Abstract
Background
A randomized trial suggested that reducing left‐sided subthalamic stimulation amplitude could improve axial dysfunction.
Objectives
To explore open‐label tolerability and associations between trial outcomes and asymmetry data.
Methods
We collected adverse events in trial participants treated with open‐label lateralized settings for ≥3 months. We explored associations between trial outcomes, location of stimulation and motor asymmetry.
Results
14/17 participants tolerated unilateral amplitude reduction (left‐sided = 10, right‐sided = 4). Two hundred eighty‐four left‐sided and 1113 right‐sided stimulated voxels were associated with faster gait velocity, 81 left‐sided and 22 right‐sided stimulated voxels were associated with slower gait velocity. Amplitude reduction contralateral to shorter step length was associated with 2.4‐point reduction in axial MDS‐UPDRS. Reduction contralateral to longer step length was associated with 10‐point increase in MDS‐UPDRS.
Conclusions
Left‐sided amplitude reduction is potentially more tolerable than right‐sided amplitude reduction. Right‐sided more than left‐sided stimulation could be associated with faster gait velocity. Shortened step length might reflect contralateral overstimulation.
Keywords: Parkinson's disease, deep brain stimulation, axial motor function, lateralization, asymmetry
In patients with Parkinson's disease (PwPD) who develop axial dysfunction after bilateral subthalamic deep brain stimulation (STN‐DBS), lateralized stimulation (unilateral amplitude reduction) could reduce stimulation‐induced axial dysfunction while maintaining the benefits of bilateral STN‐DBS. 1 , 2 , 3 , 4 , 5 , 6 , 7 In a randomized trial, we found that 50% left‐sided STN‐DBS amplitude reduction was associated with a 2.5‐point reduction in the axial subscale of the Movement Disorders Society‐sponsored Unified Parkinson's Disease Rating Scale (MDS‐UPDRS). 1 Thus, we conducted an open‐label extension of the trial. Since axial dysfunction in PwPD has been associated with motor and gait asymmetry, 2 , 3 we conducted exploratory analyses examining associations between trial outcomes, location of stimulation and motor asymmetry data.
Methods
Participant characteristics and methodology of the randomized trial are reported separately. 1 Briefly, 22 PwPD and treatment‐resistant axial dysfunction after bilateral STN‐DBS were blindly and randomly crossed over between bilateral, left‐sided and right‐sided lateralized STN‐DBS (unilateral 50% amplitude reduction) for ≥21 days for each intervention. The primary outcome was gait velocity change. Secondary outcomes were changes in selected measures of quality of life, motor, axial motor and cognitive function.
Open‐Label Extension
The 17 of 22 participants who experienced axial benefits during the blinded phase were offered treatment with the most beneficial lateralized settings in an optional, 3‐month open‐label extension. Lateralized settings associated with the greatest increase in gait velocity were considered the most beneficial. Open‐label lateralized settings were programmed or continued immediately after conclusion of the blinded phase. Adverse events were systematically collected (Fig. 1).
Figure 1.
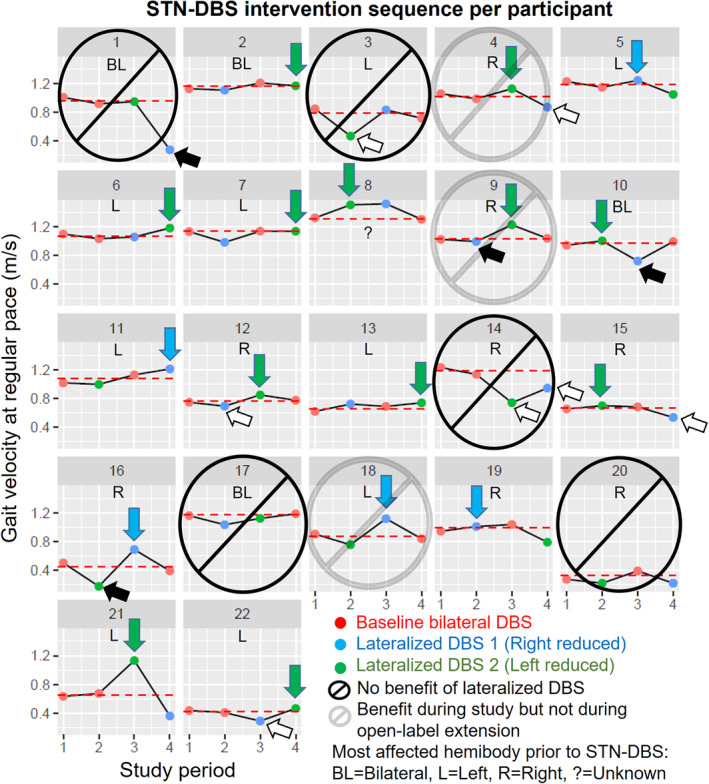
Summary of the blinded and open‐label phases of the lateralized STN‐DBS study. 1 Dashed red line: Average values of the bilateral‐STN‐DBS periods. White arrows: Medication adjustments. Black arrows: Treatment failures. Green arrows: Left‐sided amplitude reduction beneficial. Blue arrows: Right‐sided amplitude reduction beneficial.
