Abstract
Background:
Prognostic factors for total shoulder arthroplasty (TSA) clinical outcomes are incompletely understood. This study investigates the associations of preoperative patient, disease-specific, and surgical factors with 1-year postoperative PENN Shoulder Score (PSS) in patients undergoing primary TSA.
Methods:
Cleveland Clinic patients undergoing primary anatomic TSA (aTSA) or reverse TSA (rTSA) for glenohumeral osteoarthritis (GHOA) or rotator cuff tear arthropathy (CTA) between February 2015 and August 2019, and having complete preoperative and 1-year postoperative patient-reported outcome measures (PROMs), were included. Twenty preselected preoperative patient, disease-specific, and surgical factors were used to fit multivariable models for 1-year PSS and its subscores.
Results:
Of 1427 eligible primary TSAs, 1174 had 1-year follow-up by PROMs (82%), with 1042 analyzed after additional exclusions, including 30% rTSAs for CTA (n = 308), 26% rTSAs for GHOA (n = 275), and 44% aTSAs for GHOA (n = 459). All PROMs showed statistically significant improvements postoperatively, with 89% of patients reaching an acceptable symptom state. Lower 1-year PSS was associated with younger age, female sex, current smoking, chronic pain diagnosis, history of prior surgery, worker’s compensation claim, lower preoperative mental health, lower baseline PSS, absence of glenoid bone loss, and diagnosis-arthroplasty type (CTA-rTSA < GHOA-rTSA < GHOA-aTSA). The most important prognostic factors associated with 1-year PSS were diagnosis-arthroplasty type, baseline mental health status, and insurance status.
Conclusions:
Disease diagnosis, arthroplasty type, and several other baseline factors are strongly and individually associated with PROMs following primary TSA, with patients undergoing aTSA for GHOA demonstrating the highest PROM scores at 1-year follow-up. Patient, disease-specific, and surgical factors can be used to guide postoperative prognosis following primary TSA for improved preoperative patient counseling regarding expected outcomes of these procedures.
Level of evidence:
Level II; Prospective Cohort Comparison; Prognosis Study
Keywords: Shoulder arthroplasty, glenohumeral osteoarthritis, cuff tear arthropathy, patient-reported outcome measures, outcomes, multivariable model, beta regression model
Annual shoulder arthroplasties in the United States are expected to increase from currently about 100,0002 to 175,000–350,000 in the coming years.31,48 Surgical outcomes following total shoulder arthroplasty (TSA) vary by arthroplasty type (anatomic [aTSA] or reverse [rTSA])21,45,34 and design, and preoperative factors such as diagnosis,51,40 rotator cuff status,33,24 degree of glenoid or humeral bone loss,50,12,11 and prior shoulder surgery.40,24,26,41,9 Although the prognostic factors for aTSA and rTSA clinical outcomes remain incompletely understood, recent evidence specifically from patients undergoing aTSA or rTSA for glenohumeral osteoarthritis (GHOA) with intact rotator cuffs suggests these 2 arthroplasty types yield similar short-term outcomes for this diagnosis.34,42,57,19 However, most such reports are based on small retrospective studies, highlighting the need for larger comprehensive cohort studies allowing multivariable analyses of factors that may predict clinical outcomes, including diagnosis and arthroplasty type.
The Cleveland Clinic Health System’s (CCHS’s) Outcomes Management and Evaluation (OME)30 database is an established, valid tool for prospective collection of standardized shoulder arthroplasty data; including baseline demographic, disease-specific and surgical data, and shoulder-specific validated baseline and 1-year patient-reported outcome measures (PROMs).39 PROMs offer a means to reliably quantify a patient’s assessment of their shoulder’s physical and/or functional condition, and have gained emphasis because of a shift in focus toward patient experience and engagement in the evaluation of surgical outcomes. Using an OME cohort of 788 cases of patients with GHOA or rotator cuff tear arthropathy (CTA) undergoing primary shoulder arthroplasty, we previously found that female sex, less education, worse mental health status, and preoperative opioid use were associated with lower preoperative PENN Shoulder Score (PSS).38
In this study, we now investigate the associations of patient, disease-specific, and surgical factors with 1-year postoperative PSS in an expanded OME cohort undergoing primary TSA for GHOA or CTA. One year has been shown to be an appropriate landmark for postoperative PROMs following shoulder arthroplasty as PROMs do not substantially change between 1 and 2 years post-operatively.23,54,4,10 Based on our prior study and the literature, we hypothesized that worse preoperative mental health status, preoperative opioid use, worse baseline PSS, prior shoulder surgery, glenoid bone loss, CTA (vs. GHOA), and rTSA (vs. aTSA) would be associated with lower 1-year PSS and its pain, function, and satisfaction subscores. Correlations between 1-year PSS, American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form (ASES), and Single Assessment Numeric Evaluation (SANE) scores were also evaluated to clarify the relationships among these different PROMs.
Materials and methods
Primary shoulder arthroplasty surgical cohort
Patients enrolled in the OME database30 (Institutional Review Board 06–196) for primary shoulder arthroplasty (hemiarthroplasty, aTSA, and rTSA) between February 2015 and August 2019 with diagnosis of GHOA or CTA were eligible for inclusion. Patients undergoing arthroplasty for other diagnoses, with a history of joint infection in the operative shoulder, or having incomplete preoperative or 1-year postoperative PROMs were excluded.
Variable selection
PSS was the primary and its subscores (pain, function, and satisfaction) the secondary outcomes for analysis.20 Several additional outcomes (ASES score, SANE score, 1-year Patient Acceptable Symptom State [PASS], return to work by 1 year, and additional surgery within 1 year) were also described.
No outcome-driven variable selection was performed. We prespecified, for multivariable modeling of 1-year PSS and its subscores, 20 preoperative patient and disease-specific/surgical factors as possible predictors: age, sex, race, body mass index, smoking status, preoperative opioid use, years of education, Area Deprivation Index (ADI),46,18 insurance status, mental health as assessed by Veterans Rand 12-Item Health Survey (VR-12) mental component summary (VR-12 MCS), psychiatric diagnosis, comorbidities (Charlson Comorbidity Index [CCI]),36 chronic pain diagnosis, baseline PSS, prior ipsilateral shoulder surgery, glenoid bone loss, shoulder diagnosis, arthroplasty type, humeral component fixation (uncemented, cemented), and superior-posterior rotator cuff repair during shoulder arthroplasty. Preoperative opioid use was based on an opioid prescription in the electronic medical record between 12 months and 24 hours before surgery. Chronic pain was determined by International Classification of Diseases, Ninth Revision (ICD-9), diagnosis of 338.2 (chronic pain) and/or 304.0x (opioid dependence), and psychiatric diagnosis by ICD-9 diagnosis of depression, anxiety, posttraumatic stress disorder, psychosis, or bipolar disorder, in the electronic medical record (Supplementary Table S1). ADI, which ranks neighborhoods at census block levels by socioeconomic disadvantage, was obtained from the University of Wisconsin neighborhood atlas.46,18
Statistical analysis
Continuous variables were summarized by medians (and quartiles) and categorical variables by frequency counts (%). Associations among predictors were assessed a priori, without reference to outcomes, for opportunities to group clinically related categories. For multivariable analysis, rotator cuff pathology was reduced to a composite “RC status” variable describing combinations of superior-posterior rotator cuff and subscapularis status, as previously described38 (Table I). Because of almost complete collinearity between RC status and diagnosis (99.4% [806 of 811] RC status group 1 having GHOA and 93.1% [337 of 362] RC status groups 2–4 having CTA), only diagnosis was included in the predictive models and the 30 cases with discordant RC status and diagnosis combinations (GHOA with a large/massive rotator cuff tear or CTA with an intact rotator cuff) were excluded. As no CTA cases underwent aTSA, diagnosis and arthroplasty type were combined in multivariable analysis into a 3-category composite “diagnosis-arthroplasty” variable (GHOA-aTSA, GHOA-rTSA, and CTA-rTSA). Glenoid bone loss, categorized in OME by region of the glenoid (central, anterior, posterior, or superior) based on surgeon assessment of preoperative imaging and intraoperative findings, was also condensed into a “yes/no” categorization for multivariable analysis, as previously described.38
Table I.
Definition of the "rotator cuff (RC) status" composite variable
| Superior-posterior RC status |
||||
|---|---|---|---|---|
| Intact | Small or medium tear | Large or massive tear | ||
|
| ||||
| Subscapularis status | Intact | Group 1 | Group 2 | |
| Small or medium tear | Group 3 | |||
| Large or massive tear | Group 5 | Group 4 | ||
Rotator cuff pathology was assessed intraoperatively by the operating surgeon and classified, separately for each of the superior-posterior rotator cuff and the subscapularis, as intact or full-thickness tear (small: 0–1 cm, medium: 1–3 cm, large: 3–5 cm, or massive: >5 cm). CTA patients with RC status 1 (n = 5), GHOA patients with RC status 2–4 (n = 25), and all RC status 5 (n = 2) were excluded from analyses.
