Abstract
Pulmonary large cell neuroendocrine carcinoma (LCNEC) has a poor prognosis, and there is no consensus on optimal treatment strategies for pulmonary LCNEC. Certain patients with pulmonary LCNEC may benefit from targeted and traditional programmed cell death protein-1 (PD-1) monoclonal antibody therapies, however, for most patients, only a few drugs are effective after chemotherapy. The present report describes the case of a 68-year-old man with advanced pulmonary LCNEC treated with cadonilimab and anlotinib after becoming resistant to PD-1 monoclonal antibody therapy and multiple chemotherapies. Computed tomography was used to evaluate treatment response. The treatment was efficacious and met the partial response criteria after three treatment cycles, and the coughing and dyspnea resolved. The primary mass and lymph node metastases continued to shrink after five treatment cycles. Therefore, the present case report suggests that a combination of cadonilimab and anlotinib is a potential treatment strategy for patients with pulmonary LCNEC.
Keywords: pulmonary large cell neuroendocrine carcinoma, cadonilimab, anlotinib
Introduction
Pulmonary large cell neuroendocrine carcinoma (LCNEC) is a rare and highly invasive lung cancer with a poor prognosis, constituting 1–3% of primary lung tumor (1). For stage I LCNEC, the 5-year survival rate is only 18% and the overall 5-year survival rate for LCNEC in all stages is 13% (2). While surgery is the primary treatment for early-stage LCNEC, adjuvant chemotherapy post-lobar resection improves survival (3). However, there is a lack of large-scale controlled clinical studies exploring the optimal treatment for advanced LCNEC, and certain studies have shown that chemotherapy remains the most common treatment option (4–6).
Immune checkpoint inhibitors (ICIs) have markedly improved the prognosis of patients with small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) (7,8). Furthermore, there have been case reports and retrospective studies of clinical data on the use of ICIs in LCNEC treatment (9–17). Nevertheless, reports on alternative treatment methods in patients resistant to traditional programmed cell death protein-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) monoclonal antibodies are limited.
Case report
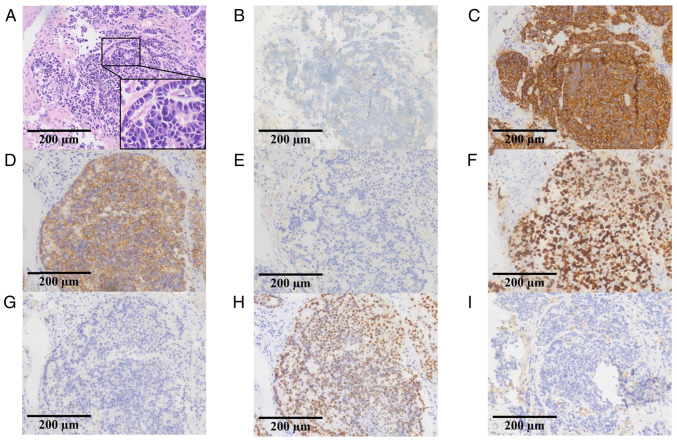
In December 2020, a 68-year-old man with a significant smoking history who underwent lung adenocarcinoma resection and four cycles of adjuvant chemotherapy 15 years prior at Affiliated Hospital of Guilin Medical University in Guilin, (Guangxi Zhuang Autonomous Region, China), presented with a cough and hemoptysis. Computed tomography (CT) revealed a neoplasm near the carina and multiple enlarged lymph nodes in the right hilum and mediastinum (data not shown). Pathological samples obtained through fiber bronchoscopy were stained and immunohistochemically analyzed by the Department of Pathology, Affiliated Hospital of Guilin Medical University, and the results showed that chromogranin A (+), synaptophysin (+), CD56 (+), NapsinA (−), Ki-67 (+, 80%), P40 (−), and thyroid transcription factor-1 (+) (Fig. 1), these results were consistent with LCNEC. Subsequently, the biopsy tissue samples were used for next-generation sequencing (NGS) targeting 139 cancer-relevant genes, performed by Nanjing Geneseeq Technology Inc. The mean coverage depth was 300× for controls and 1,000× for tissue samples. The results revealed no mutations in epidermal growth factor receptor, anaplastic lymphoma kinase, ROS proto-oncogene 1, receptor tyrosine kinase, MET proto-oncogene, receptor tyrosine kinase, KRAS proto-oncogene, GTPase, ret proto-oncogene, neurotrophic tropomyosin-receptor kinase, B-Raf proto-oncogene and serine/threonine kinase or fusion. However, RB transcriptional corepressor 1 (RB1; c.137 +1del; abundance, 55.1%) and tumor protein P53 (TP53; c.404G>T; abundance, 64.2%/c.393C>G; abundance, 63.7%) mutations were identified, and PD-L1 was positive (tumor proportion score, 2%). The clinical stage was determined to be T1bN2M0 (IIIA), according to the Eighth Edition Lung Cancer Stage Classification (18).
