Abstract
The process by which cerebral blood flow (CBF) remains approximately constant in response to short-term variations in arterial blood pressure (ABP) is known as cerebral autoregulation. This classic view, that it remains constant over a wide range of ABP, has however been challenged by a growing number of studies. To provide an updated understanding of the static cerebral pressure-flow relationship and to characterise the autoregulation curve more rigorously, we conducted a comprehensive literature research. Results were based on 143 studies in healthy individuals aged 18 to 65 years. The mean sensitivities of CBF to changes in ABP were found to be 1.47 ± 0.71%/% for decreased ABP and 0.37 ± 0.38%/% for increased ABP. The significant difference in CBF directional sensitivity suggests that cerebral autoregulation appears to be more effective in buffering increases in ABP than decreases in ABP. Regression analysis of absolute CBF and ABP identified an autoregulatory plateau of approximately 20 mmHg (ABP between 80 and 100 mmHg), which is much smaller than the widely accepted classical view. Age and sex were found to have no effect on autoregulation strength. This data-driven approach provides a quantitative method of analysing static autoregulation that can be easily updated as more experimental data become available.
Keywords: Cerebral autoregulation, static autoregulation, cerebral blood flow, arterial blood pressure, autoregulatory curve
Introduction
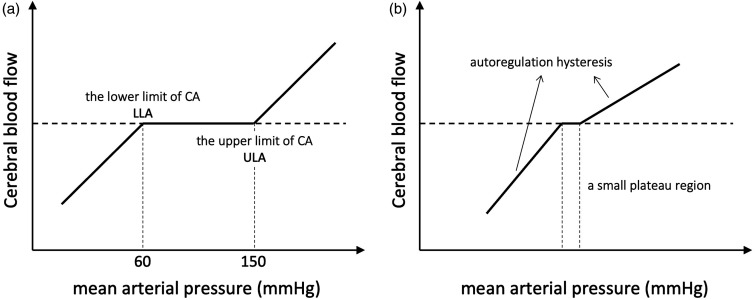
The brain is one of the most tightly regulated organs in the human body. Healthy cerebral blood flow (CBF) and metabolism are essential for the proper functioning of the human brain. CBF regulation is closely related to changes in arterial blood pressure. As early as the 1930s, studies demonstrated in animal models that cerebral blood vessels dilate or constrict in response to reduced or enhanced arterial blood pressure. 1 In 1959, Niels Lassen first constructed the relationship between mean arterial blood pressure (MAP) and CBF using data taken from 11 studies. 2 Based on this analysis, it was proposed that there is a plateau region above the lower limit of autoregulation (LLA), where CBF remains essentially constant across a relatively wide range of MAP (approximately 60–150 mmHg, Figure 1(a)). Due to the fact that Lassen's original publication did not include data at higher blood pressure values, the concept of the upper limit of autoregulation (ULA) was later proposed and validated.3 –5 This physiological relationship was termed static cerebral autoregulation (CA). Subsequent studies in animals, non-human primates and humans have replicated Lassen's curve and confirmed the substantial autoregulatory capacity of the cerebral circulation.5 –8 Prior to this, CBF was assumed to vary passively with perfusion pressure.
Figure 1.
(a) Classical view of autoregulation, first proposed by Lassen. 2 It should be noted that the ULA was only demonstrated later in hypercapnic dogs in 1971. 3 (b) A typical contemporary view of autoregulation showing a small plateau region and the directional sensitivity of cerebral autoregulation. 47
Despite the fact that Lassen's autoregulation curve continues to be cited and described in numerous publications and textbooks, increasing evidence has challenged the existence of this wide cerebral autoregulation plateau.9 –12 In 1983, Heistad and Kontos pointed out that the original study 2 had incorrectly plotted several points in the figure and that some of the experimental data were invalid due to incorrect experimental design. 13 When these erroneous experimental data were corrected, the autoregulation plateau disappeared. Additionally, recent evidence suggests that in healthy humans, the range of MAP within which CBF is maintained constant is much smaller than originally proposed (only ±10% △MAP). 9 Furthermore, due to differences in baseline blood pressure, the upper and lower limits of autoregulation, as well as the plateau region, also vary significantly among individuals. One study captured MAP/CBF relationships individually from 10 hypertensive patients and found that the extent of autoregulatory range varied between patients. 5 Therefore, it remains unknown whether the group-averaged autoregulatory curve would apply to all individuals. 14
Assessment of CA requires simultaneous measurements of CBF and blood pressure (BP). Over the past 20 years, there has been significant development in the study of the human cerebral pressure-flow relationship, with the introduction of CBF quantification techniques that provide high temporal resolution. The use of non-invasive techniques such as transcranial Doppler ultrasound (TCD) and digital arterial volume clamp (such as Finapres) to continuously measure CBF velocity (CBFv) and MAP allowed the dynamic relationship between these two variables to be quantified. CA measurement methods are thus generally divided into two categories: static and dynamic CA. 15 Static CA (sCA) is the autoregulatory response to steady-state changes in ABP or ICP (when mean blood pressure changes to a new level and remains stable for several minutes or hours), providing information on the CPP range in which CA is active. 1 The dynamic cerebral autoregulation (dCA) refers to the instantaneous change in CBF in response to rapid changes in MAP (in a time scale of seconds). Although sCA and dCA operate on different time scales, the CBF regulatory mechanisms involved in these two types of CA may be the same or have some common basis. The differences and similarities between them have been reported in many studies.16,17
However, few attempts have been made to characterise the cerebral pressure-flow relationship over a wide range of MAP due to the lack of methods to alter blood pressure effectively. In the most comprehensive study to date, Numan et al. 18 reviewed 459 studies on static autoregulation through PubMed and Scopus searches. After an exclusion procedure, 49 experiments were included and divided into groups of decreasing MAP (n = 23) and increasing MAP (n = 26). The mean sensitivities to changes in MAP were found to be 0.82 ± 0.77%/% for decreased MAP and 0.21 ± 0.47%/% for increased MAP, which were altered to 0.64 ± 1.16%/% and 0.39 ± 0.30%/% respectively when changes in PaCO2 were corrected for. Their findings suggested that the efficacy of regulating CBF seemed to differ depending on the direction of blood pressure changes, with a more effective capacity to maintain stable CBF during acute (transient) periods of hypertension compared to hypotension. This directional sensitivity of CBF response to MAP will cause the slope of the autoregulation curve to be greater below the LLA than above the ULA, as shown in Figure 1(b). However, the mechanisms governing this are not entirely understood.
It is worth noting that most of the convincing evidence for this asymmetry is based on studies of dCA.19 –22 While Numan et al. 18 also observed directional sensitivity in sCA, several studies included in the review used blood pressure manipulation drugs (such as Dexmedetomidine and Sevoflurane), as well as included some dCA experiments (such as supine-to-stand and cold pressor test), leading to potential confounding factors such as medication effects, autonomic activation, and differences in PaCO2. Thus, the presence of autoregulatory asymmetry in sCA needs to be reconsidered. In addition, in this study, they only considered linear changes in CBF, and did not consider the range of the autoregulatory plateau. They also did not consider the effects of sex or age on the results, largely due to the size of the dataset. With more sCA experimental datasets becoming available, a more detailed analysis can be performed to better understand the directional sensitivity, which may provide better guidance for clinical arterial pressure management.
Therefore, to provide an updated understanding of the static relationship between MAP and CBF, and to characterise the autoregulation curve more rigorously, we conducted a comprehensive literature review and data-driven analysis of static cerebral autoregulation. Firstly, we re-examined the experiments on sCA in Numan et al. 18 and excluded experiments that might involve dCA, and updated more experiments from 2013 to 2023, to explore the relative changes in cerebral pressure-flow response in healthy humans more rigorously. Based on these results, we will investigate the directional sensitivity of sCA and whether this asymmetry persists after CO2 correction, by exploring the CBF response to MAP increases and decreases separately. Secondly, we developed an autoregulation curve in humans using regression analysis in absolute CBF units to quantitatively determine the LLA, ULA and CA plateau. Finally, the effects of sex and age on the cerebral pressure-flow relationship are also investigated.
Methods
In the original analysis by Numan et al., 18 a total of 459 studies published before 2012 were obtained through a PubMed search. 40 studies (49 experiments) were included after exclusion criteria, with 23 experiments lowering MAP and 26 experiments raising MAP. In this updated and expanded review, we re-examined the 459 studies published before 2012. Additionally, we used PubMed, Scopus, and Springer to search for studies with ‘arterial pressure’, ‘cerebral blood flow’ and ‘healthy subjects’ published between January 2013 and September 2022. Non-human studies and non-English studies were excluded, and the selected population was limited to healthy subjects aged 18 to 65. The updated search resulted in the review of an additional 946 studies. As we were also interested in the relationship between absolute CBF and MAP, studies reporting baseline MAP and CBF values in healthy subjects were included in our analysis. Due to the fact that Numan et al.'s 18 study included some dCA experiments, we applied more stringent controls and updates to the exclusion criteria. Based on this, we re-examined the 40 studies already selected by Numan et al. 18 and screened the additional 946 studies to ensure that all experiments included in this analysis strictly followed the sCA assessment.
