Abstract
Study objective:
We sought to determine changes in continuous mean and systolic blood pressure and heart rate in Blood pressure a cohort of non-cardiac surgical patients recovering on the postoperative ward. Furthermore, we estimated the Continuous proportion of vital signs changes that would remain undetected with intermittent vital signs checks.
Design:
Retrospective cohort.
Setting:
Post-operative general ward.
Patients:
14,623 adults recovering from non-cardiac surgical procedures.
Interventions & measurements:
Using a wireless, noninvasive monitor, we recorded postoperative blood pressure and heart rate at 15-s intervals and encouraged nursing intervention as clinically indicated.
Main results:
7% of our cohort of 14,623 patients spent >15 sustained minutes with a MAP <65 mmHg, and 23% had MAP <75 mmHg for 15 sustained minutes. Hypertension was more common, with 67% of patients spending at least 60 sustained minutes with MAP >110 mmHg. Systolic pressures <90 mmHg were present for 15 sustained minutes in about a fifth of all patients, and 40% of patients had pressures >160 mmHg sustained for 30 min. 40% of patients were tachycardic with heart rates >100 beats/min for at least continuous 15 min and 15% of patients were bradycardic at a threshold of <50 beats/min for 5 sustained minutes. Conventional vital sign assessments at 4-h intervals would have missed 54% of mean pressure episodes <65 mmHg sustained >15 min, 20% of episodes of mean pressures >130 mmHg sustained >30 min, 36% of episodes of heart rate > 120 beats/ min sustained <10 min, and 68% of episodes of heart rate sustained <40 beats per minute for >3 min.
Conclusions:
Substantial hemodynamic disturbances persisted despite implementing continuous portable ward monitoring coupled with nursing alarms and interventions. A significant proportion of these changes would have gone undetected using traditional intermittent monitoring. Better understanding of effective responses to alarms and appropriate interventions on hospital wards remains necessary.
Keywords: Blood pressure, Continuous, Heart rate, Monitoring, Vital signs
1. Introduction
Intraoperative mortality is now so rare that it is now hard to quantify [1,2]. In contrast, 30-day mortality after noncardiac surgery for inpatients over 45 years ranges between 0.5 and 4% [3–7]. About 70% of postoperative deaths occur during the initial hospitalization, that is under direct medical care in our highest-level healthcare facilities. About half of all adverse events in hospitalized patients occur in hospital wards rather than in higher-acuity units [8–10]. Major bleeding, myocardial injury, and sepsis, together account for about half of all in-hospital deaths [11]. Intraoperative and postoperative hypotension are associated with myocardial and renal injury, and with death [12,13–16]. Postoperative hypertension and tachycardia are also associated with myocardial injury, stroke, and bleeding risk [8,17,18].
Most acute cardiorespiratory events do not occur suddenly, instead are preceded by hours of progressively worsening vital signs [19]. The difficulty is that many disturbances are missed when vital signs are only assessed at standard 4–6-h intervals as is typical. Consequently, postoperative blood pressure and heart rate perturbations are often sustained for long periods without recognition [20]. However, the extent to which postoperative patients experience vital sign abnormalities, and the extent to which they are missed with conventional monitoring remains unclear because previous studies have been small, restricted to selected populations, and sometimes blinded clinicians to supplemental monitoring [20,21–23].
The current study evaluated continuous ward vital signs to determine the incidence and severity of abnormalities in a broad general surgical population. Specifically, we describe the incidence and severity of blood pressure (defined as changes in mean arterial pressure and systolic pressure) and heart rate abnormalities based on pre-defined thresholds in patients recovering from noncardiac surgery in routine hospital wards. We also estimated the proportion of patients who experienced potentially meaningful hemodynamic disturbances that would not have been detected by intermittent vital signs assessments at 4-hour intervals.
2. Methods
With institutional board review approval (IRB 00065685) and waived consent, we obtained data from adults who had noncardiac surgery with general anesthesia and were subsequently admitted to conventional surgical wards between January 1, 2016, to October 31, 2019 at the Atrium Health Wake Forest Baptist Medical Center. (Fig. 1) Patients in our surgical wards are continuously monitored with untethered ViSi units (Sotera Wireless, Inc. San Diego, California) that continuously record saturation, heart rate, and noninvasive blood pressure. Continuous monitoring is instituted at the time of ward admission and sustained until discharge, transfer to a higher level of care, or death.
Fig. 1.
Consort chart.