Preoperative differences of patients with and without 1-year PROMs were tested by Wilcoxon rank sum and χ2 tests, and preoperative to postoperative changes in outcomes by Wilcoxon signed-rank tests. Multivariable identity-link beta regression models were fit to 1-year PSS, PSS pain, and PSS function, and a multivariable proportional-odds model to 1-year PSS satisfaction. Missing predictor data were multiply imputed using multivariate imputation by chained equations (mice R package3), and results were pooled/aggregated using Rubin standard formula.37 Age, body mass index, CCI, years of education, ADI, VR-12 MCS, and PSS were treated as continuous variables with additive linear effects; other variables were modeled categorically. Differences in means (beta regression models) or odds ratios (proportional-odds model) were presented with 95% confidence intervals and P values. Separately comparing multiple groups (“diagnosis-arthroplasty”), or multiple pairs of levels of factors with more than 2 levels (eg, insurance status), separately testing relationships of each variable of interest with multiple outcome measures (PSS total score and 3 subscores), are conducive to false positive findings. We therefore limited false positive errors by employing multiple degree of freedom omnibus tests rather than paired comparisons to assess differences among multiple groups or levels of multicategorical variables, and by using the Bonferroni-Holm multiple comparison adjustment, with a family-wise Type I error rate of 0.05, to conduct simultaneous tests of each variable in relation to the multiple groups or outcome measures. The false discovery rate resulting from examinations of hypotheses about numerous variables was controlled at a threshold of 20% or less using the Benjamini-Yeuketeli method.1
Relative importances of each variable in explaining variation in postoperative PROMs in this cohort were assessed by calculating and ranking the increases in the Akaike information criterion13 on removal of that variable from the full model. Pairwise Pearson correlations of 1-year PROMs (PSS, ASES, and SANE scores) were also calculated. As sensitivity analyses, we fit additional models each including only 1 of 6 variables considered potential mediators of effects of the others (ADI, VR-12 MCS, chronic pain, preoperative opioid use, smoking status, insurance status), to assess possible overadjustment.47
Data management and analysis used R, version 4.0,43 particularly the betareg and rms packages. Hypothesis testing was 2-sided and considered significant if P < .05.
Results
Primary shoulder arthroplasty surgical cohort
Of 1920 primary shoulder arthroplasties performed, 493 met exclusion criteria (Fig. 1). Of the remaining 1427 cases, 1174 had 1-year follow-up by PROMs (82%), and were compared to the 253 cases without 1-year PROMs (Supplementary Table S2). Nonrespondents were more commonly CTA-rTSA patients and tended to be younger, less often White, with more comorbidities, less education, higher ADI, lower baseline PSS and VR-12 scores, more preoperative opioid use, and more often with chronic pain or psychiatric diagnosis. Hemiarthroplasty (n = 27) and RC status group 5 (n = 2) were excluded from analysis because of infrequency, and cases with discordant diagnosis and RC status combinations (CTA with RC status group 1 [n = 5], and GHOA with RC status groups 2–4 [n = 25]) were also excluded (Table I). Second primary TSAs in patients undergoing bilateral primary TSAs (n = 75) were excluded to avoid confounding related to the first surgery, leaving 1042 cases for analysis (Fig. 1).
Figure 1.
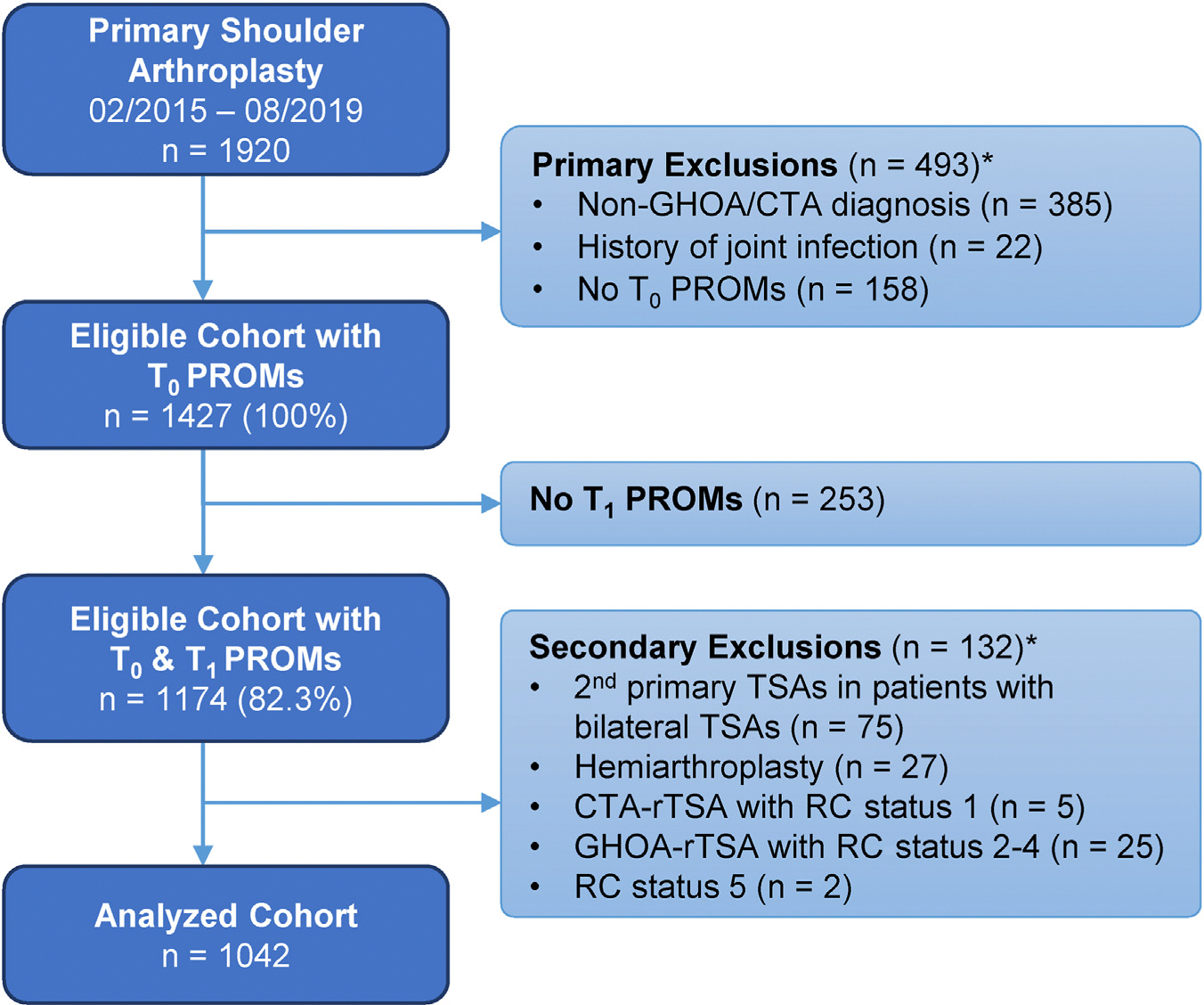
Patient selection flow diagram for the primary shoulder arthroplasty surgical cohort. *Total number of cases matching 1 or more exclusion criteria. PROMs, patient-reported outcome measures; GHOA, glenohumeral osteoarthritis; CTA, cuff tear arthropathy; TSA, total shoulder arthroplasty; rTSA, reverse total shoulder arthroplasty; RC, rotator cuff.
Preoperative patient- and disease-specific characteristics
Table II presents the patient and disease/surgical characteristics of the overall sample and 3 diagnosis-arthroplasty subgroups: GHOA-rTSA (n = 275, 26%), CTA-rTSA (n = 308, 30%), and GHOA-aTSA (n = 459, 44%). Five baseline variables were missing for some patients, all at low rates (0.1% VR-12, 0.4% insurance status, 1% CCI, 3.2% ADI, 4.1% race). Overall, patients had a median age of 69 years (quartiles 63, 75), median body mass index of 30 (26.8, 34.3), and the large majority were White (93.5%) and used Medicare for insurance (70.2%). Notable differences (P < .001) were observed among the 3 groups in age, sex, insurance status, prior shoulder surgery, glenoid bone loss, and humeral component fixation. CTA-rTSA cases more often were female (55.2%) with prior shoulder surgery (31.8%), and more commonly required a cemented humeral component (21.4%). GHOA-aTSA cases were more often male (59.9%), younger (median age, 65 years), and had private insurance (36.3%). GHOA-rTSA cases more commonly had glenoid bone loss (80.7%).
Table II.