Figure 1.
Pathology and immunohistochemistry results of the biopsy specimen. (A) Hematoxylin and eosin staining revealed the presence of large cell neuroendocrine carcinoma. Immunohistochemistry results for (B) chromogranin A, (C) synaptophysin, (D) CD56, (E) NapsinA, (F) Ki-67, (G) P40, (H) thyroid transcription factor-1 and (I) programmed cell death-ligand 1 staining.
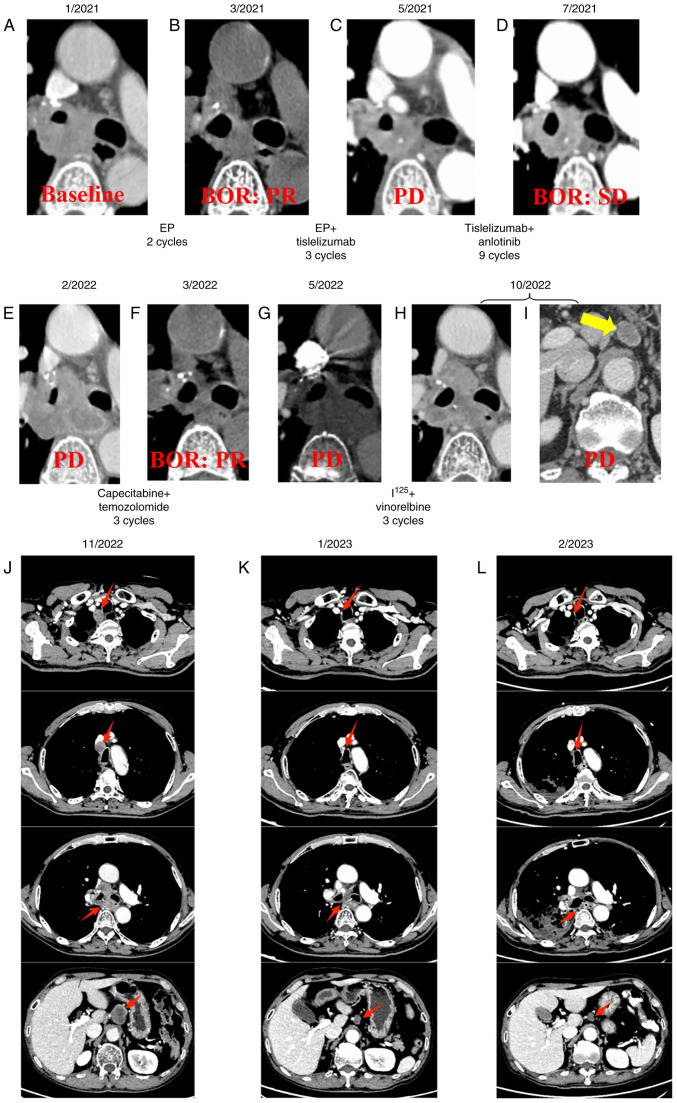
Despite the relatively small tumor size (29.5×19.1 mm), radical resection and conventional radiotherapy could not be performed due to the presence of severe restrictive ventilation disorder (diagnosed by pulmonary function testing). In January 2021, the patient received first-line treatment with etoposide combined with cisplatin (EP), etoposide 100 mg/m2 and cisplatin 25 mg/m2 were injected intravenously from day 1 to day 3, and repeated every 3 weeks. Partial response (PR) was achieved compared with baseline according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (19) (Fig. 2A and B). Tisleslizumab (an anti-PD-1 antibody drug), administered intravenously at a dose of 200 mg every 3 weeks, was added to the EP regimen for another three cycles. However, progressive disease (PD) was observed during an imaging evaluation in May 2021 (Fig. 2C). Subsequently, anlotinib (an anti-vascular multi-targeted tyrosine kinase receptor inhibitor), at a dose of 10 mg, was taken orally from day 1 to day 14 every 3 weeks, in combination with tisleslizumab (200 mg), serving as a second-line treatment for nine cycles. The best of response (BOR) during the treatment was stable disease (Fig. 2D). Unfortunately, disease progression was again observed in February 2022 after seven months of treatment (Fig. 2E). Following this, temozolomide (150 mg/m2, taken orally from day 10 to day 14, every 4 weeks) combined with capecitabine (750 mg/m2 twice daily, taken orally from day 1 to day 14, every 4 weeks) were administered, with a PR as BOR (Fig. 2F), and PD was observed again in May 2022 (Fig. 2G). In June 2022, radioactive iodine-125 seeds were implanted, followed by the administration of vinorelbine (40 mg, administered orally every Monday, Wednesday and Friday) as a beat regimen. PD was observed again in October 2022 due to the development of retroperitoneal lymph node