Exclusion criteria
Parallel changes in other critical variables such as carbon dioxide (CO2), brain activation and sympathetic tone may also be present when manipulating blood pressure. Exclusion procedures were tightly controlled to attenuate and eliminate the adverse effects of these variables on CBF and thereby more accurately determine the cerebral pressure-flow relationship. Multiple experiments have shown that hypocapnia or hypercapnia can affect the range of autoregulation as well as the LLA and ULA. 23 Studies have also shown that middle cerebral artery diameter can be altered in severe hypercapnia (+15 mmHg) resulting in non-negligible errors in CBF measurements, but not in mild hypercapnia (+7.5 mmHg).24,25 Therefore, experimental procedures known to affect the partial pressure of arterial carbon dioxide (PaCO2) levels (e.g. hyperventilation, exercise, altitude and heat stress) and/or experiments with changes in PaCO2 of more than 7.5 mmHg were excluded. In addition, experiments involving cognitive and functional tasks were excluded because blood flow to specific brain regions may be increased through neurovascular coupling. Furthermore, studies that involved dynamic changes in MAP and CBF (e.g., squat-stand manoeuvres, handgrip test or Valsalva manoeuvre), and/or CBF and MAP recordings less than 2 minutes after blood pressure (BP) manipulation were excluded as they do not reflect a steady-state scenario. Only stable steady-state levels of MAP and CBF were included to evaluate the static CA.
Inclusion criteria
The methods for inducing BP changes can basically be divided into pharmacological-induced and postural manoeuvres-induced, but the optimal protocol for studying sCA remains undetermined and the advantages/disadvantages of different methods are still under debate. Postural manoeuvres, mainly achieved through tilting or changes in circulatory blood volume in the study of sCA, have the advantages of being non-invasive and more consistent with physiological conditions, but have limitations such as limited amplitude of BP changes and difficulty in controlling the magnitude and stability of induced changes. Using pharmacological agents to manipulate BP can better control the stability and duration of BP changes. Drugs such as phenylephrine for inducing hypertension or nitroprusside for inducing hypotension have been widely used in experiments evaluating sCA. However, pharmacological methods have limitations due to their invasive nature, and many drugs can activate the autonomic nervous system, potentially interfering with the changes in CBF produced.
To exclude the influence of normal MAP fluctuations on CBF and ensure a reasonable stimulus to activate autoregulation mechanisms, the minimum change in MAP (ΔMAP) was set to ±5% from baseline. Studies should include baseline measurements of CBF and MAP, as well as at least one stable step change in MAP to calculate the relative change slope. Experiments inducing BP changes by postural manoeuvres required stable recording of MAP and CBF(CBFv) for at least 5 minutes to ensure that BP changes reached a stable state. Pharmacological agents used to induce BP changes have been confirmed not to have a direct effect on cerebral vasculature. Detailed descriptions of all blood pressure manipulation methods can be found in Supplementary Table S1.
As shown in Figure 2, after exclusion criteria were applied, a total of 143 studies were included. Multiple experiments within a single study were considered separately, resulting in a total of 148 experiments. Of the 148 experiments, 104 only included baseline CBF and MAP values to explore the relationship between absolute CBF and MAP. 39 studies (44 experiments) with MAP changes (27 experiments with MAP decrease and 17 experiments with MAP increase) were included to investigate the relationship between relative CBF and MAP.
Figure 2.
Flow chart of excluding procedure.
Correction for CO2
Although studies with induced hypercapnia or hypocapnia have been excluded, the effect of uncontrolled changes in PaCO2 persisted in some of the included studies. To eliminate the effect of CO2, CBF or CBFv measured in the middle cerebral artery (MCAv) will be recalculated for studies that report changes in PaCO2. Considering the exclusion process, which limited the variation in PaCO2 within 7.5 mmHg for the included experiments, and the minor differences observed in the baseline PaCO2 of the subjects ranging from 38–42 mmHg, a simple linear correction method is justified. The correction factor used for cerebrovascular reactivity to PaCO2 was 4%/mmHg in hypercapnia and 3%/mmHg in hypocapnia. 26 Following the calculation of CO2-corrected CBF, the CBF/MAP slope will be recalculated.
Statistical analysis
All statistical analyses were performed using SPSS (IBM SPSS Statistics Version 25.0) and presented as mean ± standard deviation (SD). For each experiment, the relative changes in MAP and CBF from baseline was calculated. The slopes were calculated with linear regression between changes in %MAP and %CBF, and mmHg (MAP) and %CBF. The derived ‘average slope line’ was weighted to the number of subjects in each study. Specifically, each study slope line was multiplied by the sample size. These values were then summed and divided by the pooled sample size (total number of pooled subjects). The Shapiro-Wilk test was applied to verify the normal distribution for each variable. Differences between the slopes during increased or decreased MAP were evaluated using a Mann–Whitney U-test. We used p-values to determine if there was a statistically significant difference between the means of two groups, and used Cohen's d to measure the effect size and indicate how meaningful the relationship between variables or the difference between groups is. All p-values of <0.05 were considered statistically significant. Pearson's product-moment correlation coefficients were used to determine the correlation between the different variables. Based on our physiological understanding of autoregulation characteristics, we chose linear regression and a third-order polynomial function to fit the relationship between the absolute CBF and MAP to maximise goodness of fit. The Curve Fitting Toolbox in MATLAB was used for conducting regression analysis, while the fitting tool in SPSS was used for secondary validation.
Results
From all the articles reviewed, 143 studies were included in this study. Experiments with changes in MAP (n = 44) were divided into decreasing MAP (n = 27) and increasing MAP (n = 17) to investigate the directional sensitivity of CA. All experimental data were used to fit the autoregulation curve.
Methods for continuous arterial pressure measurement can vary among studies. Most studies recorded blood pressure non-invasively using photoplethysmography (Finapres). The Finapres device uses a finger cuff and provides an estimation of the continuous ABP waveform based on the principle of arterial volume clamping. 27 Other methods included auto-inflatable cuff (non-invasive) or intravascular catheters (invasive). Although there are various techniques for measuring CBF, over 90% studies used TCD to explore the cerebral pressure-flow response. Other methods included positron emission tomography (PET) and Kety Schmidt technique. The combination of TCD and photoplethysmography (Finapres) measurements was the primary choice for examining changes in CBF caused by BP alterations. In all included experiments, blood pressure was altered within a relatively limited extent (see Table 1 for details), varying only between 54 and 138 mmHg. Note that this range did not reach the upper limit of the classical autoregulation curve. 4
Table 1.
Summary of the included studies on sCA.