Preoperative patient demographic, disease-specific, and surgical characteristics of the 1042 patients undergoing primary shoulder arthroplasty
| Variable | All (N = 1042) | GHOA-rTSA (n = 275) | CTA-rTSA (n = 308) | GHOA-aTSA (n = 459) | P value |
|---|---|---|---|---|---|
|
| |||||
| General patient characteristics | |||||
| Age, yr, median (IQR) | 69 (63, 75) | 72 (67, 77) | 72 (66.8, 76) | 65 (59, 71) | <.001 |
| BMI, median (IQR) | 30 (26.8, 34.3) | 29.6 (26.4, 33.3) | 29.6 (25.8, 34.2) | 30.5 (27.4, 34.8) | .003 |
| CCI, median (IQR) | 0 (0, 2) | 0 (0, 2) | 1 (0, 2) | 0 (0, 1) | .0027 |
| Education, yr, median (IQR) | 14 (12, 16) | 14 (12, 16) | 13 (12, 16) | 14 (12, 16) | <.001 |
| ADI, median (IQR) | 46 (28, 63) | 45 (27, 64) | 49 (31, 65) | 44 (25, 61) | .068 |
| Sex, n (%) | |||||
| Female | 489 (47) | 135 (49.1) | 170 (55.2) | 184 (40.1) | <.001 |
| Male | 553 (53) | 140 (50.9) | 138 (44.8) | 275 (59.9) | |
| Race, n (%) | |||||
| White | 930 (93) | 259 (95.2) | 270 (92.2) | 401 (92.4) | .316 |
| Black | 57 (6) | 12 (4.41) | 17 (5.8) | 28 (6.5) | |
| Other | 12 (1) | 1 (0.37) | 6 (2.0) | 5 (1.1) | |
| Smoking status, n (%) | |||||
| Current | 74 (7) | 17 (6.2) | 30 (9.7) | 27 (5.9) | .240 |
| Quit | 434 (42) | 117 (42.5) | 130 (42.2) | 187 (40.7) | |
| Never | 534 (51) | 141 (51.3) | 148 (48.1) | 245 (53.4) | |
| Preoperative opioid use, n (%) | |||||
| Yes, within 3 mo | 317 (30) | 76 (27.6) | 108 (35.1) | 133 (29.0) | .0017 |
| Yes, but not within 3 mo | 219 (21) | 57 (20.7) | 80 (26.0) | 82 (17.9) | |
| None in 12 mo | 506 (49) | 142 (51.6) | 120 (39.0) | 244 (53.2) | |
| Chronic pain: yes, n (%) | 195 (19) | 48 (17.5) | 67 (21.8) | 80 (17.4) | .265 |
| Insurance status, n (%) | |||||
| Private | 263 (25.4) | 51 (18.7) | 46 (15.0) | 166 (36.3) | <.001 |
| Medicare | 729 (70.2) | 217 (79.5) | 247 (80.5) | 265 (57.9) | |
| Medicaid | 32 (3.1) | 2 (0.7) | 7 (2.3) | 23 (5.0) | |
| Worker's compensation | 14 (1.4) | 3 (1.1) | 7 (2.3) | 4 (0.9) | |
| Psychiatric diagnosis: yes, n (%) | 314 (30) | 69 (25.1) | 104 (33.8) | 141 (30.7) | .070 |
| Disease and surgical characteristics, n (%) | |||||
| Prior shoulder surgery: yes | 189 (18) | 30 (10.9) | 98 (31.8) | 61 (13.3) | <.001 |
| Glenoid bone loss: yes | 590 (57) | 222 (80.7) | 105 (34.1) | 263 (57.3) | <.001 |
| Rotator cuff status | |||||
| 1 | 734 (70.4) | 275 (100) | — | 459 (100) | <.001 |
| 2 | 187 (18.0) | — | 187 (60.7) | — | |
| 3 | 66 (6.3) | — | 66 (21.4) | — | |
| 4 | 55 (5.3) | — | 55 (17.9) | — | |
| Humeral component | |||||
| Uncemented | 914 (88) | 264 (96.0) | 242 (78.6) | 408 (88.9) | <.001 |
| Cemented | 128 (12) | 11 (4.0) | 66 (21.4) | 51 (11.1) | |
| Superior-posterior rotator cuff repair: yes | 18 (1.7) | 1 (0.4) | 13 (4.2) | 4 (0.9) | .001 |
IQR, interquartile range; BMI, body mass index; CCI, Charlson Comorbidity Index; ADI, Area Deprivation Index; GHOA, glenohumeral osteoarthritis; rTSA, reverse total shoulder arthroplasty; CTA, rotator cuff tear arthropathy; aTSA, primary anatomic total shoulder arthroplasty.
Results are presented as median (IQR) for continuous variables and as counts (%) for categorical variables. All statistically significant variables at α = 0.05 remain so after Bonferroni-Holm multiple comparison adjustment.
PROMs and multivariable modeling
Tables III and IV present the preoperative and postoperative PROMs and secondary outcomes in the 1042 patients. Six 1-year outcome variables were missing for some patients, all at low rates (0.9% additional surgery, 1.5% VR-12, 2.0% SANE, 2.2% PSS, 2.2% return to work, 2.9% PASS). All PROMs showed statistically significant improvements from preoperative to 1 year postoperatively, with 89% of patients reaching an acceptable symptom state. CTA-rTSA patients had relatively smaller increases in shoulder PROMs with lower proportions reaching PASS and returning to work. One-year PSS total and pain and function subscores were highly correlated with their ASES counterparts (respectively, r = 0.94, 0.87, and 0.97), as was PSS total with SANE score (r = 0.81).
Table III.
Preoperative and 1-year postoperative PROMs in the 1042 patients undergoing primary shoulder arthroplasty
| Variable | All |
GHOA-rTSA |
CTA-rTSA |
GHOA-aTSA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative value | 1-yr value | 1-yr change | Preoperative value | 1-yr value | 1-yr change | Preoperative value | 1-yr value | 1-yr change | Preoperative value | 1-yr value | 1-yr change | |
|
| ||||||||||||
| PSS total | 32 (22, 42.9) | 87.5 (75, 94) | 50.1 (48.8, 51.4) | 31 (21, 43) | 88.4 (80.4, 94.6) | 53.7 (51.5, 55.9) | 30.4 (20.7, 41.1) | 77.9 (61.7, 89.3) | 42 (39.4, 44.6) | 33 (24, 44) | 91 (81, 96.5) | 52.9 (51, 54.7) |
| PSS pain | 11 (7, 15) | 28 (25, 30) | 15.5 (15, 16) | 11 (7, 15) | 29 (27, 30) | 16.5 (15.5, 17) | 11 (7, 16) | 27 (22.5, 29) | 13.5 (12.5, 14.5) | 11 (7, 15) | 28 (26, 30) | 16 (15.5, 16.5) |
| PSS function | 19 (12.6, 27) | 51.1 (42.5, 56) | 28.3 (27.5, 29.1) | 19 (11.1, 26.7) | 51.6 (45.6, 56) | 30.7 (29.3, 32.1) | 17.9 (11.2, 25.4) | 44.2 (34, 52.6) | 23.5 (21.7, 25.1) | 20 (14, 28) | 54 (47, 57.8) | 29.9 (28.6, 31) |
| PSS satisfaction | 1 (0, 3) | 9 (8, 10) | 7.5 (7, 7.5) | 1 (0, 2) | 10 (8, 10) | 7.5 (7.5, 8) | 1 (0, 3) | 9 (5, 10) | 6.5 (6, 7) | 1 (0, 3) | 10 (8, 10) | 7.5 (7, 7.5) |
| ASES total | 36.7 (23.3, 49.1) | 88.8 (76.7, 95) | 46.9 (45.6, 48.3) | 36.7 (21.8, 46.7) | 90 (82, 95) | 51 (48.5, 53.6) | 36.9 (25, 50) | 80.4 (67, 90) | 39.4 (36.8, 42) | 38 (25, 50) | 91.7 (81.3, 96.7) | 49.2 (47.3, 51.2) |
| ASES pain | 20 (10, 30) | 50 (40, 50) | 25 (25, 25) | 20 (10, 30) | 50 (45, 50) | 27.5 (25, 27.5) | 20 (10, 30) | 45 (40, 50) | 22.5 (20, 25) | 20 (10, 30) | 50 (45, 50) | 25 (25, 27.5) |
| ASES function | 15 (10, 21.7) | 41.7 (33.3, 46.3) | 23.2 (22.5, 23.9) | 14.8 (9.39, 21.7) | 41.7 (36.1, 45.8) | 24.9 (23.7, 26.2) | 14.8 (10, 20) | 35.2 (26.7, 42.6) | 18.8 (17.4, 20.2) | 16.7 (11.1, 21.7) | 43.3 (37, 46.7) | 24.8 (23.7, 25.8) |
| SANE | 30 (20, 50) | 90 (80, 95) | 55 (52.5, 55) | 30 (15, 50) | 90 (80, 98) | 57.5 (55, 60) | 30 (17.2, 50) | 80 (70, 95) | 47.5 (45, 50) | 30 (20, 50) | 90 (80, 98) | 56 (54.5, 57.5) |
| VR-12 MCS | 52.9 (43.9, 60.7) | 56.1 (47, 60.9) | 1.3 (0.7, 2) | 56 (47.2, 61.8) | 57.2 (48.9, 61.5) | 0.7 (−0.4, 1.7) | 49.3 (41.3, 58.4) | 53.6 (44.3, 60.5) | 2 (0.7, 3.4) | 52.9 (43.7, 61.1) | 56.2 (47.1, 60.6) | 1.4 (0.4, 2.3) |
| VR-12 PCS | 31.2 (25.5, 37.7) | 43.3 (34.1, 51.5) | 10.4 (9.8, 11.1) | 30.6 (25.1, 37.3) | 43.2 (35.2, 51.5) | 11.5 (10.3, 12.7) | 30.3 (24.7, 35.8) | 39 (31.2, 46.5) | 8 (6.8, 9.2) | 31.9 (26.5, 39) | 45.9 (37.3, 52.9) | 11.4 (10.5, 12.3) |
PROMs, patient-reported outcome measures; PSS, PENN Shoulder Score; ASES, American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form; SANE, Single Assessment Numeric Evaluation; VR-12, Veterans RAND 12-Item Health Survey; MCS, mental component summary; PCS, physical component summary; GHOA, glenohumeral osteoarthritis; rTSA, reverse total shoulder arthroplasty; CTA, rotator cuff tear arthropathy; aTSA, primary anatomic total shoulder arthroplasty.
Preoperative and 1-yr values are presented as medians (quartiles). 1-yr changes are presented as medians (95% confidence intervals).
Table IV.
