Abstract
Background
As a feed additive, medium-chain fatty acids (MCFAs)/medium-chain fatty acid triglycerides (MCTs) have been used in ruminant production, but mostly added in the form of mixed esters. Studies have shown that MCTs may have a positive effect on feed intake or oxidative stress in animals, but it is unclear which MCT could play a role, and the mechanism has not been elucidated. In this study, the effects of individual MCT on growth performance, serum intake-related hormones, and oxidative stress indices in finishing bulls were investigated and further studied the effects of MCT supplementation on gastrointestinal tract bacteria and rumen fluid metabolomics.
Results
Four ruminally fistulated Yanbian cattle (bulls) were selected in 4 × 4 Latin square designs and allocated to four treatment groups: a control group (CON) fed a basal diet (total mixed ration, TMR), three groups fed a basal diet supplemented with 60 g/bull/day glycerol monocaprylin (GMC, C8), glycerol monodecanoate (GMD, C10), and glycerol monolaurate (GML, C12), respectively. Compared with the CON group, GMD tended to increase the dry matter intake (DMI) of finishing bulls (P = 0.069). Compared with the CON group, GMD significantly increased the concentration of ghrelin O-acyl transferase (GOAT), total ghrelin (TG), acylated ghrelin (AG), and orexins (P < 0.05) and significantly decreased the concentrations of hydrogen peroxide (H2O2), malondialdehyde, reactive oxygen species (ROS), and lipopolysaccharides (LPS) in the serum of finishing bulls (P < 0.05). Compared with the CON group, GMD and GML significantly increased the concentrations of total antioxidant capacity (T-AOC), catalase, glutathione peroxidase (GSH-PX), glutathione reductase (GR), and nitric oxide (NO) in the serum of finishing bulls (P < 0.05). Compared with the CON group, there were 5, 14, and 6 significantly different bacteria in the rumen digesta in the C8, C10, and C12 groups, respectively; there were 3, 10, and 5 significantly different bacteria in the rumen fluid in the C8, C10, and C12 groups, respectively; and only one differential bacteria (genus level) in the feces among the four treatment groups. Compared with the CON group, there were 3, 14, and 15 significantly differential metabolites identified under positive ionization mode in the C8, C10, and C12 groups, respectively, while under negative ionization mode were 3, 11 and 14, respectively. Correlation analysis showed that there was a significant correlation between DMI, GOAT, AG, GSH-PX, LPS, gastrointestinal tract bacteria, and rumen fluid metabolites.
Conclusions
Our findings revealed that different types of MCTs have different application effects in ruminants. Among them, GMD may improve the feed intake of finishing bulls by stimulating the secretion of AG. GMD and GML may change gastrointestinal tract microorganisms and produce specific rumen metabolites to improve the oxidative stress of finishing bulls, and ghrelin may also be involved. This study enlightens the potential mechanisms by which MCT improves feed intake and oxidative stress in finishing bulls.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-024-01946-2.
Keywords: Feed intake, Gastrointestinal tract bacteria, Medium-chain fatty acid triglycerides, Metabolome, Oxidative stress, Yanbian cattle
Background
Medium-chain fatty acids (MCFAs) are fatty acids with chain lengths between 6 and 12 carbon atoms, mainly including caprylic acid (C8:0), decanoic acid (C10:0), and lauric acid (C12:0), which are easy to be digested and absorbed in animal intestines [1]. At present, as a safe and efficient additive, MCFAs have been used in ruminants to improve performance (such as milk performance, immune function, rumen fermentation, etc.), nutrients digestibility, and inhibit methane (CH4) production [2–7]. Despite this, MCFAs are mostly added to the diet in the form of mixed esters (such as palm oil and coconut oil), but it is unclear which MCFA could play a role.
MCFAs can be esterified by glycerol to form medium-chain fatty acid triglycerides (MCTs), mainly including glycerol monocaprylin (GMC), glycerol monodecanoate (GMD), and glycerol monolaurate (GML). Some studies have shown that MCTs can improve feed intake and oxidative stress in monogastric animals. The addition of 1% 1: 1: 1 blend of C6:0, C8:0, and C10:0 in nursery pig diets increased average daily feed intake (ADFI) compared with control-fed pigs [8]. Moreover, MCFAs treatment (C8:0, C10:0, and C12:0) significantly increased the total antioxidant capacity (T-AOC), and expression of antioxidant-related genes in AML12 cells under oxidative stress conditions, and reduced reactive oxygen species (ROS) production [9]. Dietary decanoate acid (C10:0) supplementation improved cyclophosphamide-induced intestinal inflammation and oxidative stress in miniature pigs [10], and mustard oil rich in decanoate acid (C10:0) could enhance the antioxidant protection of rat liver and brain [11]. The above results indicated that MCFAs/MCTs may play a positive role in improving feed intake and oxidative stress in animals. However, it is unknown whether or which MCFA/MCT will affect the feed intake and oxidative stress in ruminants, and the mechanism has not been clarified.
It has been reported that MCFAs can be used for the acylation of ghrelin and increase the concentration of acylated ghrelin (AG), and AG can stimulate animal appetite [12, 13], and some studies have shown that ghrelin may also be involved in the regulation of oxidative stress [14–16], which provides new insights into the improvement of feed intake and oxidative stress by MCFAs/MCTs supplementation. Although the effects of mixed forms of MCFA on feed intake and ghrelin secretion in beef/dairy cattle have been studied [13, 17, 18], the effects of individual MCFA/MCT on feed intake, ghrelin secretion, and oxidative stress in beef cattle have not been reported, and the related mechanism has not been revealed. Different omics approaches, especially 16S rRNA gene sequencing and metabolomics, provide powerful tools to identify potential players in a high-throughput manner and to further elucidate mechanisms. Previous studies suggest that gastrointestinal tract microbial, metabolites, or metabolic pattern variations may influence host physiology and behavior via multiple direct or indirect pathways [19–22] and play crucial roles in animal health, hormone secretion, nutrient absorption and metabolism, oxidative stress, gastrointestinal tract development, and immune function [23–26].
Thus, in this study, we aimed to answer two questions. Does the individual MCT (GMC/GMD/GML) supplementation contribute to feed intake or oxidative stress in finishing bulls? If so, is this related to ghrelin and gastrointestinal tract microorganisms and metabolites? This study evaluated the effects of individual MCT (GMC/GMD/GML) supplementation on growth performance, serum intake-related hormones, serum oxidative stress indices, and further studied their effects on gastrointestinal tract bacteria and rumen fluid metabolomics of finishing bulls.
Materials and methods
The experiment was carried out from February 2023 to June 2023 in Yanbian University College of Agriculture Animal Experimental Teaching Base, Jilin Province, China. All procedures involving animals were performed with the approval (approval ID: 20221015) of the Yanbian University Institutional Animal Care and Use Committee.
Animals, feeding, experimental designs, and growth performance
The experiment was conducted by using four ruminally fistulated Yanbian cattle (18-month-old bulls) with initial body weight (BW) 367 ± 32 kg in 4 × 4 Latin square designs. These bulls were allocated to four treatment groups: a group (CON) fed a basal diet (total mixed ration, TMR), a group (C8) fed a basal diet supplemented with 60 g/bull/day GMC, a group (C10) fed a basal diet supplemented with 60 g/bull/day GMD, and a group (C12) fed a basal diet supplemented with 60 g/bull/day GML. Three monoglycerides (purity ≥ 99%) were purchased from Shandong Binzhou Jinsheng New Material Technology Co., Ltd. The selection of the addition amount was based on the appropriate concentration determined in the previous in vitro study [27] and converted according to the volume of rumen fluid in adult cattle. The ingredients and chemical composition of the basal diets are shown in Table 1.
Table 1.
Ingredient and nutritional composition of basal diets (%, dry matter basis)
| Ingredient composition | Content (% of DM) | Nutritional compositionb | Content (% of DM) |
|---|---|---|---|
| Corn meal | 45.00 | NEgc (Mcal/kg DM) | 1.16 |
| Cornstalk | 40.00 | Crude protein | 11.47 |
| Soybean meal | 6.75 | Crude fat | 3.17 |
| Corn germ meal | 2.25 | Crude ash | 5.62 |
| DDGS | 2.25 | Neutral detergent fibers | 34.89 |
| Calcium carbonate | 0.68 | Acid detergent fibers | 20.62 |
| Molasses cane | 0.60 | Calcium | 0.51 |
| Sodium bicarbonate | 0.45 | Phosphorus | 0.34 |
| Calcium hydrogen phosphate | 0.37 | Sodium chloride | 0.45 |
| Urea | 0.45 | ||
| Salt | 0.45 | ||
| Premixa | 0.75 | ||
| Total | 100.00 |
aPremix provided the following per kg of the diet: Fe 400 mg, Cu 600 mg, Zn 400 mg, Mn 20 mg, Se 10 mg, Co 10 mg, vitamin A 350 000 IU, vitamin D 300 000 IU, vitamin E 5000 IU
bConventional nutrients in feed were calculated based on the actual content of feed ingredients
cNEg (net energy for gain) was estimated from the analyzed value of the dietary ingredients (based on Ministry of Agriculture of P.R. China (2018))
Before the start of the trial, all bulls were ear-tagged, dewormed, and tethered in a tie stall using neck straps. During the study, each bull was kept in an individual pen and fed individually twice a day (0500 and 1500 h) and had free access to fresh water. Each experimental period was composed of 28 days. The first 21 days were considered the adaptation period, and the final 7 days were the sampling period.
Dry matter intake (DMI) was individually measured based on the differences between the amount of diet offered and refused daily. Initial and final BW measurements were repeated on two consecutive days before morning feeding, and then the average of the 2 days was used. Average daily gain (ADG) was calculated as the difference between initial and final live weight divided by 28 days. Feed conversion efficiency (feed:gain) was the ratio of individual DMI to ADG.
Sample collection
Feed and fecal sample collection
Representative feed samples of approximately 200 g were collected during the sampling period. Fecal grab samples were collected once a day for seven consecutive days on days 22–28 of each experimental period by grab sampling after the morning feeding. The feed and feces samples collected from each bull within 7 days were mixed and stored at − 20°C for measuring the contents of DM, crude protein (CP), ether extract (EE), ash, neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid insoluble ash (AIA).
Blood sample collection
At the last day of each experimental period, about 10 mL of blood was collected from each bull before morning feeding by venipuncture with vacuum tubes without anticoagulant and were centrifuged at 3186 × g for 20 min at 4°C to harvest serum. The serum samples were immediately stored at − 20°C for measurement intake-related hormones, antioxidant indices, oxidative stress indices, and endotoxin lipopolysaccharides (LPS). Intake-related hormones included GOAT, total ghrelin (TG), deacylated ghrelin (DAG), growth hormone (GH), insulin (INS), orexins, and corticotropin-releasing hormone (CRH). Antioxidant indices included T-AOC, superoxide dismutase (SOD), catalase, glutathione peroxidase (GSH-PX), and glutathione reductase (GR). Oxidative stress indices included hydrogen peroxide (H2O2), malondialdehyde, nitric oxide (NO), and ROS.
Gastrointestinal tract sample collection
At the end of each experimental period, rumen samples were collected from four different (the ventral sac, the atrium or reticulum, and two samples from the feed mat) locations in the rumen, filtered through four layers of gauze to separate solid and fluid fractions; the collecting methods were referred to Pitta et al. [28]. Rumen fluid was snap frozen in liquid nitrogen and stored at − 80°C for measurement fermentation parameters (such as pH, ammoniacal nitrogen [NH3-N], lactic acid, LPS, and volatile fatty acids [VFAs]), bacterial diversity, and liquid chromatography-mass spectrometry (LC–MS) metabolomics. Moreover, rumen digesta (solid) and fresh rectum fecal samples were also snap frozen in liquid nitrogen, and stored at − 80°C for measurement of bacterial diversity.