The ViSi Mobile System is a portable wrist-mounted system that is cleared by the United States Food and Drug Administration. The system includes a 3- or 5-lead electrocardiogram and an oscillometric blood pressure monitor, which is used to calibrate the continuous noninvasive blood pressure monitor at least once daily. Continuous blood pressure is estimated from pulse arrival time, specifically the time that elapses between R wave being detected and arrival of the resulting pulse at the SpO2 finger sensor. According to the manufacturer, the estimated maximal mean error is 5 mmHg with a standard deviation ≤8 mmHg.
ViSi monitors were calibrated at least daily and connected to the hospital’s wireless network. Vital signs abnormalities exceeding established thresholds for the local hospital system (Supplementary Table 1) generated alerts that were distributed to a central station and to the nurses’ hospital-supplied phones (ASCOM, Morrisville, NC). Alarms that were not addressed by the primary nurses escalated to other floor nurses, and thereafter to the unit manager. This system has been in place several years in our hospital system and has been organized to improve workflow and outcomes [24,25].
2.1. Data analysis
Data were collected prospectively per hospital procedure and were retrieved retrospectively from the ViSi data cloud. We excluded patients who had <12 h of continuous monitoring, and those who had monitoring gaps exceeding 4 h, or who had vital signs missing for >30% of their total ward monitoring period [20]. (Fig. 1).
We summarized the following clinical outcomes across a range of potential harm thresholds:
Hypotension defined by mean arterial pressures <80, <75, <70, and < 65 mmHg and a systolic pressure of <90, and < 80 mmHg;
Hypertension defined by mean arterial pressures >110, >115, >120, >125 mmHg and a systolic pressure of >140,160, and 180 mmHg;
Tachycardia defined by heart rates >100, >110, and > 120 bpm;
Bradycardia defined by heart rates <50, and < 45 bpm.
2.2. Definition of events
We estimated the fraction of patients who spent various percentages of time beyond each pre-defined potential harm threshold. The percentage of time spent beyond a given threshold was calculated as (time spent beyond threshold) / (total time followed - missing time). That is, the fraction of the continuous monitoring period during which values passed a given threshold.
Next, we summarized the incidence of episodic events for each clinical outcome defined by various potential harm thresholds. More specifically, we defined episodic events to be a sustained periods spent beyond defined thresholds. Episodic events were deemed to have ended after 1 min of recovery. In sensitivity analyses, we considered the effect of requiring 2 and 3 min of recovery.
Monitoring gaps within an episodic event lasting <5 min were considered to be part of the sustained duration episode when both the last observed measurement before the gap started and the first observed measurement after the gap exceeded the designated threshold. When gaps during an episodic event exceeded 5 min, the duration of an event was considered to have ended when the gap started.
We simulated traditional intermittent assessment of patient vital signs by assessing 1-min snapshots every 4 h. Each 1-min snapshot consisted of 4 measurements at 15-s intervals. Analysis was based on all four individual values, with any value exceeding a threshold being considered a deviation for estimating the intermittent detection rate and proportion detected for hypotension, hypertension, tachycardia, and bradycardia. Specifically, we estimated the fraction of episodes defined by continuous monitoring that would have also been detected by intermittent routine vital sign assessments at 4-h intervals. Sustained episodes of hypotension were identified as longest contiguous periods of time of 15min or more of hypotension with MAP <65 mmHg, 30 min or more of contiguous hypertension with MAP >130 mmHg, 10 min or more of tachycardia with heart rate > 120 bpm, and 3 min or more of bradycardia with heart rate < 40 bpm. As an exploratory analysis we also assessed blood pressure thresholds (MAP <75 mmHg and MAP>130 mmHg) and heart rate thresholds (HR < 50/min and HR > 110/min) each for a continuous 15 min and quantitated clinically relevant outcomes of hospital length of stay (LOS), ICU admission, ICU LOS, RRT calls, in-hospital mortality and ICU mortality for all of these thresholds. We also reported these outcomes on patients who had missing monitoring data or where continuous monitoring was not done. In addition, we determined the association of baseline patient characteristics, including demographics and comorbid illness with duration of episodes, using a linear mixed effects model nested within patients via random intercepts. Here we fitted 4 linear mixed effects models, one for each outcome: hypertension, hypotension, tachycardia, and bradycardia. For each outcome, we modeled length of time of episode duration for MAP >130 mmHg, MAP <65 mmHg, HR > 120/min, and HR < 40/min each as a continuous variable. Episodes needed to be at least 2 continuous minutes with MAP >130 mmHg, MAP <65 mmHg, or HR >120/min, or at least 1 min of HR <40/min, to be included. Included as main fixed effects were, time since monitoring began (in hours), age, sex, ethnicity, race, length of stay in days, CCI, hypertension, diabetes, CKD, COPD, AFIB, stroke, MI, end stage renal failure, and surgery duration, nested within patient via random intercepts.