One-year postoperative secondary outcomes in the 1042 patients undergoing primary shoulder arthroplasty
| Variable | All | GHOA-rTSA | CTA-rTSA | GHOA-aTSA |
|---|---|---|---|---|
|
| ||||
| PASS | 900 (89) | 248 (93) | 243 (81) | 409 (92) |
| Additional surgery | 33 (3.2) | 6 (2.2) | 9 (2.9) | 18 (4.0) |
| Return to work | 294 (77) | 64 (77) | 54 (61) | 176 (83) |
PASS, patient acceptable symptom state; GHOA, glenohumeral osteoarthritis; rTSA, reverse total shoulder arthroplasty; CTA, rotator cuff tear arthropathy; aTSA, primary anatomic total shoulder arthroplasty.
Results are presented as counts (%).
Tables V and VI, and Figure 2 show the results from multivariable models for 1-year PSS and each PSS subscore. As hypothesized, preoperative mental health (VR-12 MCS), baseline PSS, prior ipsilateral shoulder surgery, diagnosis-arthroplasty type, and glenoid bone loss were significantly associated with 1-year PSS. A 7.5-point lower baseline VR-12 MCS and 12.1-point lower baseline PSS predicted a mean 1-point lower 1-year PSS (P = .011 and .014, respectively). Prior ipsilateral surgery predicted a 3.7-point lower 1-year PSS (P = .01). Compared with GHOA-rTSA cases, GHOA-aTSA predicted a 3.3-point higher 1-year PSS and CTA-rTSA a 4.9-point lower 1-year PSS (P < .001). Contrary to our hypothesis, glenoid bone loss predicted a 2.6-point higher 1-year PSS compared with no glenoid bone loss (P = .006). To clarify this unexpected association, we tested for interactions of glenoid bone loss with diagnosis-arthroplasty type, but these were not statistically significant in any model.
Table V.
Estimated effects and odds ratios of patient demographic and surgical characteristics on 1-year PENN Shoulder Score (PSS), and 95% confidence intervals for each predictor in the full models in patients undergoing primary shoulder arthroplasty
| Variable | Level | Estimated difference | P value |
|---|---|---|---|
|
| |||
| General patient characteristics | |||
| Age | 12-yr increase | 1.8 (0.27, 3.33) | .021 |
| BMI | 7.5-point increase | 0.38 (−0.68, 1.44) | .485 |
| CCI | 2-unit increase | 0.06 (−1.08, 1.20) | .915 |
| Education | 4-yr increase | 0.22 (−0.84, 1.27) | .686 |
| ADI | 35-unit increase | −0.34 (−1.85, 1.17) | .659 |
| Baseline VR-12 MCS | 16.9-point increase | 2.26 (0.56, 3.95) | .011 |
| Sex (vs. Male) | Female | −2.31 (−4.44, −0.18) | .035 |
| Race (vs. White) | Black | −3.97 (−9.37, 1.42) | .339 |
| Other | 1.66 (−5.67, 9.01) | ||
| Smoking status (vs. never) | Quit | −0.57 (−2.31, 1.17) | .01 |
| Current | −5.29 (−9.29, −1.29) | ||
| Preoperative opioid use (vs. none in 12 mo) | From 12 to 3 mo | 0.77 (−1.34, 2.89) | .474 |
| Within 3 mo | −0.58 (−2.53, 1.36) | ||
| Chronic pain (vs. no) | Yes | −3.02 (−5.52, −0.53) | .019 |
| Psychiatric diagnosis (vs. no) | Yes | 0.30 (−1.74, 2.35) | .771 |
| Insurance status (vs. private) | Worker's compensation | −19.82 (−31.14,-8.49) | .005 |
| Medicaid | −3.07 (−10.58, 4.43) | ||
| Medicare | −0.60 (−2.92, 1.71) | ||
| Baseline PSS total | 20.9-point increase | 1.73 (0.35, 3.10) | .014 |
| Disease and surgical characteristics | |||
| Prior shoulder surgery (vs. no) | Yes | −3.73 (−6.57, −0.89) | .01 |
| Superior-posterior rotator cuff repair (vs. no) | Yes | 0.25 (−6.56, 7.07) | .942 |
| Glenoid bone loss (vs. no) | Yes | 2.62 (0.77, 4.47) | .006 |
| Humeral component (vs. uncemented) | Cemented | −1.19 (−3.69, 1.31) | .351 |
| Diagnosis-arthroplasty (vs. GHOA-rTSA) | CTA-rTSA | −4.89 (−7.57, −2.21) | <.001 |
| GHOA-aTSA | 3.34 (1.35, 5.33) | ||
BMI, body mass index; CCI, Charlson Comorbidity Index; ADI, Area Deprivation Index; VR-12 MCS, Veterans RAND 12-Item Health Survey mental component summary; GHOA, glenohumeral osteoarthritis; rTSA, reverse total shoulder arthroplasty; CTA, rotator cuff tear arthropathy; aTSA, primary anatomic total shoulder arthroplasty.
The effects for continuous variables (Age, BMI, Education, ADI, baseline PSS, baseline VR-12 MCS, and CCI) are comparing the quartiles (75th vs. 25th percentiles) indicated in Table II. The false discovery rate for all statistically significant (α = 0.05) potential predictors in this table except sex is controlled at 20% or less by the Benjamini-Yeuketelimethod.1
Examples of interpretation of PSS total scores (identity-link beta regression models):
• CTA-rTSA patients have 1-year PSS total cores that are 4.89 points lower on average respectively than GHOA-rTSA patients, after controlling for all other variables.
• A patient with baseline VR-12 MCS of 60.7 (75th percentile) has 1-year PSS total scores that are 2.26 points higher on average respectively than a patient with baseline VR-12 MCS of 43.9 (25th percentile), after controlling for all other variables.
Table VI.
Estimated effects and odds ratios of patient demographic and surgical characteristics on pain, function, and satisfaction subscores of 1-year PENN Shoulder Score (PSS), and 95% confidence intervals for each predictor in the full models in patients undergoing primary shoulder arthroplasty
| Variable | Level | 1-yr PSS pain |
1-yr PSS function |
1-yr PSS satisfaction |
|||
|---|---|---|---|---|---|---|---|
| Estimated difference | P value | Estimated difference | P value | Estimated odds ratio | P value | ||
|
| |||||||
| General patient characteristics | |||||||
| Age | 12-yr increase | 0.78 (0.42, 1.15) | <.001 | 0.98 (0.01, 1.95) | .048 | 1.06 (0.84, 1.34) | .608 |
| BMI | 7.5-point increase | 0.25 (0.02, 0.48) | .033 | −0.04 (−0.65, 0.56) | .888 | 1.11 (0.96, 1.29) | .149 |
| CCI | 2-unit increase | −0.09 (−0.37, 0.18) | .515 | 0.11 (−0.63, 0.86) | .762 | 0.96 (0.81, 1.13) | .604 |
| Education | 4-yr increase | 0.06 (−0.21, 0.33) | .666 | −0.11 (−0.85, 0.63) | .768 | 0.97 (0.82, 1.16) | .768 |
| ADI | 35-unit increase | −0.09 (−0.43, 0.24) | .579 | −0.43 (−1.35, 0.49) | .358 | 1.06 (0.88, 1.28) | .515 |
| Baseline VR-12 MCS | 16.9-point increase | 0.58 (0.27, 0.93) | .001 | 1.79 (0.95, 2.63) | <.001 | 1.08 (0.89, 1.30) | .427 |
| Sex (vs. Male) | Female | −0.29 (−0.75, 0.16) | .205 | −1.64 (−2.85, −0.43) | .008 | 1.11 (0.85, 1.44) | .463 |
| Race (vs. White) | Black | −1.55 (−3.07, −0.04) | .122 | −2.84 (−5.56, −0.11) | .116 | 0.58 (0.32, 1.05) | .092 |
| Other | 0.39 (−1.58, 2.36) | 0.94 (−3.78, 5.67) | 0.52 (0.19, 1.43) | ||||
| Smoking status (vs. never) | Quit | −0.01 (−0.41, 0.39) | .219 | −0.26 (−1.30, 0.77) | .009 | 1.12 (0.87, 1.43) | .009 |
| Current | −0.63 (−1.64, 0.38) | −3.40 (−5.94, −0.86) | 0.53 (0.33, 0.84) | ||||
| Preoperative opioid use (vs. none in 12 mo) | From 12 to 3 mo | 0.31 (−0.17, 0.79) | .199 | 0.13 (−1.20, 1.45) | .853 | 1.16 (0.85, 1.59) | .447 |
| Within 3 mo | −0.32 (−0.82, 0.19) | −0.14 (−1.34, 1.06) | 0.94 (0.70, 1.25) | ||||
| Chronic pain (vs. no) Psychiatric diagnosis (vs. no) | Yes | −0.36 (−0.91, 0.18) | .192 | −1.83 (−3.28, −0.39) | .013 | 0.60 (0.44, 0.81) | .001 |
| Yes | 0.08 (−0.41, 0.57) | .753 | −0.10 (−1.36, 1.16) | .877 | 0.99 (0.74, 1.31) | .92 | |
| Insurance status (vs. private) | Worker's compensation | −5.69 (−9.30, −2.09) | .004 | −12.22(−19.35, −5.09) | .004 | 0.34 (0.13, 0.84) | .051 |
| Medicaid | −2.27 (−4.45, −0.10) | −3.65 (−7.91, 0.61) | 0.99 (0.44, 2.26) | ||||
| Medicare | −0.10 (−0.62, 0.41) | −0.55 (−1.90, 0.79) | 1.20 (0.86, 1.67) | ||||
| Baseline PSS total | 20.9-point increase | 0.90 (0.54, 1.26) | <.001 | 1.30 (0.41, 2.19) | .005 | 0.82 (0.68, 1.00) | .046 |
| Disease and surgical characteristics | |||||||
| Prior shoulder surgery (vs. no) | Yes | −1.04 (−1.97, −0.1) | .031 | 2.26 (−4.14, −0.37) | .019 | 0.67 (0.49, 0.92) | .013 |
| Superior-posterior rotator cuff repair (vs. no) | Yes | 0.50 (−0.95, 1.95) | .499 | 0.29 (−4.00, 4.58) | .895 | 0.72 (0.31, 1.72) | .465 |
| Glenoid bone loss (vs. no) | Yes | 0.50 (0.05, 0.94) | .029 | 1.38 (0.26, 2.49) | .015 | 1.53 (1.17, 2.00) | .002 |
| Humeral component (vs. uncemented) | Cemented | −0.25 (−0.88, 0.39) | .447 | −0.85 (−2.48, 0.78) | .309 | 1.07 (0.73, 1.57) | .729 |
| Diagnosis-arthroplasty (vs. GHOA-rTSA) | CTA-rTSA | −0.73 (−1.33, −0.13) | .008 | −4.00 (−5.71, −2.29) | <.001 | 0.53 (0.38, 0.76) | <.001 |
| GHOA-aTSA | 0.15 (−0.31, 0.62) | 2.55 (1.30, 3.80) | 1.11 (0.80, 1.54) | ||||
BMI, body mass index; CCI, Charlson Comorbidity Index; ADI, Area Deprivation Index; VR-12 MCS, Veterans RAND 12-Item Health Survey mental component summary; GHOA, glenohumeral osteoarthritis; rTSA, reverse total shoulder arthroplasty; CTA, rotator cuff tear arthropathy; aTSA, primary anatomic total shoulder arthroplasty.