Analysis of nutrients' apparent digestibility
The feed and fecal samples collected above were dried at 65°C for 72 h and ground to pass through a 1-mm screen for nutrients’ apparent digestibility analysis. DM, CP, EE, ash, NDF, ADF, and AIA contents of feed and feces were determined according to standard procedures [29]. The apparent digestibility of DM, CP, EE, organic matter (OM), NDF, and ADF was calculated by the endogenous indicator (AIA) method according to Luan et al. [30].
Analysis of serum intake-related hormones
The serum GOAT, TG, DAG, GH, INS, orexins, and CRH were determined using commercial ELISA Kits—YJ623074, YJ446287, YJ233015, YJ921050, YJ016134, YJ180841, and YJ405202, respectively—according to the manufacturer’s instructions (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China), and a microplate reader (CMax Plus, Molecular Devices, SV, USA). Using the same measurement method and principle, details about the method description of the measurement refer to Luan et al. [30]. The intra-assay and inter-assay coefficients of variation (CV) were < 10 and 15%, respectively.
Analysis of antioxidant and oxidative stress indices
Antioxidant indices in the serum samples of bulls were determined in a service corporation (Beijing Sino-UK of Biological Technology, Beijing, China) using commercial kits (Beijing Sino-UK of Biological Technology, Beijing, China) by the method of colorimetry and an A6 semi-automatic biochemistry analyzer (Beijing Shiningsun Technology Inc., China). Details about the method description of the measurement of T-AOC, SOD, catalase, and GSH-PX refer to Luan et al. [31]. GR was determined using an HY-60010 commercial kit. Briefly, under the condition that reduced coenzyme II (nicotinamide adenine dinucleotide phosphate, NADPH) provides hydrogen, GR can reduce oxidized glutathione (GSSG) to reduced glutathione (GSH). Meanwhile, NADPH forms NADP+. NADPH has an absorption peak at 340 nm, and the amount of NADPH production is proportional to the activity of GR. Therefore, the activity of GR can be calculated by measuring the absorbance of samples at 340 nm. For T-AOC, catalase, GSH-PX, and GR, the intra-assay coefficients of variation were less than 7.3%; the inter-assay coefficients of variation were less than 10%. For SOD, the intra-assay coefficients of variation were less than 8.3%; the inter-assay coefficients of variation were less than 10%.
Oxidative stress indices in the serum samples of bulls were determined in a service corporation (Beijing Sino-UK of Biological Technology, Beijing, China) using commercial kits (Beijing Sino-UK of Biological Technology, Beijing, China) by method of colorimetry and an A6 semi-automatic biochemistry analyzer (Beijing Shiningsun Technology Inc., China) (H2O2, malondialdehyde, and NO) or a microplate reader (DR-200BS, Wuxi Hiwell-Diatek Instruments Co., Ltd., Wuxi, China) (ROS). Details about the method description of the measurement of H2O2, malondialdehyde, NO, and ROS refer to Luan et al. [31] and Geng et al. [32]. Moreover, the measurement method and principle of LPS (YJ600359) are the same as those of serum intake-related hormones. For H2O2, malondialdehyde, and NO, the intra-assay coefficients of variation were less than 7.3%; the inter-assay coefficients of variation were less than 10%. For ROS, the intra-assay coefficients of variation were less than 8.3%, the inter-assay coefficients of variation were less than 10%. For LPS, the intra-assay coefficients of variation were less than 10%; the inter-assay coefficients of variation were less than 15%.
Analysis of rumen fluid fermentation parameters
The pH value of the rumen fluid was measured instantly by a rapid pH analyzer (ST3100, Ohaus, NJ, USA) after collection. NH3-N and lactic acid concentrations in rumen fluid were determined by a microplate reader (CMax Plus, Molecular Devices, SV, USA) according to the method of Barker and Summerson [33] and Broderick and Kang [34]. VFA was determined by gas chromatograph (GC-1120, Sunny Hengping Instrument, Shanghai, China). Rumen fluid in a volume of 1 mL was mixed with 0.2 mL 25% (w/v) metaphosphoric acid solution containing 2-ethylbutyrate and centrifuged at 10,621 × g/min for 15 min for VFA analysis.
Analysis of gastrointestinal tract bacterial diversity
High-throughput 16S rRNA gene sequencing
Total genomic DNA was extracted from rumen digesta, rumen fluid, and fecal samples using the TGuide S96 Magnetic Soil/Stool DNA Kit (Tiangen Biotech (Beijing) Co., Ltd.) according to the manufacturer’s instructions. The quality and quantity of the extracted DNA were examined using electrophoresis on a 1.8% agarose gel, and DNA concentration and purity were determined with a NanoDrop 2000 UV–vis spectrophotometer (Thermo Scientific, Wilmington, USA). After quantitative measurement of DNA samples, a total of 48 samples were qualified. There were 16 samples of rumen digesta, rumen fluid, and feces, respectively (4 samples in each group [CON, C8, C10, C12]). The hypervariable region V3–V4 of the bacterial 16S rRNA gene was amplified with primer pairs 338F: 5'- ACTCCTACGGGAGGCAGCA-3' and 806R: 5'- GGACTACHVGGGTWTCTAAT-3'. PCR products were checked on agarose gel and purified through the Omega DNA purification kit (Omega Inc., Norcross, GA, USA). The purified PCR products were collected and the paired ends (2 × 250 bp) were performed on the Illumina Novaseq 6000 platform (Beijing Biomarker Technologies Co., Ltd., Beijing, China).
Sequence data bioinformatic analysis
Analysis of sequence data followed by Gao and Geng [35]. The qualified sequences with more than 97% similarity thresholds were allocated to one operational taxonomic unit (OTU) using USEARCH (v.10.0). Taxonomy annotation of the OTUs was performed based on the Naive Bayes classifier in QIIME2 (v.2020.6) using the SILVA database (release 138.1) with a confidence threshold of 70%. The Venn diagram, rarefaction curve, Shannon curves, and rank abundance curves (OTU level) were created using R (v.3.1.1), Mothur (v.1.22.2), and Python (v.2.7.8). QIIME2 (v.2020.6) was used to determine beta diversity to evaluate the degree of similarity of microbial communities from different samples, and three-dimensional principal component analysis (3D-PCA) and principal coordinates analysis (PCoA) for analysis of beta diversity. Species distribution histograms at phylum, family, and genus levels were created using Python (v.2.7.8).
Liquid chromatography-mass spectrometry (LC–MS) metabolomics and data analysis
The pretreatment of rumen fluid samples was referred to Gao and Geng [35]. The LC–MS system for metabolomics analysis is composed of Waters Acquity I-Class PLUS ultra-high performance liquid tandem Waters Xevo G2-XS QTof high-resolution mass spectrometer with a Waters Acquity UPLC HSS T3 column (1.8 μm × 2.1 mm × 100 mm). Samples were eluted in 0.1% formic acid aqueous solution (mobile phase A) and 0.1% formic acid acetonitrile (mobile phase B), injection volume 1 μL. A flow rate of 400 μL/min was applied to the elution gradient (A%: B%) 98:2 for 0–0.25 min, 2:98 at 10–13 min, and 98:2 at 13.1–15 min. In each data acquisition cycle, dual-channel data acquisition was performed on both low collision energy and high collision energy at the same time. The low collision energy is 2 V, the high collision energy range is 10–40 V, and the scanning frequency is 0.2 s for a mass spectrum. The parameters of the ESI ion source are as follows: capillary voltage: 2000 V (positive ion mode) or − 1500 V (negative ion mode); cone voltage: 30 V; ion source temperature: 150°C; desolvent gas temperature 500°C; backflush gas flow rate: 50 L/h; desolventizing gas flow rate: 800 L/h. The raw data collected using MassLynx (v.4.2) is processed by Progenesis QI software for peak extraction, peak alignment, and other data processing operations. After normalizing the original peak area information with the total peak area, the follow-up analysis was performed. Metabolites were preliminarily identified in an in-house secondary mass spectrometry database using R (v.3.3.2), then compared with the Kyoto Encyclopedia of Genes and Genomes Database (KEGG), Human Metabolome Database (HMDB), and lipid maps to identify known metabolites.
The overall metabolic differences between groups of samples (including quality control samples) and the degree of variation between samples in the group were preliminarily understood by PCA. Additionally, partial least squares-discriminant analysis (PLS-DA) was applied to screen the variables related to the differences among groups.
Statistics and analysis
The experimental data (i.e., growth performance, serum intake-related hormones, antioxidant indices, oxidative stress indices, rumen fluid fermentation parameters, and nutrients apparent digestibility) were analyzed by the general linear model of SPSS 21.0 (SPSS Inc., Chicago, IL, USA) according to the model: Yijk = μ + Di + Aj + Sk + eijk, where Yijk is the observation of dependent variables, μ is the overall mean, Di represents the fixed effect of treatment, Aj represents the fixed effect of period, Sk represents the random effect of bulls (animal ID), and eijk is the residual error of the model. P < 0.05 means a significant difference, while differences with P > 0.05 to P < 0.10 are considered as a trend.
Moreover, for the analysis of alpha diversity indices (ACE, Chao1, Simpson, Shannon, and coverage) of gastrointestinal tract bacteria, the raw data were extracted and analyzed by the general linear model of SPSS 21.0 (SPSS Inc., Chicago, IL, USA). The raw data of relative abundance (more than 0.1%) of bacteria were extracted, and species abundance data between groups were analyzed by the general linear model of SPSS 21.0 (SPSS Inc., Chicago, IL, USA), and species were screened according to P-value. The raw data of metabolite abundance were also extracted to determine the significant differences of metabolites by the general linear model of SPSS 21.0 (SPSS Inc., Chicago, IL, USA) and plotted KEGG classification maps of significant differential metabolites. The parameter threshold for differential metabolite screening was set at VIP (variable importance in the projection) > 1, and P < 0.05. Before the data analysis, the alpha diversity indices, species abundance, and metabolite abundance were not performed data transformation, and a Shapiro–Wilk normality test found that these data conform to the normality distribution.
Partial correlation matrix (groups are used as control variables) and P-value matrix were calculated using SPSS 21.0 (SPSS Inc., Chicago, IL, USA) for the differential conventional indices (i.e., DMI, GOAT, TG, AG, Orexins, GHS-PX, MDA, NO, LPS), differential gastrointestinal tract bacteria (all levels), and differential metabolites, plotting the correlation heat maps. Correlation coefficients ranging from − 1 to + 1 represented strong negative correlations to strong positive correlations. Correlation P < 0.05 (*) and 0.01 (**) represented significant and extremely significant correlations, respectively.
Results
Growth performance
The effects of MCT supplementation on the growth performance of finishing bulls are shown in Table 2. The average initial BW of four bulls was 367 ± 32 kg, and the average final BW of four bulls was 519 ± 39 kg. Compared with the CON group, there were no significant effects on ADG and feed conversion efficiency of finishing bulls among the C8, C10, and C12 groups (P > 0.05), but the C10 group tended to increase the DMI of finishing bulls (P = 0.069).
Table 2.