metastases as new lesions (Fig. 2H and I). Subsequently, mediastinal lymph node (4R) metastases were observed as new lesions following irinotecan (60 mg/m2, administered intravenously on days 1, 8, and 15, every 4 weeks) treatment in November 2022 (Fig. 2J). Based on previous studies (20,21), patient received intravenous infusion of cadonilimab (250 mg) combined with oral administration of anlotinib (10 mg, from day 1 to day 14) every 3 weeks (initiated in November 2022).
Figure 2.
Therapy timeline of the patient. CT images of the patient in (A) January, 2021, (B) March, 2021, (C) May, 2021, (D) July, 2021, (E) February, 2022, (F) March, 2022, (G) May, 2022, (H and I) October, 2022. (J) CT findings before treatment with cadonilimab + anlotinib in November 2022. CT findings after 3 and 5 cycles of treatment with cadonilimab + anlotinib in (K) January 2023, (L) March 2023, respectively. The new lesion of retroperitoneal lymph node metastases is indicated by a yellow arrows, and red arrows indicate the changes in primary tumor and lymph node metastases before and after treatment with cadonilimab + anlotinib. BOR, best of response; PR, partial response; SD, stable disease; PD, progressive disease; EP, etoposide + cisplatin.
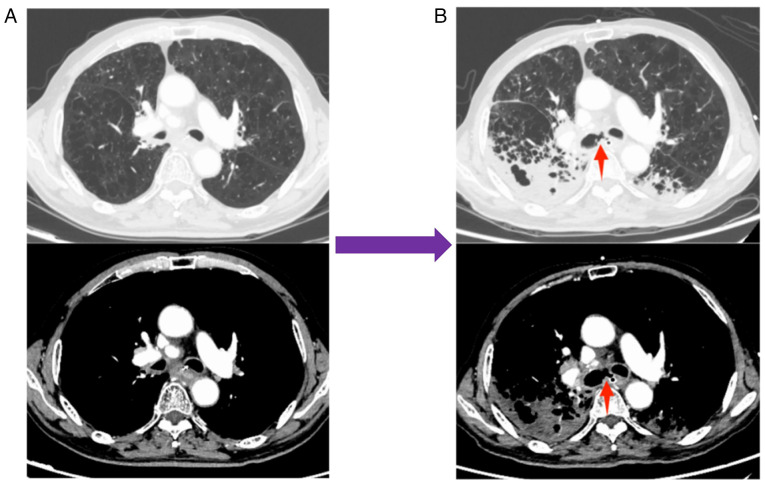
The treatment demonstrated efficacy and met PR criteria according to the RECIST 1.1 (19) after three treatment cycles (Fig. 2K), resulting in the resolution of the patient's coughing and dyspnea. After five treatment cycles, the primary mass and lymph node metastases continued to shrink (Fig. 2L). Notably, serum levels of gastrin-releasing peptide precursor (reference value: 3–77.8 pg/ml) demonstrated a marked decline (Fig. 3). However, during the fourth and fifth treatment cycles, the patient experienced moderate dyspnea during the infusion of cadonilimab but after intravenous injection of 40 mg of methylprednisolone, the dyspnea symptoms improved. In February 2023, the patient reported a severe cough lasting 2 days, prompting a return to the Affiliated Hospital of Guilin Medical University (Guangxi Zhuang Autonomous Region, China). A tracheoesophageal fistula was identified using CT (Fig. 4), and the patient died following respiratory failure caused by a lung infection in March 2023.
Figure 3.