Analyses were performed using R statistical software (version 4.10, Vienna, Austria). Continuous variables are reported as means, standard deviations, medians, and 25th and 75th percentiles. Categorical variables are reported as frequencies and percentages. Finally, we estimated the proportion of patients who experience episodic clinical events of interest using exact 95% binomial confidence intervals.
3. Results
Among 28,108 continuously monitored noncardiac surgical patients, 13,485 were excluded because they had total monitoring time < 12 h and had gaps in monitoring >4 h, and/or had >30% of missing monitoring time. 14,623 patients did have qualifying records based on monitoring time of at least 12 h and with gaps in monitoring <4 h, and/or <30% of monitoring time. (Fig. 1) Patient characteristics and types of surgeries for the included and excluded sample are shown in Table 1. Excluded patients had a longer duration of surgery, length of stay and a higher ASA score. For the cohort without missing data that was included in the analysis, mean patient age was 58 years, about 54% of patients were ASA 3, another 10% were ASA 4 and mean Charlson Comorbidity Index (CCI) was 3.0. At least 70% of our patients had surgery that lasted >2 h and among them general surgery, orthopedics, neurosurgery, and urology were the most common. Data were right-skewed, with a median duration of continuous monitoring time of 38 [interquartile range: 20, 76] hours.
Table 1.
Baseline characteristics of Study Cohort.
| Total Patients (n) | 28,108 | |
|---|---|---|
|
|
|
|
| Characteristics | Patients with gaps in monitoring <4 h and or <30% of monitoring time and with at least 12 h or more of monitoring data | Patients with gaps in monitoring >4 h and or >30% of monitoring time and with <12 h of monitoring data |
| Excluded/Included in Analysis | Included in Analysis | Excluded from Analysis |
| n (%) | 14,623 (52) | 13,485 (48) |
| Gender, Male, n (%) | 7095 (48.5) | 6938 (51.4) |
| Age, mean (SD) | 57.23 (16.23) | 57.77 (16.52) |
| ASA-PS status, n (%) | ||
| 1 | 271 (1.9) | 285 (2.1) |
| 2 | 4797 (32.8) | 2847 (21.1) |
| 3 | 7874 (53.8) | 8085 (60) |
| 4 | 1563 (10.7) | 2034 (15.1) |
| 5 | 118 (0.8) | 234 (1.7) |
| Race/Ethnicity, n (%) | ||
| Black | 3184 (21.8) | 2138 (15.9) |
| Black Hispanic/Latino | 204 (1.4) | 142 (1.1) |
| Hispanic/Latino | 569 (3.9) | 292 (2.2) |
| White Hispanic/Latino | 66 (0.5) | 74 (0.5) |
| White | 10,182 (69.6) | 10,417 (77.2) |
| Other | 380 (2.6) | 399 (3) |
| Unknown | 38 (0.3) | 23 (0.2) |
| LOS in days, median [IQR] | 2.25 [1.27–4.78] | 3.34 [2.11–5.89] |
| CCI, mean (SD) | 2.96 (3.42) | 3.03 (3.49) |
| Hypertension, n (%) | 5352 (36.6) | 5996 (44.5) |
| Diabetes, n (%) | 2295 (15.7) | 2360 (17.5) |
| Chronic Kidney Disease, n (%) | 1784 (12.2) | 1819 (13.5) |
| COPD, n (%) | 1374 (9.4) | 1503 (11.1) |
| Atrial fibrillation, n (%) | 1287 (8.8) | 1294 (9.6) |
| Stroke, n (%) | 672 (4.6) | 592 (4.4) |
| Myocardial infarction, n (%) | 1140 (7.8) | 1184 (8.8) |
| End Stage Renal Disease, n (%) | 277 (1.9) | 270 (2) |
| Surgical Duration, n (%) | ||
| <1 h | 1887 (12.9) | 609 (4.5) |
| 1–2 Hours | 2654 (18.15) | 1682 (12.5) |
| >2 Hours | 10,082 (68.95) | 11,194 (83) |
| Admitting Service, n (%) | ||
| General Surgery | 3539 (24.2) | 3298 (24.46) |
| Neurosurgery | 1357 (9.28) | 1592 (11.8) |
| Orthopedic Surgery | 4144 (28.34) | 3926 (29.1) |
| Urology | 1548 (10.59) | 1238 (9.18) |
| ENT | 1043 (7.13) | 925 (6.9) |
| Others | 2992 (20.44) | 2506 (18.56) |
3.1. Blood pressure
Mean MAP for the entire included cohort was 102 mmHg (SD = 11.2) and median was 101 mmHg with a minimum of 64.3 mmHg and a maximum of 147 mmHg. (Supplemental Table 5). About 20% of our patients spent at least 10% of their monitoring time with a MAP <80 mmHg. Slightly fewer than 10% of patients spent at least 10% of their time with MAP <75 mmHg, and fewer than 5% of patients spent at least 5% of time with MAP <70 mmHg (Fig. 2A). About a fifth of our patients had a MAP <70 mmHg for at least 15 sustained minutes, whereas another 7% of patients spent at least 15 sustained minutes with MAP <65 mmHg (Fig. 2B).