Location of Stimulation
We computed bilateral volumes of tissue activated (VTAs) corresponding to high amplitude (VTAH) and low amplitude (VTAL) settings per participant. Brain MRI studies sufficient for VTA computation were available for 20 of 22 participants (1 excluded due to motion artifact, 1 due to unavailable images). Seventeen of 20 participants underwent pre‐operative 1.5 T scans. The remaining 3 underwent pre‐operative 3 T scans. Pre‐operative sequences were: three‐dimensional spoiled gradient echo (3D‐SPGR) acquired with 3.0 T GE Signa HDxt scanner: voxel size = 1 × 1 × 1 mm, TR = 9.0 ms, TE = 3.7 ms, flip angle = 12°(n = 3) and 3D‐SPGR acquired with 1.5 T GE Signa Excite scanner: voxel size = 1 × 1 × 1 mm, TR = 12.4 ms, TE = 5.3 ms, flip angle = 20°(n = 17). Post‐operative sequences were: 3D‐SPGR acquired with 1.5 T GE Signa Excite scanner: voxel size = 1 × 1 × 1 mm, TR = 11.9 ms, TE = 5.0 ms, flip angle = 20°(n = 20). T1‐weighted MRI acquisitions were processed with the publicly available Lead‐DBS pipeline (www.lead-dbs.org), which was used to perform electrode localization, native‐to‐standard space normalization, and VTA modeling. 8 Each VTA (binary label) was assigned a corresponding gait velocity value based on the participant and amplitude setting (VTAH or VTAL) from which it was drawn. Subsequently, we used a voxel‐wise linear mixed effect model to explore whether stimulation of a voxel (denoted by VTA overlap with each voxel) was associated with the individual change in gait velocity (R software 3.4.4, R Core Team, R Foundation for Statistical Computing, 2017 and RMINC, https://github.com/Mouse-Imaging-Centre/RMINC). Given the exploratory nature of this analysis, we established an uncorrected significance threshold of P < 0.05.
Using the Wilcoxon signed rank test (IBM SPSS, IBM Corp., Armonk, NY, USA), we compared selected right‐sided and left‐sided intraoperative data for each participant (mean STN length, mean firing rate, burst index, local field potential frequency bands and power). We corrected for the order of DBS lead insertion (right or left first) and the number of recording trajectories per hemisphere.
Motor Asymmetry
We obtained asymmetry scores using the formula “100% × [right–left sided items]/[right+left sided items]” for MDS‐UPDRS part III and lower extremity MDS‐UPDRS part III. Step length was measured by a 6‐meter walkway and quantitative gait analysis system (Zeno walkway and PKMAS system, ProtoKinetics, Havertown, PA, USA). Shorter and longer step length at baseline were defined by comparing the average right and left step length obtained during both bilateral STN‐DBS conditions. Step length asymmetry was calculated using the formula “100% × [right–left step length]/[right+left step length]”. Asymmetric step length at baseline was defined as >2% difference in favor of right or left step length. Step length ratio was calculated using the formula “right/left step length”. Using the same linear effects model employed for primary and secondary outcome analyses, 1 , 9 we explored correlations between trial outcomes and measures of motor and step length asymmetry at baseline and during each trial intervention.