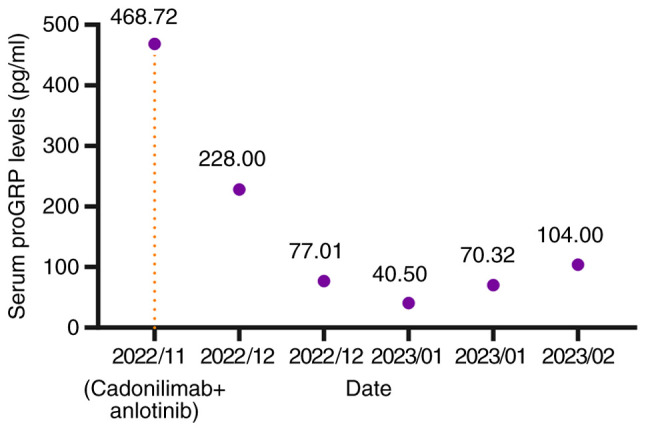
Changes in serum proGRP levels before and after treatment with cadonilimab + anlotinib. The orange line corresponds to the start time of cadonilimab + anlotinib treatment. proGRP, pro-Gastrin-Releasing Peptide.
Figure 4.
CT images before and after the occurrence of tracheoesophageal fistula. (A) CT images before the occurrence of tracheoesophageal fistula in January 2023 and (B) CT images after the occurrence of tracheoesophageal fistula in February 2023, respectively. The site of tracheoesophageal fistula is indicated by a red arrow.
Discussion
LCNEC was first described by Travis et al in 1991 (22) and is similar to SCLC in terms of its high invasiveness, proliferation and neuroendocrine gene expression patterns (23). Therefore, the 2021 World Health Organization Classification of Lung Tumours defines LCNEC as a neuroendocrine carcinoma subtype (24). PD-1 and PD-L1 ICIs are standard treatment options for advanced NSCLC and SCLC. However, only a few studies have been published on the application of ICIs in advanced LCNEC (Table I). Furthermore, there is no consensus among clinicians on how to effectively and rationally treat patients with LCNEC, especially for the subsequent treatment of advanced LCNEC, and this issue needs more attention.
Table I.
Summary of studies of patients with advanced large cell neuroendocrine carcinoma treated with immune checkpoint inhibitors.
| First author/s, year | Journal | Type of research | Findings | (Refs.) |
|---|---|---|---|---|
| Wang et al, 2017 | Journal for ImmunoTherapy of Cancer | Case report | A patient with TMB-H LCNEC who was resistant to first-line platinum doublet achieved a marked and durable response with pembrolizumab. | (9) |
| Qin et al, 2020 | Immunotherapy | Case report | A case of advanced lung LCNEC treated with combined radiotherapy and nivolumab resulted in a durable response. | (10) |
| Sherman et al, 2020 | Lung Cancer | Retrospective cohort | Patients with advanced LCNEC treated with ICIs alone, ORR and median PFS were 33% and 4.2 months, respectively (n=23). | (11) |
| Chauhan et al, 2018 | Oncotarget | Case report | A total of 3 patients with metastatic LCNEC were treated with nivolunmab on ≥2-line therapy, of which 1 patient achieved CR, 1 patient achieved clinical and radiological response and 1 patient achieved stable disease. | (12) |
| Takimoto Sato et al, 2020 | Molecular and Clinical Oncology | Case report | A case of stage IVB LCNEC treated with-nivolunmab as a three-line treatment resulted in stable disease for ≥6 months. | (13) |
| Oda et al, 2020 | Thoracic Cancer | Case report | A case of stage IV LCNEC was treated with nivolumab, all the metastatic lesions shrunk and a partial response was maintained for >3 years. | (14) |
| Giaj Levra et al, 2017 | Journal of Thoracic Oncology | Retrospective cohort | A total of 10 LCNEC patients were treated with nivolumab or pembrolizumab, of which 6/10 achieved partial response and 1 had stable disease. The median PFS was reported to be 57 weeks. | (15) |
| Zhang et al, 2020 | OncoTargets and Therapy | Case report | A patient with TMB-H advanced LCNEC who had received nivolunmab had achieved CR for >20 months. | (16) |
| Dudnik et al, 2021 | Journal for ImmunoTherapy of Cancer | Retrospective cohort | Survival benefit was reported in patients with advanced LCNEC treated with ICIs (n=41) compared with those treated with no ICIs (n=84) in an analysis of real-world data. | (17) |
ICIs, immune checkpoint inhibitors; LCNEC, large cell neuroendocrine carcinoma; TMB-H, tumor mutation burden-high; ORR, objective response rate; PFS, progression-free survival; CR, complete response.