The effects for continuous variables (age, BMI, education, ADI, baseline PSS, baseline VR-12 MCS, and CCI) are comparing the quartiles range (75th vs. 25th percentiles) indicated in Table II. Eighteen of the 24 variables found to be statistically significantly related to PENN total score in Table V remain so after Bonferroni-Holm multiple comparison adjustment.
Examples of interpretation of PSS pain and function subscores (identity-link beta regression models):
• CTA-rTSA patients have 1-year PSS pain and function subscores that are 0.73 and 4.00 points lower on average, respectively, than GHOA-rTSA patients, after controlling for all other variables.
• A patient with baseline VR-12 MCS of 60.7 (75th percentile) has 1-year PSS pain and function subscores that are 0.58 and 1.79 points higher on average, respectively, than a patient with baseline VR-12 MCS of 43.9 (25th percentile), after controlling for all other variables.
• Examples of interpretation of PSS satisfaction subscore (proportional-odds regression model):
• The odds that CTA-rTSA patients have a 1-year PSS satisfaction score of at least X (say 5, for example) are (1–0.53) × 100 = 47% lower than the odds that GHOA-rTSA patients have a 1-year PSS satisfaction score of at least 5, after adjusting for all other variables (Note: This interpretation holds regardless of the value of X; hence proportional odds).
• The odds that a patient with a baseline PSS total of 42.9 points (75th percentile) has a PSS satisfaction score of at least X are (1–0.82) × 100 = 18% lower than the odds of a patient with a baseline PSS total of 22 points (25th percentile), after controlling for all other variables.
Figure 2.
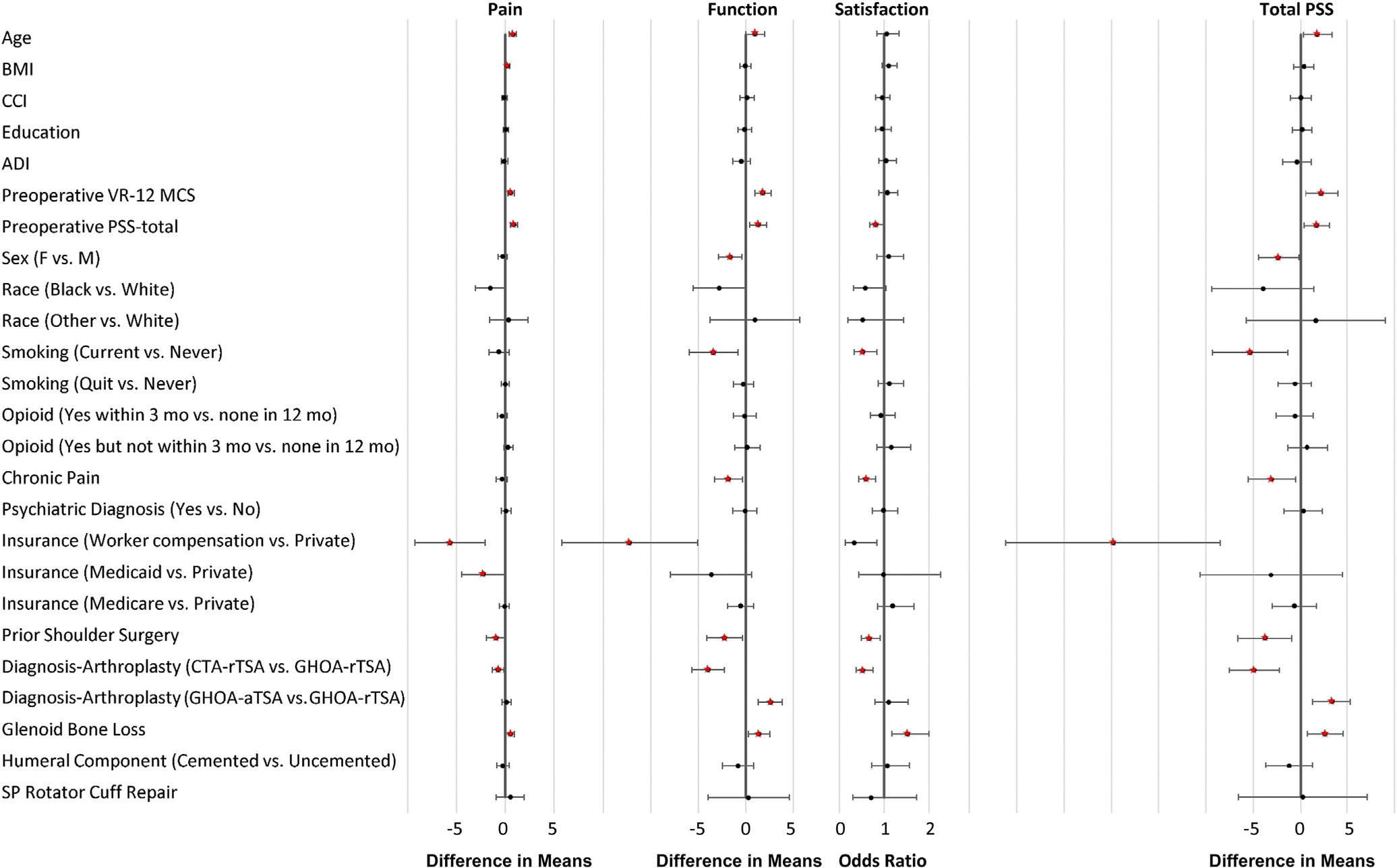
Forest plot showing the estimated difference in means for pain and function subscores and total PENN Shoulder Score (PSS) and odds ratios for the satisfaction subscore, each with 95% confidence intervals, for predictors in the full models of patients undergoing primary shoulder arthroplasty. The effects for continuous variables (age, BMI, CCI, education, ADI, baseline VR-12 MCS, and baseline PSS) are comparing the 75th vs. 25th percentiles shown in Table II. Predictors or contrasts of categories of categorical predictors are regarded as statistically significant, and their respective estimated effect sizes are depicted in red, only if the corresponding overall test simultaneously assessing associations of all categories with the respective 1-year PSS score or subscore is statistically significant. BMI, body mass index; CCI, Charlson Comorbidity Index; ADI, Area Deprivation Index; VR-12 MCS, Veterans RAND 12-Item Health Survey mental component summary; F, female; M, male; CTA, cuff tear arthropathy; rTSA, reverse total shoulder arthroplasty; GHOA, glenohumeral osteoarthritis; aTSA, anatomic total shoulder arthroplasty; SP, superior-posterior.
Lower age, female sex, current smoking, chronic pain diagnosis, and worker’s compensation claim were also significantly associated with lower 1-year PSS compared to their respective reference categories (Table V, Fig. 2). Unexpectedly, preoperative opioid use was not significantly associated with 1-year PSS (P =.474). Effects changed only minimally in sensitivity analyses (Supplementary Table S3).
Baseline PSS, prior ipsilateral shoulder surgery, diagnosis-arthroplasty type, and glenoid bone loss were the only factors significantly associated with all 3 PSS subscores (Table VI).
Akaike information criterion analysis indicated that the 3 most important factors for 1-year PSS in this cohort were diagnosis-arthroplasty type, baseline mental health status (VR-12 MCS), and insurance status (Fig. 3). Diagnosis-arthroplasty type was among the most important variables for PSS and all 3 of its subscores, but more strongly affected the PSS function and satisfaction subscores than the pain subscore. Baseline PSS was the most important factor for the PSS pain subscore. Baseline VR-12 MCS and insurance status were among the most important variables for all but the PSS satisfaction subscore (Fig. 3).
Figure 3.