| First Author | Year | BP direction | Method measure CBF(v) | Method measure BP | Method to change BP | Baseline BP (mmHg) | Baseline CBF | End BP | End CBF | △PaCO2 (mmHg) | Correct CBF | Number of subjects | Mean age subjects | Slopes (%/%) | Slopes (mmHg/%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kurazumi | 2022 | increase | TCD | Radial artery | NS-15 infusion | 79.0 | 67.3 | 86.0 | 72.1 | −1.0 | 74.3 | 12 | 21 | 0.80 | 1.02 | 48 |
| Pernice | 2022 | decrease | TCD | photoplethysmography | HUT | 98.5 | 71.5 | 92.9 | 61.0 | – | – | 13 | 27 | 2.57 | 2.60 | 49 |
| Bari | 2022 | decrease | TCD | Radial artery | HUT | 99.3 | 47.9 | 68.9 | 37.1 | 0.0 | 37.1 | 13 | 27 | 0.73 | 0.74 | 50 |
| Rosenberg | 2021 | decrease | TCD | photoplethysmography | LBNP | 94.1 | 64.5 | 85.1 | 53.0 | −6.1 | 62.7 | 52 | 26 | 1.86 | 1.98 | 51 |
| Rosenberg | 2021 | decrease | TCD | photoplethysmography | LBNP | 95.4 | 64.4 | 89.4 | 56.2 | −3.7 | 62.4 | 19 | 27 | 2.02 | 2.12 | 51 |
| Robertson | 2020 | decrease | TCD | volume-clamp method | Tilt | 80.0 | 75.0 | 70.0 | 67.0 | −2.0 | 71.0 | 13 | 21 | 0.85 | 1.07 | 52 |
| Robertson | 2020 | decrease | TCD | volume-clamp method | Tilt | 85.0 | 62.0 | 72.0 | 46.0 | −3.0 | 50.1 | 14 | 22 | 1.69 | 1.99 | 52 |
| Stok | 2019 | decrease | TCD | photoplethysmography | HUT | 77.0 | 61.0 | 60.0 | 52.0 | −4.0 | 58.2 | 22 | 40 | 0.67 | 0.87 | 53 |
| Schlotman | 2019 | decrease | TCD | photoplethysmography | LBNP | 94.0 | 67.7 | 83.0 | 51.5 | – | – | 107 | 29 | 2.04 | 2.18 | 54 |
| Schlotman | 2019 | decrease | TCD | photoplethysmography | LBNP | 92.0 | 75.4 | 81.0 | 60.3 | – | – | 85 | 27 | 1.67 | 1.82 | 54 |
| Yoshida | 2018 | increase | TCD | photoplethysmography | LBPP | 87.0 | 62.0 | 92.1 | 63.8 | 2.6 | 57.2 | 14 | 24 | 0.50 | 0.57 | 55 |
| van Helmond | 2018 | decrease | TCD | brachial artery | LBNP | 103.0 | 62.0 | 86.0 | 54.0 | −1.0 | 55.6 | 10 | 32 | 0.78 | 0.76 | 56 |
| van den Brule | 2018 | decrease | TCD | Radial artery | LPS injection | 91.0 | 69.9 | 78.9 | 59.0 | – | – | 10 | 23.3 | 1.17 | 1.29 | 57 |
| van der Scheer | 2018 | increase | TCD | upper-arm cuff | Cold stress | 85.0 | 63.0 | 90.0 | 64.0 | – | – | 6 | 40 | 0.27 | 0.32 | 58 |
| Bronzwaer | 2017 | decrease | TCD | photoplethysmography | LBNP | 96.0 | 59.0 | 88.0 | 44.0 | −5.0 | 50.6 | 14 | 56 | 3.05 | 3.18 | 59 |
| Lund | 2017 | decrease | TCD | brachial artery | HUT | 87.1 | 51.4 | 57.2 | 32.2 | – | – | 20 | 25 | 1.09 | 1.25 | 60 |
| Bronzwaer | 2017 | decrease | TCD | plethysmography | HUT | 95.0 | 70.0 | 76.0 | 60.0 | −3.3 | 65.9 | 10 | 22 | 0.71 | 0.75 | 61 |
| Ogawa | 2016 | decrease | TCD | Radial artery | Gravity | 79.0 | 65.0 | 54.0 | 60.0 | −2.0 | 63.6 | 10 | 23 | 0.24 | 0.31 | 62 |
| Kay | 2016 | decrease | TCD | photoplethysmography | LBNP | 96.5 | 61.4 | 88.1 | 44.1 | −3.3 | 48.5 | 7 | 26 | 3.24 | 3.35 | 63 |
| Rickards | 2015 | decrease | TCD | brachial artery | LBNP | 94.0 | 70.0 | 86.0 | 61.5 | −2.0 | 65.2 | 9 | 31 | 1.43 | 1.52 | 64 |
| Perry | 2014 | increase | TCD | photoplethysmography | LBPP | 83.7 | 62.0 | 93.0 | 59.7 | −1.0 | 61.5 | 15 | 28 | −0.33 | −0.40 | 65 |
| Del Pozzi | 2014 | increase | TCD | photoplethysmography | Phenylephrine | 78.0 | 71.0 | 87.0 | 75.0 | 1.0 | 73.5 | 12 | 23 | 0.49 | 0.63 | 66 |
| Shaw | 2014 | decrease | TCD | photoplethysmography | HUT | 84.2 | 61.9 | 70.4 | 40.6 | −2.5 | 43.6 | 15 | 26.3 | 2.10 | 2.49 | 67 |
| Lewis | 2014 | decrease | TCD | photoplethysmography | LBNP | 89.0 | 66.3 | 71.7 | 60.3 | 0.0 | 60.3 | 24 | 25 | 0.46 | 0.52 | 68 |
| Perry | 2013 | increase | TCD | photoplethysmography | LBPP | 81.0 | 74.0 | 94.0 | 72.0 | 0.0 | 72.0 | 15 | 26 | −0.17 | −0.21 | 69 |
| Stewart | 2013 | increase | TCD | photoplethysmography | Phenylephrine | 78.0 | 71.0 | 87.0 | 75.0 | 1.0 | 73.5 | 12 | 18–24 | 0.49 | 0.63 | 70 |
| Schlader | 2013 | decrease | TCD | brachial artery | LBNP | 89.0 | 59.6 | 71.7 | 45.2 | −6.0 | 53.3 | 8 | 29 | 1.25 | 1.40 | 71 |
| Stewart | 2013 | decrease | TCD | photoplethysmography | SNP | 78.0 | 71.0 | 61.0 | 62.0 | −3.0 | 67.6 | 12 | 18–24 | 0.58 | 0.75 | 70 |
| Guo | 2013 | decrease | TCD | auto-inflatable cuff | SNP | 80.7 | 67.8 | 75.6 | 62.7 | −4.9 | 71.9 | 5 | 24 | 1.19 | 1.47 | 72 |
| Chan | 2011 | decrease | TCD | Photoplethysmography | SNP | 81.0 | 71.0 | 59.0 | 65.0 | – | – | 14 | 25 | 0.46 | 0.61 | 73 |
| Chan | 2011 | increase | TCD | Photoplethysmography | Phenylephrine | 83.0 | 75.0 | 101.0 | 80.0 | – | – | 14 | 25 | 0.30 | 0.37 | 73 |
| Ogoh | 2011 | increase | TCD | Brachial Artery | Phenylephrine | 77.0 | 50.0 | 97.0 | 52.0 | 1.5 | 48.9 | 8 | 26 | 0.15 | 0.20 | 74 |
| Ogawa | 2010 | decrease | TCD | Radial artery | Propofol | 77.0 | 63.0 | 70.0 | 58.0 | 0.0 | 58.0 | 10 | 22 | 0.87 | 1.13 | 75 |
| Tzeng | 2010 | decrease | TCD | Photoplethysmography | SNP | 84.0 | 70.0 | 63.0 | 58.0 | – | – | 10 | 26 | 0.69 | 0.82 | 22 |
| Tzeng | 2010 | increase | TCD | Photoplethysmography | Phenylephrine | 84.0 | 70.0 | 103.0 | 77.3 | – | – | 10 | 26 | 0.46 | 0.55 | 22 |
| Brassard | 2010 | increase | TCD | Left brachial artery | Phenylephrine | 84.0 | 66.6 | 108.0 | 70.5 | 0.8 | 68.2 | 9 | 23 | 0.22 | 0.29 | 76 |
| Hussain | 2009 | decrease | PET | / | ATP | 84.3 | 56.0 | 78.8 | 54.0 | – | – | 10 | 24 | 0.55 | 0.65 | 77 |
| Zhang | 2009 | increase | TCD | Photoplethysmography | Phenylephrine | 83.0 | 63.0 | 114.0 | 70.0 | 0.0 | 70.0 | 13 | 30 | 0.30 | 0.36 | 12 |
| McCulloch | 2005 | increase | TCD | Radial artery | Phenylephrine | 81.0 | 74.0 | 101.0 | 84.4 | – | – | 12 | 21–59 | 0.57 | 0.70 | 38 |
| Lavi | 2003 | decrease | TCD | Tonometry | SNP | 85.0 | 65.0 | 78.0 | 64.0 | – | – | 17 | 27 | 0.19 | 0.22 | 78 |
| Hjorth Lassen | 2003 | increase | TCD | Sphygmomanometer | L-NMMA | 80.2 | 71.5 | 95.8 | 73.0 | 0.0 | 73.0 | 6 | 25 | 0.14 | 0.18 | 79 |
| McCulloch | 2000 | increase | TCD | Radial artery | Phenylephrine | 80.0 | 59.9 | 101.0 | 68.9 | – | – | 8 | 22–52 | 0.57 | 0.72 | 80 |
| Kawai | 1993 | increase | TCD | Sphygmomanometer | HDT | 90.0 | 55.5 | 99.0 | 63.2 | – | – | 8 | 36 | 1.39 | 1.54 | 81 |
| Jobes | 1975 | increase | Kr-uptake technique | Radial artery | Phenylephrine | 88.0 | 38.9 | 120.0 | 49.5 | 0.0 | 49.5 | 8 | / | 0.75 | 0.85 | 82 |
Mean age is averaged value or range. Slopes presented in % MAP/% CBF and mmHg MAP/% CBF. TCD: transcranial Doppler ultrasound; PET: positron emission tomography; HUT: head-up tilt; LBNP: lower body negative pressure; LBPP: lower body positive pressure; SNP: sodium nitroprusside; ATP: adenosine triphosphate.
Relative CBF
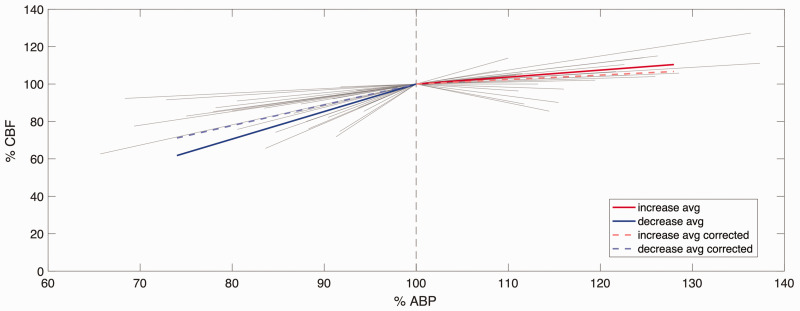
For each experiment, the percent change from baseline was calculated for MAP and CBF. The slopes were calculated with linear regression between changes in %MAP and %CBF, and mmHg (MAP) and %CBF. 18 In the studies where MAP decreased, the calculated slopes were found to lie between 0.19 and 3.24%CBF/%MAP. The average slope for decreased MAP was 1.47 ± 0.71%CBF/%MAP (or 1.61 ± 0.73%CBF/mmHg MAP). In the studies where MAP was increased, the slopes ranged between −0.33 and 1.39%CBF/%MAP with an average slope of 0.37 ± 0.38%CBF/%MAP (or 0.45 ± 0.45%CBF/mmHg MAP).
A significant difference in CBF/MAP slope was observed between increasing and decreasing MAP in both relative and absolute measures, as shown in Figure 3 (both p < 0.001; d = 1.93 for %CBF/%MAP comparison, and d = 1.91 for %CBF/mmHg MAP comparison), which is plotted in the same form as Numan et al. 18 for ease of comparison. 8 experiments in which MAP was decreased and 9 experiments in which MAP was increased had changes in MAP from baseline greater than 20%. 10 experiments in which MAP was decreased had changes in MAP between 5 and 10%, whereas 3 experiments that had increases in MAP showed a similar level of change. The remaining 9 included experiments in the decreasing MAP group and 5 remaining experiments in the increasing MAP group showed a percentage change in MAP between 10 and 20%.
Figure 3.
