Abstract
We present the case of a patient with Myxofibrosarcoma (MFS), a mesenchymal type of soft tissue sarcoma (STS) and the response to combination immunotherapy with anti PD-1 and anti-CTLA-4 therapy, following disease progression after Standard chemotherapy (SACT) and Radiotherapy (RT). We have shown a timeline of treatment and responses, as well as the overall safety profile and the management of immunotherapy related adverse events. This study demonstrates the potential of checkpoint inhibitors as therapeutic agents in the treatment of MFS.
Keywords: rare tumors, combination immunotherapy, immunotherapy outcome, myxofibrosarcoma, ipilimumab, nivolumab
Introduction
MFS represents a common STS subtype, forming mesenchymal lesions of distinct myxoid stroma and various polymorphisms. 1 Surgery +/− radiation is the mainstay of management for localized disease. Chemotherapy options for advanced/ metastatic MFS can include doxorubicin/ifosfamide, dacarbazine +/− doxorubicin and gemcitabine +/− docetaxel, however, outcomes remain poor. 1 Immunotherapy with combination ipilimumab/nivolumab has previously demonstrated promising efficacy in certain sarcoma subtypes, with a manageable safety profile. 2 Anti-PD-1 therapy has confirmed activity and safety in another STS study, with partial response achieved in one patient with MFS, 3 while other clinical trials are investigating the role of combination immunotherapy and targeted treatments for patients with MFS (envafolimab/ipilimumab 4 and ezabenlimab/MDM2 inhibitor 5 ).
Case report
We present the case of a 46-year-old male with metastatic, Grade 2 MFS, treated with third line compassionate access ipilimumab/nivolumab at the Royal Marsden Hospital. The patient presented initially with a lesion in the right pretibial region and underwent preoperative neo-adjuvant RT between April and May 2019 (50 Gy/ 25fractions), followed by right pretibial region resection with flap reconstruction in July 2019. Recurrent disease developed in December 2020, in the right thigh and right inguinal region. He received systemic treatment with ifosfamide and doxorubicin and completed six cycles in April 2021, with good response. In November 2021, the patient developed multifocal recurrence, in the right thigh, groin and popliteal fossa and was found not to be a candidate for surgery and he commenced second-line gemcitabine plus dacarbazine chemotherapy. There was clear disease progression after two cycles of treatment, with L3/4 nerve root compression, requiring RT to the spine (8 Gy in a single fraction, to L2-L5). He subsequently consented for combination ipilimumab and nivolumab treatment.
The patient initiated treatment with nivolumab 3 mg/kg and ipilimumab 1 mg/kg, three weekly, in October 2022, on a compassionate access program. After completion of four cycles of combination treatment in January 2023, he subsequently had 6 weeks off and started monotherapy nivolumab in February 2023, at a dose of 480 mg every 4 weeks, with good response and has remained on treatment with maintained stable disease until January 2024. Recently, in February 2024, the patient opted for a treatment break, and surveillance with three monthly imaging was initiated.
The patient tolerated ipilimumab and nivolumab treatment well, with Grade 1 (G1)-Grade 2 (G2) mucositis reported as the most common side effect. Symptoms improved after the regular use of mouthwash and supportive topical steroid and analgesic opioid treatment [gelclair: PVP (polyvinylpyrrolidone) and sodium hyaluronate mouthwash, benzydamine mouthwash, liquid morphine sulphate PRN, betamethasone soluble mouthwash]. The patient experienced G1 anaemia and G1 ALP elevation during the first cycle of treatment, but he did not develop any worsening symptoms or toxicities, and he was able to tolerate his next cycles of treatment.
