Abstract
Purpose of Review
Current non-invasive tests for evaluating patients with peripheral artery disease (PAD) have significant limitations for early detection and management of patients with PAD and are generally focused on the evaluation of large vessel disease. PAD often involves disease of microcirculation and altered metabolism. Therefore, there is a critical need for reliable quantitative non-invasive tools that can assess limb microvascular perfusion and function in the setting of PAD.
Recent Findings
Recent developments in positron emission tomography (PET) imaging have enabled the quantification of blood flow to the lower extremities, the assessment of the viability of skeletal muscles, and the evaluation of vascular inflammation and microcalcification and angiogenesis in the lower extremities. These unique capabilities differentiate PET imaging from current routine screening and imaging methods.
Summary
The purpose of this review is to highlight the promising role of PET in the early detection and management of PAD providing a summary of the current preclinical and clinical research related to PET imaging in patients with PAD and related advancement of PET scanner technology.
Keywords: Peripheral artery disease, Positron emission tomography, Diagnosis, Management
Introduction
Peripheral arterial disease (PAD) involves atherosclerosis of non-coronary arteries and affects approximately 8.5 million adults in the USA and > 230 million adults worldwide [1–3]. There is tremendous variation in the presentation of PAD ranging from early-stage disease with claudication (exertional calf pain) [4] to late-stage critical limb ischemia (CLI) as the most severe manifestation of lower extremity PAD, characterized by lower limb ischemic rest pain and/or the presence of necrotic tissue. Regardless of the symptomatic expression, PAD is associated with a high risk for cardiovascular events, lower extremity amputation, poor quality of life, death, and high treatment costs [5, 6].
Besides cardiovascular risk management, relief of ischemic pain, healing of wounds, preservation of functional limbs, and improvement of quality of life are the primary therapeutic goals for PAD. Ultimately, improving microvascular perfusion and non-invasively quantifying those improvements within specific regions of the ischemic limb are essential for achieving therapeutic goals. Indeed, the importance and need for standard non-invasive tools that can assess limb microvascular perfusion in the setting of CLI were recently highlighted by the American Heart Association (AHA) [7•]. However, the majority of clinically available non-invasive tools only allow for evaluation of the hemodynamic and anatomical improvements associated with therapy, while the available tools that do allow for evaluation of microvascular perfusion are restricted to superficial measurements (e.g., transcutaneous oxygen pressure (TcP02)) or a limited anatomical region of tissue (e.g., toe pressure). Non-invasive assessment of lower extremity microvascular perfusion is particularly important for patients with diabetes mellitus (DM), which exacerbates PAD by promoting microvascular disease and ultimately contributes to increased mortality and increased risk for lower extremity amputation [8–11]. A 2021 AHA scientific statement states that PAD should be recognized as an increasing global burden that has been systematically understudied and underappreciated [3].
Current non-invasive tests for lower extremity PAD have several limitations in diagnosing and managing patients with PAD, particularly their ability for early detection and monitoring the response for various treatment options. Ankle-brachial index has been historically used for screening for PAD; however, it has a limited sensitivity and specificity for diagnosing the disease and can be vulnerable to the skills of the person performing the test [12]. Duplex Doppler can provide a reasonable picture of the blood flow across large vessels but does not inform us about the microvasculature and tissue viability which is believed to be crucial for clinical decision-making. Similarly, computed tomography (CT) angiography provides a satisfactory view of the vessels and surrounding structures in the lower extremities; however, it poses a risk of causing acute renal failure in vulnerable individuals due to the use of contrast injection. This led to an increased interest in examining alternative diagnostic methods.
Magnetic resonance imaging (MRI) can provide very detailed images of the lower extremity, and several MRI tools have been developed and tested such as T2*(PIVOT) to quantify microvascular perfusion [13], and blood oxygen level-dependent (BOLD) MRI has shown a strong potential for evaluating muscle tissue oxygenation [14, 15]. Similarly, nuclear molecular imaging can offer valuable insights into the metabolic and physiological processes of the limbs that are affected by PAD including comprehensive assessment of blood flow, angiogenesis, and tissue viability. Single-photon emission computed tomography (SPECT) has been shown to be effective in assessing regional perfusion and can help guide management in patients with critical limb ischemia [16, 17]. Positron emission tomography (PET) has advantages over SPECT including higher sensitivity and better spatial resolution providing higher sensitivity and accuracy in detecting abnormalities.
In this review, we highlight on the potential role of PET in PAD by providing a comprehensive assessment of both physiological indices like flow and metabolism as well as molecular markers of inflammation, thrombosis, angiogenesis, autonomic function, fibrosis, and microvascular calcifications. We provide an overview of the latest preclinical and clinical investigations of PET imaging in this field along with the advancement of PET imaging scanners and novel acquisition protocols.
Quantification of Blood Flow in Lower Extremities
The measurement of blood flow in the lower extremities is crucial in assessing the health and viability of limbs. PET imaging can serve as a valuable non-invasive method for quantifying blood flow in the lower extremities. 15O-water PET imaging is a well-established method for quantifying blood flow in the lower extremities, particularly in the calf muscles (Fig. 1). The short half-life of 15O-water enables accurate assessment of blood flow during rest and exercise and has been shown to be useful in evaluating patients with diabetes mellitus (DM) and PAD [18]. A decrease in muscle flow reserve estimated using 15O-water PET imaging indicates poor perfusion and muscle viability, which are important factors in managing PAD patients [19, 20]. However, the costs and limited availability of 15O-water have led researchers to look for alternative radiotracers. Preclinical and clinical studies have demonstrated that 13N-ammonia imaging can also effectively measure blood flow in extremities [21, 22]. In a pilot porcine study [23], we recently demonstrated that dynamic rubidium-82 (82Rb) PET imaging of the lower abdomen and extremities can reliably assess flows in skeletal muscle following acute unilateral femoral artery occlusion. The established 82Rb kinetic parameters (K1 and k2) could play an important role in identifying skeletal muscle flow as well as viability [24]. Further investigation is necessary to optimize data acquisition protocols and denoise dynamic 82Rb images of the lower extremity. There are several challenges associated with 82Rb imaging of the lower extremities including the short half-life (75 s) causing short imaging time and precluding the ability to obtain exercise stress perfusion imaging and the relatively lower first-pass extraction fraction resulting in a non-linear relationship between radiotracer uptake with blood flow at higher flows associated with pharmacological stress or reactive hyperemia [25]. The extraction of 82Rb in skeletal muscle may be even lower than what has been observed for the heart. 18F-flurpiridaz may provide an alternative to the currently approved PET radiotracers, although it still has not received FDA approval. 18F-flurpiridaz has a longer half-life (109 min) and a higher first-pass extraction fraction which may allow for the quantification of blood flow in the lower extremity and the assessment of exercise perfusion [26].