Relationship between %MAP and %CBF in the increased MAP and decreased MAP ranges. All individual lines represent individual experiments (not corrected for CO2); the length of the line indicates the range of MAP of that experiment. Before CO2 correction, average slope (solid line) was 1.47%MAP/%CBF for the decreased MAP (n = 27), and 0.35%MAP/%CBF for increased MAP (n = 17). After CO2 correction, the average slope (dashed line) was 1.11%MAP/%CBF for decreasing MAP (n = 18) and 0.24%MAP/%CBF for increasing MAP (n = 11).
CO2 correction
18 experiments with decreasing MAP and 11 experiments with increasing MAP reported values for PaCO2 (or partial pressure of end-tidal CO2), which allowed for a post-hoc PaCO2 correction for changes in CBF. After calculating the CO2-corrected CBF, the CBF/MAP slopes were recalculated as described above. To quantify the influence of the CO2 correction factor, the CBF/MAP slopes in these selected studies were also calculated prior to correction. Following correction, the slopes for the decreasing MAP range varied between −0.96 and 2.58%CBF/%MAP (mean 1.11 ± 0.78%CBF/%MAP, 1.21 ± 0.65%CBF/mmHg MAP). The slopes in the increasing MAP range post correction varied between −0.41 and 1.39%CBF/%MAP (mean 0.24 ± 0.60%CBF/%MAP, 0.29 ± 0.50%CBF/mmHg MAP). Consistent with the uncorrected slope results, the corrected slopes also showed significant differences in the range of increasing and decreasing MAP (p = 0.003; d = 1.25), as shown by the dashed lines in Figure 3. In addition, the CO2 correction had a significantly greater effect on experiments with decreasing MAP than on experiments with increasing MAP due to the different sensitivity to CO2 in each direction.
Manipulation and measurement methods
The CBF measurement methods used in different studies were varied. Of the 44 experiments included in this analysis with MAP changes, 42 used TCD. The slope produced by measuring CBF with methods other than TCD was 0.55%CBF/%MAP (MAP decreased) and 0.75%CBF/%MAP (MAP increased), while the average slope measured using TCD was 1.49 ± 0.71%CBF/%MAP (MAP decreased) and 0.35 ± 0.38%CBF/%MAP (MAP increased). Since only 2 experiments used non-TCD methods to study CA, it is not yet known whether different CBF measurement methods lead to differences in slopes.
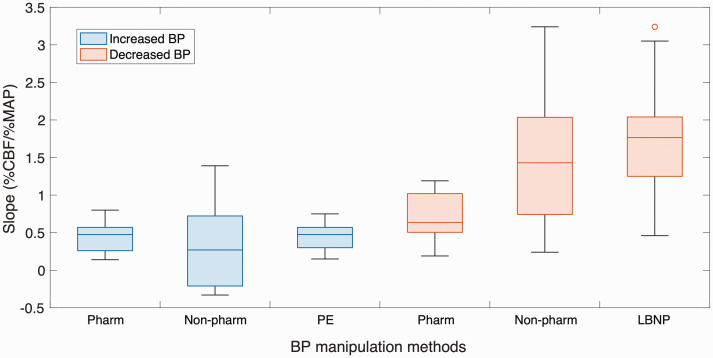
Of the 17 experiments that increased MAP, the most frequently used experimental method to manipulate blood pressure was phenylephrine (pharmacological). 5 experiments using non-pharmacological BP manipulation methods yielded mean slopes of 0.21 ± 0.58%CBF/%MAP, while 12 experiments using pharmacological BP manipulation methods yielded mean slopes of 0.45 ± 0.20%CBF/%MAP. Significant differences were found with %CBF/%MAP between the different BP increasing manipulation methods (p < 0.001; d = 0.55). Of the 27 experiments that decreased MAP, the most frequently used experimental method to manipulate blood pressure was lower body negative pressure (LBNP, non-pharmacological). 19 experiments using non-pharmacological BP manipulation methods yielded mean slopes of 1.63 ± 0.65%CBF/%MAP, while mean slopes with pharmacological BP manipulation methods were 0.63 ± 0.32%CBF/%MAP. Significant differences were found with %CBF/%MAP between the different BP decreasing manipulation methods (p < 0.001; d = 1.95).
The statistical differences observed between pharmacological and non-pharmacological BP manipulation methods indicated that different methods have different levels of reproducibility and stability. Regardless of whether the experiment induced an increase or decrease in MAP, a tighter slope distribution was observed for pharmacological manipulation methods, indicating higher reproducibility as shown in Figure 4. However, studies of dCA have also shown that manoeuvres such as the sit-to-stand are biologically acceptable and reproducible for creating oscillations in blood pressure, and are thus most representative when compared to other techniques.28,29 This suggested that using drugs to induce BP changes has an advantage in longer time-scale sCA studies, whereas postural changes to induce BP changes are more advantageous in shorter time-scale dCA studies. It should also be noted that any pharmacological approach has the inherent limitation that the drugs may have multiple unknown effects on the cerebral vasculature. Therefore, when choosing a BP manipulation method, it is important to evaluate and choose based on the specific research design and purpose.
Figure 4.
Slope distribution of different blood pressure manipulation methods. Experiments inducing BP increase are represented by blue boxplots, while experiments inducing BP decrease are represented by orange boxplots. Non-pharm, non-pharmacological BP manipulation methods; Pharm, pharmacological BP manipulation methods; PE, phenylephrine; LBNP, lower body negative pressure.
Absolute CBF
Although direct comparison of absolute CBF (or velocity) changes between measurement techniques is not possible, over 90% of all experiments included in this study used CBFv (MCAv) as an indicator of CBF measured by TCD. To obtain sufficient experimental data to fit the absolute cerebral pressure-flow relationship, we hypothesised that CBFv (MCAv) could be used as a surrogate CBF measure. The limitations of this assumption will be discussed later.
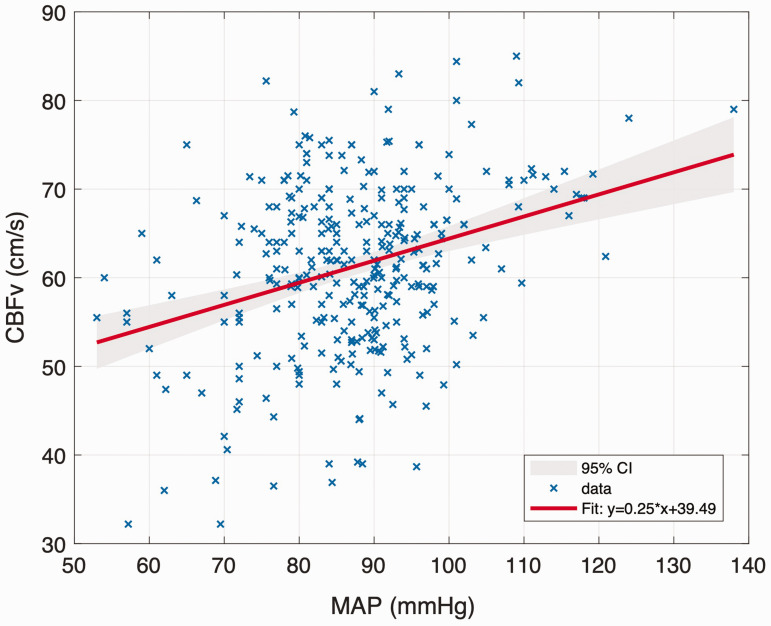
A significant positive correlation was observed between MAP and absolute CBFv (r = 0.363; p < 0.001), as shown in Figure 5. MAP varied between 53 and 138 mmHg but did not reach the upper limit of autoregulation proposed by Lassen.
Figure 5.
First-order linear regression model for MAP and CBFv. The grey shading between these bounds reflects the 95% confidence level.
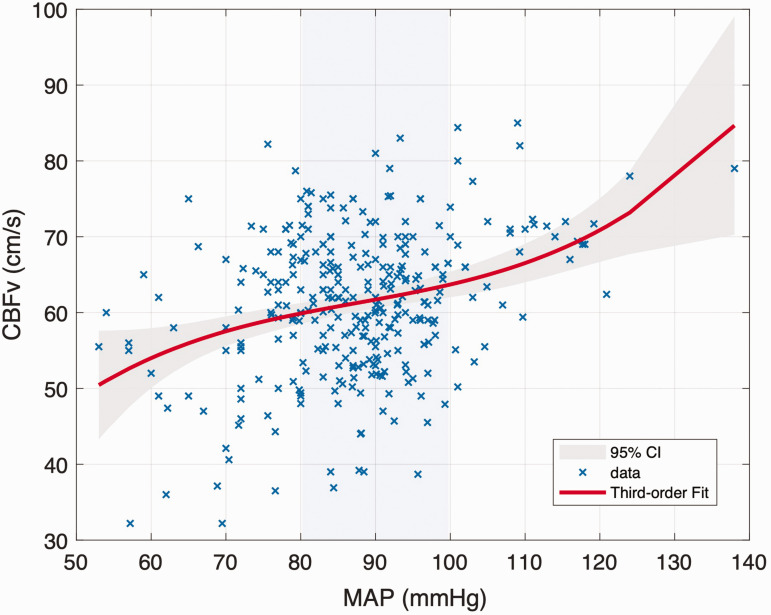
Compared to the first-order linear model, the fit quality of the third-order polynomial function was improved (first order: R2 = 0.1320 vs. third order: R2 = 0.1341), although this would be expected given the additional degrees of freedom. A relatively flat ‘autoregulatory plateau' was observed from the third-order polynomial model, as shown in Figure 6. After differentiation of the function, we found that CBF remained essentially stable when MAP varied between 80 and 100 mmHg. To further quantify this behaviour, the correlations between MAP and CBF were recalculated over different blood pressure ranges, as indicated in Supplemental Figure S4. If the relationship between pressure and flow is purely passive, the correlation coefficients should increase monotonically or remain essentially constant. However, the correlation between blood pressure and flow was first found to increase, then to decrease and then to increase again, with the two turning points being observed at 80 and 100 mmHg, as shown by the shaded area in Supplemental Figure S4. This indicates that this range of MAP appears to act as the ‘plateau’ region for static cerebral autoregulation, supporting the claims that this region is a relatively small one.