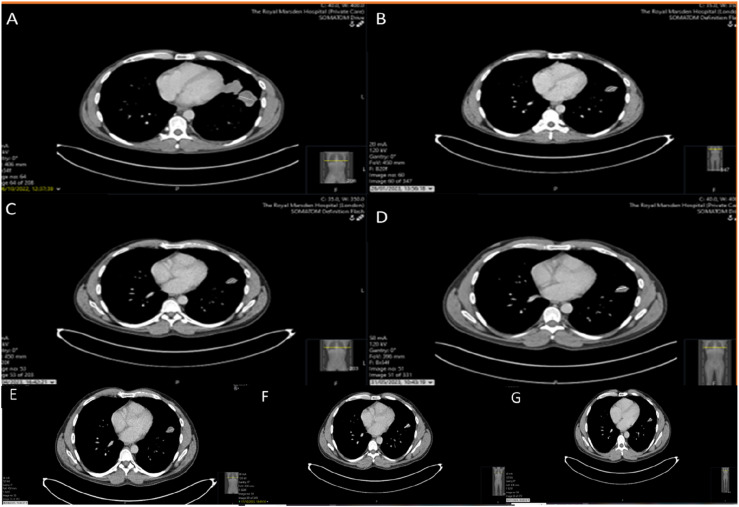
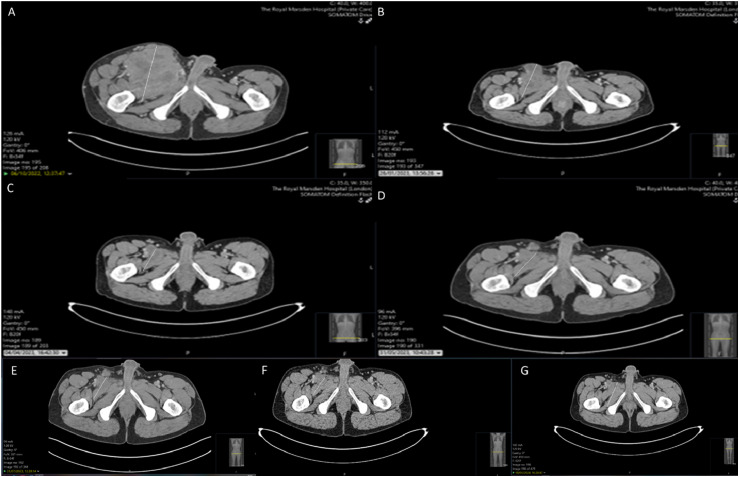
His baseline imaging in October 2022 showed evidence of widespread metastatic disease, with pulmonary, bilateral ilioinguinal nodal and skeletal disease progression. He had re-staging imaging every 3 months and imaging in January 2023 showed partial response to treatment, with a reduction in the size of pulmonary (17 mm vs 40 mm) and nodal (73 mm vs 120 mm) disease, compared to the October 2022 baseline scan (Figure 1 (a) and (b)), and the intramuscular disease within the right iliacus has regressed in size (Figure 2 (a) and (b)). His subsequent imaging in April 2023 showed some further response to therapy in the right groin (4.2 x 4 cm compared to 7.3 x 6 cm in January) and in the left lung (2.5 cm compared to 3 cm in January 2023). However, there was some progression in the right external iliac territory (5.2 x 4.1 cm compared to 2.7 x 2 cm in January 2023) (Figures 1(c) and 2(c)). He continued with nivolumab monotherapy, as there was evidence of mixed response on imaging and his next CT in May 2023 showed overall stable appearances, when compared to the most recent examination, with no features of progressive disease and overall stable pulmonary metastases, measuring up to 25 mm in the left upper lobe (Figures 1(d) and 2(d)). His subsequent imaging in July 2023 showed maintained stable disease, including the left upper lobe lung lesion and the right ilioinguinal adenopathy, with no new sites of disease, compared to previous imaging (Figures 1(e) and 2(e)). Subsequent imaging in October 2023 (Figures 1(f) and 2(f)) and January 2024 (Figures 1(g) and 2(g)) showed minimal changes in the right iliac fossa mass and unchanged appearances elsewhere.
Figure 1.
Serial CT thorax images indicating the effect of Immunotherapy in the left upper lobe lung nodule (a): October 2022, (b): January 2023, (c): April 2023, (d): May 2023, (e): July 2023, (f): October 2023, (g): January 2024.
Figure 2.
Serial CT abdomen and pelvis images indicating the effect of Immunotherapy in the intramuscular disease in the right iliac fossa (a): October 2022, (b): January 2023, (c): April 2023, (d): May 2023, (e): July 2023, (f): October 2023, (g): January 2024.