Results
Open‐Label Extension
The 17 participants who benefited from lateralized STN‐DBS during the blinded phase opted to participate in the three‐month open‐label extension. During this extension, three participants returned to baseline settings. In the 14 participants who tolerated lateralized STN‐DBS, left‐side amplitude was reduced in 10 and right‐side amplitude was reduced in 4. Medication adjustments were not required (Fig. 1, Table S1).
Location of Stimulation
There was an overall association between stimulation location and gait velocity (P < 0.05, uncorrected) (Fig. 2). Out of the voxels that survived significance threshold (t = 2.02), a larger extent of voxels associated with faster gait velocity localized to the right STN and vicinity (n = 1113) compared to those located in the left STN and vicinity (n = 284). Likewise, a larger extent of voxels associated with slower gait velocity localized to the left STN and vicinity (n = 81) compared to those located in the right STN and vicinity (n = 22). In the right hemisphere, voxels associated with faster gait velocity when stimulated (n = 1113) localized to the zona incerta, anteroventral STN, and white matter lateral to STN, while voxels associated with slower gait velocity (n = 22) localized near the superior border of the substantia nigra. In the left hemisphere, voxels associated with faster gait velocity (n = 284) localized to a circumscribed portion of the medial STN, while voxels associated with slower gait velocity (n = 81) localized to the dorsolateral STN and adjacent white matter.
Figure 2.
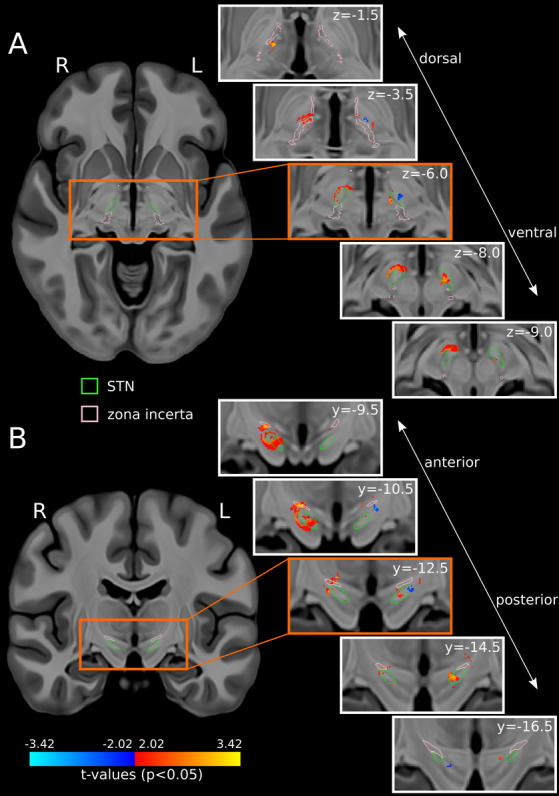
Voxels associated with gait velocity changes when stimulated (P < 0.05, uncorrected) are projected on high‐resolution T1‐weighted template slices (A: axial, B: coronal). 10 Warm colors: Faster gait velocity. Cool colors: Slower gait velocity. Green outlines: STN. Pink outlines: Zona incerta.
Intraoperative data were available for 16 participants. There were no significant differences in surgical and electrophysiological characteristics when comparing right‐sided and left‐sided data (Table S4).
Motor Asymmetry
Reducing stimulation amplitude contralateral to shorter step length resulted in 2.4‐point reduction in axial MDS‐UPDRS (P = 0.006, uncorrected) and 1.5‐point reduction in UPDRS‐PIGD (P = 0.037, uncorrected). Reducing stimulation amplitude contralateral to longer step length resulted in 10‐point increase in total MDS‐UPDRS (P = 0.029, uncorrected), 5‐point increase in motor MDS‐UPDRS (P = 0.02, uncorrected), 4.2 ± 2 cm reduction in mean step length (P = 0.038, uncorrected) and 4.1 ± 1.9 cm reduction in right step length (P = 0.042, uncorrected) (Table S2). In the 15 participants with baseline asymmetric step length (Fig. S1), reducing stimulation contralateral to shorter step length resulted in 2.5‐point reduction in axial MDS‐UPDRS (P = 0.01, uncorrected) (Table S3).