Figure 6.
Third-order polynomial function regression model for MAP and CBFv. The grey shading between these bounds reflects the 95% confidence level. The blue shading between 80∼100 mmHg reflects the autoregulatory plateau.
To better understand the relationship between MAP and CBF, correlation and linear regressions were further performed according to data points below 80 mm Hg, 80 to 100 mm Hg and above 100 mm Hg. This segmentation also ensured that sufficient experimental data were available for analysis in each blood pressure range. In the MAP range of 80 to 100 mmHg, the correlation coefficient between MAP and CBF was only 0.003 (p = 0.952), indicating that the CBF was largely unaffected by MAP and showed significant autoregulatory characteristics. However, it is worth noting that within this range, the linear regression analysis between MAP and CBF still demonstrated a small slope instead of a horizontal line with zero slope. For experimental data in the MAP range below 80 mmHg or above 100 mmHg, the correlation coefficients between MAP and CBF were found to be 0.359 and 0.332, respectively (p = 0.001, p = 0.068). This suggests that when MAP exceeds 100 mmHg or is lower than 80 mmHg, autoregulation mechanisms are no longer able to maintain CBF stability, and CBF will increase with increasing blood pressure. The slope of the linear regression analysis for the different blood pressure ranges showed that CBF was more sensitive to BP decrease than BP increase (0.515 for BP range below 80 mmHg; 0.301 for BP range above 100 mmHg;), which was consistent with the results of the relative analysis.
The influence of age
Age and biological sex remain important modifiers of cerebrovascular function in health and disease, but their impact on cerebral blood pressure and flow remains unclear. In this review, 91 experiments reported mean age for subjects and 67 experiments reported haemodynamic related data by sex, which allowed us to explore the effect of sex and age on cerebrovascular and autoregulation.
There were significant differences in baseline vascular measures between young (18–40 years old; number of subjects = 2854; mean age = 28) and older (40–65 years old; number of subjects = 1341; mean age = 50) participants. At baseline, MAP was significantly higher in older participants (young: 88.08 ± 6.72 mmHg vs. older: 90.96 ± 3.72 mmHg). MCAv was 10.2% lower in older participants (young: 63.20 ± 7.45 cm/s vs. older: 56.78 ± 6.14 cm/s).
To further explore the correlation between age and baseline blood pressure, a regression analysis was performed (see Supplemental Figure S4). The results showed that there was a statistically significant positive correlation between age and baseline MAP, i.e., as age increased, baseline MAP also increased (r = 0.198). The linear relationship established between the independent variable age and the dependent variable MAP at baseline was extremely statistically significant, but the goodness of the regression was poor (R2 = 0.038; p = 0.009). A statistically significant negative linear correlation was also found between baseline CBF and age (see Supplemental Figure S5), whereby baseline CBF decreased with increasing age (r = −0.101; p < 0.001). However, the goodness of the regression was poor (R2 = 0.131).
Both the Pearson correlation coefficient and regression analysis outputs showed there was no relationship between age and absolute and relative slopes (r = 0.009). No age differences were observed in the cerebral haemodynamic response to changes in pressure suggesting that aging itself did not alter cerebral autoregulation, in good agreement with the substantial literature on the effect of aging on dynamic CA.14,30,31
The influence of sex
17 experiments included only male subjects and 3 experiments included only female subjects, which allowed us to examine sex differences in CBF autoregulation. There were no significant differences in the demographic and haemodynamic characteristics of study participants at baseline between biological sex categories. No sex differences were found for age (male: 26 ± 4 years vs. female: 27 ± 5 years; p = 0.776; d = 0.22). Although the results were consistent with previous studies in that female subjects had a lower mean BP and a higher CBF than males, 32 no statistically significant gender differences were found (MAP: male = 88.48 ± 6.74 mmHg vs. female = 87.77 ± 6.19 mmHg; p = 0.583; d = 0.11. CBF: male = 60.11 ± 7.58 cm/s vs. female = 63.34 ± 11.14 cm/s; p = 0.070; d = 0.34). Pearson correlation coefficients also confirmed that sex was not associated with baseline MAP or CBF (r = 0.045; r = −0.056, respectively).
Since there were no experiments conducted solely on female participants to induce an increase in MAP, it is unclear whether the slope of increased BP is influenced by gender. In experiments inducing a decrease in MAP, 17 studies included only male participants while 3 studies included only female participants. In the male group, the slopes for the decreasing MAP ranged from 0.15 to 2.04%CBF/%MAP (mean 1.29 ± 0.69%CBF/%MAP, 1.42 ± 0.71%CBF/mmHg MAP). In the female group, the slopes for the decreasing MAP ranged from 0.85 to 1.69%CBF/%MAP (mean 1.58 ± 0.26%CBF/%MAP, 1.75 ± 0.26%CBF/mmHg MAP). No significant difference in CBF/MAP slope was observed between female and male in both relative and absolute measures (p = 0.382 for %CBF/%MAP comparison, p = 0.371 for %CBF/mmHg MAP comparison). Therefore, the study results indicated that the cerebrovascular response to decrease in BP has no sex differences, although given the small number of studies performed in women this finding should be treated with caution at this stage.
Discussion
Directional sensitivity of cerebral autoregulation
A stronger autoregulatory response was observed during the increase in blood pressure compared to the decrease in sCA, consistent with former studies and findings in dCA.9,18,19,21,22,33 However, our findings differ from those of Numan et al. 18 in that this asymmetry in autoregulation did not disappear after CO2 correction. Compared to 2014, we have employed stricter exclusion criteria to eliminate interference from some dCA study results, thus confirming the presence of directional sensitivity in static CA. Although CBF remained more sensitive to blood pressure reduction after CO2 correction, the CO2 correction eliminated the significant difference in CBF response to increased and decreased MAP (the CBF to changes in ABP were found to be 1.47 ± 0.71%/% for decreased ABP and 0.37 ± 0.38%/% for increased ABP, which were altered to 1.11 ± 0.78%/% and 0.24 ± 0.60%/% after CO2 correction). The greater effect of CO2 correction on CBF regulation for reduced MAP may be related to the activation of metabolic pathways during hypoperfusion. It should be noted that a positive correlation between changes in blood gas levels and the capacity of autoregulation was observed both before and after CO2 correction. A larger magnitude of PaCO2 variations was associated with an increased slope of %CBF/%MAP. This finding suggests that different CBF regulatory mechanisms do not operate in isolation, as metabolic pathways can influence autoregulation. The interdependence of CBF regulation highlights the robustness of the cerebrovascular system in ensuring precise control of CBF, while also emphasizing the challenges in isolating and studying specific CBF regulatory mechanisms.
Several potential mechanisms have been proposed to explain the directional sensitivity of CA, including selective cerebral vasoconstriction secondary to activation of the perivascular cerebral sympathetic nerves, as well as intrinsic differences in myogenic responses to falling and rising MAP. 22 Another possible explanation might be that compensatory vasodilatation during MAP decrease may increase intracranial pressure, aggravating MAP decrease and reducing the benefit of lowered blood flow resistance. 34 Previous studies have shown that a substantial MAP change of approximately 50% from baseline is required to trigger sympathetic nervous system activation. 35 However, our research findings suggest that directional sensitivity of CA is independent of the magnitude of MAP changes, consistent with the findings of Panerai et al. 36 regarding dynamic CA. The activation of cerebral sympathetic nerve activity alone is therefore insufficient to explain this phenomenon. Directional sensitivity is more likely determined by the intrinsic properties of vascular smooth muscle cells, which serves to protect the brain from the risk of hyperperfusion during MAP increases and subsequently prevent oedema and capillary damage.
Although the physiological mechanisms of autoregulatory asymmetry remain unclear and part of the explanation is based on evidence from animal studies, the finding of autoregulatory asymmetry has clinical implications. The differential CA response between increasing and decreasing MAP in humans was initially reported in patients with traumatic brain injury, 33 suggesting that directional sensitivity may be enhanced in pathological conditions. This indicates the potential for quantifying CA directional sensitivity as a clinical indicator for predicting cerebral diseases. Given the brain heterogeneity and the significant individual variability in autoregulation, further research and clinical trials are needed to make CA assessment a reliable clinical tool.
Limitations
Potential limitations of this study should be considered. We used the linear regression slope between MAP and CBF (MCAv) to compare the effectiveness of autoregulation for falling and rising blood pressure, which assumed a linear response of cerebral circulation to blood pressure perturbations. This simple approach is a compromise to the limited studies describing the relationship between MAP and CBF. The lack of experimental data is mainly due to the technical limitations in manipulating blood pressure and recording CBF.