Cadonilimab is a tetravalent PD-1/cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) bispecific antibody that specifically binds PD-1 and CTLA-4. It exhibits superior antitumor effects compared with mono-specific anti-PD-1 antibody (25) and was approved in China in June 2022 for patients with relapsed or metastatic cervical cancer who have progressed or after platinum-based chemotherapy (26). Previous studies have reported that certain patients with LCNEC may benefit from targeted or traditional PD-1 monoclonal antibody therapies (27,28). In the present case, an elderly male patient was treated with and responded to cadonilimab combined with anlotinib after developing resistance to PD-1 monoclonal antibodies and several chemotherapeutic drugs. However, considering the complexity of the treatment process, the anlotinib and radioactive iodine-125 seeds combination may have affected the efficacy of the ICIs (29,30). Nonetheless, cadonilimab exhibited considerable short-term efficacy and markedly improved the quality of life of the patient. However, it is still unclear whether CTLA-4 monoclonal antibodies will benefit patients who are resistant to conventional PD-1 monoclonal antibodies, and to the best of our knowledge, cadonilimab does not have fragment crystallizable which is necessary for antibody-dependent cell-mediated cytotoxicity (31). It may exert antitumor effects by other means or combined blocking of immune checkpoints (32,33).
Although the patient in the present report experienced grade 2 cadonilimab-related infusion reactions, the treatment plan was successfully completed with routine management. During nearly 3 months of cadonilimab treatment, no immune-related adverse event was observed. After the multi-disciplinary team discussion, it was speculated that the formation of a tracheoesophageal fistula may have been related to the radioactive seed implantation, antitumor therapy or the therapeutic side effects. Considering the excessive cumulative dose of radiation in the esophagus, the fistula was most likely caused by the radioactive seeds. Previous studies have mixed views on the effect of antiangiogenic tyrosine kinase inhibitors (TKIs) on tracheoesophageal fistula, with certain studies suggesting that TKIs can cause this condition, whilst others indicating they may improve it (34,35). Moreover, the pneumonia that led to the death of the patient in the present report had definite evidence of infection, and no imaging features typical of immune-related pneumonitis were observed during treatment.
Moreover, NGS results have reported that LCNEC is a biologically heterogeneous group of tumors comprising distinct subsets of SCLC, NSCLC and highly proliferative carcinoids with distinct genomic signatures (36). Although LCNEC is rare, few randomized controlled clinical trials have been reported. The NGS results of the patient in the present report revealed RB1 and TP53 mutations at baseline. Based on previous reports (36,37), the present case is characterized as the SCLC-like subtype of LCNEC. It was reported that a case of a patient with advanced LCNEC who received cadonilimab as first-line treatment and achieved complete response with duration of response over 20 months (20). In the present study, it was notable that progression-free survival was still reached >3 months after multiple drug resistance and objective remission was achieved. The short-term efficacy of the cadonilimab and anlotinib combination observed suggests that combined inhibition of PD-1 and CTLA-4 may have efficacy in treating certain neuroendocrine cancers and may be a potential treatment option for advanced LCNEC after resistance to PD-1/PD-L1 monoclonal antibodies. However, additional biochemical and clinical studies are required to assess the efficacy of this combination.
Currently, several registered clinical studies are using PD-1 or PD-L1 monoclonal antibodies to treat LCNEC (Table II), whilst certain clinical studies have concentrated on subsequent treatment options after initial ICI resistance, possibly due to the low incidence of LCNEC and the difficulty in recruiting patients.
Table II.