Discussion
This study was designed as exploratory. Thus, we did not correct for multiple comparisons and all results should be considered hypothesis‐generating.
During the open‐label phase, 14 of 17 participants tolerated lateralized STN‐DBS without medication adjustments. Consistent with the blinded phase, 1 left‐sided amplitude was reduced in 10 of those 14 participants. During both phases, 9 of the 12 lateralized STN‐DBS interventions that were not tolerated had right‐sided amplitude reduction (Fig. 1). Although 3 months is a relatively short duration to infer longer‐term effects, this suggests that left STN‐DBS amplitude reduction is potentially more tolerable than right STN‐DBS amplitude reduction. Medication adjustments during the blinded phase were associated with lack of tolerability, regardless of the side of amplitude reduction (Fig. 1, Table S1).
DBS offers the opportunity to study interhemispheric network interactions. 11 , 12 Our imaging and intraoperative analyses explored right versus left‐sided differences potentially contributing to lateralized STN‐DBS outcomes. VTAs were generated with the E‐field norm finite element method, 8 which resembles gold standard models when estimating VTAs for monopolar settings but may not be as reliable for complex DBS settings used in PwPD and axial dysfunction. 13 Since 10 of 14 participants had left‐sided amplitude reduction, a greater number of analyzed voxels localized to the right hemisphere. Considering these limitations, more stimulation regions associated with faster gait velocity localized to the right STN and neighboring regions, and more stimulation regions associated with slower gait velocity localized to the left STN and neighboring regions (Fig. 2). These findings suggest that right‐sided stimulation may contribute to faster gait velocity and left‐sided overstimulation may contribute to slower gait velocity, which is consistent with prior studies suggesting right hemispheric dominance for locomotion. 14 , 15 , 16 , 17 , 18
In PwPD treated with bilateral STN‐DBS, maintaining optimized right‐sided stimulation while avoiding left‐sided overstimulation could prevent stimulation‐induced axial dysfunction, independent of handedness and appendicular asymmetry. 1 , 4 , 5 , 6 , 19 , 20 We did not find left vs. right differences between averaged electrophysiological data corresponding to STN trajectories (Table S4). However, we found that bilateral regions associated with faster gait velocity localized outside of the dorsolateral STN. Moreover, there were voxels associated with slower gait velocity that localized to the left dorsolateral STN. These findings could imply that dorsolateral STN stimulation, particularly left‐sided, might be detrimental for gait velocity. Unilateral pallidal stimulation could also have axial and bilateral effects. 21 In fact, left‐sided pallidal‐subthalamic connectivity has predicted the overall motor response to bilateral STN‐DBS. 19 Future work could analyze left and right‐sided neuronal or regional activity, including remote measurements to assess for continuous and task‐related changes while correcting for tolerability and response to right versus left‐sided amplitude reduction. Future work could include PwPD treated with subthalamic and pallidal DBS, and compare voxel laterality after standardizing for discrepancies between lateralized VTAs but not number of participants or side of amplitude reduction. Combining patient‐specific connectivity with directional stimulation could allow individualized stimulation of right‐sided and left‐sided regions most important for axial motor control. 22 , 23
Amplitude reduction contralateral to longer step length was associated with increase in total and motor MDS‐UPDRS. Conversely, amplitude reduction contralateral to shorter step length was associated with reduction in axial MDS‐UPDRS (Tables S2 and S3, Fig. S1). Future studies could analyze changes in motor fluctuations using the MDS‐UPDRS part IV. Evaluating gait asymmetry in these patients is challenging due to combined disease, medication and stimulation effects. Restoring gait symmetry could normalize gait coordination but its benefits are unclear. 3 , 24 , 25 Stimulation‐induced effects could play a significant role in gait dysfunction because continuous, bilateral STN‐DBS might not adequately modulate alternating oscillations that occur during each contralateral step cycle. 26 , 27