Difficulties in blood pressure manipulation
It is challenging to manipulate MAP over a sufficiently wide range in human subjects. The experimental methods of manipulating blood pressure included in this study can be basically divided into physical and pharmacological methods. These experimental methods can produce fluctuations in mean arterial pressure of about 5 to 30 mmHg, which are largely buffered via cerebral autoregulation. Normal baroreflex function limits the effective range within which MAP can be altered normally. Therefore, we could only analyse the cerebral pressure-blood flow relationship based on experimental data of MAP between 53 and 138 mmHg, which cannot fully cover the autoregulatory plateau of 60 to 150 mmHg proposed by Lassen 2 and Paulson et al. 4
Pharmacological methods can perturb blood pressure by intravenous injection of vasodilator and/or vasoconstrictor drugs, such as nitroprusside and phenylephrine. The obvious advantage of the pharmacological approaches is that the response of CBF to rising and falling MAP can be studied simultaneously by injecting different drugs. In addition, pharmacological methods allow blood pressure to be altered over a relatively long period of time, as opposed to physical actions that tend to stimulate the cerebral circulation abruptly, resulting in more stable and accurate measurements of MAP and CBF. However, many studies have pharmacologically manipulated CBF with anaesthetics, angiotensin and α-adrenergic receptor agonists and/or nitric oxide donors,10,12,37,38 and their effects on cerebrovascular tone are controversial; for example, the drugs used to manipulate MAP may have as yet unknown effects on the autoregulation response by directly altering cerebrovascular resistance downstream from the middle cerebral arteries.
Physical methods of blood pressure manipulation include tilt (head-up tilt), LBNP, etc. Of these, tilt tests can induce the greatest fluctuations in MAP, up to 43% change in MAP. Physical methods can only induce unidirectional hypotension or hypertension, and therefore cannot reliably explore autoregulatory asymmetry. In addition, the stability and reproducibility of blood pressure changes induced by physical methods are poor. However, physical methods are still widely used in dCA studies as they can noninvasively and quickly change blood pressure, and have minimal direct effects on vascular tone or indirect effect on arterial CO2 or cerebral metabolism, independently from cerebral autoregulation. Although accurate measurement of MAP and CBF is crucial for clinical purposes, so far there is no “gold standard” for autoregulation assessment. There is also no evidence to suggest significant differences in conclusions obtained from different assessment methods.
Accuracy of CBF measurements
CBF measurement techniques commonly used for static autoregulation studies in humans include TCD, Kety Schmidt technique, 133Xenon clearance or other indicator methods, arterio-venous O2 difference, near-infrared spectroscopy and X-ray imaging.18,39 Notably, the experiments reviewed in this study primarily used TCD to measure CBF. Using MCAv as an index of CBF, requires the assumption that the diameter of the artery remains constant. Multiple studies in humans have shown that the diameters of the middle cerebral artery and other large cerebral vessels remain relatively constant despite changes in ABP and blood gases.40 –42 Furthermore, changes in MCAv were highly correlated with CBF changes measured by two MRI techniques using the tracer gadolinium and arterial spin labelling, again validating TCD as a representative assessment of CBF. 43 Given the strict exclusion criteria employed in this study to limit the influence of factors such as blood gases changes on cerebrovascular, it can be assumed that the diameter of cerebral arteries remains relatively constant. Therefore, in the present study, we suggest that the cerebral blood flow velocity measured by TCD may accurately reflect the response to CBF.
Another limitation of TCD is that it measures regional changes in blood flow velocity rather than global CBF. 44 Given the complex physiological mechanisms of autoregulation, it is not yet known whether monitoring CA based on flow measurements in a large artery is reflective of the autoregulation behaviour in the entire brain (including regions perfused by other large arteries) as well as at the microcirculation level. In addition to the physiological variables directly affecting CBF, such as PaCO2 and brain activation, there are many other factors that can influence CBF through metabolic coupling (such as haematocrit, temperature), making it challenging to find appropriate methods to assess cerebral autoregulation.
This also leads to few studies attempting to reproduce the entire autoregulation curve. In most cases, researchers induce changes in MAP to determine the lower or upper limits of autoregulation or assess whether specific patients retain intact autoregulatory capacity. Patients with impaired or absent autoregulation (such as severe head injury or subarachnoid haemorrhage) are at a significantly higher risk of cerebral ischemia or secondary brain damage caused by hyperperfusion. Cerebral autoregulation assessment is crucial in the clinical management of such patients. Therefore, more efforts are needed to improve the reproducibility and stability of autoregulation assessment to facilitate its clinical application.
Other limitations
In addition to the difficulties of manipulation and measurement, another major limitation of this review is the direct comparison of experimental results using different BP manipulation methods and different CBF measurement methods. Although no significant differences in slopes were found for experimental data using different manipulation and measurement methods, only a very small number of experiments induced both an increase and a decrease in MAP. It remains unclear whether the asymmetry in autoregulation may be partially caused by using different BP manipulation methods and CA assessment methods. Furthermore, not all studies reported end-tidal or arterial partial pressure of carbon dioxide, so we could only correct the effect of CO2 on CBF based on PaCO2 values from 29 experiments. A fixed correction coefficient was employed to eliminate the confounding effect of CO2, but due to the considerable variability in cerebrovascular reactivity at different CO2 concentrations, this approach may have limitations of under- or over-corrections. To address these limitations, future research should aim to standardize BP manipulation methods and CBF measurement techniques across studies to improve comparability and enhance the robustness of meta-analyses.
Accurately defining the range of autoregulation is currently challenging from the limited available studies, as many studies focus only on one end of the autoregulatory curve or a limited range of blood pressure variations. It is also important to note that the exact lower and upper limits of cerebral autoregulation vary considerably between individuals and can be influenced by factors such as age, resting blood pressure, antihypertensive/hypotensive treatments, and potential cerebral diseases. Even with future research that may help us quantify and understand the autoregulatory curve better, a group-averaged range is unlikely to be applicable to all individuals. 14 Therefore, although cerebral autoregulation can provide important information in the management of brain injury, stroke, and other conditions affecting brain function, it should be measured and used with caution in clinical applications.
Future research on static cerebral autoregulation assessment
The availability of measurement techniques that allow high temporal resolution for continuous recording of CBF changes has made dynamic CA the focus of most current research and clinical assessment. It is worth emphasizing that our findings are highly consistent with other studies on dCA. This strong consistency suggests a close relationship between these two phenomena. While dynamic testing overcomes some limitations of static methods, it is important to note that the interchangeability of the two approaches is still unclear. Previous studies have produced conflicting results regarding the association between dynamic and static autoregulation: de Jong et al. 17 found no correlation, whereas Tiecks et al. 16 found a strong linear relationship between them. Several factors may explain the opposite findings, including methodological differences, heterogeneity of study subjects, and variations in data processing and analysis methods. Additionally, the time frame over which changes in BP occur also influences the effectiveness and stabilization of CBF by autoregulation. 45
For clinical applications, it is conceivable that patients with impaired autoregulation may exhibit slowed adaptation to rapid blood pressure changes (i.e., impaired dynamic autoregulation), while their adaptation to slow blood pressure changes remains intact (i.e., normal static autoregulation).14,46 Further research is needed to determine the consistency and complementarity between dynamic and static autoregulation. The combination of these two approaches may provide a more comprehensive assessment of cerebral autoregulation in different physiological and clinical contexts.
Considering the diversity of experimental designs and clinical practices, proposing a “gold standard” for cerebral autoregulation testing is challenging. However, the following guidelines can be recommended: a minimum change of 10% from baseline in MAP, a duration of each assessment of >5 minutes, minimizing autonomic nervous system stimulation, controlling brain activation, measuring PaCO2 simultaneously with ABP and CBF, and focusing on individual data for each subject. These guidelines not only contribute to improving the reproducibility and inter-method agreement of autoregulation assessment but also enhance the accuracy of different tests on individuals. Despite some limitations, the present study provides a further update on the static cerebral pressure-flow relationship. With further research and technological advancements, we can expect a better understanding and application of dynamic and static cerebral autoregulation assessment methods.
Conclusion
Although the concept of cerebral autoregulation has been widely accepted after its proposal, increasing evidence suggests that the classic view of autoregulation may be incomplete or inaccurate. We performed a reanalysis of the dataset originally published by Numan et al., 18 adding papers published between 2013 and 2022, to provide new insights for updating the classical cerebral autoregulation curve. A total of 143 experiments (including Numan's original dataset) were included in the analysis. The cerebral pressure-flow relationship was comprehensively explored by multiple analyses of absolute and relative CBF values. The main findings of this paper are:
The cerebrovascular system in healthy subjects does have autoregulatory ability, but CBF cannot maintain constant over the wide MAP range of 60 to 150 mmHg proposed by Lassen. 2 First-order linear regression and third-order polynomial functions were used to fit the relationship between absolute CBF (MCAv) and MAP. An autoregulatory plateau region of only approximately 20 mmHg (MAP between 80 and 100 mmHg) was found using a data-driven approach, indicating a considerably narrower autoregulation range than previously accepted. Furthermore, within the autoregulatory plateau region, the cerebral pressure-flow relationship is not a straight line but with a slight positive slope.
The results of the relative CBF analysis showed asymmetry in the autoregulatory curve: the slope of %ΔCBF/%ΔMAP were found to be 1.47 ± 0.71 for decreasing blood pressure and 0.37 ± 0.38 for increasing blood pressure, which were altered to 1.11 ± 0.78%/% and 0.24 ± 0.60%/% respectively after CO2 correction. The significant difference in CBF directional sensitivity suggests that the cerebral vasculature defends more effectively against MAP increase than MAP decrease.