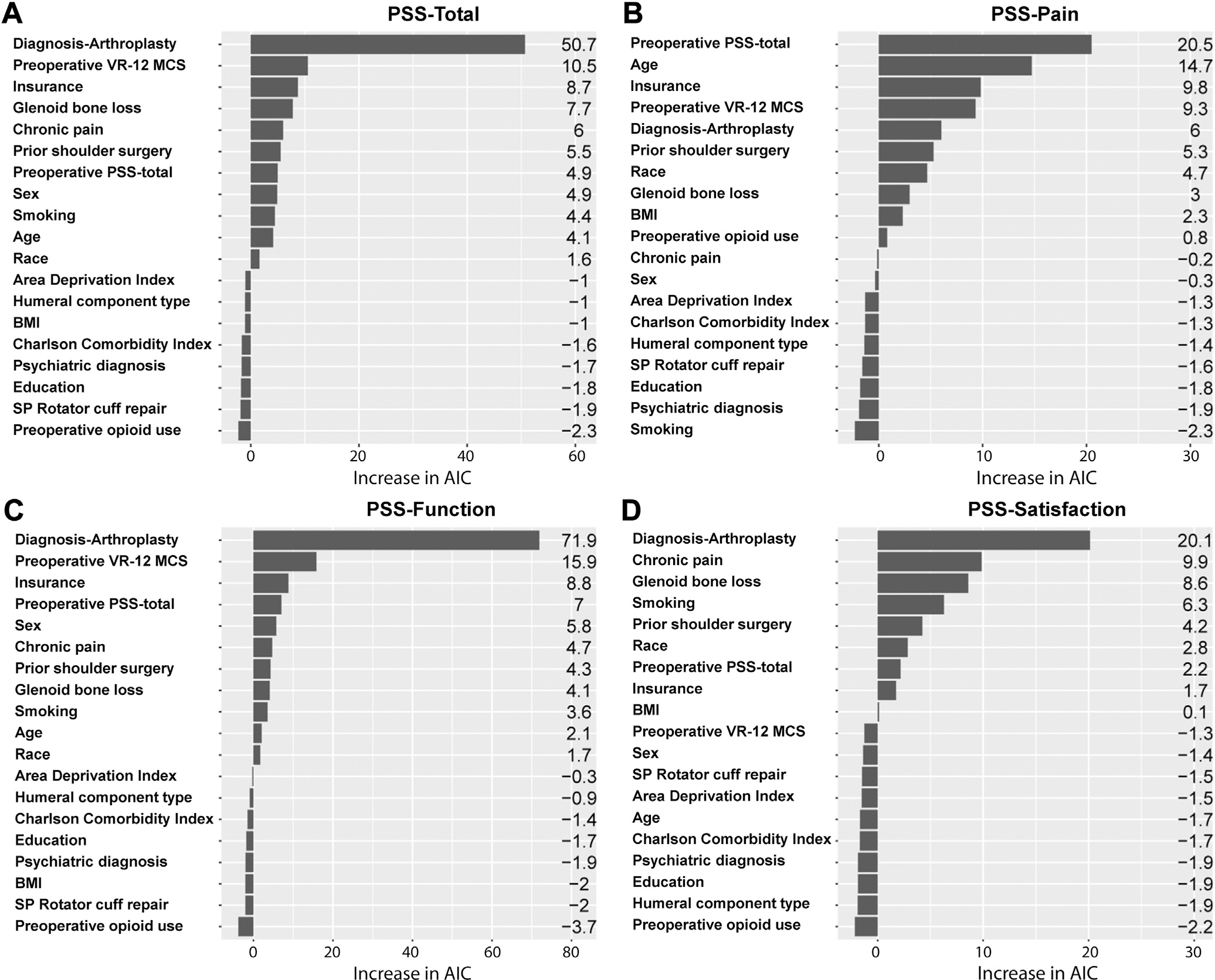
Relative variable importance of patient demographic, disease-specific, and surgical characteristics on 1-year PSS total (A) and its subscores (Pain [B], Function [C], Satisfaction [D]), based on the increase in Akaike information criterion (AIC) on removal from the full model. Variables with the largest contribution to outcomes are listed at the top of the respective charts. VR-12 MCS, Veterans RAND 12-Item Health Survey mental component summary; PSS, PENN Shoulder Score; BMI, body mass index; SP, superior-posterior.
Discussion
Consistent with prior literature, patients showed excellent PROMs following aTSA and rTSA for GHOA or CTA. Significant 1-year improvement was seen in PSS, ASES, and SANE scores, with 89% of patients reporting an acceptable symptom state. The most important factors associated with 1-year PSS were diagnosis-arthroplasty type, baseline mental health status, and insurance status. Although not modeled in this study, the strong correlations of 1-year PROMs suggest that predictors associated with 1-year PSS will also predict 1-year ASES and SANE scores.
Among diagnosis-arthroplasty types, 1-year PSS was highest in patients undergoing aTSA for GHOA and lowest in those undergoing rTSA for CTA. Some studies comparing outcomes following aTSA and rTSA have reported better PROMs following aTSA.21,7 and others have reported no differences.16,8 However, past studies have often involved small cohorts and bivariate analyses that lack simultaneous control for confounding by diagnosis and rotator cuff status; that is, CTA is associated with rotator cuff deficiency whereas GHOA is not. To overcome such confounding, some studies have restricted analysis to patients with GHOA and intact rotator cuffs, including a recent matched-pairs (n = 367 pairs) cohort analysis from a large international database, and found no significant difference in postoperative PROMs following aTSA or rTSA.19,17,25 Other studies limited to rTSA have shown better outcomes following GHOA than following CTA.51,40 To our knowledge, however, no study has prospectively investigated outcomes considering both diagnosis and arthroplasty type across the 3 commonly treated surgical groups (GHOA-aTSA, GHOA-rTSA, and CTA-rTSA), nor simultaneously controlled for such a comprehensive collection of possible confounders with multivariable analysis.
Our Akaike information criterion analysis showed that the diagnosis-arthroplasty factor more strongly affected the PSS function and satisfaction subscores than the pain subscore (Fig. 3). This finding suggests that the differential outcome in overall 1-year PSS across diagnosis-arthroplasty types may be most related to the negative impact of rotator cuff deficiency on function and satisfaction in CTA-rTSA patients compared with the other 2 surgical groups (GHOA-rTSA and GHOA-aTSA) that are not rotator cuff deficient. In contrast, pain improvement may be similar across the groups.
Preoperative mental health instruments like VR-12 MCS56 have been shown to be associated with arthroplasty outcomes; however, VR-12 MCS captures just a 4-week snapshot of recent mental status, and is also complicated by its calculation of patient-reported activity limitations that could be caused because of physical or emotional health issues. Therefore, some studies have looked at associations of outcomes with established diagnoses of mental illness.54,53,47 We included both in our models and observed that preoperative VR-12 MCS, but not mental illness diagnosis, was significantly associated with 1-year PSS. The mechanism by which mental health is associated with PROMs is not fully understood. We previously38 found that worse preoperative VR-12 MCS, female sex, less education, and preoperative opioid use were associated with lower preoperative PSS in patients undergoing shoulder arthroplasty. The current study found lower preoperative PSS to be a significant predictor of 1-year PSS. Interestingly, worse preoperative VR-12 MCS and female sex continued to be significantly associated with lower 1-year PSS, but education and preoperative opioid use were not, whereas age, smoking, chronic pain, worker’s compensation status, glenoid bone loss, prior ipsilateral surgery, and diagnosis-arthroplasty type emerged as predictors. These results suggest that some preoperative factors may act on postoperative PSS through preoperative PSS, whereas others may directly affect postoperative PSS. The negligible association between preoperative opioid use and 1-year PSS contrasts with prior studies linking its use to worse outcomes following TSA.27,28,5,44 Preoperative opioid use may be reflected in other baseline covariates such as lower preoperative PSS, chronic pain diagnosis, and current smoking status, which have been found to be associated with opioid use elsewhere52,15 and were significantly associated with lower 1-year PSS in this study.
Higher baseline PSS was associated with significantly higher 1-year PSS, although clinical significance was modest. A similar small association between baseline and 2-year postoperative ASES score has been reported in patients with rTSA after multivariable analysis adjusting for age, sex, prior shoulder surgery, and shoulder diagnosis (GHOAvs. CTA).40 Conversely, some studies have reported an inverse relationship between baseline and postoperative shoulder PROMs24,26,9,56 possibly because of ceiling effects: patients with high preoperative scores have less room for improvement. We used multivariable beta-regression, which is based on the large class of inherently bounded and primarily skewed beta distributions,14 to address the limitations of ordinary least squares regression from ceiling effects and skewed distributions of postoperative PROMs.33,24
We found associations of worse postoperative outcomes with younger age,49,32,35 female sex,55,22,29 current smokers,9,52 chronic pain diagnosis, lower preoperative VR-12 MCS,56 presence of worker’s compensation claim,47,6 and prior ipsilateral shoulder surgery40,24,26,41,9 consistent with previous reports. However, we also observed unexpected associations of glenoid bone loss, present in respectively 34%, 57%, and 81% of our CTA-rTSA, GHOA-aTSA, and GHOA-rTSA subgroups, with higher 1-year PSS and each of its subscores. Since the location and pattern of glenoid bone loss may differ and have different implications in these groups for how it is surgically addressed, we tested for interactions to assess if glenoid bone loss had differential effects on outcomes in the groups, but no significant interactions were observed. This finding may still relate to differing surgical methods (eg, eccentric reaming, bone grafting, and augmented components) used with aTSA and rTSA in the setting of varying degrees of glenoid bone loss associated with different diagnoses (GHOA vs. CTA), but such granular data were not available for analysis in the current study. Further investigation is needed to confirm and, if confirmed, better understand these associations.
