Abstract
Myeloperoxidase of phagocytic leucocytes is thought to utilize H2O2 to oxidize halides, which then react with and kill ingested microbes. This hypothesis was based largely on the incorporation of radiolabelled iodide into cells that had phagocytosed bacteria. The present studies investigated the stoichiometry of these reactions and the subcellular localization and electrophoretic pattern of the cellular components that became iodinated. 1. The stoichiometry of the reactions are such that only a small proportion (less than 0.3%) of the total oxygen consumed is utilized for iodination. Iodination after stimulation with the soluble stimulus phorbol myristate acetate (PMA), which is not known to involve the azurophil granules and their contained myeloperoxidase, was comparable with that occurring after bacterial ingestion. 2. Analytical subcellular fractionation of cells that had phagocytosed bacteria localized about 25% of the radioactivity to the membranes, and most of the residual radioactivity distributed with the bacteria and dense granules. In cells stimulated with PMA, more of the radioactivity was associated with the membranes, but about half was still associated with the dense granules. 3. Autoradiographs after dodecyl sulphate/polyacrylamide-gel electrophoresis of cells stimulated with opsonized bacteria gave a similar distribution of iodinated components to that obtained with cells that had been stimulated with PMA or iodinated with Iodogen. These patterns of iodination were very different from those obtained when bacteria alone were iodinated with Iodogen or myeloperoxidase and H2O2. Preparations in which bacteria had been phagocytosed did not show evidence of iodination of bacterial proteins or coating opsonins. Thus positive evidence for the iodination of bacteria has not been produced, and the role of iodination in the microbicidal process of neutrophils remains to be established.