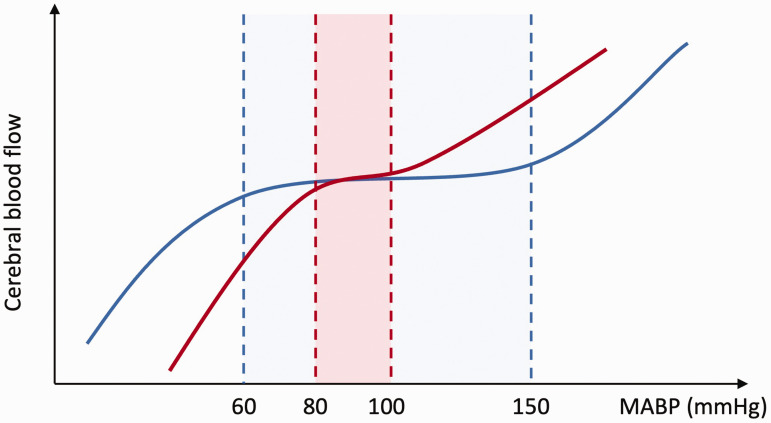
Based on the above findings, we proposed a new cerebral pressure-flow relationship, as shown in Figure 7. It should be noted that this new curve is based on the limited experimental data available so far, with many assumptions. Despite some advances in physiological measurement of cerebral blood flow and perfusion pressure, the ability to quantify cerebral autoregulation in health and disease remains limited. The physiological mechanisms that regulate CBF, how CBF changes when MAP is above 140 mmHg, and how autoregulation is altered in pathological conditions remain unclear, reminding us that more explicit assessments of CBF are needed under a range of non-pharmacological and pharmacological perturbations of blood pressure while maintaining PaCO2. Only by understanding these mechanisms can we clarify the role of impaired autoregulation in numerous cerebrovascular diseases, such as hypertension, Alzheimer's disease and dementia,10,12,37,38 thus providing more insights for clinical application.
Figure 7.
Classical view and updated view of the cerebral autoregulation curve. The blue line and shaded area represent the autoregulation curve and plateau proposed by Lassen 2 and Paulson et al. 4 The red line and shaded area represent the new autoregulation curve and plateau proposed in this study, based on the dataset from Numan et al. 18 and studies from 2013 to 2022. It should be noted that the new curve is asymmetric (with a lower slope for MAP > 100 mmHg than for MAP < 80 mmHg) and has a slight slope between the “autoregulation plateau” of MAP and CBF.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231210430 for Static autoregulation in humans by Yufan Wang and Stephen J Payne in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SJP is supported by a Yushan Fellowship from the Ministry of Education, Taiwan (# 111V1004-2).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material: Supplemental material for this article is available online.
References
- 1.Fog M. Cerebral circulation: the reaction of the pial arteries to a fall in blood pressure. Arch Neurpsych 1937; 37: 351–364. [Google Scholar]
- 2.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959; 39: 183–238. [DOI] [PubMed] [Google Scholar]
- 3.Ekström-Jodal B, Häggendal E, Linder L-E, et al. Cerebral blood flow autoregulation at high arterial pressures and different levels of carbon dioxide tension in dogs. ENE 1971; 6: 6–10. [DOI] [PubMed] [Google Scholar]
- 4.Paulson O, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990; 2: 161–192. [PubMed] [Google Scholar]
- 5.Strandgaard S, Olesen J, Skinhøj E, et al. Autoregulation of brain circulation in severe arterial hypertension. Br Med J 1973; 1: 507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heistad DD, Marcus ML, Piegors DJ, et al. Regulation of cerebral blood flow in atherosclerotic monkeys. Am J Physiol 1980; 239: H539–H544. [DOI] [PubMed] [Google Scholar]
- 7.Harper SL, Bohlen HG, Rubin MJ. Arterial and microvascular contributions to cerebral cortical autoregulation in rats. Am J Physiol 1984; 246: H17–H24. [DOI] [PubMed] [Google Scholar]
- 8.Fitch W, Ferguson GG, Sengupta D, et al. Autoregulation of cerebral blood flow during controlled hypotension in baboons. J Neurol Neurosurg Psychiatry 1976; 39: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brassard P, Labrecque L, Smirl JD, et al. Losing the dogmatic view of cerebral autoregulation. Physiol Rep 2021; 9: e14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Zhu Y-S, Hill C, et al. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension 2013; 62: 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas SJE, Tzeng YC, Galvin SD, et al. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension 2010; 55: 698–705. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Behbehani K, Levine BD. Dynamic pressure–flow relationship of the cerebral circulation during acute increase in arterial pressure. J Physiol 2009; 587: 2567–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heistad DD, Kontos HA. Cerebral circulation. In: Comprehensive physiology. John Wiley & Sons, Ltd, pp. 137–182. [Google Scholar]
- 14.Claassen JAHR, Thijssen DHJ, Panerai RB, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev 2021; 101: 1487–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin 2016; 34: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 1995; 26: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 17.de Jong DLK, Tarumi T, Liu J, et al. Lack of linear correlation between dynamic and steady‐state cerebral autoregulation. J Physiol 2017; 595: 5623–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Numan T, Bain AR, Hoiland RL, et al. Static autoregulation in humans: a review and reanalysis. Med Eng Phys 2014; 36: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 19.Brassard P, Ferland-Dutil H, Smirl JD, et al. Evidence for hysteresis in the cerebral pressure-flow relationship in healthy men. Am J Physiol Heart Circ Physiol 2017; 312: H701–H704. [DOI] [PubMed] [Google Scholar]
- 20.Labrecque L, Drapeau A, Rahimaly K, et al. Dynamic cerebral autoregulation and cerebrovascular carbon dioxide reactivity in middle and posterior cerebral arteries in young endurance-trained women. J Appl Physiol (1985) 2021; 130: 1724–1735. [DOI] [PubMed] [Google Scholar]
- 21.Panerai RB, Barnes SC, Nath M, et al. Directional sensitivity of dynamic cerebral autoregulation in squat-stand maneuvers. Am J Physiol Regul Integr Comp Physiol 2018; 315: R730–R740. [DOI] [PubMed] [Google Scholar]
- 22.Tzeng Y-C, Willie CK, Atkinson G, et al. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 2010; 56: 268–273. [DOI] [PubMed] [Google Scholar]
- 23.Meng L, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology 2015; 122: 196–205. [DOI] [PubMed] [Google Scholar]
- 24.Verbree J, Bronzwaer A-SGT, Ghariq E, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 2014; 117: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 25.Coverdale NS, Gati JS, Opalevych O, et al. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol (1985) 2014; 117: 1090–1096. [DOI] [PubMed] [Google Scholar]
- 26.Willie CK, Macleod DB, Shaw AD, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 2012; 590: 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molhoek GP, Wesseling KH, Settels JJM, et al. Evaluation of the penàz servo-plethysmo-manometer for the continuous, non-invasive measurement of finger blood pressure. Basic Res Cardiol 1984; 79: 598–609. [DOI] [PubMed] [Google Scholar]
- 28.Labrecque L, Rahimaly K, Imhoff S, et al. Diminished dynamic cerebral autoregulatory capacity with forced oscillations in mean arterial pressure with elevated cardiorespiratory fitness. Physiological Reports 2017; 5: e13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smirl JD, Hoffman K, Tzeng Y-C, et al. Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J Appl Physiol (1985) 2015; 119: 487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carey BJ, Eames PJ, Blake MJ, et al. Dynamic cerebral autoregulation is unaffected by aging. Stroke 2000; 31: 2895–2900. [DOI] [PubMed] [Google Scholar]
- 31.van Beek AH, Claassen JA, Rikkert MGO, et al. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab 2008; 28: 1071–1085. [DOI] [PubMed] [Google Scholar]
- 32.Barnes JN. Sex specific factors regulating pressure and flow. Exp Physiol 2017; 102: 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aaslid R, Blaha M, Sviri G, et al. Asymmetric dynamic cerebral autoregulatory response to cyclic stimuli. Stroke 2007; 38: 1465–1469. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt B, Czosnyka M, Klingelhöfer J. Asymmetry of cerebral autoregulation does not correspond to asymmetry of cerebrovascular pressure reactivity. Perspect Med 2012; 1: 285–289. [Google Scholar]
- 35.Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1255–R1261. [DOI] [PubMed] [Google Scholar]
- 36.Panerai RB, Barnes SC, Batterham AP, et al. Directional sensitivity of dynamic cerebral autoregulation during spontaneous fluctuations in arterial blood pressure at rest. J Cereb Blood Flow Metab 2023; 43: 552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krejcy K, Wolzt M, Kreuzer C, et al. Characterization of angiotensin-II effects on cerebral and ocular circulation by noninvasive methods. Br J Clin Pharmacol 1997; 43: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCulloch TJ, Boesel TW, Lam AM. The effect of hypocapnia on the autoregulation of cerebral blood flow during administration of isoflurane. Anesth Analg 2005; 100: 1463–1467. [DOI] [PubMed] [Google Scholar]
- 39.Panerai RB. Assessment of cerebral pressure autoregulation in humans – a review of measurement methods. Physiol Meas 1998; 19: 305–338. [DOI] [PubMed] [Google Scholar]
- 40.Lindegaard KF, Lundar T, Wiberg J, et al. Variations in middle cerebral artery blood flow investigated with noninvasive transcranial blood velocity measurements. Stroke 1987; 18: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 41.Greisen G, Johansen K, Ellison PH, et al. Cerebral blood flow in the newborn infant: comparison of doppler ultrasound and 133xenon clearance. J Pediatr 1984; 104: 411–418. [DOI] [PubMed] [Google Scholar]