Effects of medium-chain fatty acid triglycerides supplementation on growth performance of finishing bulls
| Itemsa | Dietary treatmentb | SEMc | P-value | |||
|---|---|---|---|---|---|---|
| CON | C8 | C10 | C12 | |||
| ADG (kg/day) | 1.39 | 1.26 | 1.52 | 1.25 | 0.167 | 0.393 |
| DMI (kg/day) | 9.73 | 9.98 | 10.51 | 9.50 | 0.304 | 0.069 |
| Feed conversion efficiency (feed: gain) | 7.15 | 8.05 | 7.15 | 7.62 | 0.723 | 0.574 |
aADG average daily gain, DMI dry matter intake
bCON, the control group cattle fed control diets; C8, the treatment group cattle fed control diets containing glycerol monocaprylin; C10, the treatment group cattle fed control diets containing glycerol monodecanoate; C12, the treatment group cattle fed control diets containing glycerol monolaurate
cSEM standard error of the means
eSerum intake-related hormones
The effects of MCT supplementation on serum intake-related hormones of finishing bulls are shown in Table 3. Compared with the CON group, the C8 and C10 groups significantly increased the concentration of GOAT in the serum of finishing bulls (P < 0.05). The C10 group significantly increased the concentrations of TG and AG in the serum of finishing bulls compared with the other three groups (P < 0.05). The C10 group significantly increased the AG/TG and the concentration of orexins in the serum of finishing bulls compared with the CON and C8 groups (P < 0.05). There were no significant effects on serum DAG, GH, INS, and CRH of finishing bulls among the four treatment groups (P > 0.05).
Table 3.
Effects of medium-chain fatty acid triglycerides supplementation on serum intake-related hormones of finishing bulls
| Itemsc | Dietary treatmentd | SEMe | P-value | |||
|---|---|---|---|---|---|---|
| CON | C8 | C10 | C12 | |||
| GOAT (ng/mL) | 2.471b | 2.916a | 3.000a | 2.818ab | 0.183 | 0.043 |
| TG (pg/mL) | 139.231b | 156.649b | 188.471a | 158.082b | 14.244 | 0.018 |
| AG (pg/mL) | 37.635b | 30.785b | 66.714a | 43.093b | 6.875 | 0.001 |
| DAG (pg/mL) | 101.596 | 125.864 | 121.757 | 114.989 | 13.624 | 0.327 |
| AG/TG | 0.250b | 0.190b | 0.353a | 0.272ab | 0.040 | 0.005 |
| GH (ng/mL) | 2.435 | 2.435 | 2.538 | 2.358 | 0.102 | 0.392 |
| INS (mIU/L) | 4.472 | 5.017 | 5.460 | 4.917 | 0.387 | 0.118 |
| Orexins (ng/mL) | 1.312b | 1.254b | 1.538a | 1.385ab | 0.077 | 0.008 |
| CRH (pg/mL) | 11.648 | 10.800 | 10.170 | 11.796 | 1.210 | 0.511 |
a,bMeans bearing different superscripts in the same row differ significantly (P-value < 0.05)
cGOAT ghrelin O-acyl transferase, TG total ghrelin, AG acyl ghrelin, DAG des-acyl ghrelin, GH growth hormone, INS insulin, CRH corticotropin-releasing hormone
dCON, the control group cattle fed control diets, C8 the treatment group cattle fed control diets containing glycerol monocaprylin, C10 the treatment group cattle fed control diets containing glycerol monodecanoate, C12 the treatment group cattle fed control diets containing glycerol monolaurate
eSEM standard error of the means
Serum antioxidant and oxidative stress indices, and endotoxin LPS
Effects of MCT supplementation on serum antioxidant and oxidative stress indices, and endotoxin LPS of finishing bulls are shown in Table 4. Compared with the CON group, the C10 and C12 groups significantly increased the concentrations of TAOC, GHS-PX, and NO in the serum of finishing bulls (P < 0.05). The C12 group significantly increased the concentration of SOD in the serum of finishing bulls compared with the CON and C8 groups (P < 0.05). Compared with the CON group, the concentrations of catalase and GR significantly increased in the serum of finishing bulls among the other three groups (P < 0.05). The C10 and C12 groups significantly decreased the concentrations of H2O2, malondialdehyde, and ROS in the serum of finishing bulls compared with the CON group (P < 0.05). The C10 group significantly decreased the concentrations of LPS in the serum of finishing bulls compared with the CON and C8 groups (P < 0.05).
Table 4.
Effects of medium-chain fatty acid triglycerides supplementation on serum antioxidant and oxidative stress indices, and endotoxin LPS of finishing bulls
| Itemse | Dietary treatmentf | SEMg | P-value | |||
|---|---|---|---|---|---|---|
| CON | C8 | C10 | C12 | |||
| TAOC (U/mL) | 7.50c | 8.21bc | 9.13ab | 9.87a | 0.607 | 0.032 |
| SOD (U/mL) | 49.49b | 57.12b | 68.22ab | 82.72a | 7.855 | 0.024 |
| Catalase (U/mL) | 33.42d | 41.99c | 46.23b | 53.69a | 1.124 | < 0.001 |
| GHS-PX (U/mL) | 115.78b | 142.97b | 205.95a | 220.70a | 15.508 | 0.001 |
| GR (U/L) | 3.65c | 5.21b | 6.04a | 6.72a | 0.326 | < 0.001 |
| H2O2 (U/mL) | 56.08a | 50.35ab | 44.60bc | 39.30c | 3.025 | 0.007 |
| Malondialdehyde (nmol/mL) | 4.14a | 3.66b | 3.17c | 2.32d | 0.189 | < 0.001 |
| NO (µmol/L) | 49.75c | 49.76c | 55.42b | 59.65a | 0.817 | < 0.001 |
| ROS (Fluorescence intensity/mL) | 1150.09a | 986.48b | 686.51c | 511.23d | 44.987 | < 0.001 |
| LPS (EU/mL) | 1.131a | 1.040a | 0.698b | 0.868ab | 0.145 | 0.033 |
a−dMeans bearing different superscripts in the same row differ significantly (P-value < 0.05)
eT-AOC total antioxidant capacity, SOD superoxide dismutase, GSH-PX glutathione peroxidase, GR glutathione reductase, H2O2 hydrogen peroxide, NO nitric oxide, ROS reactive oxygen species, LPS lipopolysaccharides
fCON the control group cattle fed control diets, C8, the treatment group cattle fed control diets containing glycerol monocaprylin; C10, the treatment group cattle fed control diets containing glycerol monodecanoate; C12, the treatment group cattle fed control diets containing glycerol monolaurate
gSEM, standard error of the means
Rumen fermentation parameters
The effects of MCT supplementation on rumen fermentation parameters of finishing bulls are shown in Table S1. Compared with the CON group, the C10 group significantly increased the pH in the rumen fluid of finishing bulls (P < 0.05), the C10 and C12 groups significantly decreased the concentrations of NH3-N in the rumen fluid of finishing bulls (P < 0.05), and the C8 and C10 groups significantly decreased the concentrations of lactic acid in the rumen fluid of finishing bulls (P < 0.05). There were no significant effects on the concentrations of LPS, TVFA, individual VFA, and A/P in the rumen fluid of finishing bulls among the four treatment groups (P > 0.05).
Diversity of gastrointestinal tract bacteria
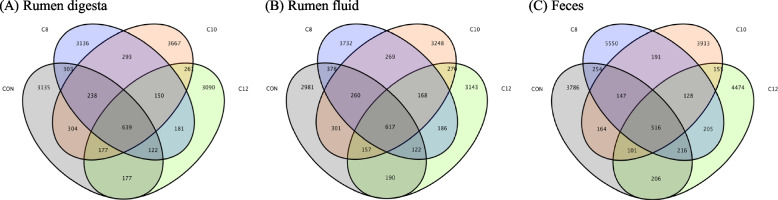
Each sample produced at least 67,815 clean reads, with an average of 85,989 ± 23,684 clean reads (Supplementary Table 3). The results of the OTUs were used to create histograms (Supplementary Fig. 1 A, B, C) and Venn diagrams (Fig. 1A, B, C), showing the numbers of gastrointestinal tract microbes and variances of different groups of finishing bulls.
Fig. 1.
The number of operational taxonomic units (OTUs) of gastrointestinal tract bacteria in finishing bulls. Venn diagram (A) rumen digesta, (B) rumen fluid, and (C) feces. CON, the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4)
Alpha diversity analysis showed that the rarefaction curves of each group first raised sharply and then tended to be gentle (Supplementary Fig. 2 A, D, G), the Shannon curve of each group had a plateau period (Supplementary Fig. 2 B, E, H), and the rank abundance curves were relatively wide (Supplementary Fig. 2 C, F, I), indicating that the sampling was sufficient. To measure the diversity within the microbial community of each sample, we compared the alpha diversity of the gastrointestinal tract bacteria in all samples by calculating the ACE, Chao1, Simpson, and Shannon indices (Table 5). The coverage of all samples in this experiment was higher than 99.9%, indicating that the sequencing coverage was sufficient for further analysis. For rumen digesta, the Shannon index of the C12 group was significantly decreased compared with the other three groups (P < 0.05), but there were no significant effects on ACE, Chao 1, and Simpson indices among the four treatment groups (P > 0.05). For rumen fluid, the ACE and Chao 1 indices of the C8 group were significantly increased compared with the CON and C12 groups (P < 0.05), Shannon index of the C12 group was significantly decreased compared with the other three groups (P < 0.05), but there were no significant effects on Simpson index among four treatment groups (P > 0.05). For feces, The alpha diversity was not significantly different among the four treatment groups (P > 0.05).
Table 5.