Fig. 1.
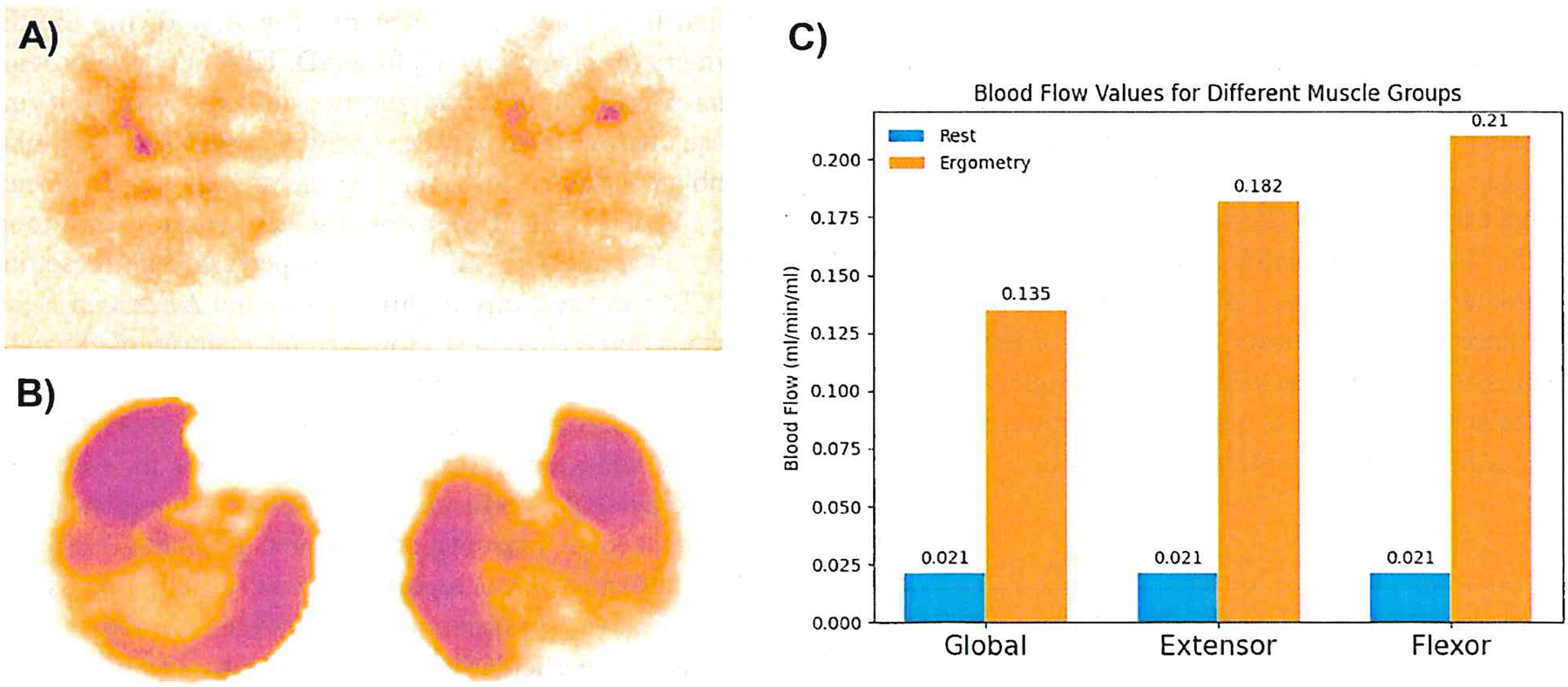
Blood flow in a healthy participant. A At rest. B During pedal ergometry, increasing flow in the anterior tibial and medial gastrocnemius muscles. A and B use logarithmic scaling for a wide range of flow values. C Calf muscle blood flow comparison in five volunteers at rest and during pedal ergometry: extensors vs. flexors, with a non-significant p-value at rest and a p-value of 0.022 during ergometry (adapted from [18])
PET imaging techniques for evaluating relative or absolute blood flow have great potential for advancing our understanding of PAD and developing more efficient management strategies. However, the application of PET imaging for the evaluation of absolute blood flow faces many challenges including the derivation of an image-derived arterial input function from a large enough vascular structure that is not complicated by partial volume effects. This might be accomplished with newer whole-body PET scanners or the use of shuttling of the scanner back and forth from the heart or abdomen to the lower extremities for the derivation of a reliable arterial input function [23].
Evaluation of Skeletal Muscle Metabolism
Under normal conditions, skeletal muscles rely on different metabolic pathways depending on the physiological state. During fasting, muscles predominantly utilize fat oxidation for energy production, whereas, during insulin-stimulated conditions, glucose oxidation is favored. However, these patterns may be altered in individuals with insulin insensitivity or PAD [27, 28].
18F-fluorodeoxyglucose (FDG) is a glucose analog radiotracer that cells take up and metabolize similarly to glucose. This can provide insights into the muscles’ glucose uptake and metabolism; this can be particularly helpful in detecting muscle insulin resistance. Patients with obesity-associated insulin resistance may exhibit impaired glucose uptake in major muscles, as measured by the FDG uptake, without significant changes in muscle fiber type or blood flow [29]. Similarly, patients with PAD and claudication demonstrated significantly lower calf muscle FDG uptake compared with the healthy subjects suggesting a possible correlation between muscle insulin resistance and exercise limitation [30]. Furthermore, FDG can be used to characterize muscle activity and evaluate variations of glucose metabolism within the exercising skeletal muscles [31, 32]. This glucose uptake heterogeneity may reflect heterogeneity in muscle metabolic activity during exercise with implications in patients with PAD [33].