Full text
PDF





Images in this article
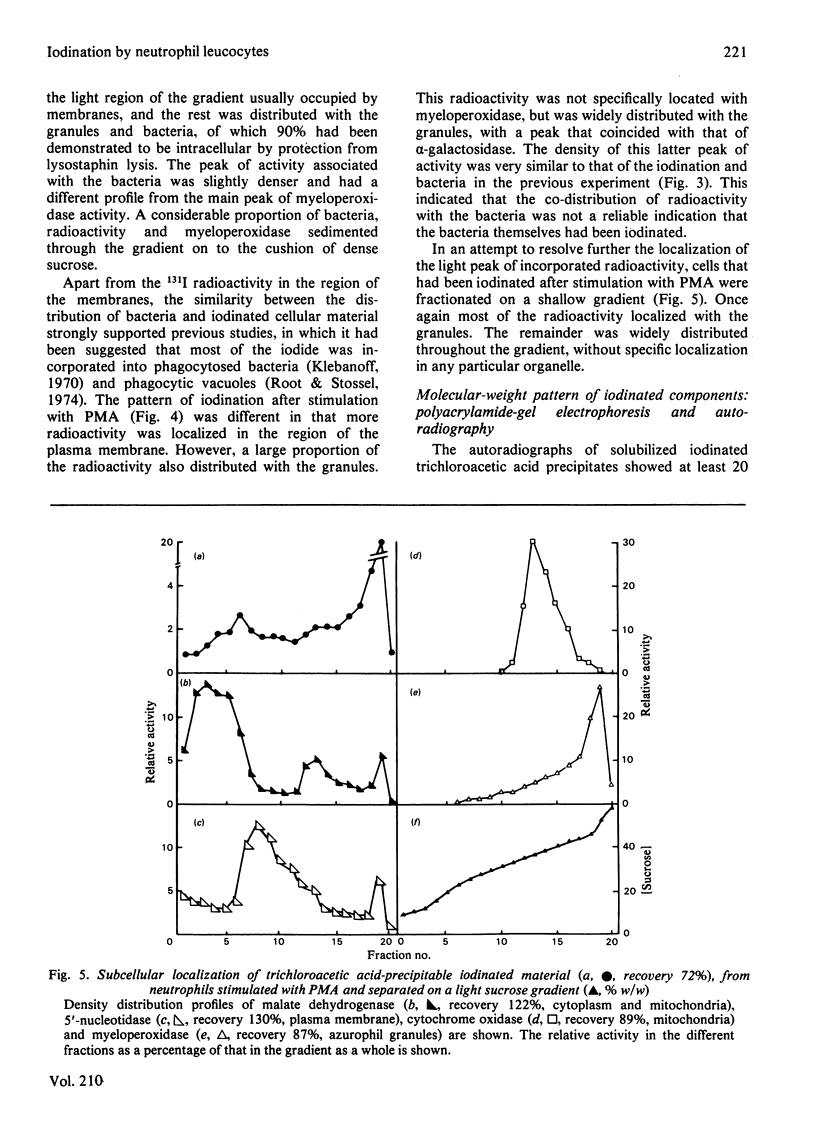
Selected References
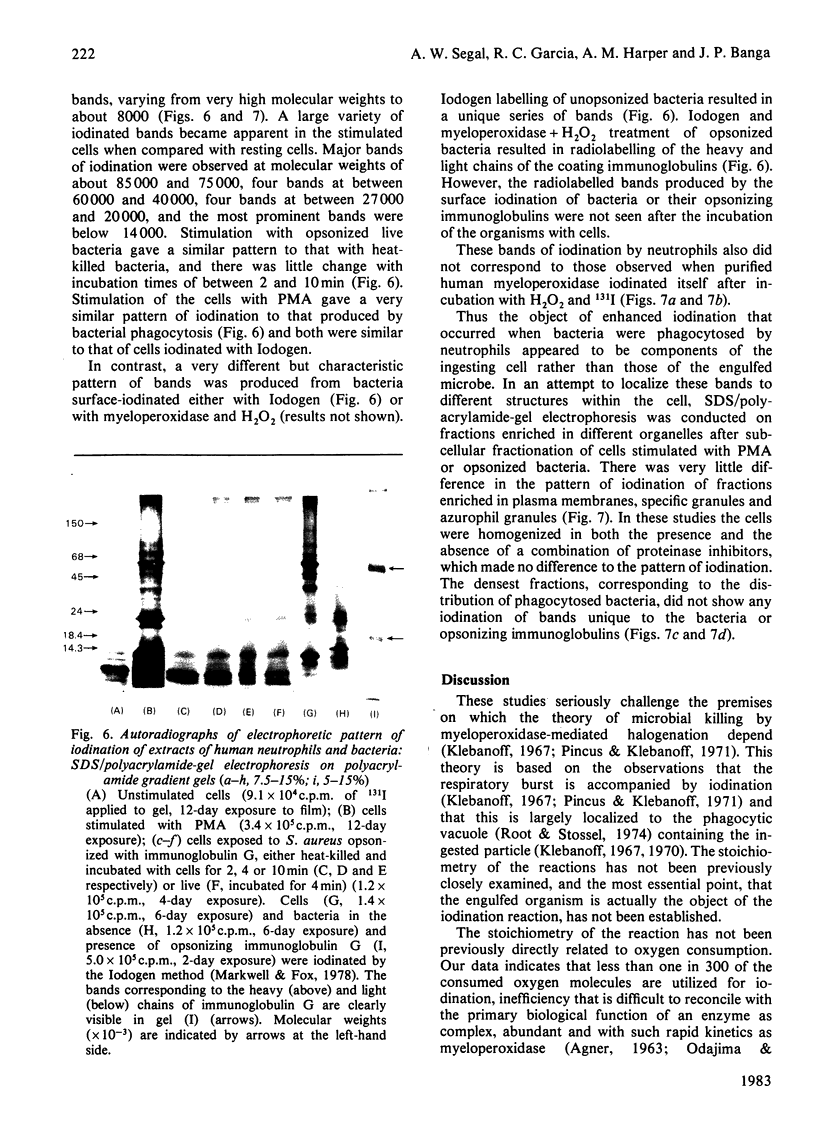
These references are in PubMed. This may not be the complete list of references from this article.
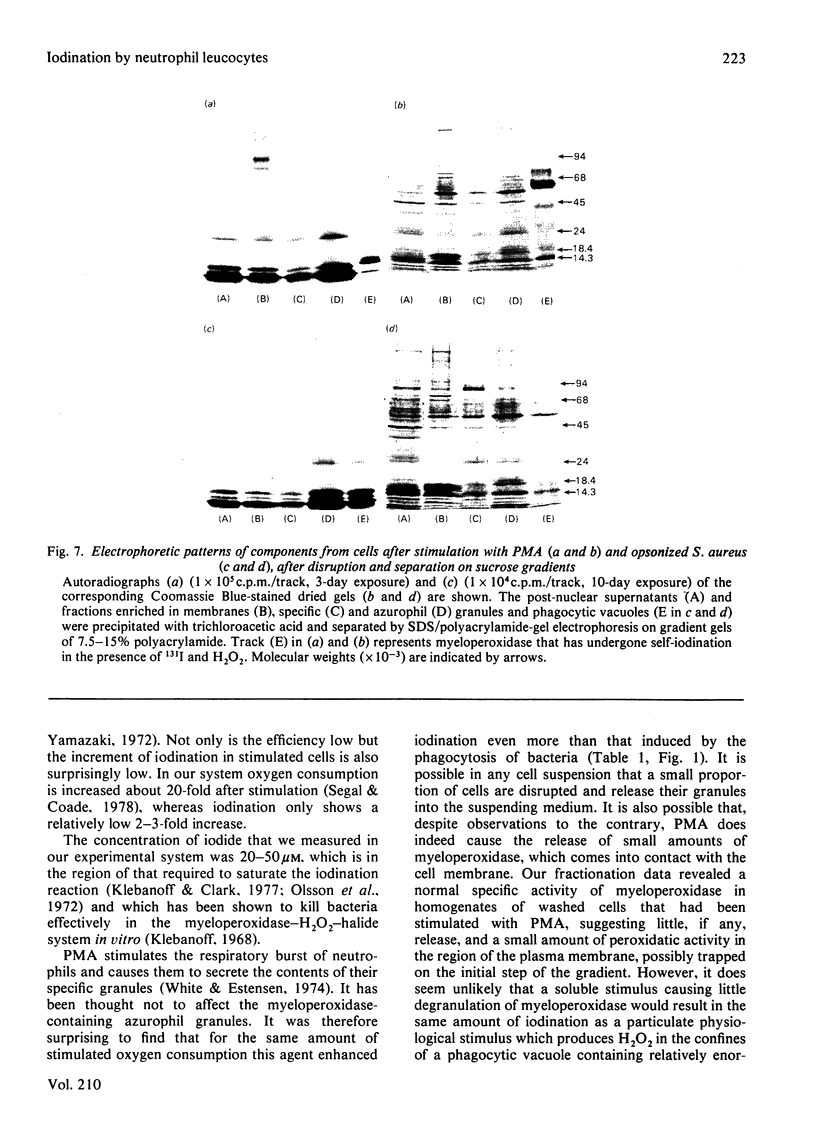
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- Douglas A. P., Kerley R., Isselbacher K. J. Preparation and characterization of the lateral and basal plasma membranes of the rat intestinal epithelial cell. Biochem J. 1972 Aug;128(5):1329–1338. doi: 10.1042/bj1281329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. P., Peters T. J. Analytical subcellular fractionation of human granulocytes with reference to the localization of vitamin B12-binding proteins. Clin Sci Mol Med. 1975 Aug;49(2):171–182. doi: 10.1042/cs0490171. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- Morrison M., Schonbaum G. R. Peroxidase-catalyzed halogenation. Annu Rev Biochem. 1976;45:861–888. doi: 10.1146/annurev.bi.45.070176.004241. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Steinman R. M., Cohn Z. A. The membrane proteins of the vacuolar system I. Analysis of a novel method of intralysosomal iodination. J Cell Biol. 1980 Jul;86(1):292–303. doi: 10.1083/jcb.86.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloperoxidase of the leukocyte of normal blood. V. The spectral conversion of myeloperoxidase to a cytochrome oxidase like derivative. Biochim Biophys Acta. 1972 Oct 12;284(2):368–374. doi: 10.1016/0005-2744(72)90132-5. [DOI] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Pincus S. H., Klebanoff S. J. Quantitative leukocyte iodination. N Engl J Med. 1971 Apr 8;284(14):744–750. doi: 10.1056/NEJM197104082841402. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Subcellular distribution of glycosidases in human polymorphonuclear leucocytes. Biochem J. 1978 Jul 15;174(1):53–59. doi: 10.1042/bj1740053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra A. J., Selvaraj R. J., Paul B. B., Poskitt P. K., Zgliczynski J. M., Mitchell G. W., Jr, Louis F. Biochemical, functional, and structural aspects of phagocytosis. Int Rev Exp Pathol. 1976;16:249–271. [PubMed] [Google Scholar]