Effects of medium-chain fatty acid triglycerides supplementation on alpha diversity of gastrointestinal tract bacteria of finishing bulls
| Alpha diversity indices | Dietary treatmentd | SEMe | P-value | |||
|---|---|---|---|---|---|---|
| CON | C8 | C10 | C12 | |||
| Rumen digesta | ||||||
| ACE | 1598.27 | 1575.59 | 1807.17 | 1480.44 | 107.443 | 0.100 |
| Chao1 | 1583.60 | 1561.65 | 1791.29 | 1467.60 | 106.676 | 0.101 |
| Simpson | 0.9963 | 0.9961 | 0.9966 | 0.9952 | 0.00061 | 0.256 |
| Shannon | 9.162a | 9.149a | 9.211a | 8.953b | 0.066 | 0.030 |
| Coverage | 0.9992 | 0.9993 | 0.9993 | 0.9993 | 0.00012 | 0.689 |
| Rumen fluid | ||||||
| ACE | 1570.03bc | 1745.06a | 1647.51ab | 1485.83c | 40.029 | 0.003 |
| Chao1 | 1559.93bc | 1730.22a | 1635.17ab | 1472.76c | 40.696 | 0.004 |
| Simpson | 0.9972 | 0.9968 | 0.9970 | 0.9944 | 0.00132 | 0.213 |
| Shannon | 9.344a | 9.335a | 9.312a | 8.889b | 0.140 | 0.045 |
| Coverage | 0.9994 | 0.9991 | 0.9993 | 0.9993 | 0.00011 | 0.284 |
| Feces | ||||||
| ACE | 1588.49 | 2098.54 | 1581.11 | 1728.13 | 317.253 | 0.396 |
| Chao1 | 1581.93 | 2089.61 | 1573.65 | 1721.08 | 317.524 | 0.400 |
| Simpson | 0.9950 | 0.9925 | 0.9948 | 0.9934 | 0.001 | 0.291 |
| Shannon | 8.869 | 8.853 | 8.822 | 8.759 | 0.172 | 0.918 |
| Coverage | 0.9997 | 0.9996 | 0.9995 | 0.9997 | 0.00009 | 0.497 |
a−cMeans bearing different superscripts in the same row differ significantly (P-value < 0.05)
dCON the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4)
eSEM standard error of the means
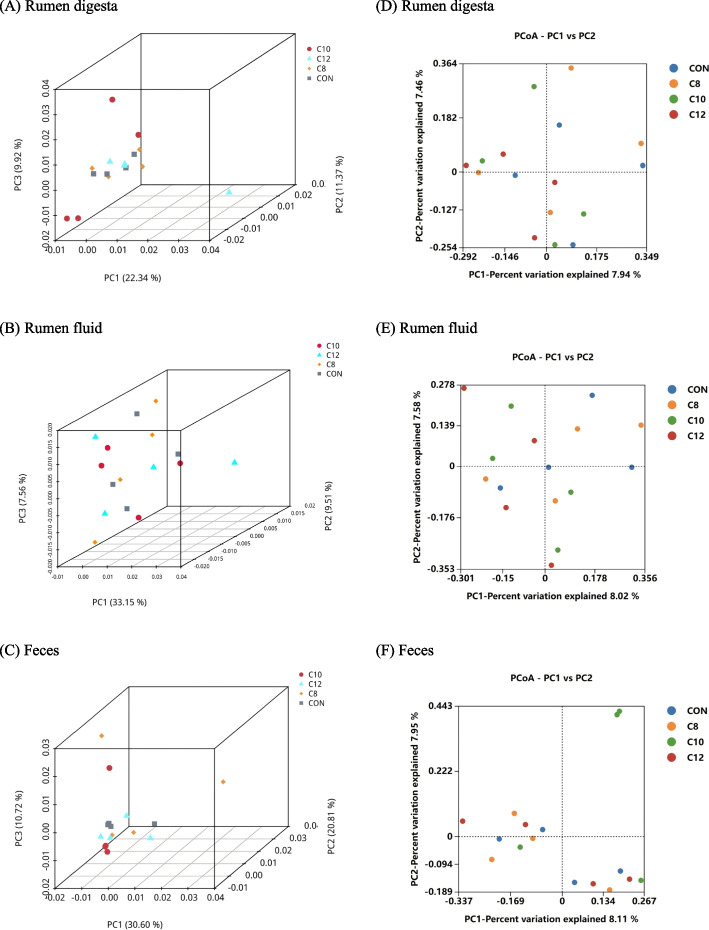
Beta diversity was used to compare the microbial community in different samples by calculating the 3D-PCA and PCoA. As shown in Fig. 2A, B, C, the 3D-PCA showed that the points representing gastrointestinal tract microorganisms in the four treatment groups were not independent, which indicated that the community structure of gastrointestinal tract bacteria had no difference among treatments. In the PCoA plots (Fig. 2D, E, F), the points representing gastrointestinal tract bacteria in four treatment groups were clustered in separate quadrants.
Fig. 2.
Beta diversity analysis of gastrointestinal tract bacteria. Three-dimensional principal component analysis (3D-PCA) (A) rumen digesta, (B) rumen fluid, and (C) feces, and principal coordinates analysis (PCoA) (D) rumen digesta, (E) rumen fluid, and (F) feces. CON the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4)
Composition of gastrointestinal tract bacteria
At the phylum level, Firmicutes and Bacteroidota were the dominant phylum among the four treatment groups and occupied more than 80% of the microbial community (Supplementary Fig. 3 A, D, G). At the family level, for rumen digesta and rumen fluid, the bacteria with relative abundance greater than 10% were Prevotellaceae and Lachnospiraceae, while for feces were Lachnospiraceae, Oscillospiraceae and, Rikenellaceae (Supplementary Fig. 3 B, E, H). At the genus level, for rumen digesta, the bacteria with relative abundance in the top 5 were Prevotella, uncultured_rumen_bacterium, Rikenellaceae_RC9_gut_group, Succiniclasticum, and Saccharofermentans (Supplementary Fig. 3C); for rumen fluid, the bacteria with relative abundance in the top 5 were Prevotella, uncultured_rumen_bacterium, Rikenellaceae_RC9_gut_group, NK4A214_group, and Succiniclasticum (Supplementary Fig. 3 F); for feces, the bacteria with relative abundance in the top 5 were unclassified_Lachnospiraceae, UCG_005, unclassified_UCG_010, Rikenellaceae_RC9_gut_group, and Succinivibrio (Supplementary Fig. 3I).
Furthermore, the significantly different gastrointestinal tract bacteria among the four treatment groups were analyzed by the general linear model. As shown in Table 6, compared with the CON group, there were 5 (including 2 order level, 2 family level, and 1 genus level), 14 (including 1 phylum level, 2 class level, 2 order level, 4 family level, and 5 genus level) and 6 (including 1 phylum level, 1 class level, 1 order level, 1 family level, and 2 genus level) significantly different bacteria in the rumen digesta in the C8, C10, and C12 groups, respectively; there were 3 (including 1 class level, 1 order level, and 1 genus level), 10 (including 1 phylum level, 1 class level, 3 order level, 2 family level, and 3 genus level) and 5 (including 1 order level, 2 family level, and 2 genus level) significantly different bacteria in the rumen fluid in the C8, C10, and C12 groups, respectively; but only one differential bacteria at the genus level (g_Ruminococcus) was observed in the feces of the four treatment groups (P < 0.05).
Table 6.
Effects of medium-chain fatty acid triglycerides supplementation on significantly differential gastrointestinal tract bacteria composition of finishing bulls
| Itemsd | Dietary treatmente | SEMf | P-value | |||
|---|---|---|---|---|---|---|
| CON | C8 | C10 | C12 | |||
| Rumen digesta (%, relative abundance) | ||||||
| p_Proteobacteria | 0.0116b | 0.0148b | 0.0110b | 0.0460a | 0.0092 | 0.023 |
| p_Spirochaetota | 0.0112ab | 0.0126a | 0.0035c | 0.0100b | 0.0009 | < 0.001 |
| c_Gammaproteobacteria | 0.0107b | 0.0136b | 0.0103b | 0.0454a | 0.0089 | 0.020 |
| c_Negativicutes | 0.0595b | 0.0594b | 0.0697a | 0.0564b | 0.0031 | 0.021 |
| c_Spirochaetota | 0.0112ab | 0.0126a | 0.0035c | 0.0100b | 0.0009 | < 0.001 |
| o_Enterobacterales | 0.0095b | 0.0128b | 0.0092b | 0.0445a | 0.0089 | 0.019 |
| o_Oscillospirales | 0.2041bc | 0.2190a | 0.2158ab | 0.1916c | 0.0059 | 0.012 |
| o_RF39 | 0.0023b | 0.0036a | 0.0009c | 0.0020b | 0.0003 | < 0.001 |
| o_Spirochaetales | 0.0112ab | 0.0126a | 0.0035c | 0.0100b | 0.0009 | < 0.001 |
| f_Bacteroidales_RF16_group | 0.0012b | 0.0016b | 0.0027a | 0.0017b | 0.0004 | 0.047 |
| f_Oscillospiraceae | 0.0679b | 0.0790ab | 0.0903a | 0.0679b | 0.0066 | 0.039 |
| f_Prevotellaceae | 0.2061a | 0.1687b | 0.2079a | 0.1827ab | 0.0118 | 0.042 |
| f_Rikenellaceae | 0.0659b | 0.0787a | 0.0536c | 0.0748ab | 0.0048 | 0.007 |
| f_Spirochaetaceae | 0.0112ab | 0.0126a | 0.0035c | 0.0100b | 0.0009 | < 0.001 |
| f_Succinivibrionaceae | 0.0092b | 0.0126b | 0.0088b | 0.0442a | 0.0089 | 0.019 |
| g_Christensenellaceae_R_7_group | 0.0239b | 0.0272a | 0.0288a | 0.0267ab | 0.0012 | 0.038 |
| g_Desulfovibrio | 0.0019b | 0.0028ab | 0.0032a | 0.0025ab | 0.00035 | 0.047 |
| g_Lachnospiraceae_UCG_006 | 0.0020b | 0.0020b | 0.0024a | 0.0025a | 0.00016 | 0.040 |
| g_Lachnospiraceae_XPB1014_group | 0.0172a | 0.0153a | 0.0163a | 0.0071b | 0.00128 | < 0.001 |
| g_NK4A214_group | 0.0386b | 0.0455ab | 0.0532a | 0.0382b | 0.0038 | 0.023 |
| g_Rikenellaceae_RC9_gut_group | 0.0610ab | 0.0730a | 0.0495b | 0.0701a | 0.0051 | 0.014 |
| g_Treponema | 0.0110ab | 0.0125a | 0.0034c | 0.0098b | 0.00089 | < 0.001 |
| Rumen fluid (%, relative abundance) | ||||||
| p_Fibrobacterota | 0.0026bc | 0.0039ab | 0.0054a | 0.0018c | 0.00079 | 0.016 |
| p_Synergistota | 0.0012ab | 0.0016a | 0.0013ab | 0.0008b | 0.00021 | 0.033 |
| c_Fibrobacterota | 0.0026bc | 0.0039ab | 0.0054a | 0.0018c | 0.00079 | 0.016 |
| c_Gracilibacteria | 0.0095b | 0.0175a | 0.0091b | 0.0063b | 0.0024 | 0.016 |
| c_Synergistota | 0.0012ab | 0.0016a | 0.0013ab | 0.0008b | 0.00021 | 0.033 |
| o_Absconditabacteriales__SR1 | 0.0095b | 0.0175a | 0.0091b | 0.0063b | 0.0024 | 0.016 |
| o_Erysipelotrichales | 0.0101ab | 0.0087bc | 0.0078c | 0.0116a | 0.0008 | 0.012 |
| o_Fibrobacterales | 0.0026bc | 0.0039ab | 0.0054a | 0.0018c | 0.00079 | 0.016 |
| o_Peptococcales | 0.0013a | 0.0010ab | 0.0009b | 0.0005c | 0.0002 | 0.009 |
| o_Synergistales | 0.0012ab | 0.0016a | 0.0013ab | 0.0008b | 0.00021 | 0.033 |
| f_Fibrobacteraceae | 0.0026bc | 0.0039ab | 0.0054a | 0.0018c | 0.0008 | 0.016 |
| f_Peptococcaceae | 0.0013a | 0.0010ab | 0.0009b | 0.0005c | 0.0002 | 0.009 |
| f_Prevotellaceae | 0.2115a | 0.1949a | 0.2007a | 0.1633b | 0.0128 | 0.040 |
| f_Synergistaceae | 0.0012ab | 0.0016a | 0.0013ab | 0.0008b | 0.0002 | 0.033 |
| g_Anaerovorax | 0.0062a | 0.0060ab | 0.0045bc | 0.0042c | 0.00065 | 0.047 |
| g_Fibrobacter | 0.0026bc | 0.0039ab | 0.0054a | 0.0018c | 0.00079 | 0.016 |
| g_Prevotellaceae_NK3B31_group | 0.0122a | 0.0061b | 0.0069b | 0.0036b | 0.00207 | 0.029 |
| Feces (%, relative abundance) | ||||||
| g_Ruminococcus | 0.0043a | 0.0042a | 0.0028b | 0.0035ab | 0.00043 | 0.043 |
a−cMeans bearing different superscripts in the same row differ significantly (P-value < 0.05)
dp phylum, c class, o order, f family, g genus
eCON the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4)
fSEM standard error of the means
Analysis of rumen metabolites
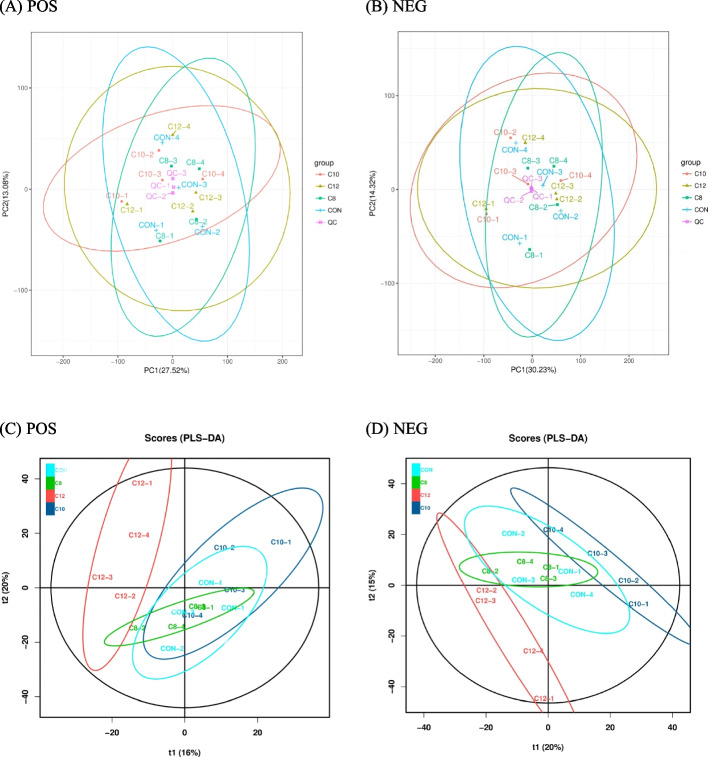
In this study, based on the LC-QTOF platform, 16 rumen fluid samples were analyzed qualitatively and quantitatively. A total of 13,655 peaks were detected, of which 3484 metabolites were annotated. Unsupervised multivariate statistical analysis (PCA) of all samples provided an overview of rumen metabolites in the four groups (Fig. 3A, B). Differential rumen metabolites across the groups were clearly distinguished using a supervised discriminant analysis method (PLS-DA) (Fig. 3C, D).