Fig. 2.
(A): Percent of time spent under various MAP thresholds. Each line represents Y% of patients (Y-axis) that spent X% of time (X-axis) under the specified line threshold given by the MAP color. (B): Percent of patients below a range of MAP thresholds by maximum continuous or sustained time spent under each threshold. Each line represents Y% of patients (Y-axis) that spent X threshold of MAP (X-axis) under the specified levels given by sustained time in various colors.
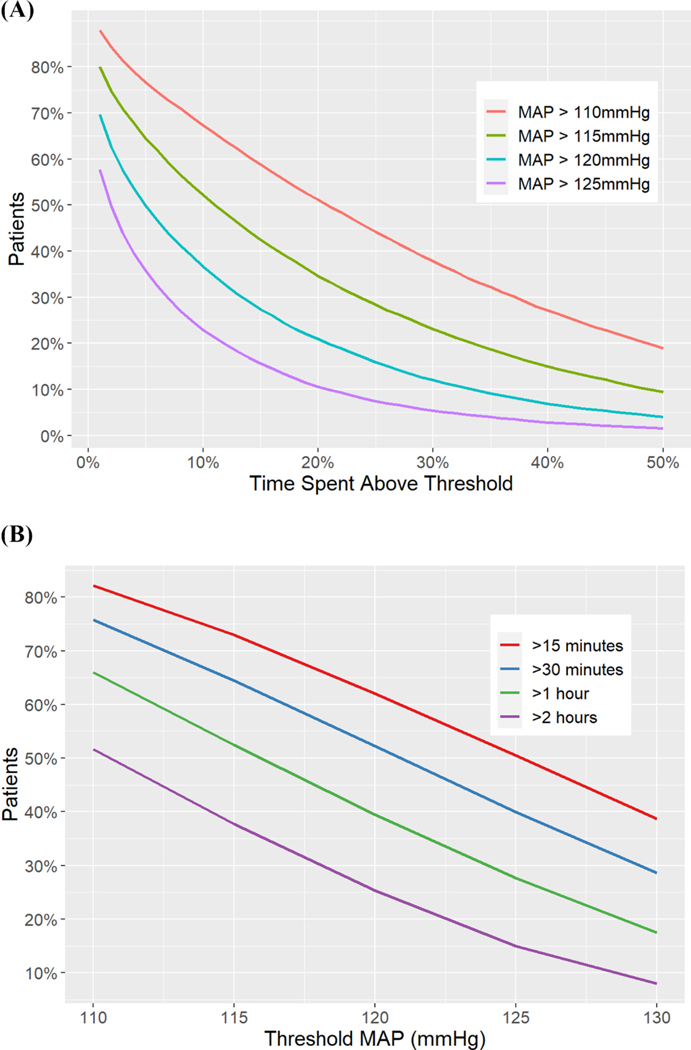
Hypertension was more common than hypotension, with approximately half of all patients spending at least a fifth of monitoring time with a MAP exceeding 110 mmHg. About 20% of patients spent 10% of their time with a MAP >125 mmHg (Fig. 3A). A fifth of patients spent at least 60 continuous minutes with a MAP >130 mmHg and nearly 67% of patients spent a sustained hour of time with a MAP >110 mmHg (Fig. 3B).
Fig. 3.
(A): Percent of time spent above various MAP thresholds. Each line represents Y% of patients (Y-axis) that spent X% of time (X-axis) above the specified line threshold given by the MAP color. (B): Percent of patients above a range of MAP thresholds by maximum continuous or sustained time spent above each threshold. Each line represents Y% of patients (Y-axis) that spent X threshold of MAP (X-axis) above the specified levels given by sustained time in various colors.