This study has several strengths. It used a large cohort of consecutive patients with prospectively collected standardized data within a large tertiary health care system with a high rate of follow-up for 1-year PROMs (82%). Factors cited or judged to influence postoperative outcomes were prespecified for multivariable modeling, and we used beta-regression models that accommodate skewed data with ceiling effects. Although the effects of several significant variables were small and perhaps not clinically significant in isolation, these variables occur in patients in combinations with additive effects in our multivariable models. For example, a female patient, current smoker with low baseline mental health status, chronic pain diagnosis, and prior shoulder surgery undergoing rTSA for CTA would have, on average, a 25-point (2.3 + 5.3 + 2.3 + 3.0 + 3.7 + 8.2) lower 1-year PSS score compared with a male patient, nonsmoker with high baseline mental health status, no chronic pain diagnosis or prior shoulder surgery undergoing aTSA for GHOA, assuming common values of all other modeled factors. For purposes of demonstration and development of a tool for preoperative patient counseling regarding expected outcomes, we have developed an online calculator to predict 1-year PSS following primary TSA based on the multivariable model developed in this study (available online at: https://riskcalc.org/Predicting1YearPROMSAfterTotalShoulderArthroplasty/).
This study also has limitations. Our patients were from 1 tertiary health care system, and we excluded patients who did not complete 1-year PROMs, assuming their data were missing at random. However, non-respondents differed in several characteristics from respondents (Supplementary Table S2). Generalizing the results to broader patient populations should be done cautiously. The study also did not include imaging or objective functional outcomes. Although 1 year has been shown to be an appropriate landmark for postoperative PROMs and for investigating the short-term factors associated with PROMs after shoulder arthroplasty,23,54,4,10 longer follow-up and imaging may be needed for investigating other relevant outcomes such as implant survival or progressive rotator cuff failure that could impact longer-term PROMs across the surgical groups to different degrees. More granular detail than our database provides may also be needed to better understand certain associations noted in the study, such as the findings related to glenoid bone loss. Finally, our data are observational, and measures of associations from our simplistic models may be biased by omission of unknown confounders and, even if unbiased, may not reflect causal effects.
Conclusion
PROMs at 1 year following primary TSA are strongly associated with disease diagnosis and arthroplasty type, with the highest PROM scores demonstrated in patients undergoing aTSA for GHOA. Predicting postoperative outcomes following primary TSA using patient, disease and surgical factors could improve preoperative patient counseling regarding expected outcomes. Future studies are needed to determine whether predictors of short-term PROMs also predict longer-term outcomes.
Supplementary Material
Acknowledgments
We acknowledge the following 13 Cleveland Clinic surgeons who contributed cases to the OME database for investigation in this study: Vahid Entezari, Peter J. Evans, Gregory J. Gilot, Kirk Haidet, Robert J. Hampton, Joseph P. Iannotti, Anthony A. Miniaci, Eric T. Ricchetti, Vani J. Sabesan, Frank M. Sabo, William H. Seitz, Alfred Serna, and Joseph F. Styron.
We acknowledge the Orthopaedic and Rheumatologic Institute at Cleveland Clinic for support of the OME database and related infrastructure.
Cleveland Clinic Institutional Review Board (IRB) approved this study (#06–196).
Disclaimers:
Funding:
Research reported in this publication was supported by departmental resources and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR075286. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Cleveland Clinic Shoulder Group:
Kurt P. Spindler, MDd, William H. Seitz, MDa, Gregory J. Gilot, MDd, Anthony Miniaci, MDa, Peter J. Evans, MD, PhDd, Vani J. Sabesan, MDd, Greg Strnad, MSa
Footnotes
Conflicts of interest: Kurt P. Spindler and Greg Strnad have rights to royalties from OBERD for technology related to the subject of this article. The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Supplementary Data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jse.2024.01.028.
References
- 1.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 2001;29:1165–88. 10.1214/aos/1013699998 [DOI] [Google Scholar]
- 2.Best MJ, Aziz KT, Wilckens JH, McFarland EG, Srikumaran U. Increasing incidence of primary reverse and anatomic total shoulder arthroplasty in the United States. J Shoulder Elbow Surg 2021;30:1159–66. 10.1016/j.jse.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 3.van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Software 2011;45:1–67. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 4.Cabarcas BC, Gowd AK, Liu JN, Cvetanovich GL, Erickson BJ, Romeo AA, et al. Establishing maximum medical improvement following reverse total shoulder arthroplasty for rotator cuff deficiency. J Shoulder Elbow Surg 2018;27:1721–31. 10.1016/j.jse.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 5.Carducci MP, Zimmer ZR, Jawa A. Predictors of unsatisfactory patient outcomes in primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2019;28:2113–20. 10.1016/j.jse.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 6.Cvetanovich GL, Savin DD, Frank RM, Gowd AK, Sumner SA, Romeo AA, et al. Inferior outcomes and higher complication rates after shoulder arthroplasty in workers’ compensation patients. J Shoulder Elbow Surg 2019;28:875–81. 10.1016/j.jse.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Flurin PH, Tams C, Simovitch RW, Knudsen C, Roche C, Wright TW, et al. Comparison of survivorship and performance of a platform shoulder system in anatomic and reverse total shoulder arthroplasty. JSES Int 2020;4:923–8. 10.1016/j.jseint.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn L, Patrick MR, Roche C, Zuckerman JD, Flurin PH, Crosby L, et al. Anatomical and reverse shoulder arthroplasty utilizing a single implant system with a platform stem: a prospective observational study with midterm follow-up. Shoulder Elbow 2020;12:330–7. 10.1177/1758573219840675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman RJ, Eichinger J, Schoch B, Wright T, Zuckerman J, Flurin PH, et al. Preoperative parameters that predict postoperative patient-reported outcome measures and range of motion with anatomic and reverse total shoulder arthroplasty. JSES Open Access 2019;3:266–72. 10.1016/j.jses.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glanzmann MC, Kolling C, Schwyzer HK, Flury M, Audige L. Radiological and functional 24-month outcomes of resurfacing versus stemmed anatomic total shoulder arthroplasty. Int Orthop 2017;41:375–84. 10.1007/s00264-016-3310-4 [DOI] [PubMed] [Google Scholar]
- 11.Ho JC, Amini MH, Entezari V, Jun BJ, Alolabi B, Ricchetti ET, et al. Clinical and radiographic outcomes of a posteriorly augmented glenoid component in anatomic total shoulder arthroplasty for primary osteoarthritis with posterior glenoid bone loss. J Bone Joint Surg Am 2018;100:1934–48. 10.2106/JBJS.17.01282 [DOI] [PubMed] [Google Scholar]
- 12.Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am 2013;95:e82. 10.2106/JBJS.L.00336 [DOI] [PubMed] [Google Scholar]
- 13.James G, Witten D, Hastie T, Tibshirani RJ. Linear Model Selection and Regularization. An Introduction to Statistical Learning. New York: Springer; 2013. p. 203–64. [Google Scholar]
- 14.Kharroubi SA. Analysis of SF-6D Health State Utility Scores: Is Beta Regression Appropriate? Healthcare 2020;8:525. 10.3390/healthcare8040525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khazi ZM, Lu Y, Patel BH, Cancienne JM, Werner B, Forsythe B. Risk factors for opioid use after total shoulder arthroplasty. J Shoulder Elbow Surg 2020;29:235–43. 10.1016/j.jse.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 16.Kiet TK, Feeley BT, Naimark M, Gajiu T, Hall SL, Chung TT, et al. Outcomes after shoulder replacement: comparison between reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:179–85. 10.1016/j.jse.2014.06.039 [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Kim CH, Kim M, Lee W, Jeon IH, Lee KW, et al. Is reverse total shoulder arthroplasty (rTSA) more advantageous than anatomic TSA (aTSA) for osteoarthritis with intact cuff tendon? A systematic review and meta-analysis. J Orthop Traumatol 2022;23:3. 10.1186/s10195-022-00625-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N Engl J Med 2018;378:2456–8. 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirsch JM, Puzzitiello RN, Swanson D, Le K, Hart PA, Churchill R, et al. Outcomes after anatomic and reverse shoulder arthroplasty for the treatment of glenohumeral osteoarthritis: a propensity score-matched analysis. J Bone Joint Surg Am 2022;104:1362–9. 10.2106/JBJS.21.00982 [DOI] [PubMed] [Google Scholar]
- 20.Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP,Williams GR Jr. The Penn Shoulder Score: reliability and validity. J Orthop Sports Phys Ther 2006;36:138–51. 10.2519/jospt.2006.36.3.138 [DOI] [PubMed] [Google Scholar]