Alternatively, 11C-acetate PET imaging can provide a comprehensive assessment of skeletal muscle oxidative metabolism and can be useful in detecting the changes between active and inactive muscles during rest and exercise as well as examining the impact of exercise on muscle metabolic activity [34, 35]. 11C-acetate PET imaging has also been used to assess muscle function recovery after intervention [36]. These unique PET imaging techniques can be very valuable tools for assessing the metabolism and viability of skeletal muscles before and after intervention in the setting of critical limb ischemia.
Assessment of Vascular Inflammation
Vascular inflammation plays a significant role in the progression of peripheral artery disease (PAD) as it often precedes the formation of atherosclerosis. FDG PET imaging using an appropriate metabolic preparation can detect early subclinical arterial inflammation by measuring FDG uptake by inflammatory cells. This approach requires the assessment of the target-to-background ratio (TBR), derived by comparing the standardized uptake value (SUVmax) in arteries to the SUVmax in the blood pool [37, 38]. Early detection of subclinical arterial inflammation can guide medical treatment, such as prescribing statins, which have been associated with reduced inflammation in the femoral artery in people with dyslipidemia [39]. Arterial inflammation, as measured by FDG PET imaging, is significantly correlated with arterial stiffness which is independently associated with PAD [37]. FDG PET imaging can also help differentiate between infection in a vascular graft and infection in the surrounding soft tissue, which can aid in the management of such difficult clinical presentations [40].
While FDG is more readily available compared to other inflammatory markers, there are several limitations associated with FDG PET imaging. FDG is not specific for inflammatory cells and can also be taken up by other active metabolic cells, leading to false positives. Moreover, the effects of blood glucose levels on FDG uptake can reduce the accuracy of the imaging results. Alternative PET radiotracers have been developed for imaging inflammation and can potentially be used to evaluate inflammation in peripheral artery disease [41]. For example, gallium-68-pentixafor (68 Ga-pentixafor) targets C-X-C motif chemokine receptor 4 (CXCR4), which is expressed on several pro-inflammatory immune cells [42, 43]. [11C]-PK11195 targets mitochondrial translocator protein (TSPO), which is expressed in activated macrophages [44, 45]. Other potential inflammatory targets include somatostatin receptors (SSTR) and fibroblast activation protein alpha (FAP). These PET imaging probes can help assess both early and advanced stages of PAD in patients with unexplained lower extremity symptoms despite normal routine screening or known abnormal results. However, more research is needed regarding the application of PET inflammation imaging in the setting of PAD.
Evaluation of Arterial Microcalcification
Prolonged inflammation can contribute to the development of arterial microcalcification and thrombosis, signifying disease progression and the need for a more aggressive treatment approach. A prospective study of 10 patients with early type 2 diabetes, but no history of cardiovascular disease, found a strong correlation between the baseline mean TBR FDG and the 5-year follow-up mean TBR 18F-sodium fluoride (18F-NaF) suggesting that systemic arterial inflammation plays a critical role in the development of systemic arterial microcalcification, which in turn affects thrombosis risk [46•].
In patients with type 2 diabetes and arterial disease, 18F-NaF uptake in femoral arteries was linked to modifiable cardiovascular risk factors, such as cholesterol, triglycerides, and HbAlc levels [47]. Controlling these factors may reduce arterial calcification. A separate study found that PAD patients with diabetes or CKD had more active microcalcification in their lower extremity arteries than those without. Statin use was shown to decrease microcalcification in the femoral-popliteal artery [48]. A recent cohort study of 50 patients underwent imaging of the superficial femoral artery using FDG and 18F-NaF before and 6 weeks after angioplasty. The study found that patients with higher baseline levels of femoral arterial inflammation and microcalcification were more likely to develop restenosis. Both 18F-FDG and 18F-NaF uptake were effective in predicting 1-year restenosis, suggesting their potential use in identifying high-risk patients and improving the management of PAD and CLI (Fig. 2) [49•].