Systolic pressures are used more commonly than a MAP to titrate therapeutics on the hospital wards. We therefore also considered systolic blood pressures and found similar results. About 5% of patients had at least 10% of total monitoring time at a systolic pressure of <90 mmHg, and a systolic pressure of >180 mmHg. Looking at continuous durations of time, pressures <90 mmHg were present for 15 sustained minutes in about a fifth of all patients, and in 40% of patients with a sustained 30 min of systolic pressure >160 mmHg (Supplemental Figs. 1, 2, 3, and 4).
3.2. Heart rate
Mean heart rate for the entire included cohort was 78.9/min (SD = 12) while median heart rate was 78 with a minimum of 42.6/min and a maximum of 135/min. (Supplemental Table 5) About 15% of patients spent a fifth of their time with a heart rate exceeding 100 beats/min (Fig. 4A), but only 2% of patients spent as much time at heart rates >120 beats/min. Just over 45% of patients had sustained heart rates >100 beats/min, and 10% patients had sustained rates >120 beats/min for >15 min (Fig. 4B).
Fig. 4.
(A): Percent of time spent above various heart rate (HR) thresholds. Each line represents Y% of patients (Y-axis) that spent X% of time (X-axis) above the specified line threshold given by the HR color. (B): Percent of patients above a range of heart rate (HR) thresholds by maximum continuous or sustained time spent above each threshold. Each line represents Y% of patients (Y-axis) that spent X threshold of HR (X-axis) above the specified levels given by sustained time in various colors.
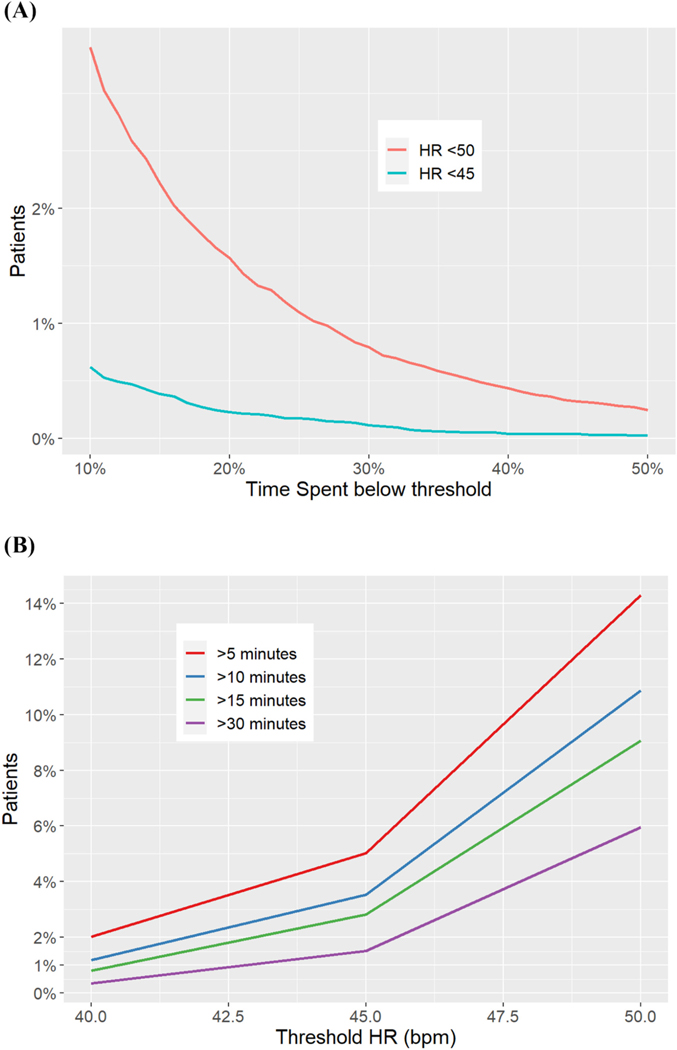
Sustained bradycardia was uncommon in our population. About 2% of patients had heart rates <50 beats/min, and < 1% of the population had rates <45 beats/min for at least 15% of total monitoring time (Fig. 5A). About 15% of patients had heart rates <50 beats/min for a sustained 5 min while that dropped to 5% of patients at a threshold of <45 beats/min for 5 min (Fig. 5B).
Fig. 5.
