications, including our previous studies5,6, considered plasma amino acid levels in the context of the dopaminergic, serotonergic, and glutamatergic hypotheses of schizophrenia. The current findings could reflect the changes in the whole metabolism, and they may not be restricted to the abnormalities in the brain. Previous studies investigated the serum and urine biomarkers of schizophrenia and FEP patients suggested that an energy metabolism disorder; an increased energy demand and production of glucose, and upregulation in fatty acid catabolism as an alternate source23.
Further analysis of the patient characteristics provided additional insights into the subgroups. For instance, disorganized patients had more severe symptoms than paranoid patients, and they benefitted from treatment more. However, EAA levels or the calculated parameters did not show any interaction with these subtypes of psychosis. Interestingly, olanzapine-treated disorganized patients had higher levels of Val compared to risperidone or other antipsychotic-treated patients, although the patient group had lower levels of Val, and it remained low after treatment. The same groups also had a higher Fisher’s ratio, probably linked with the increased Val levels, which is a branched-chain amino acid. In a recent study, various amino acid levels were measured before and during antipsychotic treatment (for an average of 42 days) in 80 FEP patients24. They did not report the patient subgroups, but similar to our findings, Val increased with olanzapine, but not risperidone, treatment. Val and Fisher’s ratio can increase as a result of proteolysis and reduced protein synthesis in skeletal muscles, and has been reported in obesity and type 1 and 2 diabetes25. The metabolic effects of olanzapine have been well documented. Olanzapine-treated patients demonstrate the highest maximal weight gain, marked hyperglycemia, and increased diabetes risk22. Our findings imply that the metabolic effects of olanzapine could be detected even after 10 weeks of treatment in a subgroup of patients. The utility of Val and Fisher’s Ratio to detect the metabolic risk created by olanzapine treatment could be a topic for further investigation.
The present study is not without limitations. Firstly, we measured the amino acid levels in peripheral blood, and it is not evident how extend they reflect brain levels. Even cerebrospinal fluid samples collected from the lumbar area hardly represent the brain metabolism26. However, we demonstrated significant and consistent correlations between several amino acids and assessment scores, providing valuable insights that changes in the metabolism of EAA can be effectively detected from peripheral blood. This method is not only more practical but also less invasive, making it an advantageous approach for clinical applications. The second limitation of the study was the short follow-up period of only 10 weeks, which may not have been sufficient to observe changes in clinical manifestations related to changes in EAA levels. Longer follow-up periods with multiple measurement points are needed to better track the clinical progress of patients. Thirdly, Phe and Trp levels in the healthy controls were missing, thus we could not compare their levels and the ratios that were calculated using them (Phe/Tyr, Phe/Trp, Tyr/LNAA, and Trp/LNAA) with the patient group. Finally, the diet status of the patients could have an impact on the blood amino acid levels at the first admission, but, we could not collect consistent information about the diet status of the participants. During the follow-up, sample collection was performed after an overnight fasting which provided a clearer profile for the peripheral amino acid assessment.
Supplementary information
Acknowledgements
This work was supported by research grants from Gulhane Military Medical Academy (grant number: AR-2013/03). Grant source has no authority on the study design, analyses, results, and the publication of the data. The blood samples collected for the present study were obtained in a parallel study in the same patient and healthy control groups and studied in the same analytical system at a later time point. These studies have been independently approved by the same committee. The initial results of the parallel study have been previously published by refs. 5,6. The authors would like to thank Prof. Ozcan Uzun for his valuable contributions during the planning and designing of the study.
Author contributions
B.G.: Conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, validation, and writing. J.Y.K.: Resources, supervision, writing, reviewing, and editing. H.K.: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; supervision; validation; visualization; writing, and editing.
Data availability
The data that support the findings of this study are available openly as of Nov 1st, 2024 (Mendeley Data, V1, 10.17632/dr5ccc34ns.1).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-024-00528-3.