Fig. 2.
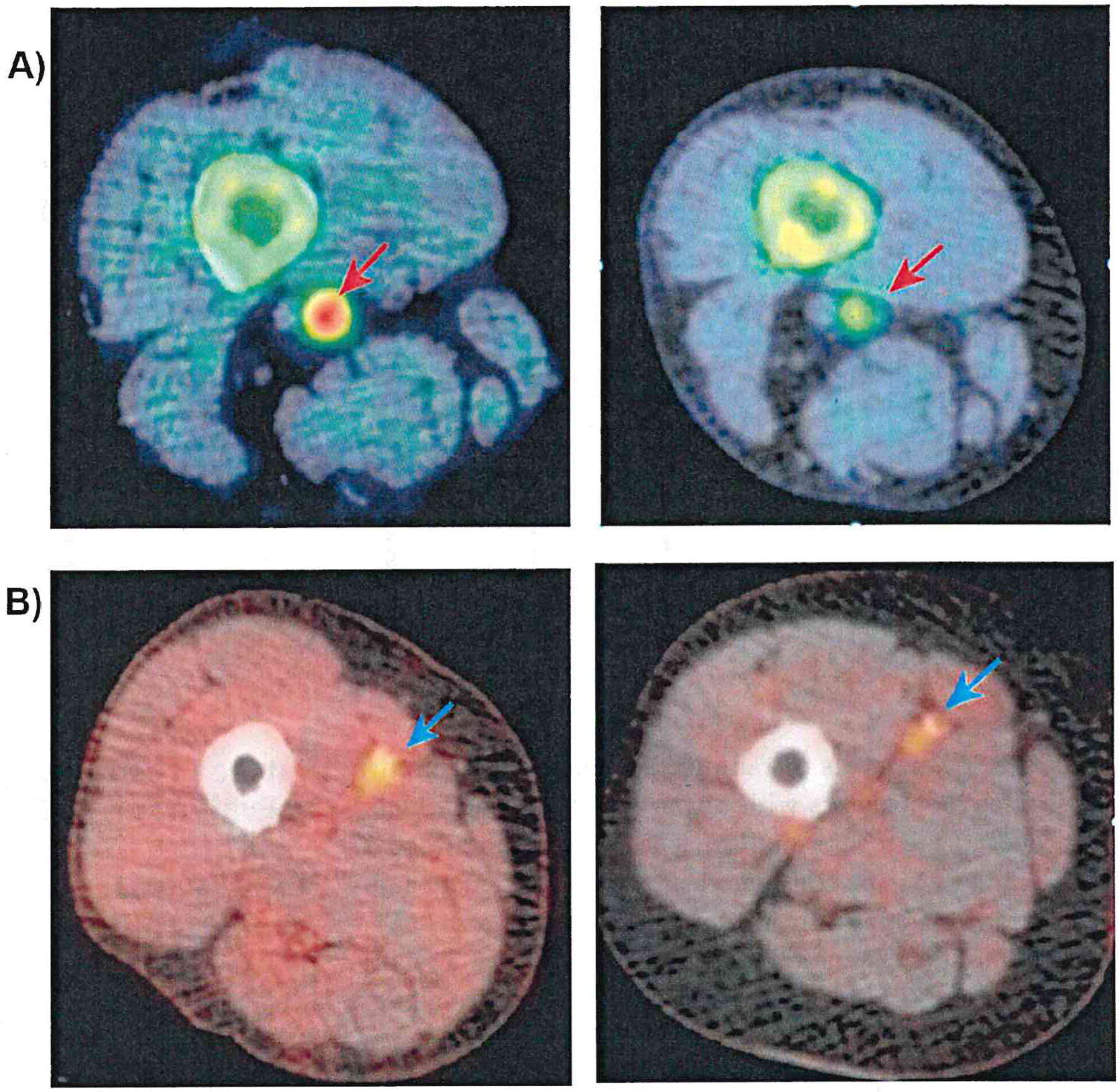
A 18F-NaF PET/CT reveals significant uptake in the superficial femoral artery at the adductor canal level (upper left, red arrow). Post-angioplasty imaging at 6 weeks shows reduced signal (upper right, red arrow) in a patient without restenosis. B The FDG PET/CT also displays notable uptake in the mid-thigh region of the superficial femoral artery (lower left, blue arrow). Persistent signal is observed at 6 weeks post-angioplasty (lower right, blue arrow) in a patient who developed restenosis (adapted from [49•])
Evaluation of Arterial Thrombosis
Other PET tracers have also been investigated for their potential to visualize different aspects of thrombosis including 68 Ga-DOTA-fibrin-binding-probe (68 Ga-FBP) which specifically targets fibrin, a major component of thrombus formation [50, 51] and 18F-GP1 which binds with the GPIIb/IIIa receptor found in activated platelets and involved in platelet aggregation during thrombus formation [52]. The use of thrombus-targeted radiotracers can help identify patients with unstable plaques and elevated thrombotic risk, guiding adjustments in their medical treatment approaches or early preventive intervention.
Assessment of Angiogenesis
Angiogenesis is a defensive mechanism in which new blood vessels are formed in response to vascular injury and tissue hypoxia. Monitoring angiogenesis can be crucial in determining the efficacy of proangiogenic therapies in diabetic and PAD patients. Various tracers have been developed and have shown efficacy in monitoring angiogenesis in preclinical models of hind limb ischemia. For instance, [18F]-RGD was used for the early detection of the proangiogenic effects of simvastatin and metformin in a diabetic murine model of the hindlimb [53]. Similarly, 64Cu-NOTA-TRC105 showed that tracer uptake in the ischemic hindlimb was significantly higher in the treatment group than in the control group on day 10, indicating increased CD105 expression and higher levels of angiogenesis upon pravastatin treatment [54]. Several other 64Cu-labeled angiogenesis-targeted tracers have been developed including one that is conjugated to RGD and target alpha(v)beta(3) integrin which plays a role in cell adhesion and endothelial cell proliferation in the early stages of angiogenesis (Fig. 3) [55–57]. Diabetic patients have impaired wound healing due to the disturbed angiogenic balance [58]; therefore, these PET tracers that target angiogenesis can help detect the degree of impairment and evaluate the response to various treatments.
Fig. 3.
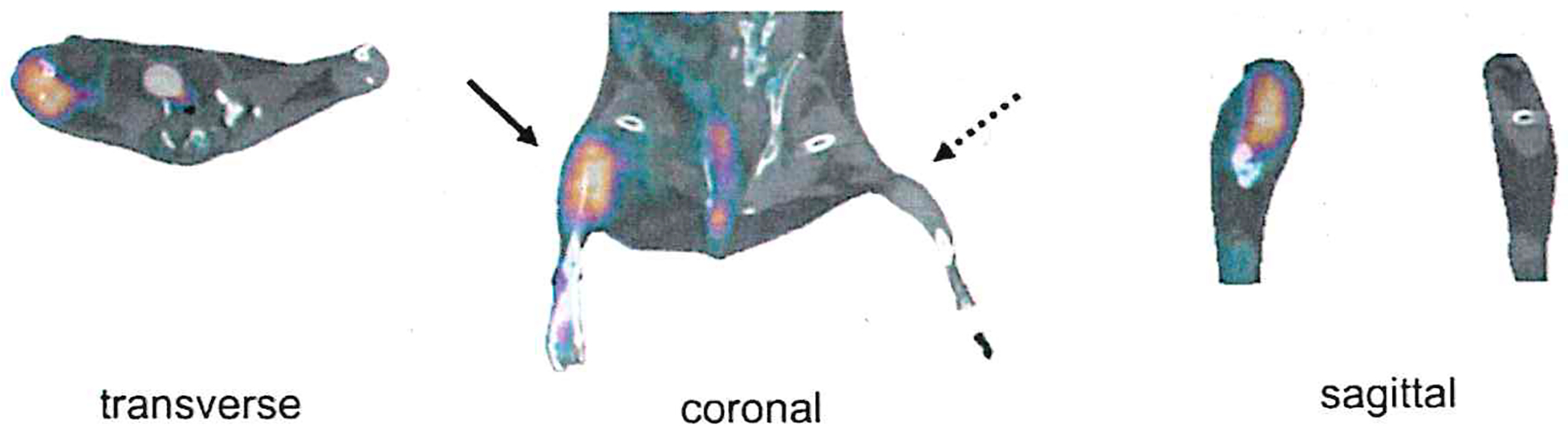
Illustrates a PET-CT imaging analysis. Specifically, it shows in vivo PET-CT images of peripheral angiogenesis 1 week post right femoral artery surgical ligation, where a significant uptake of the 64Cu-NOTA-PEG4-cRGD2 radiotracer was observed in the ischemic hindlimb after 1 h of intravascular injection (adapted from [57])
Evaluation of Sympathetic Innervation
Although sympathetic innervation has been proven to be valuable in assessing various cardiac diseases [59], sympathetic imaging may also have clinical use in evaluating lower extremity innervation in patients with diabetic neuropathy and PAD. One study used 6-(18F)-fluorodopamine to demonstrate a regional reduction in sympathetic innervation in the painful feet of patients with diabetic neuropathy in comparison to healthy individuals [60]. Other tracers, including 11C-hydroxyephedrine (11C-HED) and 18F-LMI1195, have the potential to evaluate autonomic innervation dysregulation in patients with diabetes mellitus and PAD and may provide new insight regarding the pathophysiology and progression of the disease [61, 62]. While the non-invasive evaluation of sympathetic changes in the lower extremities of patients with diabetes mellitus and PAD is currently limited, the approach has significant clinical potential.