Fig. 3.
Principal component analysis (PCA) POS (positive ionization mode) (A), NEG (negative ionization mode) (B), and partial least squares discriminant analysis (PLS-DA) POS (positive ionization mode) (C), NEG (negative ionization mode) (D) of rumen fluid metabolites. QC quality control samples. CON the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4)
Furthermore, the significantly different metabolites in rumen fluid among the four treatment groups were analyzed by the general linear model. Compared with the CON group, the C8 group significantly up-regulated S-(2-hydroxyethyl)glutathione, bis(glutathionyl)spermine, significantly down-regulated 25-hydroxyvitamin D3 under positive ionization mode; the C10 group significantly up-regulated 6 metabolites (e.g., L-threonine, 22-hydroxydocosanoic acid, cortisol etc.), significantly down-regulated 8 metabolites (e.g., S-(2-hydroxyethyl)glutathione, (-)-jasmonic acid, (S)-2-aceto-2-hydroxybutanoate, etc.) under positive ionization mode; the C12 group significantly up-regulated 7 metabolites (e.g., imidazole-4-acetate, linoleic acid, 3-(3'-methylthio)propylmalic acid, etc.), significantly down-regulated 8 metabolites (e.g., S-(2-hydroxyethyl)glutathione, isomaltotriose, 3-methylbutanoic acid, etc.) under positive ionization mode (Supplementary Table 4). Compared with the CON group, the C8 group significantly up-regulated gamma-hydroxy-3-pyridinebutanoate, significantly down-regulated dopamine quinone, 12-Oxo-9(Z)-dodecenoic acid under negative ionization mode; the C10 group significantly up-regulated 7 metabolites (e.g., creatine, succinic acid, 11(R)-HPETE, etc.), significantly down-regulated 4 metabolites (e.g., L-dopachrome, dopamine quinone, docosanedioate, etc.) under negative ionization mode; the C12 group significantly up-regulated 10 metabolites (e.g., creatine, 3-hexenal, 11(R)-HPETE, etc.), significantly down-regulated 5 metabolites (e.g., dopamine quinone, docosanedioate, zidovudine, etc.) under negative ionization mode (Supplementary Table 5).
KEGG classification maps showed that under positive ionization mode, the differential metabolite pathway was rich in amino acid metabolism, biosynthesis of other secondary metabolites, cancer: overview, cancer: specific types, carbohydrate metabolism, digestive system, endocrine and metabolic disease, endocrine system, energy metabolism, excretory system, glycan biosynthesis and metabolism, infectious disease: bacterial, lipid metabolism, membrane transport, metabolism of cofactors and vitamins, metabolism of other amino acids, nervous system, nucleotide metabolism, sensory system, signaling molecules and interaction, translation, and xenobiotics biodegradation and metabolism (Supplementary Fig. 4A). Besides, under negative ionization mode, the differential metabolite pathway was rich in amino acid metabolism, biosynthesis of other secondary metabolites, cancer: overview, carbohydrate metabolism, digestive system, endocrine system, energy metabolism, infectious disease: bacterial, lipid metabolism, membrane transport, metabolism of cofactors and vitamins, metabolism of terpenoids and polyketides, nervous system, neurodegenerative disease, nucleotide metabolism, signal transduction, and xenobiotics biodegradation and metabolism (Supplementary Fig. 4 B).
Correlation analysis of differential conventional indicators, differential gastrointestinal bacteria, and differential metabolites
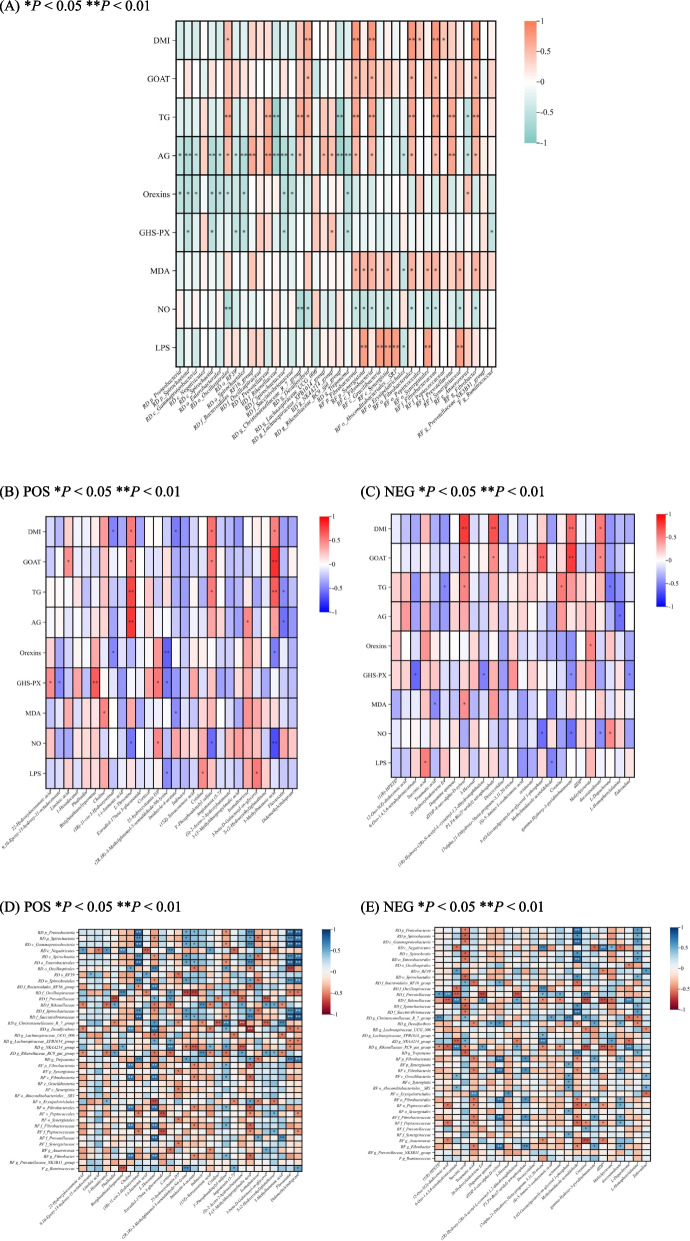
Correlation of differential conventional indices and differential gastrointestinal tract bacteria (all levels) showed that DMI and GOAT were positively associated with rumen digesta g_Desulfovibrio, rumen fluid p_Fibrobacterota, c_Fibrobacteria, o_Fibrobacterales, f_Fibrobacteraceae, and g_Fibrobacter. AG was positively associated with rumen digesta o_Oscillospirales, f_Prevotellaceae, g_Christensenellaceae_R_7_group, rumen fluid p_Fibrobacterota, c_Fibrobacteria, o_Fibrobacterales, f_Fibrobacteraceae, f_Prevotellaceae, and g_Fibrobacter, and negatively associated with rumen digesta p_Proteobacteria, p_Spirochaetota, c_Gammaproteobacteria, c_Spirochaetia, o_Enterobacterales, o_Spirochaetales, f_Spirochaetaceae, f_Succinivibrionaceae, and g_Treponema. LPS was positively associated with ruemen fluid p_Synergistota, c_Gracilibacteria, c_Synergistia, o_Absconditabacteriales__SR1, o_Synergistales, f_Synergistaceae, and negatively associated with rumen fluid o_Erysipelotrichales (Fig. 4A).
Fig. 4.
Correlation analysis between significantly differential conventional indices and differential gastrointestinal tract bacteria (A); significantly differential conventional indices and differential metabolites under positive ionization mode (B), and negative ionization mode (C); significantly differential gastrointestinal tract bacteria and differential metabolites under positive ionization mode (D), and negative ionization mode (E). RD rumen digesta, RF rumen fluid, F feces. POS positive ionization mode, NEG negative ionization mode. Correlation coefficients ranging from − 1 to + 1 represented strong negative correlations to strong positive correlations. Correlation P < 0.05 (*) and 0.01 (**) represented significant and extremely significant correlations, respectively. In this analysis, the bacteria with a relative abundance of 0.1% or higher were considered; rumen metabolites VIP (variable importance in the projection) > 1, and P < 0.05 were considered significant
Correlation of differential conventional indices and differential metabolites showed that, under positive ionization mode, DMI and GOAT were positively associated with L-threonine, 3'-phosphoadenylyl sulfate, and 3-methylbutanoic acid. AG was positively associated with L-threonine, isomaltotriose, and negatively associated with fluvastatin. LPS was positively associated with cytidine, 3-beta-D-galactosyl-sn-glycerol, and negatively associated with (2R,3R)-3-methylglutamyl-5-semialdehyde-N6-lysine (Fig. 4B).Under negative ionization mode, DMI and GOAT were positively associated with dTDP-4-oxo-alpha-D-xylose, P1,P4-Bis(5'-uridyl) tetraphosphat, gamma-hydroxy-3-pyridinebutanoate, and docosanedioate. AG was negatively associated with L-homophenylalanine. Orexins was positively associated with Maleylpyruvate. LPS was positively associated with succinic acid and negatively associated with methylimidazole acetaldehyde (Fig. 4C).
Besides, the results of the correlation of differential gastrointestinal tract bacteria (all levels) and differential metabolites are shown in Fig. 4D and E.