(A): Percent of time spent under various heart rate (HR) thresholds. Each line represents Y% of patients (Y-axis) that spent X% of time (X-axis) under the specified line threshold given by the HR color. (B): Percent of patients below a range of heart rate (HR) thresholds by maximum continuous or sustained time spent under each threshold. Each line represents Y% of patients (Y-axis) that spent X threshold of HR (X-axis) under the specified levels given by sustained time in various colors.
3.3. Intermittent monitoring detection rates
Across the varying thresholds used to define clinical conditions, we found that intermittent assessments at 4-h intervals would have failed to capture many patients who experienced clinically meaningful sustained periods beyond a potential harm threshold (Table 2). For example, among patients with hypotension, only about 45% would have had an observed MAP <65 mmHg in at least one of their 4-h intermittent assessments. In other words, about half of patients with hypotensive episodes defined by MAP <65 would have been missed by intermittent assessments. Hypertension was more likely to be detected than hypotension, and about 80% of patients would have been detected through intermittent assessments every 4 h. Tachycardia would have gone undetected in about 35% of patients, and bradycardia would have been undetected in nearly 70% of the patient cohort. A full description of these estimated detection rates is provided in Table 2.
Table 2.
Proportion of episodes detected by continuous monitoring that would have been captured by intermittent monitoring.
| Number of Patients with at Least 1 Episode(s) Detected by Continuous Monitoring (%) | Intermittent Monitoring Detection Rate | Proportion (%) Detected with Intermittent Monitoring (95% CI) | |
|---|---|---|---|
| Hypotension for > 15 min (mmHg) | |||
| MAP <80 | 5897 (38.11%) | 4509 / 5897 | 76.5% (75.4%, 77.5%) |
| MAP <75 | 3630 (23.46%) | 2445 / 3630 | 67.4% (65.8%, 68.9%) |
| MAP <70 | 1798 (11.62%) | 1002 / 1798 | 55.7% (53.4%, 58%) |
| MAP <65 | 676 (4.37%) | 308 / 676 | 45.6% (41.8%, 49.3%) |
| MAP <60 | 140 (0.9%) | 43 / 140 | 30.7% (23.7%, 38.8%) |
| MAP <55 | 27 (0.17%) | 11/27 | 40.7% (24.5%, 59.3%) |
| MAP <50 | 1 (0.01%) | 0 / 1 | 0% (0%, 94.9%) |
| Hypertension for > 30 min (mmHg) | |||
| MAP >110 | 10,371 (67.02%) | 9810 / 10,371 | 94.6% (94.1%, 95%) |
| MAP >115 | 8414 (54.38%) | 7769 / 8414 | 92.3% (91.7%, 92.9%) |
| MAP >120 | 6482 (41.89%) | 5795 / 6482 | 89.4% (88.6%, 90.1%) |
| MAP >125 | 4660 (30.12%) | 3986 / 4660 | 85.5% (84.5%, 86.5%) |
| MAP >130 | 3172 (20.5%) | 2551 / 3172 | 80.4% (79%, 81.8%) |
| MAP >135 | 1957 (12.65%) | 1479 / 1957 | 75.6% (73.6%, 77.4%) |
| MAP >140 | 1120 (7.24%) | 779 / 1120 | 69.6% (66.8%, 72.2%) |
| Tachycardia for > 10 min (beats/min) | |||
| HR > 100 | 6268 (40.51%) | 5557 / 6268 | 88.7% (87.8%, 89.4%) |
| HR > 110 | 3169 (20.48%) | 2485 / 3169 | 78.4% (76.9%, 79.8%) |
| HR > 120 | 1256 (8.12%) | 807 / 1256 | 64.3% (61.6%, 66.9%) |
| HR > 140 | 179 (1.16%) | 83 / 179 | 46.4% (39.2%, 53.7%) |
| HR > 160 | 30 (0.19%) | 13 / 30 | 43.3% (27.4%, 60.8%) |
| Bradycardia for > 3 min (beats/min) | |||
| HR < 50 | 1975 (12.76%) | 1111 / 1975 | 56.3% (54.1%, 58.4%) |
| HR < 45 | 671 (4.34%) | 292 / 671 | 43.5% (39.8%, 47.3%) |
| HR < 40 | 258 (1.67%) | 85 / 258 | 32.9% (27.5%, 38.9%) |
| HR < 35 | 149 (0.96%) | 39 / 149 | 26.2% (19.8%, 33.8%) |
| HR < 30 | 99 (0.64%) | 24 / 99 | 24.2% (16.9%, 33.5%) |
3.4. Sensitivity analysis
We assessed whether our results were consistent when the recovery of clinical event occurred at 2 min and 3 min as the required length of time for defining the end of a single event, compared with our standard definition of 1 min separation to define events (Supplementary Tables 2 and 3). Results were consistent with those from our main analysis.