- 21.Levy JC, Everding NG, Gil CC Jr, Stephens S, Giveans MR. Speed of recovery after shoulder arthroplasty: a comparison of reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1872–81. 10.1016/j.jse.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 22.Lindbloom BJ, Christmas KN, Downes K, Simon P, McLendon PB, Hess AV 2nd, et al. Is there a relationship between preoperative diagnosis and clinical outcomes in reverse shoulder arthroplasty? An experience in 699 shoulders. J Shoulder Elbow Surg 2019;28:S110–7. 10.1016/j.jse.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 23.Mahendraraj KA, Carducci MP, Galvin JW, Golenbock SW, Grubhofer F, Jawa A. Reassessing the minimum two-year follow-up standard after total shoulder arthroplasty-is one year sufficient? Shoulder Elbow 2021;13:527–33. 10.1177/1758573220922845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahony GT, Werner BC, Chang B, Grawe BM, Taylor SA, Craig EV, et al. Risk factors for failing to achieve improvement after anatomic total shoulder arthroplasty for glenohumeral osteoarthritis. J Shoulder Elbow Surg 2018;27:968–75. 10.1016/j.jse.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 25.Marigi EM, Hao KA, Friedman RJ, Greene AT, Roche CP, Wright TW, et al. Equinoxe Exactech anatomic versus reverse total shoulder arthroplasty for primary osteoarthritis: case controlled comparisons using the machine learning derived shoulder arthroplasty score. J Shoulder Elbow Surg 2023;32:793–802. 10.1016/j.jse.2022.09.029 [DOI] [PubMed] [Google Scholar]
- 26.Matsen FA 3rd, Russ SM, Vu PT, Hsu JE, Lucas RM, Comstock BA. What factors are predictive of patient-reported outcomes? A prospective study of 337 shoulder arthroplasties. Clin Orthop Relat Res 2016;474:2496–510. 10.1007/s11999-016-4990-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris BJ, Laughlin MS, Elkousy HA, Gartsman GM, Edwards TB. opioid use and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:11–6. 10.1016/j.jse.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 28.Morris BJ, Sciascia AD, Jacobs CA, Edwards TB. Preoperative opioid use associated with worse outcomes after anatomic shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:619–23. 10.1016/j.jse.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 29.Okoroha KR, Muh S, Gabbard M, Evans T, Roche C, Flurin PH, et al. Early outcomes of shoulder arthroplasty according to sex. JSES Open Access 2019;3:43–7. 10.1016/j.jses.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.OME Cleveland Clinic Orthopaedics. Implementing a scientifically valid, cost-effective, and scalable data collection system at point of care: the Cleveland Clinic OME cohort. J Bone Joint Surg Am 2019;101:458–64. 10.2106/JBJS.18.00767 [DOI] [PubMed] [Google Scholar]
- 31.Palsis JA, Simpson KN, Matthews JH, Traven S, Eichinger JK, Friedman RJ. Current trends in the use of shoulder arthroplasty in the United States. Orthopedics 2018;41:e416–23. 10.3928/01477447-20180409-05 [DOI] [PubMed] [Google Scholar]
- 32.Patel RB, Muh S, Okoroha KR, Wright TW, Flurin PH, Roche C, et al. Results of total shoulder arthroplasty in patients aged 55 years or younger versus those older than 55 years: an analysis of 1135 patients with over 2 years of follow-up. J Shoulder Elbow Surg 2019;28:861–8. 10.1016/j.jse.2018.09.029 [DOI] [PubMed] [Google Scholar]
- 33.Petri M, Euler SA, Dornan GJ, Greenspoon JA, Horan MP, Katthagen JC, et al. Predictors for satisfaction after anatomic total shoulder arthroplasty for idiopathic glenohumeral osteoarthritis. Arch Orthop Trauma Surg 2016;136:755–62. 10.1007/s00402-016-2452-6 [DOI] [PubMed] [Google Scholar]
- 34.Polisetty TS, Colley R, Levy JC. Value analysis of anatomic and reverse shoulder arthroplasty for glenohumeral osteoarthritis with an intact rotator cuff. J Bone Joint Surg Am 2021;103:913–20. 10.2106/JBJS.19.01398 [DOI] [PubMed] [Google Scholar]
- 35.Poondla RK, Sheth MM, Heldt BL, Laughlin MS, Morris BJ, Elkousy HA, et al. Anatomic and reverse shoulder arthroplasty in patients 70 years of age and older: a comparison cohort at early to midterm follow-up. J Shoulder Elbow Surg 2021;30:1336–43. 10.1016/j.jse.2020.08.030 [DOI] [PubMed] [Google Scholar]
- 36.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 37.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc 1996;91:473–89. 10.1080/01621459.1996.10476908 [DOI] [Google Scholar]
- 38.Sahoo S, Derwin KA, Zajichek A, Cleveland Clinic Shoulder Group, Entezari V, Imrey PB, et al. Associations of preoperative patient mental health status and sociodemographic and clinical characteristics with baseline pain, function, and satisfaction in patients undergoing primary shoulder arthroplasty. J Shoulder Elbow Surg 2021;30:e212–24. 10.1016/j.jse.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahoo S, Rodriguez JA, Serna M, Cleveland Clinic Shoulder G, Spindler KP, Derwin KA, et al. Effectiveness of a web-based electronic prospective data collection tool for surgical data in shoulder arthroplasty. Semin Arthroplasty 2021;31:422–9. 10.1053/j.sart.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saini SS, Pettit R, Puzzitiello RN, Hart PA, Shah SS, Jawa A, et al. Clinical outcomes after reverse total shoulder arthroplasty in patients with primary glenohumeral osteoarthritis compared with rotator cuff tear arthropathy: does preoperative diagnosis make a difference? J Am Acad Orthop Surg 2022;30:e415–22. 10.5435/JAAOS-D-21-00797 [DOI] [PubMed] [Google Scholar]
- 41.Shields EJW, Koueiter DM, Maerz T, Schwark A, Wiater JM. Previous rotator cuff repair is associated with inferior clinical outcomes after reverse total shoulder arthroplasty. Orthop J Sports Med 2017;5:2325967117730311. 10.1177/2325967117730311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steen BM, Cabezas AF, Santoni BG, Hussey MM, Cusick MC, Kumar AG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg 2015;24:1433–41. 10.1016/j.jse.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 43.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. https://www.R-project.org [Google Scholar]
- 44.Thompson KM, Hallock JD, Smith RA, Brolin TJ, Azar FM, Throckmorton TW. Preoperative narcotic use and Inferior outcomes after anatomic total shoulder arthroplasty: a clinical and radiographic analysis. J Am Acad Orthop Surg 2019;27:177–82. 10.5435/JAAOS-D-16-00808 [DOI] [PubMed] [Google Scholar]
- 45.Triplet JJ, Everding NG, Levy JC, Moor MA. Functional internal rotation after shoulder arthroplasty: a comparison of anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:867–74. 10.1016/j.jse.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 46.University of Wisconsin School of Medicine and Public Health. 2015 area deprivation index v2.0 [database on the Internet]. 2015. Available at: https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed September 1, 2019.
- 47.Vajapey SP, Cvetanovich GL, Bishop JY, Neviaser AS. Psychosocial factors affecting outcomes after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2020;29:e175–84. 10.1016/j.jse.2019.09.043 [DOI] [PubMed] [Google Scholar]
- 48.Wagner ER, Farley KX, Higgins I, Wilson JM, Daly CA, Gottschalk MB. The incidence of shoulder arthroplasty: rise and future projections compared with hip and knee arthroplasty. J Shoulder Elbow Surg 2020;29:2601–9. 10.1016/j.jse.2020.03.049 [DOI] [PubMed] [Google Scholar]
- 49.Wagner ER, Houdek MT, Schleck CD, Harmsen WS, Sanchez-Sotelo J, Cofield R, et al. The role age plays in the outcomes and complications of shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1573–80. 10.1016/j.jse.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 50.Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg 2012;21:1526–33. 10.1016/j.jse.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 51.Waterman BR, Dean RS, Naylor AJ, Otte RS, Sumner-Parilla SA, Romeo AA, et al. Comparative clinical outcomes of reverse total shoulder arthroplasty for primary cuff tear arthropathy versus severe glenohumeral osteoarthritis with intact rotator cuff: a matched-cohort analysis. J Am Acad Orthop Surg 2020;28:e1042–8. 10.5435/JAAOS-D-19-00493 [DOI] [PubMed] [Google Scholar]
- 52.Wells DB, Holt AM, Smith RA, Brolin TJ, Azar FM, Throckmorton TW. Tobacco use predicts a more difficult episode of care after anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 2018;27:23–8. 10.1016/j.jse.2017.06.033 [DOI] [PubMed] [Google Scholar]
- 53.Werner BC, Wong AC, Chang B, Craig EV, Dines DM, Warren RF, et al. Depression and patient-reported outcomes following total shoulder arthroplasty. J Bone Joint Surg Am 2017;99:688–95. 10.2106/JBJS.16.00541 [DOI] [PubMed] [Google Scholar]
- 54.Wong SE, Colley AK, Pitcher AA, Zhang AL, Ma CB, Feeley BT. Mental health, preoperative disability, and postoperative outcomes in patients undergoing shoulder arthroplasty. J Shoulder Elbow Surg 2018;27:1580–7. 10.1016/j.jse.2018.02.066 [DOI] [PubMed] [Google Scholar]
- 55.Wong SE, Pitcher AA, Ding DY, Cashman N, Zhang AL, Ma CB, et al. The effect of patient gender on outcomes after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1889–96. 10.1016/j.jse.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 56.Wong SE, Zhang AL, Berliner JL, Ma CB, Feeley BT. Preoperative patient-reported scores can predict postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:913–9. 10.1016/j.jse.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 57.Wright MA, Keener JD, Chamberlain AM. Comparison of clinical outcomes after anatomic total shoulder arthroplasty and reverse shoulder arthroplasty in patients 70 years and older with glenohumeral osteoarthritis and an intact rotator cuff. J Am Acad Orthop Surg 2020;28:e222–9. 10.5435/JAAOS-D-19-00166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