Current trials involving immune checkpoint inhibitors for the treatment of large cell neuroendocrine carcinoma.
| Trial | Phase | Tumors | n | Experimental arm | Primary endpoint | Status |
|---|---|---|---|---|---|---|
| NCT05470595 (LCNEC-ALPINE) | II | Locally advanced or metastatic LCNEC | 67 | Atezolizumab | OS | Recruiting |
| NCT03591731 (NIPINEC) | II | Gastroenteropancreatic or pulmonary poorly differentiated neuroendocrine tumors (including LCNEC) | 185 | Arm A: Nivolumab; Arm B: Nivolumab + ipilimumab | ORR | Active, not recruiting |
| NCT03976518 (CHANCE) | II | Advanced non-small cell lung cancer with rare histologies (including LCNEC) | 43 | Atezolizuma b | DCR | Recruiting |
| NCT03728361 | II | Small cell lung cancer or advanced neuroendocrine cancer (including LCNEC) | 55 | Nivolumab + temozolomide | ORR | Active, not recruiting |
| EudraCT 2020-005942-41 (DUPLE) | II | Metastatic LCNEC | 49 | Durvalumab with carboplatin + etoposide followed by durvalumab maintenance | OS rate at 1 year | Ongoing |
| EudraCT 2020-002683-31 | II | Advanced LCNEC | 67 | Atezolizumab with platinum + etoposide | OS | Ongoing |
| ChiCTR 2300068111 | II | Locally advanced/metastatic LCNEC | 64 | Serplulimab with carboplatin + etoposide | ORR | Prospective registration |
LCNEC, large cell neuroendocrine carcinoma; OS, overall survival; ORR, objective response rate; DCR, disease control rat.
In a patient with advanced LCNEC who was resistant to traditional PD-1 antibody and multiple lines of chemotherapy, the application of cadonilimab and anlotinib showed impressive efficacy. Systemic treatment of advanced LCNEC requires additional biochemical studies and clinical data.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- LCNEC
large cell neuroendocrine carcinoma
- ICIs
immune checkpoint inhibitors
- SCLC
small cell lung cancer
- NSCLC
non-small cell lung cancer
- PD-1
programmed cell death protein-1
- PD-L1
programmed cell death-ligand 1
- CT
computed tomography
- RECIST 1.1
Response Evaluation Criteria in Solid Tumors version 1.1
- CTLA-4
cytotoxic T lymphocyte-associated antigen-4
- TKIs
antiangiogenic tyrosine kinase inhibitors
- NGS
next-generation sequencing
Funding Statement
The present work was supported by the National Natural Science Foundation of China (grant nos. 82260498 and 82160471), Quzhou City Qujiang District Life Oasis Public Welfare Service Center; Health Development Promotion Project-Cancer Research Project (grant no. BJHA-CRP-033), Guangxi Medical and Health Key Discipline Construction Project, 2021 Guilin City Science Research and Technology Development Plan Project (grant no. 20210227-7-9) and Beijing Xisike Clinical Oncology Research Foundation (grant no. Y-QL202101-0214).
Availability of data and materials
The NGS data generated in the present study may be found in the China National Center for Bioinformation under accession number HRA008073 or at the following URL: https://ngdc.cncb.ac.cn/gsa-human/browse/HRA008073. Other data generated in the present study may be requested from the corresponding author.
Authors' contributions
XQ and YL participated in study design and data collection, data analysis and writing of the manuscript. LZ contributed to data collection, and was involved in drafting and revising the manuscript. LX contributed to acquisition, analysis and interpretation of image data. JL, YM and MK contributed to the conception and design of the study. FX was involved in drafting the manuscript, revising it critically for important intellectual content, data analysis and gave final approval of the version to be published. FX and XQ confirmed the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present case report was approved by the Medical Ethics Committee of the Affiliated Hospital of Guilin Medical University (Guilin, China; approval no. 2023QTLL-17).