3.5. Exploratory analysis
Assessing hemodynamic thresholds (MAP <75 mmHg and MAP>130 mmHg) and heart rate thresholds (HR < 50/min and HR > 110/min) each for a continuous 15 min, we saw that hospital length of stay was longer, ICU admission rate was higher at these thresholds compared to the entire continuously monitored cohort. (Supplemental Table 4) When compared with those that were excluded from the final analysis because they had incomplete monitoring data or were not monitored at all, in-hospital and ICU mortality was higher than in those patients who were continuously monitored and stayed 15 min at these critical thresholds. (Supplemental Table 4).
In the analyses to determine the effects of baseline patient characteristics with duration of episodes of hemodynamic disturbances (Supplemental Tables 6–9), we saw that sex and age were both significantly related to length of hypotensive episodes, such that males experience hypotensive episodes 1.42 min less than females (95% CI: −0.72, −2.13), and each additional 10 year’s in age was associated with a 0.38 min (22.8 s) decrease in hypotensive episode length (95% CI: 0.123, 0.65). Time since admission was significantly associated with a slight decrease in hypertension episode length, such that for every additional 10 h since admission time, the length of hypertension episodes decreases of 0.09 min. Additionally, patients of black race had longer hypertensive episodes by 0.88 min relative to white patients. Patients without noted hypertension had shorter hypertensive episodes by 0.59 min (95% CI: 0.08, 1.10), patients without COPD had longer hypertensive episodes by 0.83 min (95% CI: 0.11, 1.55), and patients without end stage renal disease had shorter hypertensive episodes by 1.85 min (95% CI: 0.40, 3.30). Time since admission was associated a decrease in length of tachycardia episodes, such that for each additional 10 h sinces admission, tachycardia episodes decreased by 0.25 min (95% CI: 022, 0.28). Patients without atrial fibrillation had shorter tachycardia episodes by 9.75 min (95% CI: 4.02, 15.48). For each additional 10 years in age of a patient, bradycardia episode time increased by 0. 263 min (95% CI: 0.03, 0.49). Patients of unknown ethnicity had longer bradycardia times than patients of Hispanic race. Patients without chronic kidney disease had lower bradycardia times by 0.94 min (95% CI: 0.04, 1.84).
4. Discussion
We evaluated nearly 15,000 largely unselected adults recovering from noncardiac surgery on general surgical wards. Our large population, about 30 times the size of previous continuous ward monitoring studies, presumably resulted in precise estimates of ward hemodynamic disturbances. Blood pressure and heart rate abnormalities were common and often persistent. For example, about 23% of patients had MAP <75 mmHg for at least 15 sustained minutes, 67% of patients spent at least 60 min with a MAP >110 mmHg, and 40% of patients were tachycardic with heart rates >100 beats/min sustained for at least 15 min.
Turan and colleagues used continuous monitoring in about 500 patients and reported that about 20% of their patients experienced mean pressures <65 mmHg for >15 min [20]. Another study conducted in a high-dependency postoperative ward reported that about half their patients experienced MAP <65 mmHg for 15 cumulative minutes [26]. Both estimates far exceeded the 7% incidence in our patients. However, our patients were essentially unselected, whereas, patients in many previous studies were required to have major and prolonged surgery. Nonetheless, the mean age of our patients was nearly 60 years, and three-quarters of our cohort was designated as American Society of Anesthesiologists physical status ≥3 or greater and > 80% had surgery lasting >2 h. Our results thus apply to major tertiary centers that primarily care for high-risk patients having major surgery. Presumably, ward vital sign abnormalities are considerably less common in more typical surgical settings. The extent to which vital sign abnormalities occur after discharge in ambulatory surgical patients is essentially unknown.
Possibly a more important explanation for less severe postoperative hemodynamic perturbations is that previous studies were clinician-blind whereas continuous monitoring values were available to our clinicians and were coupled to threshold-based alarms to encourage indicated corrective interventions. Presumably, this strategy reduced the duration and severity of vital sign perturbations, especially at extremes of blood pressure and heart rate. It thus seems likely that unblinded monitoring with alarm-based interventions was the major factor contributing to fewer and shorter hemodynamic perturbations in our patients than in previous reports [20,26].