- 42.Bishop CC, Powell S, Rutt D, et al. Transcranial doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 1986; 17: 913–915. [DOI] [PubMed] [Google Scholar]
- 43.Sorond F, Hollenberg NK, Panych LP, et al. Brain blood flow and velocity: correlations between magnetic resonance imaging and transcranial doppler. J Ultrasound Med 2010; 29: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willie CK, Colino FL, Bailey DM, et al. Utility of transcranial doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 2011; 196: 221–237. [DOI] [PubMed] [Google Scholar]
- 45.Birch AA, Dirnhuber MJ, Hartley-Davies R, et al. Assessment of autoregulation by means of periodic changes in blood pressure. Stroke 1995; 26: 834–837. [DOI] [PubMed] [Google Scholar]
- 46.Panerai RB, Robinson TG, Minhas JS. The upper frequency limit of dynamic cerebral autoregulation. J Physiol 2019; 597: 5821–5833. [DOI] [PubMed] [Google Scholar]
- 47.Tan CO. Defining the characteristic relationship between arterial pressure and cerebral flow. J Appl Physiol (1985) 2012; 113: 1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurazumi T, Ogawa Y, Takko C, et al. Short-Term volume loading effects on estimated intracranial pressure in human volunteers. Aerosp Med Hum Perform 2022; 93: 347–353. [DOI] [PubMed] [Google Scholar]
- 49.Pernice R, Sparacino L, Bari V, et al. Spectral decomposition of cerebrovascular and cardiovascular interactions in patients prone to postural syncope and healthy controls. Auton Neurosci 2022; 242: 103021. [DOI] [PubMed] [Google Scholar]
- 50.Bari V, Barbarossa L, Gelpi F, et al. Exploring metrics for the characterization of the cerebral autoregulation during head-up tilt and propofol general anesthesia. Auton Neurosci 2022; 242: 103011. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg AJ, Kay VL, Anderson GK, et al. The impact of acute central hypovolemia on cerebral hemodynamics: does sex matter? J Appl Physiol (1985) 2021; 130: 1786–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robertson AD, Papadhima I, Edgell H. Sex differences in the autonomic and cerebrovascular responses to upright tilt. Auton Neurosci 2020; 229: 102742. [DOI] [PubMed] [Google Scholar]
- 53.Stok WJ, Karemaker JM, Berecki-Gisolf J, et al. Slow sinusoidal tilt movements demonstrate the contribution to orthostatic tolerance of cerebrospinal fluid movement to and from the spinal dural space. Physiol Rep 2019; 7: e14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlotman TE, Akers KS, Nessen SC, et al. Differentiating compensatory mechanisms associated with low tolerance to central hypovolemia in women. Am J Physiol Heart Circ Physiol 2019; 316: H609–H616. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida H, Hamner JW, Ishibashi K, et al. Relative contributions of systemic hemodynamic variables to cerebral autoregulation during orthostatic stress. J Appl Physiol (1985) 2018; 124: 321–329. [DOI] [PubMed] [Google Scholar]
- 56.van Helmond N, Johnson BD, Holbein WW, et al. Effect of acute hypoxemia on cerebral blood flow velocity control during lower body negative pressure. Physiol Rep 2018; 6: e13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Brule JMD, Stolk R, Vinke EJ, et al. Vasopressors do not influence cerebral critical closing pressure during systemic inflammation evoked by experimental endotoxemia and sepsis in humans. Shock 2018; 49: 529–535. [DOI] [PubMed] [Google Scholar]
- 58.van der Scheer JW, Kamijo Y-I, Leicht CA, et al. A comparison of static and dynamic cerebral autoregulation during mild whole-body cold stress in individuals with and without cervical spinal cord injury: a pilot study. Spinal Cord 2018; 56: 469–477. [DOI] [PubMed] [Google Scholar]
- 59.Bronzwaer A-S, Verbree J, Stok WJ, et al. Aging modifies the effect of cardiac output on Middle cerebral artery blood flow velocity. Physiol Rep 2017; 5: e13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lund A, Sørensen H, Jensen TW, et al. Muscle oxygen saturation increases during head-up tilt-induced (pre)syncope. Acta Physiol (Oxf) 2017; 221: 74–80. [DOI] [PubMed] [Google Scholar]
- 61.Bronzwaer A-S, Verbree J, Stok WJ, et al. The cerebrovascular response to lower-body negative pressure vs. head-up tilt. J Appl Physiol (1985) 2017; 122: 877–883. [DOI] [PubMed] [Google Scholar]
- 62.Ogawa Y, Yanagida R, Ueda K, et al. The relationship between widespread changes in gravity and cerebral blood flow. Environ Health Prev Med 2016; 21: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kay VL, Rickards CA. The role of cerebral oxygenation and regional cerebral blood flow on tolerance to central hypovolemia. Am J Physiol Regul Integr Comp Physiol 2016; 310: R375–R383. [DOI] [PubMed] [Google Scholar]
- 64.Rickards CA, Johnson BD, Harvey RE, et al. Cerebral blood velocity regulation during progressive blood loss compared with lower body negative pressure in humans. J Appl Physiol (1985) 2015; 119: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry BG, Lucas SJE, Thomas KN, et al. The effect of hypercapnia on static cerebral autoregulation. Physiol Rep 2014; 2: e12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Del Pozzi AT, Pandey A, Medow MS, et al. Blunted cerebral blood flow velocity in response to a nitric oxide donor in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 2014; 307: H397–H404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaw BH, Loughin TM, Mackey DC, et al. The effect of orthostatic stress type on cardiovascular control. Blood Press Monit 2014; 19: 327–338. [DOI] [PubMed] [Google Scholar]
- 68.Lewis NCS, Bain AR, MacLeod DB, et al. Impact of hypocapnia and cerebral perfusion on orthostatic tolerance. J Physiol 2014; 592: 5203–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perry BG, Schlader ZJ, Raman A, et al. Middle cerebral artery blood flow velocity in response to lower body positive pressure. Clin Physiol Funct Imaging 2013; 33: 483–488. [DOI] [PubMed] [Google Scholar]
- 70.Stewart JM, Medow MS, DelPozzi A, et al. Middle cerebral O2 delivery during the modified oxford maneuver increases with sodium nitroprusside and decreases during phenylephrine. Am J Physiol Heart Circ Physiol 2013; 304: H1576–H1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schlader ZJ, Seifert T, Wilson TE, et al. Acute volume expansion attenuates hyperthermia-induced reductions in cerebral perfusion during simulated hemorrhage. J Appl Physiol (1985) 2013; 114: 1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo S, Ashina M, Olesen J, et al. The effect of sodium nitroprusside on cerebral hemodynamics and headache in healthy subjects. Cephalalgia 2013; 33: 301–307. [DOI] [PubMed] [Google Scholar]
- 73.Chan GSH, Ainslie PN, Willie CK, et al. Contribution of arterial windkessel in low-frequency cerebral hemodynamics during transient changes in blood pressure. J Appl Physiol (1985) 2011; 110: 917–925. [DOI] [PubMed] [Google Scholar]
- 74.Ogoh S, Sato K, Fisher JP, et al. The effect of phenylephrine on arterial and venous cerebral blood flow in healthy subjects. Clin Physiol Funct Imaging 2011; 31: 445–451. [DOI] [PubMed] [Google Scholar]
- 75.Ogawa Y, Iwasaki K, Aoki K, et al. The different effects of midazolam and propofol sedation on dynamic cerebral autoregulation. Anesth Analg 2010; 111: 1279–1284. [DOI] [PubMed] [Google Scholar]
- 76.Brassard P, Seifert T, Wissenberg M, et al. Phenylephrine decreases frontal lobe oxygenation at rest but not during moderately intense exercise. J Appl Physiol 2010; 108: 7. [DOI] [PubMed] [Google Scholar]
- 77.Hussain R, Tsuchida T, Kudo T, et al. Vasodilatory effect of adenosine triphosphate does not change cerebral blood flow: a PET study with 15O-water. Ann Nucl Med 2009; 23: 717–723. [DOI] [PubMed] [Google Scholar]
- 78.Lavi S, Egbarya R, Lavi R, et al. Role of nitric oxide in the regulation of cerebral blood flow in humans. Circulation 2003; 107: 1901–1905. [DOI] [PubMed] [Google Scholar]
- 79.Hjorth Lassen L, Klingenberg Iversen H, Olesen JJ. A dose–response study of nitric oxide synthase inhibition in different vascular beds in man. Eur J Clin Pharmacol 2003; 59: 499–505. [DOI] [PubMed] [Google Scholar]
- 80.McCulloch TJ, Visco E, Lam AM. Graded hypercapnia and cerebral autoregulation during sevoflurane or propofol anesthesia. Anesthesiology 2000; 93: 1205–1209. [DOI] [PubMed] [Google Scholar]
- 81.Kawai Y, Murthy G, Watenpaugh DE, et al. Cerebral blood flow velocity in humans exposed to 24 h of head-down tilt. J Appl Physiol 1993; 74: 3046–3051. [DOI] [PubMed] [Google Scholar]
- 82.Jobes DR, Kennell E, Bitner R, et al. Effects of morphine–nitrous oxide anesthesia on cerebral autoregulation. Anesthesiology 1975; 42: 30–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231210430 for Static autoregulation in humans by Yufan Wang and Stephen J Payne in Journal of Cerebral Blood Flow & Metabolism