Discussion
As a safe and efficient additive, MCTs have been shown to have a positive effect on growth performance and health status in animals [6–8, 36, 37], but the reports of direct addition of individual MCT (GMC/GMD/GML) are limited. Murayama et al. [38] found that MCTs (C8:0 and C10:0) supplementation in the milk replacer may improve the growth performance and gut health of dairy calves. However, some studies have shown that supplementation of glycerides of MCFAs (C8:0, C10:0, and C12:0) had no effects on the health and growth of calves [39], and even decreased DMI when supplementing coconut oil (~ 75% MCFAs) [40] or lauric acid [41]. In the present study, compared with the CON group, there were no significant effects on ADG or feed conversion efficiency of finishing bulls among the treatment groups, similar to the results of Masmeijer et al. [39]. However, compared with the CON group, the C10 group tended to increase the DMI of finishing bulls (P = 0.069), indicating that the addition of GMD may have a positive effect on feed intake of finishing bulls. The differences in growth performance of livestock responses to MCTs supplementation in different studies may be due to differences in the types and doses of MCT, age and breed, animal stress, or supplementation period in different trials.
At present, although the mechanism of MCTs on the feed intake of beef cattle has not been fully elucidated, it may be related to MCFAs’ involvement in the acylation modification of ghrelin [42]. Ghrelin is a 28-amino acid acylated peptide mainly synthesized and secreted by X/A-like cells of the gastric fundus mucosa, and it acts as a brain–gut peptide hormone that stimulates feed intake [43, 44]. It mainly exists in the form of acylated and deacylated in ruminants, and AG is its active form, which has been proved that stimulate animal appetite through orexins [12, 45]. Studies have shown that MCFAs mainly act on the stomach and can participate in the acylation of ghrelin in the stomach, which is achieved by GOAT [46]. Not only the amount of fatty acids but also the length of fatty acids can impact the efficiency of ghrelin acylation, and fatty acids derived from C6:0 to C14:0 may affect the acylation process of ghrelin [47]. In the present study, compared with the CON group, the C8 and C10 groups significantly increased the concentration of GOAT in the serum of finishing bulls, the C10 group significantly increased the AG/TG, and the concentrations of TG, AG, and orexins in the serum of finishing bulls. Studies have shown that supplementation of MCFAs-Ca (containing 25% C8:0, 20% C10:0, and 55% C12:0) in the diet could significantly increase the level of AG in dairy cows [13], which is consistent with the result of AG in blood after feeding mice with MCFAs (mainly C6:0, C8:0, and C10:0) [42]. Our results are consistent with the above results. It was reported that exogenous AG can increase the DMI in beef cattle [48], and AG concentration and AG/TG were positively correlated with DMI in beef cattle [49]. This may be the reason for the increasing trend compared to the CON group of DMI in the C10 group.
It has been reported that the high-concentrate diet mode can lead to the imbalance of oxidation and anti-oxidation in the ruminants, which makes the animals in an oxidative stress state and causes oxidative damage [50]. At present, some studies have shown that MCFAs/MCTs can improve oxidative stress in organs or tissues of mice/pigs [9, 51, 52], but there are relatively few reports in the ruminants. In the present study, compared with the CON group, the C10 and C12 groups significantly increased the concentrations of TAOC, catalase, GHS-PX, GR, and NO in the serum of finishing bulls, while the C10 group significantly decreased the concentrations of H2O2, malondialdehyde, ROS, and LPS. This indicated that supplementation of GMD/GML may improve oxidative stress in finishing bulls under high-concentrate feeding conditions, but the mechanism is still unclear.
Słupecka et al. [14] showed that the intestinal administration of AG could enhance the autophagy of piglet intestinal epithelial cells, promote the renewal of intestinal mucosa in newborn piglets, and protect the intestine. Ghrelin can improve Na2S2O5-induced apoptosis and oxidative stress-induced gastric mucosal injury in rats [53]. Previous and our studies have found that MCFAs have a stimulating effect on the secretion of ghrelin in the ruminants [13, 30], which provides a new idea to further reveal the mechanism of MCTs improving oxidative stress in beef cattle. In this study, we found that AG concentration was significantly negatively correlated with the oxidative stress marker ROS (Supplementary Fig. 5). This indicated that MCFAs may mediate ghrelin acylation through GOAT, and AG is involved in the regulation of oxidative stress in high-concentrate-fed finishing bulls. This may be one of the reasons why GMD/GML may improve oxidative stress in finishing bulls in this trial.
There are complex and diverse microbial communities in the gastrointestinal tract of ruminants. The C10 group significantly increased the relative abundance of g_Christensenellaceae_R_7_group in the rumen digesta, p_Fibrobacterota and its subordinate classification in the rumen fluid. Fibrobacterota is considered the main bacteria that degrade dietary starch polysaccharides and wood fiber [54]. Moreover, Christensenellaceae_R_7_group belongs to the Firmicutes [55], which are mainly involved in the degradation of cellulose and hemicellulose in the rumen [56, 57]. This may be the reason for the increasing trend compared to the CON group of NDF digestibility in the C10 group of finishing bulls in this trial (Supplementary Table 2, P = 0.057). Increased abundance and activity of cellulose-degrading bacteria are reported to decrease NH3-N concentration in the rumen [58]; this also explained the reason why the C10 and C12 groups significantly reduced NH3-N concentration in the rumen fluid of finishing bulls (Supplementary Table 1). Furthermore, GMD supplementation reduced the relative abundance of g_Rikenellaceae_RC9_gut_group in the rumen digesta, which may affect intestinal permeability, oxidative stress and energy metabolism, and might contribute to the pathogenesis of inflammation [59]. Additionally, although Treponema includes harmless commensal bacterial species, they are primarily known as potential pathogens [60]; one species of g_Treponema (T. brennaborense) was found to be associated with digital dermatitis in dairy cows [61]. Our study found that the addition of GMD significantly reduced the relative abundance of g_Treponema in the rumen digesta. These results indicated that the addition of MCTs did not adversely affect the function and structure of the gastrointestinal tract in finishing bulls.
Studies have found that some metabolites produced by gastrointestinal tract microorganisms in mammals can affect mitochondrial balance, ROS levels, and gastrointestinal tract health [62]. In the rumen, amino acids are degradation products of dietary or microbial proteins, precursors of peptide and protein synthesis, and regulate certain metabolic pathways in the body [63]. Lysine is the first or second limiting amino acid in beef cattle [64]. Lysine in the rumen fluid is derived mainly from dietary degradation and synthesis by rumen microorganisms [65]. In this study, the metabolites (2R,3R)-3-methylglutamyl-5-semialdehyde-N6-lysine under the lysine biosynthesis pathway (ko00300) in the rumen fluid of the C10 group was significantly down-regulated; this may suggest the up-regulation of the lysine synthesis pathway, which may have a positive effect on the growth of beef cattle. L-Threonine participates in the glycine, serine and threonine metabolism (ko00260), biosynthesis of amino acids (ko01230), protein digestion and absorption (ko04974), and other metabolic pathways. As the reaction substrate of L-threonate 3-dehydrogenase, it can improve the animal’s antioxidant status [35]. Therefore, the up-regulation of rumen fluid metabolite L-threonine in the C10 group may be the reason for the improvement of oxidative stress compared to the CON group.
Dopamine quinone is the oxidation product of dopamine oxidation and is an important biologically active intermediate. It plays an important role in physiological processes such as nerve conduction and oxidative stress in organisms, which may lead to oxidative damage and cytotoxicity [66]. Therefore, the down-regulation of dopamine quinone in the rumen fluid of finishing bulls can also explain the improvement of oxidative stress by the C10 and C12 groups. Besides, tyrosinase catalyzes the conversion of L-tyrosine to L-DOPA and then to dopachrome, L-DOPA can increase the secretion of growth hormone in steers [67]. In this study, L-dopachrome in the rumen fluid of the C10 group was significantly down-regulated, but it did not adversely affect the growth of beef cattle. Moreover, bis(glutathionyl)spermine in antioxidant-related pathway glutathione metabolism (ko00480) in the rumen fluid of the C10 group was significantly up-regulated. The changes in above rumen metabolites indicated that the improvement of MCTs (GMD/GML) on oxidative stress in beef cattle may also be related to its regulation on metabolites. However, how MCTs regulate metabolites remains to be further studied.
The interaction between gastrointestinal tract flora and oxidative stress depends on a variety of metabolites. Accumulating evidence indicates that the interaction of gastrointestinal tract microorganisms and rumen fluid metabolites can shape phenotypic traits in the host [21, 68, 69]. In the present study, bis(glutathionyl) spermine, a metabolite annotated to the glutathione metabolic pathway (an antioxidant-related pathway) was significantly positively correlated with rumen digesta c_Negativicutes (r = 0.657, P = 0.004), o_Oscillospirales (r = 0.621, P = 0.007), g_NK4A214_group (r = 0.485, P = 0.034) and significantly negatively correlated with feces g_Ruminococcus (r = − 0.618, P = 0.007). Therefore, we speculated that GMD/GML may also improve the oxidative stress of finishing bulls under high-concentrate feeding conditions by reshaping the gastrointestinal tract microorganisms and producing specific metabolites related to oxidative stress.
In addition, positive ionization metabolites L-threonine, 3'-phosphoadenylyl sulfate, negative ionization metabolites dTDP-4-oxo-alpha-D-xylose, P1,P4-bis(5'-uridyl) tetraphosphate, gamma-hydroxy-3-pyridinebutanoate, and docosanedioate were significantly correlated with rumen digesta g_Desulfovibrio and rumen fluid Fibrobacteres, and they were all significantly positively correlated with GOAT. This indicated that gastrointestinal tract microorganisms may also produce specific metabolites closely related to GOAT, indirectly affecting GOAT-mediated ghrelin acylation, and thus improving the feed intake or oxidative stress of finishing bulls. It also indicated that ghrelin, as a brain–gut peptide, may be an important link of information transmission between gastrointestinal tract microorganisms, metabolites and the body function on the brain–gut axis, and participated in the regulation of the body by MCT. However, these assumptions need to be further verified.
Moreover, studies have shown that ghrelin and its receptors are widely expressed in the gastrointestinal tract and various tissues and organs, and can regulate related signaling pathways (such as p38-MAPK; TLR4/NFκB; PI3K/Akt, etc.), reduce oxidative stress and inflammatory response of animal brain or other tissues and organs [16, 70–73]. These important signaling pathways can activate nuclear factor erythroid 2-related factor 2 (Nrf2), regulate the related factors in the Nrf2 pathway, and improve the damage caused by oxidative stress to animal bodies [16, 73, 74]. However, in this experiment, it is unknown whether GMD/GML activated the Nrf2 signaling pathway through GOAT-mediated ghrelin acylation. All in all, our results provided a foundation for the rational use of individual MCT in ruminant and provided new ideas for the improvement of feed intake and oxidative stress in finishing bulls through the microbiota–gut–brain axis. In the future, some in-depth research on the effect of MCTs on feed intake and oxidative stress should be carried out to explain more about its mechanism in finishing bulls.
Conclusion
Under the dietary conditions of our study, the addition of MCTs (GMC/GMD/GML) did not have an adverse effect on the growth of finishing bulls. Compared to the CON group, GMD tended to increase the DMI of finishing bulls, GMD increased the concentration of GOAT, TG, AG, and orexins in the serum of finishing bulls, GMD and GML increased the concentrations of TAOC, catalase, GHS-PX, GR, and NO in the serum of finishing bulls, while GMD decreased the concentrations of H2O2, malondialdehyde, ROS, and LPS. This indicated that different types of MCTs have different application effects in ruminants. Among them, GMD may improve the feed intake of finishing bulls by stimulating the secretion of AG. GMD and GML may change gastrointestinal tract microorganisms and produce specific rumen metabolites to improve the oxidative stress of finishing bulls, and ghrelin may also be involved. This study enlightens the potential mechanisms by which MCT improves feed intake and oxidative stress in finishing bulls.