In conclusion, PwPD who develop axial dysfunction after bilateral STN‐DBS may tolerate left more than right‐sided lateralized STN‐DBS. Right more than left‐sided stimulation could be associated with faster gait velocity and shortened step length might reflect contralateral overstimulation. Further research is necessary to study whether delivering individualized stimulation in a lateralized or alternating fashion could benefit PwPD and axial dysfunction. 22 , 26 , 27
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
K.J.L.: 1A, 1B, 1C, 2A, 2C, 3A
B.G.: 1B, 1C, 2C, 3B
T.M.A.: 1B, 1C, 2A, 2B, 3B
M.C.: 1A, 2C, 3B
G.T.: 1C, 2A, 2B, 3B
A.B.: 1A, 1B, 1C, 2A, 2B, 3A
G.J.B.E.: 1A, 1B, 1C, 2A, 2B, 3A
J.G.: 1A, 1B, 1C, 2A, 2B, 3A
D.S.: 1C, 2C, 3B
S.K.K.: 1B, 2C, 3B
M.H.: 1B, 2C, 3B
R.P.M.: 1B, 1C, 2C, 3B
C.M.: 1A, 2C, 3B
W.D.H.: 1A, 1B, 2A, 2C, 3B
A.M.L.: 1B, 2C, 3B
A.E.L.: 1A, 1B, 2C, 3B
A.F.: 1A, 1B, 1C, 2C, 3B
Disclosures
Ethical compliance statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. This study was approved by the Research Ethics Board of the University Health Network, University of Toronto (REB175785). The study was conducted in accordance with the declaration of Helsinki. Each study participant provided written informed consent.
Funding Sources and Conflicts of Interest: This work was supported by the Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic, Toronto Western Hospital, University Hospital Network, Toronto, Ontario, Canada (AEL, KJL), by the Chair in Neuromodulation at the University of Toronto and University Hospital Network, Toronto, Ontario, Canada (AF), and by the Canadian Institutes of Health Research Banting Fellowship (#471913) (JG). The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for Previous 12 Months: KJL has consulted for Takeda; has received honoraria from the International Parkinson and Movement Disorder Society; and has received research support from Acadia, Bluerock, the Del Monte Institute for Neuroscience at the University of Rochester, the National Institutes of Health (National Institute of Neurological Disorders and Stroke), Roche and Takeda. MC has received research support from the Canadian Institutes of Health Research and the Michael J. Fox Foundation. AB has received research support from the National Institutes of Health (National Institute of Neurological Disorders and Stroke). SKK has consulted for Abbott, Boston Scientific and Medtronic; and has received honoraria from Abbott, Boston Scientific and Medtronic. MH has received research support from the Temerty Center for AI in Research, Education and Medicine. CM has received research support from the International Parkinson and Movement Disorder Society, the Michael J. Fox Foundation and the Parkinson's Foundation. WDH has received research support from the Dystonia Medical Research Foundation Canada and the National Science and Engineering Council. AML has consulted for Abbott, Boston Scientific, Insightec, Medtronic, and Functional Neuromodulation (Scientific Director). AEL has consulted for AbbVie, Amylyx, Aprinoia, Biogen, BioAdvance, Biohaven, Biovie, BlueRock, BMS, Denali, Janssen, Lilly, Pharma 2B, Sun Pharma, UCB; has received honoraria from AbbVie, Sun Pharma and Sunovion; has received research support from Brain Canada, the Canadian Institutes of Health Research, the Edmond J. Safra Philanthropic Foundation, the Michael J. Fox Foundation, the Ontario Brain Institute, the Parkinson Foundation, Parkinson Canada and the W. Garfield Weston Foundation; and has received royalties from Elsevier, Saunders, Wiley‐Blackwell, Johns Hopkins Press and Cambridge University Press. AF has consulted for Abbvie, Abbott, Boston Scientific, Inbrain, Ipsen, Medtronic, Sunovion and Syneos Health; has been on advisory committees for Abbvie, Boston Scientific, Ceregate, Inbrain and Ipsen; has received honoraria from Abbvie, Abbott, American Academy of Neurology, Boston Scientific, Brainlab, Ipsen, Medtronic, Merz, International Parkinson and Movement Disorder Society, Paladin Labs, Sunovion and UCB; has received royalties from Springer; and has received research support from Abbvie, Boston Scientific, the Dystonia Medical Research Foundation, the University of Toronto, the Michael J. Fox Foundation, Medtronic, the MSA coalition, Praxis and ES. All other authors report no financial disclosures.