Advancements in PET Scanner Technology
PET scanner technology has advanced significantly in recent years, with new detector technology, including digital photon counting, which allows improved sensitivity to detect lower levels of radiotracers and time of flight facilitated by scanners with faster detection time which improves image resolution [63]. The advent of total-body PET scanners can be considered a major advancement and might play an important role in the application of PET imaging for the evaluation of PAD given the large field of view required to image the whole lower extremity along with the abdomen or heart to derive a simultaneous image-derived arterial input function (Fig. 4) [64•]. Furthermore, the integration of PET with computed tomography (CT) and PET with magnetic resonance imaging (MRI) can provide valuable anatomical details that help in the assessment of PAD [65]. Current PET image acquisitions of the lower extremities include two principal methods including using a static large field-of-view (FOV) scanner versus dynamic shuttling of the scanner over the body to derive whole-body dynamic PET images. The dynamic shuttling techniques consist of acquiring multiple sequential images of smaller regions of interest that are then combined to form a larger image; this provides improved image quality but is associated with longer acquisition times and potential patient motion or discomfort [66]. The advancement in image reconstruction and the application of artificial intelligence can reduce noise, speed up reconstructions and further improve image quality and reduce imaging acquisition time.
Fig. 4.
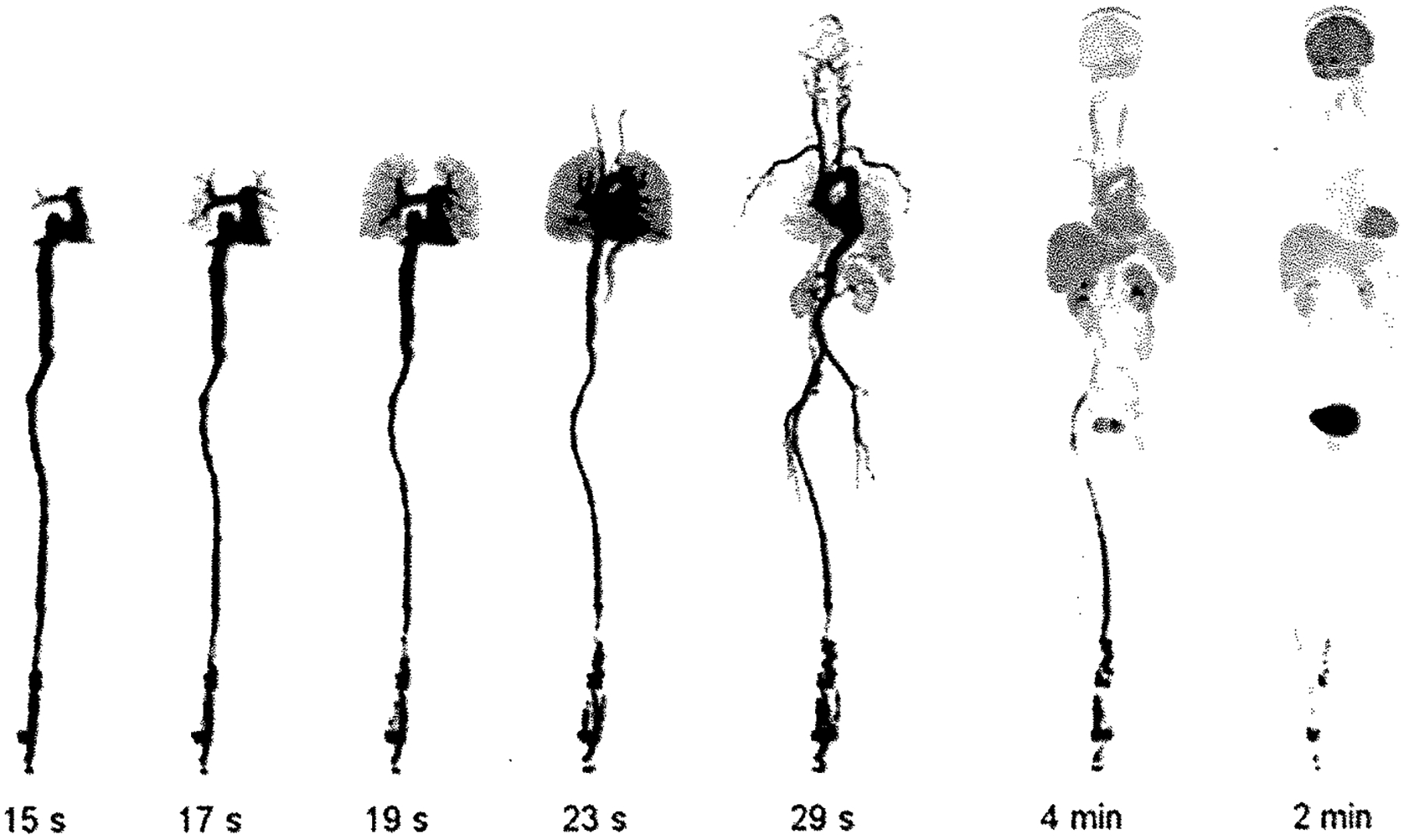
Presents selected frames from first human imaging studies with the EXPLORER total-body pet scanner taken from the rotating maximum intensity projections with most frame durations being 1 s, except for the two leftmost images, which have a 1-min frame duration. The passage of the bolus from the heart to the lungs, back to the heart, and into the arterial tree is clearly visible (adapted from [64•])
Future Prospects of PET Imaging in Evaluation of PAD
While PET imaging in a patient with PAD offers major advances in the evaluation and monitoring of this disease, PET imaging has a few limitations in comparison with other imaging technologies when it comes to assessing PAD. These limitations include the high costs, limited availability of suitable radiotracers, suboptimal spatial resolution compared to CT or MRI, and availability of PET scanners. Despite these constraints, PET imaging can serve as a valuable tool for understanding the unknown mechanism and pathophysiology of PAD, including the physiological control or modulation of blood flow in the setting of acute and/or chronic ischemia, the role of inflammation, and the autonomic dysfunction in the progression of the disease, and identifying protective factors to maintain the viability of the lower extremities. Thus, the routine application of physiological and molecular targeted PET imaging could potentially help optimize the care and the management of patients with PAD and facilitate precision and individualized patient care.