References
- 1.Perälä, J. et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch. Gen. Psychiatry64, 19 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Abel, K. M., Drake, R. & Goldstein, J. M. Sex differences in schizophrenia. Int. Rev. Psychiatry22, 417–428 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Queirazza, F., Semple, D. M. & Lawrie, S. M. Transition to schizophrenia in acute and transient psychotic disorders. Br. J. Psychiatry204, 299–305 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Carlsson, A. Antipsychotic drugs, neurotransmitters, and schizophrenia. Am. J. Psychiatry135, 165–173 (1978). [DOI] [PubMed] [Google Scholar]
- 5.Garip, B., Kayir, H. & Uzun, O. L-Arginine metabolism before and after 10 weeks of antipsychotic treatment in first-episode psychotic patients. Schizophr. Res.206, 58–66 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Garip, B. & Kayir, H. Alteration in NMDAR-related amino acids in first episode psychosis. Synapse73, 1–8 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Yao, J. K. et al. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol. Psychiatry15, 938–953 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernstrom, J. D. Dietary precursors and brain neurotransmitter formation. Annu. Rev. Med.32, 413–425 (1981). [DOI] [PubMed] [Google Scholar]
- 9.Yudkoff, M. et al. Brain amino acid requirements and toxicity: the example of leucine. J. Nutr.135, 1531S–1538SS (2005). [DOI] [PubMed] [Google Scholar]
- 10.McCunn, P., Chen, X., Gimi, B., Green, A. I. & Khokhar, J. Y. Glutamine and GABA alterations in cingulate cortex may underlie alcohol drinking in a rat model of co-occurring alcohol use disorder and schizophrenia: an 1H-MRS study. Schizophrenia8, 67 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu, W.-J., Chen, H. & He, J. Application of LC-MS/MS analysis of plasma amino acids profiles in children with autism. J. Clin. Biochem. Nutr.51, 248–249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou, B., Xiao, J. F., Tuli, L. & Ressom, H. W. LC-MS-based metabolomics. Mol. Biosyst.8, 470–481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanger, T. M. et al. Olanzapine versus haloperidol treatment in first-episode psychosis. Am. J. Psychiatry156, 79–87 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Leppik, L. et al. Profiling of amino acids and their derivatives biogenic amines before and after antipsychotic treatment in first-episode psychosis. Front. Psychiatry9, 155 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avigdor, B. E. et al. Characterization of antipsychotic medications, amino acid signatures, and platelet-activating factor in first-episode psychosis. Biomark. Neuropsychiatry5, 100045 (2021).
- 16.Fernstrom, J. D. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids45, 419–430 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Amato, D., Kruyer, A., Samaha, A. N. & Heinz, A. Hypofunctional dopamine uptake and antipsychotic treatment-resistant schizophrenia. Front. Psychiatry10, 314 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millan, M. J., Fone, K., Steckler, T. & Horan, W. P. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol.24, 645–692 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Comasco, E. et al. Genetic and functional study of L-type amino acid transporter 1 in schizophrenia. Neuropsychobiology74, 96–103 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Kanamori, K., Ross, B. D. & Kondrat, R. W. Rate of glutamate synthesis from leucine in rat brain measured in vivo by 15N NMR. J. Neurochem.70, 1304–1315 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Jin, C. Y., Anichtchik, O. & Panula, P. Altered histamine H 3 receptor radioligand binding in post-mortem brain samples from subjects with psychiatric diseases. Br. J. Pharmacol.157, 118–129 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieberman, J. A. Metabolic changes associated with antipsychotic use. Prim. Care Companion J. Clin. Psychiatry4, 239 (2002). [PMC free article] [PubMed] [Google Scholar]
- 23.Yang, J. et al. Potential metabolite markers of schizophrenia. Mol. Psychiatry18, 67–78 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, X., Wang, L., Xue, Y. & Li, Y. Effects of antipsychotics on amino acid levels in patients with first-episode schizophrenia: a prospective study. Eur. J. Psychiatry38, 100229 (2024). [Google Scholar]
- 25.Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab.15, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal, M. B., Preston, J. E., Collis, C. S. & Zlokovic, B. V. Kinetics and Na independence of amino acid uptake by blood side of perfused sheep choroid plexus. Am. J. Physiol.258, F1288-94 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available openly as of Nov 1st, 2024 (Mendeley Data, V1, 10.17632/dr5ccc34ns.1).