Supporting information
FIGURE S1. Definition and selection of the 15 participants with asymmetric step length at baseline.
TABLE S1. Number and percentage of adverse events during the blinded phase and open label extension of the lateralized subthalamic deep brain stimulation trial.
TABLE S2. Changes in primary and secondary outcomes associated with blinded phase interventions contralateral to shorter and longer step length.
TABLE S3. Changes in primary and secondary outcomes associated with amplitude reduction of subthalamic deep brain stimulation contralateral to shorter and longer step length in the 15 participants with asymmetric step length at baseline.
TABLE S4. Comparative surgical and electrophysiological data obtained during intraoperative microelectrode recordings of left and right subthalamic nucleus for 16 participants.
Acknowledgments
The authors thank Drs. Sreeram Prasad, Musleh Algarni, Vijayashankar Paramanandam and Lais Machado de Oliveira for the care provided to study participants. Part of the study design was developed during the 2017 Clinical Trials Methodology Course sponsored by the National Institute of Neurological Disorders and Stroke (NINDS) (NIH R25:NS088248). KJL is thankful for the feedback received on this study during the 2019–2021 program Training in Research for Academic Neurologists to Sustain Careers and Enhance the Numbers of Diverse Scholars (TRANSCENDS) sponsored by the NINDS (NIH R25:NS098999).
References
- 1. Lizarraga KJ, Gnanamanogaran B, Al‐Ozzi TM, et al. Lateralized subthalamic stimulation for axial dysfunction in Parkinson's disease: a randomized trial. Mov Disord 2022;37(5):1079–1087. 10.1002/mds.28953. [DOI] [PubMed] [Google Scholar]
- 2. Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson's disease related to asymmetric motor function? Ann Neurol 2005;57(5):656–663. 10.1002/ana.20452. [DOI] [PubMed] [Google Scholar]
- 3. Fasano A, Herzog J, Seifert E, et al. Modulation of gait coordination by subthalamic stimulation improves freezing of gait. Mov Disord 2011;26(5):844–851. 10.1002/mds.23583. [DOI] [PubMed] [Google Scholar]
- 4. Castrioto A, Meaney C, Hamani C, et al. The dominant‐STN phenomenon in bilateral STN DBS for Parkinson's disease. Neurobiol Dis 2011;41(1):131–137. 10.1016/j.nbd.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 5. Lizarraga KJ, Jagid JR, Luca CC. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation on gait kinematics in Parkinson's disease: a randomized, blinded study. J Neurol 2016;263(8):1652–1656. 10.1007/s00415-016-8191-3. [DOI] [PubMed] [Google Scholar]
- 6. Rizzone MG, Ferrarin M, Lanotte MM, Lopiano L, Carpinella I. The dominant‐subthalamic nucleus phenomenon in bilateral deep brain stimulation for Parkinson's disease: evidence from a gait analysis study. Front Neurol 2017;8:575. 10.3389/fneur.2017.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lizarraga KJ, Naghibzadeh M, Boutet A, Elias GJB, Fasano A. Management of Pisa syndrome with lateralized subthalamic stimulation. J Neurol 2018;265(10):2442–2444. 10.1007/s00415-018-8991-8. [DOI] [PubMed] [Google Scholar]
- 8. Horn A, Li N, Dembek TA, et al. Lead‐DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019;184:293–316. 10.1016/j.neuroimage.2018.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed‐effects models using lme4. J Stat Soft 2015;67:1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 10. Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 2018;170:271–282. 10.1016/j.neuroimage.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 11. Sirica D, Hewitt AL, Tarolli CG, et al. Neurophysiological biomarkers to optimize deep brain stimulation in movement disorders. Neurodegener Dis Manag 2021;11(4):315–328. 10.2217/nmt-2021-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lizarraga KJ, Saravanamuttu J, Baarbe JK, Lang AE, Chen R. Interhemispheric pathways in agenesis of the corpus callosum and Parkinson's disease. Brain Stimul 2020;13(2):360–362. 10.1016/j.brs.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 13. Duffley G, Anderson DN, Vorwerk J, Dorval AD, Butson CR. Evaluation of methodologies for computing the deep brain stimulation volume of tissue activated. J Neural Eng 2019;16(6):066024. 10.1088/1741-2552/ab3c95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H. Mechanisms underlying gait disturbance in Parkinson's disease: a single photon emission computed tomography study. Brain 1999;122(Pt 7):1271–1282. 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- 15. Bartels AL, de Jong BM, Giladi N, et al. Striatal dopa and glucose metabolism in PD patients with freezing of gait. Mov Disord 2006;21(9):1326–1332. 10.1002/mds.20952. [DOI] [PubMed] [Google Scholar]