Funding
Alaa Alashi and Billy C. Vermillion report an NIH training grant (NIH-NRSA T32:HL 098069). Albert J. Sinusas reports NIH funding related to this article (R01 HL163640).
Conflict of Interest
Albert J. Sinusas reports Institutional grants from Jubilant and Siemens; individual consulting fees unrelated to PET imaging compounds from MicroVide, LLC; patents related to SPECT imaging agent RP805 for MicroVide, LLC; member Cardiovascular Council of SNMMI (no funds received); receipt of equipment, materials, drugs, or other services from Lantheus (MTA LMI1195) and Jubilant (Rb-82 generator). The other authors declare that they have no conflict of interest.
Footnotes
Human and Animal Rights and Informed Consent All reported studies and experiments involving human or animal subjects conducted by the authors mentioned in this article have been previously published and adhered to all relevant ethical standards, including the Helsinki Declaration and its amendments, institutional and national research committee norms, as well as international, national, and institutional guidelines.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet Lond Engl. 2013;382(9901): 1329–40. 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4): e38–360. 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015; 116(9): 1509–26. 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11): 1317–24. 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 5.Färber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg. 2016; 151(11): 1070–7. 10.1001/jamasurg.2016.2018. [DOI] [PubMed] [Google Scholar]
- 6.Teraa M, Conte MS, Moll FL, et al. Critical limb ischemia: current trends and future directions. J Am Heart Assoc. 2016;5(2): e002938. 10.1161/JAHA.115.002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.•.Misra S, Shishehbor MH, Takahashi EA, et al. Perfusion assessment in critical limb ischemia: principles for understanding and the development of evidence and evaluation of devices: a scientific statement from the American Heart Association. Circulation. 2019;140(12): e657–72. 10.1161/CIR.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]; This scientific statement discusses various noninvasive assessments of limb perfusion in critical limb ischemia (CLI), addressing limitations and opportunities for improvement.
- 8.Armstrong DG, Wrobel J, Robbins JM. Guest editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(4): 286–7. 10.1111/j.1742-481X.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JA, Duncan MS, Damrauer SM, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140(6): 449–58. 10.1161/CIRCULATIONAHA.119.040672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowling FL, Rashid ST, Boulton AJM. Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol. 2015; 11(10): 606–16. 10.1038/nrendo.2015.130. [DOI] [PubMed] [Google Scholar]
- 11.Howard DPJ, Banerjee A, Fairhead JF, et al. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015; 132(19): 1805–15. 10.1161/CIRCULATIONAHA.115.016424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stein R, Hriljac I, Halperin JL, et al. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vase Med Lond Engl. 2006;11(1): 29–33. 10.1191/1358863x06vm663oa. [DOI] [PubMed] [Google Scholar]
- 13.Englund EK, Langham MC, Li C, et al. Combined measurement of perfusion, venous oxygen saturation, and skeletal muscle T2* during reactive hyperemia in the leg. J Cardiovasc Magn Reson. 2013; 15(1): 70. 10.1186/1532-429X-15-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utz W, Jordan J, Niendorf T, et al. Blood oxygen level–dependent MRI of tissue oxygenation. Arterioscler Thromb Vase Biol. 2005;25(7): 1408–13. 10.1161/01.ATV.0000170131.13683.d7. [DOI] [PubMed] [Google Scholar]
- 15.Stacy MR, Qiu M, Papademetris X, et al. Application of BOLD magnetic resonance imaging for evaluating regional volumetric foot tissue oxygenation: a feasibility study in healthy volunteers. Eur J Vase Endovasc Surg Off J Eur Soc Vase Surg. 2016;51(5): 743–9. 10.1016/j.ejvs.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou T-H, Alvelo JL, Janse S, et al. Prognostic value of radiotracer-based perfusion imaging in critical limb ischemia patients undergoing lower extremity revascularization. JACC Cardiovasc Imaging. 2021; 14(8): 1614–24. 10.1016/jjcmg.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou T-H, Janse S, Sinusas AJ, et al. SPECT/CT imaging of lower extremity perfusion reserve: a non-invasive correlate to exercise tolerance and cardiovascular fitness in patients undergoing clinically indicated myocardial perfusion imaging. J Nucl Cardiol. 2020;27(6): 1923–33. 10.1007/s12350-019-02019-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burchert W, Schellong S, van den Hoff J, et al. Oxygen-15-water PET assessment of muscular blood flow in peripheral vascular disease. J Nucl Med Off Publ Soc Nucl Med. 1997;38(1):93–8. [PubMed] [Google Scholar]
- 19.Schmidt MA, Chakrabarti A, Shamim-Uzzaman Q (Afifa), et al. Calf flow reserve with H2150 PET as a quantifiable index of lower extremity flow. J Nucl Med 2003;44(6):915–9. [PubMed] [Google Scholar]
- 20.Scremin OU, Figoni SF, Norman K, et al. Preamputation evaluation of lower-limb skeletal muscle perfusion with H(2) (15)O positron emission tomography. Am J Phys Med Rehabil. 2010;89(6):473–86. 10.1097/PHM.0b013e3181d89b08. [DOI] [PubMed] [Google Scholar]
- 21.Peñuelas I, Aranguren XL, Abizanda G, et al. (13)N-ammonia PET as a measurement of hindlimb perfusion in a mouse model of peripheral artery occlusive disease. J Nucl Med Off Publ Soc Nucl Med. 2007;48(7):1216–23. 10.2967/jnumed.106.039180. [DOI] [PubMed] [Google Scholar]