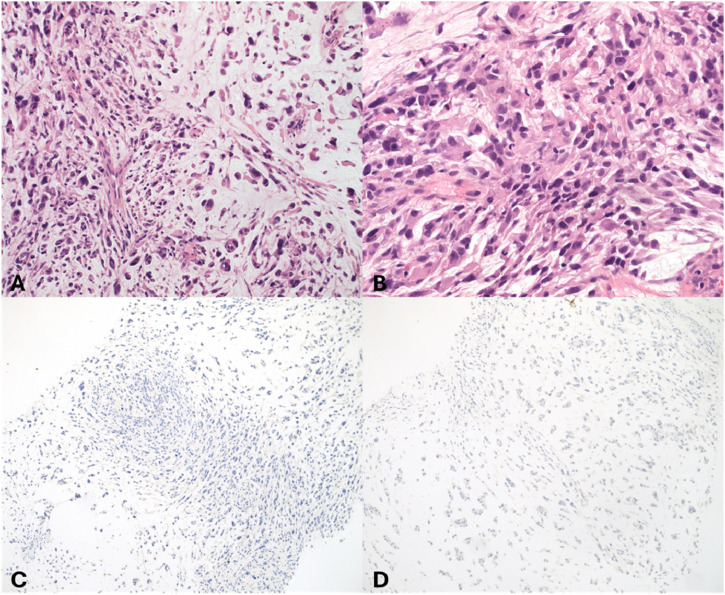
An extensive immunohistochemistry (IHC) panel was undertaken, showing negativity for a wide panel of markers, including CAM5.2, MNF116, CK903, CKAE1/3 (pictured-Figure 3), TTF1, p16, S100, CD34, SMA, desmin (pictured-Figure 3), myogenin, caldesmon and EMA, excluding therefore the diagnosis of sarcomatoid carcinoma, melanoma and sarcomas with specific lines of differentiation, such as leiomyosarcoma and confirming the diagnosis of MFS. Representative immunohistochemistry findings (AE1/AE3 and desmin) have also been included (Figure 3). Overall, the Eosin and Hematoxylin (H&E) images depicted are describing morphological features of the tumour, which were all entirely consistent with a diagnosis of MFS, with evidence of moderate to severely pleomorphic ovoid to spindled cells, embedded in a diffusely myxoid stroma, with scattered intervening curvilinear vessels, in addition to brisk mitotic activity (Figure 3).
Figure 3.
Immunohistochemistry confirming diagnosis of MFS (a) H&E (20X): A tumour composed of moderately pleomorphic spindled cells with eosinophilic cytoplasm, arranged in vague fascicles and embedded in a diffusely myxoid stroma. There are occasional curvilinear vessels also noted. These morphological features support a diagnosis of MFS. (b) H&E (40x): Higher power view demonstrating the degree of atypia seen within the tumour. Occasional mitoses are present. The overall grade is 2: differentiation score = three (i.e. undifferentiated tumour supported by no specific immunophenotype), mitoses score = 2, necrosis score = 0 = 5 (grade 2). (c) AE1/AE3 (IHC, x10): Immunohistochemistry shows no expression of broad spectrum cytokeratins helping to exclude the possibility of sarcomatoid carcinoma. (d) Desmin (IHC, x10): Immunohistochemistry shows no expression of desmin helping to exclude other soft tissue sarcoma subtypes including leiomyosarcoma and rhabdomyosarcoma.
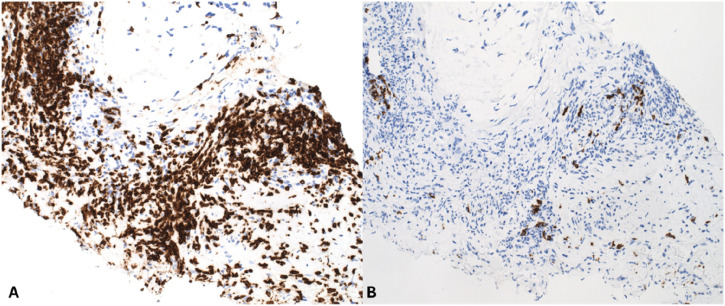
The morphology of the tumour was also investigated, in order to evaluate the grade and mitotic count of the tumour, which was grade 2 (differentiation score = 3, mitoses score = 2, necrosis score = 0: total count = 5), mitotic count 15/10HPF (including atypical forms) and no evidence of coagulative tumour necrosis. CD3 and CD20 immunohistochemistry analysis was also performed, in order to evaluate the degree of immune cell infiltration in this tumour. It was found that there were numerous CD3 positive T-lymphocytes present throughout the tumour, with isolated CD20 positive B-cells also identified (Figure 4).
Figure 4.
Immunohistochemistry of CD3 and CD20 evaluates the degree of immune cell infiltration in the tumour (a) IHC, CD3: CD3 immunohistochemistry showing a dense infiltrate of CD3 positive T-cells. (b) IHC, CD20: CD20 immunohistochemistry showing only scattered CD20 positive B-cells.