- 16. Peterson DS, Pickett KA, Duncan R, Perlmutter J, Earhart GM. Gait‐related brain activity in people with Parkinson disease with freezing of gait. PLoS One 2014;9(3):e90634. 10.1371/journal.pone.0090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 2013;136(Pt 8):2405–2418. 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dai S, Lemaire C, Piscicelli C, Perennou D. Lateropulsion prevalence after stroke: a systematic review and meta‐analysis. Neurology 2022;98(15):e1574–e1584. 10.1212/WNL.0000000000200010. [DOI] [PubMed] [Google Scholar]
- 19. Younce JR, Campbell MC, Hershey T, et al. Resting‐state functional connectivity predicts STN DBS clinical response. Mov Disord 2021;36(3):662–671. 10.1002/mds.28376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seuthe J, Hermanns H, Hulzinga F, et al. Gait asymmetry and symptom laterality in Parkinson's disease: two of a kind? J Neurol 2024;271:4373–4382. 10.1007/s00415-024-12379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayashi Y, Mishima T, Fujioka S, Morishita T, Inoue T, Nagamachi S, Tsuboi Y. Unilateral GPi‐DBS improves ipsilateral and axial motor symptoms in Parkinson's disease as evidenced by a brain perfusion single photon emission computed tomography study. Front Hum Neurosci 2022;16:888701. 10.3389/fnhum.2022.888701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Temiz G, des Neiges Santin M, Olivier C, et al. Freezing of gait depends on cortico‐subthalamic network recruitment following STN‐DBS in PD patients. Parkinsonism Relat Disord 2022;104:49–57. 10.1016/j.parkreldis.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 23. Fan H, Guo Z, Jiang Y, et al. Optimal subthalamic stimulation sites and related networks for freezing of gait in Parkinson's disease. Brain Commun 2023;5(5):fcad238. 10.1093/braincomms/fcad238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meoni S, Debu B, Pelissier P, et al. Asymmetric STN DBS for FOG in Parkinson's disease: a pilot trial. Parkinsonism Relat Disord 2019;63:94–99. 10.1016/j.parkreldis.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 25. Lizarraga KJ, Patelaki E, Mesmer H, Hewitt A, Wensel A, Foxe JJ, Freedman EG. Mobile brain‐body imaging markers of treatment‐related responses in a man with Parkinson's disease. Clin Neurophysiol 2023;152:90–92. 10.1016/j.clinph.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 26. Anidi C, O'Day JJ, Anderson RW, Afzal MF, Syrkin‐Nikolau J, Velisar A, Bronte‐Stewart HM. Neuromodulation targets pathological not physiological beta bursts during gait in Parkinson's disease. Neurobiol Dis 2018;120:107–117. 10.1016/j.nbd.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fischer P, Chen CC, Chang Y, et al. Alternating modulation of subthalamic nucleus beta oscillations during stepping. J Neurosci 2018;38(22):5111–5121. 10.1523/JNEUROSCI.3596-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Definition and selection of the 15 participants with asymmetric step length at baseline.
TABLE S1. Number and percentage of adverse events during the blinded phase and open label extension of the lateralized subthalamic deep brain stimulation trial.
TABLE S2. Changes in primary and secondary outcomes associated with blinded phase interventions contralateral to shorter and longer step length.
TABLE S3. Changes in primary and secondary outcomes associated with amplitude reduction of subthalamic deep brain stimulation contralateral to shorter and longer step length in the 15 participants with asymmetric step length at baseline.
TABLE S4. Comparative surgical and electrophysiological data obtained during intraoperative microelectrode recordings of left and right subthalamic nucleus for 16 participants.