Patient consent for publication
The patient in the present report provided written informed consent.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113:5–21. doi: 10.1002/cncr.23542. [DOI] [PubMed] [Google Scholar]
- 2.Dresler CM, Ritter JH, Patterson GA, Ross E, Bailey MS, Wick MR. Clinical-pathologic analysis of 40 patients with large cell neuroendocrine carcinoma of the lung. Ann Thorac Surg. 1997;63:180–185. doi: 10.1016/S0003-4975(96)01058-2. [DOI] [PubMed] [Google Scholar]
- 3.Raman V, Jawitz OK, Yang CJ, Tong BC, D'Amico TA, Berry MF, Harpole DH., Jr Adjuvant therapy for patients with early large cell lung neuroendocrine cancer: A National Analysis. Ann Thorac Surg. 2019;108:377–383. doi: 10.1016/j.athoracsur.2019.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Treut J, Sault MC, Lena H, Souquet PJ, Vergnenegre A, Le Caer H, Berard H, Boffa S, Monnet I, Damotte D, Chouaid C. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: the GFPC 0302 study. Ann Oncol. 2013;24:1548–1552. doi: 10.1093/annonc/mdt009. [DOI] [PubMed] [Google Scholar]
- 5.Niho S, Kenmotsu H, Sekine I, Ishii G, Ishikawa Y, Noguchi M, Oshita F, Watanabe S, Nakajima R, Tada H, Nagai K. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: A multicenter phase II study. J Thorac Oncol. 2013;8:980–984. doi: 10.1097/JTO.0b013e31828f6989. [DOI] [PubMed] [Google Scholar]
- 6.Xia L, Wang L, Zhou Z, Han S. Treatment outcome and prognostic analysis of advanced large cell neuroendocrine carcinoma of the lung. Sci Rep. 2022;12:16562. doi: 10.1038/s41598-022-18421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, Ji Y, Dvorkin M, Shi J, Pan Z, et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients With Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA. 2022;328:1223–1232. doi: 10.1001/jama.2022.16464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al. Camrelizumab Plus Carboplatin and Pemetrexed as First-Line Treatment for Advanced Nonsquamous NSCLC: Extended Follow-Up of CameL Phase 3 Trial. J Thorac Oncol. 2023;18:628–639. doi: 10.1016/j.jtho.2022.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Wang VE, Urisman A, Albacker L, Ali S, Miller V, Aggarwal R, Jablons D. Checkpoint inhibitor is active against large cell neuroendocrine carcinoma with high tumor mutation burden. J Immunother Cancer. 2017;5:75. doi: 10.1186/s40425-017-0281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin Y, Yu M, Zhou L, Jiang L, Huang M. Durable response to combination radiotherapy and immunotherapy in EP-resistant lung large-cell neuroendocrine carcinoma with B2M and STK11 mutations: A case report. Immunotherapy. 2020;12:223–227. doi: 10.2217/imt-2019-0166. [DOI] [PubMed] [Google Scholar]
- 11.Sherman S, Rotem O, Shochat T, Zer A, Moore A, Dudnik E. Efficacy of immune check-point inhibitors (ICPi) in large cell neuroendocrine tumors of lung (LCNEC) Lung Cancer. 2020;143:40–46. doi: 10.1016/j.lungcan.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan A, Arnold SM, Kolesar J, Thomas HE, Evers M, Anthony L. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: current status. Oncotarget. 2018;9:14738–14740. doi: 10.18632/oncotarget.24553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takimoto Sato M, Ikezawa Y, Sato M, Suzuki A, Kawai Y. Large cell neuroendocrine carcinoma of the lung that responded to nivolumab: A case report. Mol Clin Oncol. 2020;13:43–47. doi: 10.3892/mco.2020.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oda R, Okuda K, Yamashita Y, Sakane T, Tatematsu T, Yokota K, Endo K, Nakanishi R. Long-term survivor of pulmonary combined large cell neuroendocrine carcinoma treated with nivolumab. Thorac Cancer. 2020;11:2036–2039. doi: 10.1111/1759-7714.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giaj Levra M, Mazieres J, Audigier Valette C, Molinier O, Planchard D, Frappat V, Ferrer L, Claire Toffart A, Moro-Sibilot D. Efficacy of immune checkpoint inhibitors in large cell neuroendocrine lung cancer: Results from a French Retrospective Cohort: Topic: Drug treatment alone and in combination with radiotherapy. J Thor Oncol. 2017;12:1556–0864. [Google Scholar]
- 16.Zhang X, Sun Y, Miao Y, Xu S. Immune checkpoint inhibitor therapy achieved complete response for drug-sensitive EGFR/ALK mutation-negative metastatic pulmonary large-cell neuroendocrine carcinoma with high tumor Mutation Burden: A case report. Onco Targets Ther. 2020;13:8245–8250. doi: 10.2147/OTT.S259893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudnik E, Kareff S, Moskovitz M, Kim C, Liu SV, Lobachov A, Gottfried T, Urban D, Zer A, Rotem O, et al. Real-world survival outcomes with immune checkpoint inhibitors in large-cell neuroendocrine tumors of lung. J Immunother Cancer. 2021;9:e001999. doi: 10.1136/jitc-2020-001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Frentzas S, Kwek KY, Konpa A, Jin X. Efficacy and safety of AK104, an anti-PD-1/CTLA-4 bispecific antibody, in a patient with large cell neuroendocrine carcinoma of the lung. J Thor Oncol. 2020;16:506. doi: 10.1016/j.jtho.2021.01.887. [DOI] [Google Scholar]