Supplementary Information
Additional file 1: Table S1. Effects of medium-chain fatty acid triglycerides supplementation on ruminal fermentation parameters of finishing bulls. Table S2. Effects of medium-chain fatty acid triglycerides supplementation on nutrient digestibility of finishing bulls. Table S3. Summary of sequence statistics for each sample. Table S4. Significant differential metabolites in rumen fluid of four treatment groups (n = 4) under positive ionization mode. Table S5. Significant differential metabolites in rumen fluid of four treatment groups (n = 4) under negative ionization mode.
Additional file 2: Fig. S1. The number of operational taxonomic units (OTUs) of gastrointestinal tract bacteria in finishing bulls. (A) rumen digesta, (B) rumen fluid, and (C) feces. CON, the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4). Fig. S2. Alpha diversity curve. Rumen digesta (A) rarefaction curves, (B) shannon curves, (C) rank abundance curves, rumen fluid (D) rarefaction curves, (E) shannon curves, (F) rank abundance curves, and feces (G) rarefaction curves, (H) shannon curves, (I) rank abundance curves (OUT level). CON, the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4). Fig. S3. The relative abundances of gastrointestinal tract bacterial community at rumen digesta (A) phylum, (B) family, (C) genus, rumen fluid (D) phylum, (E) family, (F) genus, and feces (G) phylum, (H) family, (I) genus levels (top 20). The different colors of the bars represent different species, and the length of the bars represents the proportion of the species. CON, the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4). Fig. S4. KEGG (Kyoto Encyclopedia of Genes and Genomes) classification maps of significant differential metabolites in the rumen fluid of four treatment groups (n = 4) under positive ionization mode (A) and negative ionization mode (B). Fig. S5. Correlation between serum acylated ghrelin (AG) level and serum reactive oxygen species (ROS) level in finishing bulls. r, correlation coefficient.
Acknowledgements
Not applicable.
Authors’ contributions
CYG, JML and XF conceived and designed the study. JML, XF, YLD and DXY conducted the experiments and collected the samples. JML and XF analyzed the data and wrote the manuscript. CYG revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 32060763 and 31660669), Jilin Science and Technology Development Program (grant numbers YDZJ202203CGZH042 and 20220202048NC).
Data availability
The gastrointestinal tract microbiome sequencing reads (including rumen digesta, rumen fluid, and feces) are available in the Sequence Read Archive (SRA) of NCBI under accession project number PRJNA1111285 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA1111285?reviewer = uo63pvg3kuigl56msror69nrb0).
Declarations
Ethics approval and consent to participate
All procedures involving animals were performed with the approval (approval ID: 20221015) of the Yanbian University Institutional Animal Care and Use Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaming Luan and Xin Feng contributed equally and should be regarded as co-first authors.
References
- 1.Ramírez M, Amate L, Gil A. Absorption and distribution of dietary fatty acids from different sources. Early Hum Dev. 2001;65(Suppl):S95–101. 10.1016/s0378-3782(01)00211-0. [DOI] [PubMed] [Google Scholar]
- 2.Burdick M, Zhou M, Guan LL, Oba M. Effects of medium-chain fatty acid supplementation on performance and rumen fermentation of lactating Holstein dairy cows. Animal. 2022;16(4):100491. 10.1016/j.animal.2022.100491. [DOI] [PubMed] [Google Scholar]
- 3.Hill TM, Vandehaar MJ, Sordillo LM, Catherman DR, Bateman HG 2nd, Schlotterbeck RL. Fatty acid intake alters growth and immunity in milk-fed calves. J Dairy Sci. 2011;94(8):3936–48. 10.3168/jds.2010-3935. [DOI] [PubMed] [Google Scholar]
- 4.Panyakaew P, Boon N, Goel G, et al. Effect of supplementing coconut or krabok oil, rich in medium-chain fatty acids on ruminal fermentation, protozoa and archaeal population of bulls. Animal. 2013;7(12):1950–8. 10.1017/S1751731113001766. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen SH, Hegarty RS. Effects of defaunation and dietary coconut oil distillate on fermentation, digesta kinetics and methane production of Brahman heifers. J Anim Physiol Anim Nutr. 2017;101(5):984–93. 10.1111/jpn.12534. [DOI] [PubMed] [Google Scholar]
- 6.Panahiha P, Mirzaei-Alamouti H, Kazemi-Bonchenari M, Aschenbach JR. Growth performance, nutrient digestibility, and ruminal fermentation of dairy calves fed starter diets with alfalfa hay versus corn silage as forage and soybean oil versus palm fatty acids as fat source. J Dairy Sci. 2022;105(12):9597–609. 10.3168/jds.2022-22165. [DOI] [PubMed] [Google Scholar]
- 7.Beckett LM, Malacco VMR, Hilger S, Casey TM, Donkin SS. Effects of an hourly bolus postruminal infusion of flaxseed oil or palm oil on circulating fatty acid concentrations and hepatic expression of pyruvate carboxylase and phosphoenolpyruvate carboxykinase in dairy cattle. Animals. 2023;13(22):3572. 10.3390/ani13223572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas LL, Woodworth JC, Tokach MD, et al. Evaluation of different blends of medium-chain fatty acids, lactic acid, and monolaurin on nursery pig growth performance. Transl Anim Sci. 2020;4(2):txaa024. 10.1093/tas/txaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Chen J, Sun H, Chen W, Yang X. MCFA alleviate H2O2 -induced oxidative stress in AML12 cells via the ERK1/2/Nrf2 pathway. Lipids. 2022;57(3):153–62. 10.1002/lipd.12339. [DOI] [PubMed] [Google Scholar]
- 10.Lee SI, Kang KS. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci Rep. 2017;7(1):16530. 10.1038/s41598-017-16561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sengupta A, Ghosh M. Comparison of native and capric acid-enriched mustard oil effects on oxidative stress and antioxidant protection in rats. Br J Nutr. 2012;107(6):845–9. 10.1017/S0007114511003874. [DOI] [PubMed] [Google Scholar]
- 12.Schellekens H, Finger BC, Dinan TG, Cryan JF. Ghrelin signalling and obesity: at the interface of stress, mood and food reward. Pharmacol Ther. 2012;135(3):316–26. 10.1016/j.pharmthera.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Fukumori R, Sugino T, Shingu H, et al. Ingestion of medium chain fatty acids by lactating dairy cows increases concentrations of plasma ghrelin. Domest Anim Endocrinol. 2013;45(4):216–23. 10.1016/j.domaniend.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Słupecka M, Woliński J, Pierzynowski SG. The effects of enteral ghrelin administration on the remodeling of the small intestinal mucosa in neonatal piglets. Regul Pept. 2012;174(1–3):38–45. 10.1016/j.regpep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Mao Y, Li Z, Chen K, et al. Antinociceptive effect of ghrelin in a rat model of irritable bowel syndrome involves TRPV1/opioid systems. Cell Physiol Biochem. 2017;43(2):518–30. 10.1159/000480478. [DOI] [PubMed] [Google Scholar]
- 16.Jiao ZT, Luo Q. Molecular mechanisms and health benefits of ghrelin: a narrative review. Nutrients. 2022;14(19):4191. 10.3390/nu14194191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan E, Lovett DK, Monahan FJ, Callan J, Flynn B, O’Mara FP. Effect of refined coconut oil or copra meal on methane output and on intake and performance of beef heifers. J Anim Sci. 2006;84(1):162–70. 10.2527/2006.841162x. [DOI] [PubMed] [Google Scholar]
- 18.Sutter F, Casutt MM, Ossowski DA, Scheeder MR, Kreuzer M. Comparative evaluation of rumen-protected fat, coconut oil and various oilseeds supplemented to fattening bulls. 1. Effects on growth, carcass and meat quality. Archiv fur Tierernahrung. 2000;53(1):1–23. 10.1080/17450390009381935. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Duan Z. The local defender and functional mediator: gut microbiome. Digestion. 2018;97(2):137–45. 10.1159/000484687. [DOI] [PubMed] [Google Scholar]
- 20.Zalar B, Haslberger A, Peterlin B. The role of microbiota in depression - a brief review. Psychiatr Danub. 2018;30(2):136–41. 10.24869/psyd.2018.136. [DOI] [PubMed] [Google Scholar]
- 21.Xue MY, Sun HZ, Wu XH, Liu JX, Guan LL. Multi-omics reveals that the rumen microbiome and its metabolome together with the host metabolome contribute to individualized dairy cow performance. Microbiome. 2020;8(1):64. 10.1186/s40168-020-00819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aburto MR, Cryan JF. Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat Rev Gastroenterol Hepatol. 2024;21(4):222–47. 10.1038/s41575-023-00890-0. [DOI] [PubMed] [Google Scholar]
- 23.Yeoman CJ, White BA. Gastrointestinal tract microbiota and probiotics in production animals. Annu Rev Anim Biosci. 2014;2:469–86. 10.1146/annurev-animal-022513-114149. [DOI] [PubMed] [Google Scholar]
- 24.Cui Z, Wu S, Li J, et al. Effect of alfalfa hay and starter feeding intervention on gastrointestinal microbial community, growth and immune performance of yak calves. Front Microbiol. 2020;11:994. 10.3389/fmicb.2020.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leeuwendaal NK, Cryan JF, Schellekens H. Gut peptides and the microbiome: focus on ghrelin. Curr Opin Endocrinol Diabetes Obes. 2021;28(2):243–52. 10.1097/MED.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu F, Zhu S, Hou J, et al. The hindgut microbiome contributes to host oxidative stress in postpartum dairy cows by affecting glutathione synthesis process. Microbiome. 2023;11(1):87. 10.1186/s40168-023-01535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luan J, Feng X, Yang D, Yang M, Zhou J, Geng C. Effects of medium-chain fatty acids (MCFAs) on in vitro rumen fermentation, methane production, and nutrient digestibility under low- and high-concentrate diets. Anim Sci J. 2023;94(1):e13818. 10.1111/asj.13818. [DOI] [PubMed] [Google Scholar]
- 28.Pitta DW, Indugu N, Melgar A, et al. The effect of 3-nitrooxypropanol, a potent methane inhibitor, on ruminal microbial gene expression profiles in dairy cows. Microbiome. 2022;10(1):146. 10.1186/s40168-022-01341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AOAC. Official methods of analysis. AOAC. Gaithersburg. USA: MD; 2000. [Google Scholar]
- 30.Luan J, Feng X, Yang D, et al. Dietary supplementation of active dry yeast (Saccharomyces Cerevisiae) to finishing bulls: effects on growth performance, blood hormones, fatty acid concentrations in the gastrointestinal tract and trace mineral elements utilisation. Ital J Anim Sci. 2023;22(1):106–15. 10.1080/1828051X.2022.2164747. [Google Scholar]
- 31.Luan J, Jin Y, Zhang T, et al. Effects of dietary vitamin E supplementation on growth performance, slaughter performance, antioxidant capacity and meat quality characteristics of finishing bulls. Meat Sci. 2023;206:109322. 10.1016/j.meatsci.2023.109322. [DOI] [PubMed] [Google Scholar]
- 32.Geng CY, Feng X, Luan JM, Ji S, Jin YH, Zhang M. Improved tenderness of beef from bulls supplemented with active dry yeast is related to matrix metalloproteinases and reduced oxidative stress. Animal. 2022;16(5):100517. 10.1016/j.animal.2022.100517. [DOI] [PubMed] [Google Scholar]
- 33.Barker SB, Summerson WH. The colorimetric determination of lactic acid in biological material. J Biol Chem. 1941;138(2):535–54. 10.1016/s0021-9258(18)51379-x. [Google Scholar]
- 34.Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. 1980;63(1):64–75. 10.3168/jds.S0022-0302(80)82888-8. [DOI] [PubMed] [Google Scholar]