Discussion
Overall, we demonstrate the case of a metastatic MFS patient, responding well to third line ipilimumab and nivolumab combination therapy and subsequent nivolumab monotherapy, with partial response initially and maintained stable disease on subsequent imaging, with minimal only changes in the primary large pelvic sidewall mass and stable sites of metastases, without new lesions seen and a manageable toxicity profile, over a period of 12 months. Previously SARC028 trial assessed the use of anti PD-1 antibody pembrolizumab, in a multicentre, single-arm, Phase 2 trial of patients with advanced STS. UPS subtype (MFS was not included in the enrolment), verified objective response in 40% of the patients. 6 ENVASARC trial investigated the role of anti PD-L1 antibody envafolimab as a single agent or in combination with ipilimumab, in locally advanced, unresectable or metastatic UPS/MFS, with preliminary data from the first 20 enrolled patients showing that envafolimab has been well tolerated, both as a single agent and when combined with ipilimumab, with outcomes pending.1,7
A recent study reported excellent long-term response to ipilimumab and nivolumab treatment in a MFS patient, 8 with no expression of PD-L1, who had previously failed multiple lines of systemic treatment. 1 Camrelizumab, a PD-1 inhibitor which was used in a patient with PD-L1 positivity in his pulmonary metastases (40%–50%), showed a PFS of 18 months. 9 Atezolizumab anti-PD-L1 antibody, in combination with oral imidazo-tetrazine alkylating agent temozolomide, has been used in the case of a patient with MFS, and has verified maintained stable disease after 22 cycles of atezolizumab, with high TMB. 10
The importance of tumour microenvironment has been highlighted in terms of heterogeneity of sarcomas and tumour infiltrating lymphocytes (TILs) and immune-related genes (IRGs) can have a role as prognostic and predictive markers of response to immunotherapy treatment. 11
Overall, there is a great need for further studies to show long term and enduring results of the use of immunotherapy, especially in the case of UPS and MFS in particular, with the hope that it can become a standard of care for the treatment of refractory disease.
Conclusion
This report indicates that the combination of ipilimumab and nivolumab is a safe and effective treatment option in the management of metastatic MFS. The results of confirmatory trials of the efficacy and safety of checkpoint inhibitors are awaited.
Acknowledgements
We would like to thank all the staff members of the sarcoma unit and RMH pharmacists and nurses for their support during the patient’s treatment journey.
Footnotes
Contributorship: FK wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employed by Royal Marsden NHS Trust.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical statement
Informed consent
Both verbal and written informed consent was obtained from the patient for their anonymised information to be published in this article.
Open access statement
The open access publication fees were covered by the gold open access route of the Institute of Cancer Research (ICR) library and we thank them for their valuable contribution.
ORCID iDs
Foteini Kalofonou https://orcid.org/0009-0006-4068-3856
Andrea Napolitano https://orcid.org/0000-0002-7509-1555
References
- 1.Vanni S, De Vita A, Gurrieri L, et al. Myxofibrosarcoma landscape: diagnostic pitfalls, clinical management and future perspectives. Ther Adv Med Oncol 2022; 14: 17588359221093973. DOI: 10.1177/17588359221093973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol 2018; 19(3): 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monga V, Skubitz KM, Maliske S, et al. A retrospective analysis of the efficacy of immunotherapy in metastatic soft-tissue sarcomas. Cancers 2020; 12(7): 1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedel R, Chawla S, Druta M, et al. ENVASARC: a pivotal trial of envafolimab and envafolimab in combination with ipilimumab in patients with advanced or metastatic undifferentiated pleomorphic sarcoma or myxofibrosarcoma who have progressed on prior chemotherapy. J Clin Oncol 2023; 41(16_suppl): TPS11583. [Google Scholar]
- 5.Yamamoto N, Hafez N, Tolcher A, et al. A phase Ia/Ib, dose-escalation/expansion study of BI 907828 in combination with BI 754091 (ezabenlimab) and BI 754111 in patients (pts) with advanced solid tumors. J Clin Oncol 2022; 40: 3095.35709436 [Google Scholar]
- 6.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 2017; 18: 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedel RF, Chawla SP, Druta M, et al. ENVASARC: a pivotal trial of envafolimab and envafolimab in combination with ipilimumab in patients with advanced or metastatic undifferentiated pleomorphic sarcoma or myxofibrosarcoma who have progressed on prior chemotherapy. J Clin Orthod 2023; 41: TPS11583. DOI: 10.1200/JCO.2023.41.16_suppl.TPS11583. [DOI] [Google Scholar]
- 8.Zhou M, Bui N, Lohman M, et al. Long-term remission with ipilimumab/nivolumab in two patients with different soft tissue sarcoma subtypes and no PD-L1 expression. Case Rep Oncol 2021; 14: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Y, Min L, Zhou Y, et al. Remarkable response to anti-PD1 immunotherapy in refractory metastatic high-grade myxofibrosarcoma patient: a case report. Medicine (Baltim) 2021; 100: e25262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambden JP, Kelsten MF, Schulte BC, et al. Metastatic myxofibrosarcoma with durable response to temozolomide followed by atezolizumab: a case report. Oncol 2021; 26: 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Recine F, Vanni S, Bongiovanni A, et al. Clinical and translational implications of immunotherapy in sarcomas. Front Immunol 2024; 15: 1378398. DOI: 10.3389/fimmu.2024.1378398. [DOI] [PMC free article] [PubMed] [Google Scholar]