- 21.Wu L, Chen B, Yao W, Li X, Xiao Z, Liu H, Kong Y, Liu L, Xu Y, Wang Q, et al. A phase Ib/II trial of AK104 (PD-1/CTLA-4 bispecific antibody) in combination with Anlotinib in advanced NSCLC. Ann Oncol. 2021;32:1006. doi: 10.1016/j.annonc.2021.08.1902. [DOI] [Google Scholar]
- 22.Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB, Jr, Nieman L, Chrousos G, Pass H, Doppman J. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–553. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Gazdar AF, Savage TK, Johnson JE, Berns A, Sage J, Linnoila RI, MacPherson D, McFadden DG, Farago A, Jacks T, et al. The comparative pathology of genetically engineered mouse models for neuroendocrine carcinomas of the lung. J Thorac Oncol. 2015;10:553–564. doi: 10.1097/JTO.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol. 2022;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, Li B. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. MAbs. 2023;15:2180794. doi: 10.1080/19420862.2023.2180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keam SJ. Cadonilimab: First Approval. Drugs. 2022;82:1333–1339. doi: 10.1007/s40265-022-01731-1. [DOI] [PubMed] [Google Scholar]
- 27.De Pas TM, Giovannini M, Manzotti M, Trifirò G, Toffalorio F, Catania C, Spaggiari L, Labianca R, Barberis M. Large-cell neuroendocrine carcinoma of the lung harboring EGFR mutation and responding to gefitinib. J Clin Oncol. 2011;29:e819–e822. doi: 10.1200/JCO.2011.36.2251. [DOI] [PubMed] [Google Scholar]
- 28.Mauclet C, Duplaquet F, Pirard L, Rondelet B, Dupont M, Pop-Stanciu C, Vander Borght T, Remmelink M, D'Haene N, Lambin S, et al. Complete tumor response of a locally advanced lung large-cell neuroendocrine carcinoma after palliative thoracic radiotherapy and immunotherapy with nivolumab. Lung Cancer. 2019;128:53–56. doi: 10.1016/j.lungcan.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng Y, Gao Y, Li K. Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 2020;11:309. doi: 10.1038/s41419-020-2511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 31.Du X, Tang F, Liu M, Su J, Zhang Y, Wu W, Devenport M, Lazarski CA, Zhang P, Wang X, et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 2018;28:416–432. doi: 10.1038/s41422-018-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsui J, Nishikawa H, Muraoka D, Wang L, Noguchi T, Sato E, Kondo S, Allison JP, Sakaguchi S, Old LJ, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 33.Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W, Allison JP. Cytotoxic T lymphocyte antigen-4 blockade enhances antitumor immunity by stimulating melanoma-specific T-cell motility. Cancer Immunol Res. 2014;2:970–980. doi: 10.1158/2326-6066.CIR-14-0104. [DOI] [PubMed] [Google Scholar]
- 34.He L, Han Q, Zhao M, Ma H, Cheng P, Yang H, Zhao Y. Case report of radiotherapy combined with anlotinib and immunotherapy for a patient with esophageal cancer and esophageal fistula. Appl Radiat Isot. 2024;205:111162. doi: 10.1016/j.apradiso.2023.111162. [DOI] [PubMed] [Google Scholar]
- 35.Adeoye O, Kozyreva O. Case of tracheoesophageal fistula formation as a rare complication of antiangiogenic tyrosine kinase inhibitor therapy for metastatic hepatocellular carcinoma. Cureus. 2023;15:e41783. doi: 10.7759/cureus.41783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, Halpenny DF, Wang H, Tian SK, Litvak AM, et al. Next-Generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res. 2016;22:3618–3629. doi: 10.1158/1078-0432.CCR-15-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuo M, Guan Y, Yang X, Hong L, Wang Y, Li Z, Chen R, Abbas HA, Chang L, Gong Y, et al. The prognostic and therapeutic role of genomic subtyping by sequencing tumor or Cell-Free DNA in pulmonary large-cell neuroendocrine carcinoma. Clin Cancer Res. 2020;26:892–901. doi: 10.1158/1078-0432.CCR-19-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The NGS data generated in the present study may be found in the China National Center for Bioinformation under accession number HRA008073 or at the following URL: https://ngdc.cncb.ac.cn/gsa-human/browse/HRA008073. Other data generated in the present study may be requested from the corresponding author.