- 22.Scholtens AM, Tio RA, Willemsen A, et al. Myocardial perfusion reserve compared with peripheral perfusion reserve: a [13N]ammonia PET study. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2011; 18(2):238–46. 10.1007/s12350-011-9339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Thorn S, Wu J, et al. Assessment of lower extremities flow using dynamic Rb-82 PET: acquisition protocols and quantification methods. J Nucl Med. 2021;62(supplement 1):53–53. [Google Scholar]
- 24.Moody JB, Hiller KM, Lee BC, et al. The utility of 82Rb PET for myocardial viability assessment: Comparison with perfusion-metabolism 82Rb-18F-FDG PET. J Nucl Cardiol. 2019;26(2):374–86. 10.1007/sl2350-019-01615-0. [DOI] [PubMed] [Google Scholar]
- 25.Arumugam P, Tout D, Tonge C. Myocardial perfusion scintigraphy using rubidium-82 positron emission tomography. Br Med Bull. 2013; 107(1):87–100. 10.1093/bmb/ldt026. [DOI] [PubMed] [Google Scholar]
- 26.Patel KK, Singh A, Bateman TM. The potential of F-18 flurpiridaz PET/CT myocardial perfusion imaging for precision imaging. Curr Cardiol Rep. 2022;24(8):987–94. 10.1007/s11886-022-01713-5. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest. 2005; 115(7): 1699–702. 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vase Med Lond Engl. 2000;5(1):55–9. 10.1177/1358836X0000500109. [DOI] [PubMed] [Google Scholar]
- 29.Koh H-CE, van Vliet S, Meyer GA, et al. Heterogeneity in insulin-stimulated glucose uptake among different muscle groups in healthy lean people and people with obesity. Diabetologia. 2021; 64(5): 1158–68. 10.1007/s00125-021-05383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pande RL, Park M-A, Perlstein TS, et al. Impaired skeletal muscle glucose uptake by [18F]fluorodeoxyglucose–positron emission tomography in patients with peripheral artery disease and intermittent claudication. Arterioscler Thromb Vase Biol. 2011;31 (1): 190–6. 10.1161/ATVBAHA.110.217687. [DOI] [PubMed] [Google Scholar]
- 31.Tashiro M, Fujimoto T, Itoh M, et al. 18F-FDG PET imaging of muscle activity in runners. J Nucl Med Off Publ Soc Nucl Med. 1999;40(1):70–6. [PubMed] [Google Scholar]
- 32.Pappas GP, Olcott EW, Drace JE. Imaging of skeletal muscle function using 18FDG PET: force production, activation, and metabolism. J Appl Physiol. 2001; 90(1):329–37. 10.1152/jappl.2001.90.1.329. [DOI] [PubMed] [Google Scholar]
- 33.Rudroff T, Kindred JH, Benson J-M, et al. Greater glucose uptake heterogeneity in knee muscles of old compared to young men during isometric contractions detected by [18F]-FDG PET/CT. Front Physiol. 2014;5. 10.3389/fphys.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trombella S, García D, Colin DJ, et al. [11C]acetate and PET/CT assessment of muscle activation in rat studies. Int J Comput Assist Radiol Surg. 2016;11(5):733–43. 10.1007/s11548-015-1260-8. [DOI] [PubMed] [Google Scholar]
- 35.van Hall G, Sacchetti M, Ràdegran G. Whole body and leg acetate kinetics at rest, during exercise and recovery in humans. J Physiol. 2002;542(Pt 1):263–72. 10.1113/jphysiol.2001.014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchcgger F, Ratib O, Willi J-P, et al. [11C]acetate PET/CT visualizes skeletal muscle exercise participation, impaired function, and recovery after hip arthroplasty; first results. Mol Imaging Biol. 2011; 13(4):793–9. 10.1007/sl1307-010-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Boer SA, Hovinga-de Boer MC, Heerspink HJL, et al. Arterial stiffness is positively associated with 18F-lluorodeoxyglucose positron emission tomography-assessed subclinical vascular inflammation in people with early type 2 diabetes. Diabetes Care. 2016;39(8): 1440–7. 10.2337/dcl6-0327. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Friera L, Fuster V, Lópcz-Mclgar B, et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J Am Coll Cardiol. 2019,73(12): 1371–82. 10.1016/j.jacc.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 39.Ishii H, Nishio M, Takahashi H, et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther. 2010;32(14):2337–47. 10.1016/j.clinthera.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Keidar Z, Engel A, Hoffman A, et al. Prosthetic vascular graft infection: the role of 18F-FDG PET/CT. J Nucl Med. 2007;48(8): 1230–6. 10.2967/jnumed.107.040253. [DOI] [PubMed] [Google Scholar]
- 41.Iking J, Staniszewska M, Kessler L, et al. Imaging inflammation with positron emission tomography. Biomedicines. 2021;9(2):212. 10.3390/biomedicines9020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gourni E, Demmer O, Schottelius M, et al. PET of CXCR4 expression by a (68)Ga-labeled highly specific targeted contrast agent. J Nucl Med Off Publ Soc Nucl Med. 2011;52(11):1803–10. 10.2967/jnumed.l11.098798. [DOI] [PubMed] [Google Scholar]
- 43.Thackeray JT, Derlin T, Haghikia A, et al. Molecular imaging of the chemokine receptor CXCR4 after acute myocardial infarction. JACC Cardiovasc Imaging. 2015;8(12): 1417–26. 10.1016/j.jemg.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Lamare F, Hinz R, Gaemperli O, et al. Detection and quantification of large-vessel inflammation with 11C-(R)-PK11195 PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2011;52(1):33–9. 10.2967/jnumed.110.079038. [DOI] [PubMed] [Google Scholar]
- 45.Pugliese F, Gaemperli O, Kinderlerer AR, et al. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. J Am Coll Cardiol. 2010;56(8):0653–61. 10.1016/jjacc.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 46.•.Reijrink M, de Boer SA, Te Velde-Keyzer CA, et al. [18F]FDG and [18F]NaF as PET markers of systemic atherosclerosis progression: a longitudinal descriptive imaging study in patients with type 2 diabetes mellitus. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2022;29(4): 1702–9. 10.1007/sl2350-021-02781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; There is a strong correlation baseline 18F-FDG uptake and 5-year follow-up 18F-NaF uptake suggesting that FDG could play a crucial role in the early detection of PAD.