- Segal A. W., Coade S. B. Kinetics of oxygen consumption by phagocytosing human neutrophils. Biochem Biophys Res Commun. 1978 Oct 16;84(3):611–617. doi: 10.1016/0006-291x(78)90749-0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Dorling J., Coade S. Kinetics of fusion of the cytoplasmic granules with phagocytic vacuoles in human polymorphonuclear leukocytes. Biochemical and morphological studies. J Cell Biol. 1980 Apr;85(1):42–59. doi: 10.1083/jcb.85.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981 Apr 2;290(5805):406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Novel cytochrome b system in phagocytic vacuoles of human granulocytes. Nature. 1978 Nov 30;276(5687):515–517. doi: 10.1038/276515a0. [DOI] [PubMed] [Google Scholar]
- Sun A. S., Poole B. Fractionation of rat fibroblasts in a zonal rotor by means of a viscosity barrier. Anal Biochem. 1975 Sep;68(1):260–273. doi: 10.1016/0003-2697(75)90704-6. [DOI] [PubMed] [Google Scholar]
- Thorpe R., Delacourte A., Ayers M., Bullock C., Anderton B. H. The polypeptides of isolated brain 10nm filaments and their association with polymerized tubulin. Biochem J. 1979 Aug 1;181(2):275–284. doi: 10.1042/bj1810275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Estensen R. D. Selective labilization of specific granules in polymorphonuclear leukocytes by phorbol myristate acetate. Am J Pathol. 1974 Apr;75(1):45–60. [PMC free article] [PubMed] [Google Scholar]
- Zgliczyński J. M., Stelmaszyńska T. Chlorinating ability of human phagocytosing leucocytes. Eur J Biochem. 1975 Aug 1;56(1):157–162. doi: 10.1111/j.1432-1033.1975.tb02218.x. [DOI] [PubMed] [Google Scholar]