- 35.Gao K, Geng C. Alterations in the rumen bacterial communities and metabolites of finishing bulls fed high-concentrate diets supplemented with active dry yeast and yeast culture. Front Microbiol. 2022;13:908244. 10.3389/fmicb.2022.908244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chwen LT, Foo HL, Thanh NT, Choe DW. Growth performance, plasma fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol. Asian-Australas J Anim Sci. 2013;26(5):700–4. 10.5713/ajas.2012.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebhardt JT, Thomson KA, Woodworth JC, et al. Effect of dietary medium-chain fatty acids on nursery pig growth performance, fecal microbial composition, and mitigation properties against porcine epidemic diarrhea virus following storage. J Anim Sci. 2020;98(1):skz358. 10.1093/jas/skz358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murayama K, Fukui T, Kushibiki S, Sakamoto K, Inouchi K, Sugino T. Effects of medium-chain fatty acids and tributyrin supplementation in milk replacers on growth performance, blood metabolites, and hormone concentrations in Holstein dairy calves. J Dairy Sci. 2023;106(7):4599–607. 10.3168/jds.2022-22957. [DOI] [PubMed] [Google Scholar]
- 39.Masmeijer C, Rogge T, van Leenen K, et al. Effects of glycerol-esters of saturated short- and medium chain fatty acids on immune, health and growth variables in veal calves. Prev Vet Med. 2020;178:104983. 10.1016/j.prevetmed.2020.104983. [DOI] [PubMed] [Google Scholar]
- 40.Hollmann M, Powers WJ, Fogiel AC, Liesman JS, Bello NM, Beede DK. Enteric methane emissions and lactational performance of Holstein cows fed different concentrations of coconut oil. J Dairy Sci. 2012;95(5):2602–15. 10.3168/jds.2011-4896. [DOI] [PubMed] [Google Scholar]
- 41.Faciola AP, Broderick GA. Effects of feeding lauric acid on ruminal protozoa numbers, fermentation, and digestion and on milk production in dairy cows. J Anim Sci. 2013;91(5):2243–53. 10.2527/jas.2012-5169. [DOI] [PubMed] [Google Scholar]
- 42.Nishi Y, Mifune H, Kojima M. Ghrelin acylation by ingestion of medium-chain fatty acids. Methods Enzymol. 2012;514:303–15. 10.1016/B978-0-12-381272-8.00019-2. [DOI] [PubMed] [Google Scholar]
- 43.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 44.Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R353–8. 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- 45.Gualillo O, Lago F, Gómez-Reino J, Casanueva FF, Dieguez C. Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003;552(2–3):105–9. 10.1016/s0014-5793(03)00965-7. [DOI] [PubMed] [Google Scholar]
- 46.Lemarié F, Beauchamp E, Drouin G, Legrand P, Rioux V. Dietary caprylic acid and ghrelin O-acyltransferase activity to modulate octanoylated ghrelin functions: What is new in this nutritional field? Prostaglandins Leukot Essent Fatty Acids. 2018;135:121–7. 10.1016/j.plefa.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Heppner KM, Chaudhary N, Müller TD, et al. Acylation type determines ghrelin’s effects on energy homeostasis in rodents. Endocrinology. 2012;153(10):4687–95. 10.1210/en.2012-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wertz-Lutz AE, Knight TJ, Pritchard RH, et al. Circulating ghrelin concentrations fluctuate relative to nutritional status and influence feeding behavior in cattle. J Anim Sci. 2006;84(12):3285–300. 10.2527/jas.2006-053. [DOI] [PubMed] [Google Scholar]
- 49.Foote AP, Hales KE, Lents CA, Freetly HC. Association of circulating active and total ghrelin concentrations with dry matter intake, growth, and carcass characteristics of finishing beef cattle. J Anim Sci. 2014;92(12):5651–8. 10.2527/jas.2014-8291. [DOI] [PubMed] [Google Scholar]
- 50.Gabai G, Testoni S, Piccinini R, et al. Oxidative stress in primiparous cows in relation to dietary starch and the progress of lactation. Anim Sci. 2004;79(1):99–108. 10.1017/S1357729800054576. [Google Scholar]
- 51.Mett J, Müller U. The medium-chain fatty acid decanoic acid reduces oxidative stress levels in neuroblastoma cells. Sci Rep. 2021;11(1):6135. 10.1038/s41598-021-85523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J, Hu J, Ma X. Sodium decanoate improves intestinal epithelial barrier and antioxidation via activating G protein-coupled receptor-43. Nutrients. 2021;13(8):2756. 10.3390/nu13082756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ercan S, Basaranlar G, Gungor NE, et al. Ghrelin inhibits sodium metabisulfite induced oxidative stress and apoptosis in rat gastric mucosa. Food Chem Toxicol. 2013;56:154–61. 10.1016/j.fct.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 54.Ransom-Jones E, Jones DL, McCarthy AJ, McDonald JE. The Fibrobacteres: an important phylum of cellulose-degrading bacteria. Microb Ecol. 2012;63(2):267–81. 10.1007/s00248-011-9998-1. [DOI] [PubMed] [Google Scholar]
- 55.Waters JL, Ley RE. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17(1):83. 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans NJ, Brown JM, Murray RD, et al. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl Environ Microbiol. 2011;77(1):138–47. 10.1128/AEM.00993-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol. 2014;6(3):703–13. 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 59.Gao X, Chang S, Liu S, et al. Correlations between α-linolenic acid-improved multitissue homeostasis and gut microbiota in mice fed a high-fat diet. mSystems. 2020;5(6):e00391-20. 10.1128/mSystems.00391-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geervliet M, de Vries H, Jansen CA, et al. Effects of Escherichia coli Nissle 1917 on the porcine gut microbiota, intestinal epithelium and immune system in early life. Front Microbiol. 2022;13:842437. 10.3389/fmicb.2022.84243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrank K, Choi BK, Grund S, et al. Treponema brennaborense sp. nov., a novel spirochaete isolated from a dairy cow suffering from digital dermatitis. Int J Syst Bacteriol. 1999;49(Pt 1):43–50. 10.1099/00207713-49-1-43. [DOI] [PubMed] [Google Scholar]
- 62.Ballard JWO, Towarnicki SG. Mitochondria, the gut microbiome and ROS. Cell Signal. 2020;75:109737. 10.1016/j.cellsig.2020.109737. [DOI] [PubMed] [Google Scholar]
- 63.Mariz LDS, Amaral PM, Valadares Filho SC, et al. Dietary protein reduction on microbial protein, amino acid digestibility, and body retention in beef cattle: 2. Amino acid intestinal absorption and their efficiency for whole-body deposition. J Anim Sci. 2018;96(2):670–83. 10.1093/jas/sky018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neves PV, Pitarelo AP, Ramos LP. Production of cellulosic ethanol from sugarcane bagasse by steam explosion: effect of extractives content, acid catalysis and different fermentation technologies. Bioresour Technol. 2016;208:184–94. 10.1016/j.biortech.2016.02.085. [DOI] [PubMed] [Google Scholar]
- 65.Kinzel JJ, Bhattacharjee JK. Role of pipecolic acid in the biosynthesis of lysine in Rhodotorula glutinis. J Bacteriol. 1979;138(2):410–7. 10.1128/jb.138.2.410-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyazaki I, Asanuma M. Approaches to prevent dopamine quinone-induced neurotoxicity. Neurochem Res. 2009;34(4):698–706. 10.1007/s11064-008-9843-1. [DOI] [PubMed] [Google Scholar]
- 67.Kasuya E, Sutoh M, Yayou KI. The effects of l-DOPA and sulpiride on growth hormone secretion at different injection times in Holstein steers. Anim Sci J. 2017;88(11):1842–8. 10.1111/asj.12850. [DOI] [PubMed] [Google Scholar]
- 68.Mao SY, Huo WJ, Zhu WY. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ Microbiol. 2016;18(2):525–41. 10.1111/1462-2920.12724. [DOI] [PubMed] [Google Scholar]
- 69.Hua C, Tian J, Tian P, et al. Feeding a high concentration diet induces unhealthy alterations in the composition and metabolism of ruminal microbiota and host response in a goat model. Front Microbiol. 2017;8:138. 10.3389/fmicb.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu T, Wang L, Zeng Q, Zhang Y, Sheng B, Han L. Ghrelin ameliorates asthma by inhibiting endoplasmic reticulum stress. Am J Med Sci. 2017;354(6):617–25. 10.1016/j.amjms.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 71.Han QQ, Huang HJ, Wang YL, et al. Ghrelin exhibited antidepressant and anxiolytic effect via the p38-MAPK signaling pathway in hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2019;93:11–20. 10.1016/j.pnpbp.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Cao L, Liu X. Ghrelin alleviates endoplasmic reticulum stress and inflammation-mediated reproductive dysfunction induced by stress. J Assist Reprod Genet. 2019;36(11):2357–66. 10.1007/s10815-019-01589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiang JG, Smith JT, Cannon G, et al. Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma. Cell Biosci. 2020;10:63. 10.1186/s13578-020-00425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma L, Liu J, Zhang X, Qi J, Yu W, Gu Y. p38 MAPK-dependent Nrf2 induction enhances the resistance of glioma cells against TMZ. Med Oncol. 2015;32(3):69. 10.1007/s12032-015-0517-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Effects of medium-chain fatty acid triglycerides supplementation on ruminal fermentation parameters of finishing bulls. Table S2. Effects of medium-chain fatty acid triglycerides supplementation on nutrient digestibility of finishing bulls. Table S3. Summary of sequence statistics for each sample. Table S4. Significant differential metabolites in rumen fluid of four treatment groups (n = 4) under positive ionization mode. Table S5. Significant differential metabolites in rumen fluid of four treatment groups (n = 4) under negative ionization mode.
Additional file 2: Fig. S1. The number of operational taxonomic units (OTUs) of gastrointestinal tract bacteria in finishing bulls. (A) rumen digesta, (B) rumen fluid, and (C) feces. CON, the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4). Fig. S2. Alpha diversity curve. Rumen digesta (A) rarefaction curves, (B) shannon curves, (C) rank abundance curves, rumen fluid (D) rarefaction curves, (E) shannon curves, (F) rank abundance curves, and feces (G) rarefaction curves, (H) shannon curves, (I) rank abundance curves (OUT level). CON, the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4). Fig. S3. The relative abundances of gastrointestinal tract bacterial community at rumen digesta (A) phylum, (B) family, (C) genus, rumen fluid (D) phylum, (E) family, (F) genus, and feces (G) phylum, (H) family, (I) genus levels (top 20). The different colors of the bars represent different species, and the length of the bars represents the proportion of the species. CON, the control group cattle fed control diets (n = 4); C8, the treatment group cattle fed control diets containing glycerol monocaprylin (n = 4); C10, the treatment group cattle fed control diets containing glycerol monodecanoate (n = 4); C12, the treatment group cattle fed control diets containing glycerol monolaurate (n = 4). Fig. S4. KEGG (Kyoto Encyclopedia of Genes and Genomes) classification maps of significant differential metabolites in the rumen fluid of four treatment groups (n = 4) under positive ionization mode (A) and negative ionization mode (B). Fig. S5. Correlation between serum acylated ghrelin (AG) level and serum reactive oxygen species (ROS) level in finishing bulls. r, correlation coefficient.
Data Availability Statement
The gastrointestinal tract microbiome sequencing reads (including rumen digesta, rumen fluid, and feces) are available in the Sequence Read Archive (SRA) of NCBI under accession project number PRJNA1111285 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA1111285?reviewer = uo63pvg3kuigl56msror69nrb0).