- 47.Takx RAP, van Asperen R, Bartstra JW, et al. Determinants of 18F-NaF uptake in femoral arteries in patients with type 2 diabetes mellitus. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol. 2021; 28(6):2700–5. 10.1007/s12350-020-02099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou T-H, Rimmerman ET, Patel S, et al. Vessel-by-vessel analysis of lower extremity 18F-NaF PET/CT imaging quantifies diabetes- and chronic kidney disease-induced active microcalcification in patients with peripheral arterial disease. EJNMMI Res. 2023; 13(1):3. 10.1186/s13550-023-00951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.•.Chowdhury MM, Tarkin JM, Albaghdadi MS, et al. Vascular positron emission tomography and restenosis in symptomatic peripheral arterial disease. JACC Cardiovasc Imaging. 2020;13(4):1008–17. 10.1016/j.jcmg.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]; The uptake of both 18F-FDG and 18F-NaF at baseline and post-intervention may assist in predicting restenosis in PAD patients undergoing percutaneous transluminal angioplasty.
- 50.Oliveira BL, Blasi F, Rietz TA, et al. Multimodal molecular imaging reveals high target uptake and specificity of 111In- and 68Ga-labeled fibrin-binding probes for thrombus detection in rats. J Nucl Med Off Publ Soc Nucl Med. 2015;56(10): 1587–92. 10.2967/jnumed.115.160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliveira BL, Caravan P. Peptide-based fibrin-targeting probes for thrombus imaging. Dalton Trans Camb Engl. 2017;46(42): 14488–508. 10.1039/c7dt02634j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohrke J, Siebeneicher H, Berger M, et al. 18F-GP1, a novel PET tracer designed for high-sensitivity, low-background detection of thrombi. J Nucl Med Off Publ Soc Nucl Med. 2017;58(7):1094–9. 10.2967/jnumed.116.188896. [DOI] [PubMed] [Google Scholar]
- 53.Goggi JL, Haslop A, Boominathan R, et al. Imaging the proangiogenic effects of cardiovascular drugs in a diabetic model of limb ischemia. Contrast Media Mol Imaging. 2019;2019:2538909. 10.1155/2019/2538909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orbay H, Hong H, Koch JM, et al. Pravastatin stimulates angiogenesis in a murine hindlimb ischemia model: a positron emission tomography imaging study with 64Cu-NOTA-TRC105. Am J Transl Res. 2013;6(1):54–63. [PMC free article] [PubMed] [Google Scholar]
- 55.Wei L, Ye Y, Wadas TJ, et al. (64)Cu-labeled CB-TE2A and diamsar-conjugated RGD peptide analogs for targeting angiogenesis: comparison of their biological activity. Nucl Med Biol. 2009;36(3):277–85. 10.1016/j.nucmedbio.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Pressly ED, Abendschein DR, et al. Targeting angiogenesis using a C-type atrial natriuretic factor-conjugated nanoprobe and PET. J Nucl Med Off Publ Soc Nucl Med. 2011;52(12):1956–63. 10.2967/jnumed.111.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hedhli J, Slania SLL, Ploska A, et al. Evaluation of a dimeric-cRGD peptide for targeted PET-CT imaging of peripheral angiogenesis in diabetic mice. Sei Rep. 2018;8(1):5401. 10.1038/s41598-018-23372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng R, Ma J. Angiogenesis in diabetes and obesity. Rev Endocr Metab Disord. 2015; 16(1):67–75. 10.1007/s11154-015-9310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fallavollita JA, Heavey BM, Luisi AJ, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63(2): 141–9. 10.1016/j.jacc.2013.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tack CJ, van Gurp PJ, Holmes C, et al. Local sympathetic denervation in painful diabetic neuropathy. Diabetes. 2002;51(12):3545–53. 10.2337/diabetes.51.12.3545. [DOI] [PubMed] [Google Scholar]
- 61.Franzius C, Hermann K, Weckesser M, et al. Whole-body PET/CT with 11lC-meta-hydroxyephedrine in tumors of the sympathetic nervous system: feasibility study and comparison with 123I-MIBG SPECT/CT. J Nucl Med. 2006;47(10):1635–42. [PubMed] [Google Scholar]
- 62.Sinusas AJ, Lazewatsky J, Brunetti J, et al. Biodistribution and radiation dosimetry of LMI1195: first-in-human study of a novel 18F-labeled tracer for imaging myocardial innervation. J Nucl Med Off Publ Soc Nucl Med. 2014;55(9):1445–51. 10.2967/jnumed.l14.140137. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Maniawski P, Knopp MV. Performance evaluation of the next generation solid-state digital photon counting PET/CT system. EJNMMI Res. 2018;8(1):97. 10.1186/s13550-018-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.•.Badawi RD, Shi H, Hu P, et al. First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med. 2019;60(3):299–303. 10.2967/jnumed.l19.226498. [DOI] [PMC free article] [PubMed] [Google Scholar]; The EXPLORER, the first PET scanner that can scan the entire body, has the ability to conduc tcomprehensive pharmacokinetic studies, which could potentially aid in the assessment of peripheral artery disease.
- 65.Beyer T, Townsend DW, Czernin J, et al. The future of hybrid imaging—part 2: PET/CT. Insights Imaging. 2011;2(3):225–34. 10.1007/s13244-011-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahmim A, Lodge MA, Karakatsanis NA, et al. Dynamic whole-body PET imaging: principles, potentials and applications. Eur J Nucl Med Mol Imaging. 2019;46(2):501–18. 10.1007/s00259-018-4153-6. [DOI] [PubMed] [Google Scholar]