Vital signs in postoperative patients on hospital wards are generally assessed intermittently at 4–6-h intervals. Most catastrophic postoperative events on the wards are preceded by several hours of subtle changes in cardio-respiratory parameters [19]. Changes in blood pressure and heart rate are therefore missed in a large fraction of patients using conventional intermittent monitoring standards. Turan and colleagues reported that at least half of their patients with a mean pressure < 65 mmHg for 15 continuous minutes were undetected with intermittent vital sign monitoring. Consistent with these results, we report that that nearly half of all monitoring sessions, with mean pressures <65 mmHg and a fifth with >130 mmHg would have been missed by intermittent assessments at 4-h intervals and presumably even more would be missed with less frequent monitoring. Undetected changes in vital signs patterns may lead to significant complications. A significant association of postoperative hypotension with myocardial injury and adverse events has been previously reported [12,13,26]. A study of >2000 post-surgical ward patients found that unplanned ICU transfers and rapid response calls were preceded by a heart rate alarm and systolic pressure alarm in nearly a fifth of all patients and a mean pressure alarm in about 10%. Implementation of continuous ward monitoring led to a significant decrease in both serious events [27].
Postoperative hypotension is associated with myocardial [12,26] and kidney injury [13], delirium [28], and mortality [13]. Similarly, postoperative systolic pressures >200 mmHg and sustained tachycardia >100 beats/min increase the risk of surgical bleeding [29], unplanned intensive care admissions, and mortality [30]. The extent to which postoperative vital sign abnormalities are causally related to outcomes remains unknown. However, patients with abnormalities are at the very least fragile and have limited reserve for dealing with acute events.
A limitation of our analysis is that data were collected over six years in a medical center where continuous monitoring was introduced in incremental steps. Thus, while all patients we report had continuous monitoring, the fraction of all patients who were monitored increased over time, and presumably nurses comfort with the system did as well. Cultural acceptability of continuous monitoring presumably also changed over time. The relative effects of nursing willingness to act on alerts versus alarm fatigue presumably changed over time and would have been challenging to factor into our analysis but could substantially alter the results. We did attempt to quantify monitoring type and hemodynamic disturbances and report clinically significant outcomes such as mortality and transfers to ICU. These analyses were merely descriptive and showed some signal that lack of continuous monitoring led to poor outcomes, as did patients who were at extremes of hemodynamic thresholds for extended periods of time. In addition, we described and summarized population characteristics of blood pressure (both as SBP and MAP) and heart rate in this very large sample of patients, however, we did not have data on the absolute maximum decrease that included a minimum blood pressure threshold for these patients. This descriptor may have been more informative, though we did not see a lot of clinically significant hypotension to those low values. Even though we described both blood pressure and heart rate we did not do a planned analysis to look at the distribution of the common occurrence of overlapping times of low blood pressure with bradycardia and higher blood pressure with tachycardia. We had to exclude about half of our patients for missing data. Our excluded sample demographic did have similar characteristics to those included except for a very small increase in those who rated at an ASA score > 3, longer duration of surgery, and in hospital length of stay. However, excluded patients did have a higher ICU and in-hospital mortality than those patients who were in the final analysis. It is possible that excluded patients who were sicker, may have had more hemodynamic disturbances; however, there were missing data since they were away from the hospital floor for periods of time for various diagnostic or therapeutic procedures. This being a report on continuous ward monitoring, we used strict criteria for missing data and believe that our included cohort of >14,000 patients appropriately describes the true patterns of blood pressure and heart rate changes in an environment of implemented continuous vital signs monitoring. We did not differentiate patients having small operations from high-risk patients having complex surgeries, who would presumably demonstrate more hemodynamic aberrations, nor did we use comparisons to baseline blood pressure or heart rate. We did use a combination of baseline and surgical characteristics in a linear mixed effect model and our results much like other previous work indicates that postoperative hemodynamic abnormalities cannot be predicted from baseline characteristics and type of surgery [31,32]. The ViSi mobile continuous monitoring device has not been validated against the arterial line gold-standard, though it has been seen to accurate when compared to the traditional non-invasive blood pressure cuff. [23] Finally, while we know that continuous vital signs alarms were activated and used, we do not have precise details on what clinical interventions were applied in response to alarms or what fraction of alarms were acted upon in a timely manner.
In summary, we observed substantial hemodynamic disturbances despite implementing continuous portable ward monitoring coupled with nursing alarms and interventions. A large fraction of potentially serious disturbances would have gone undetected using traditional intermittent monitoring. Better understanding of effective responses to alarms and appropriate interventions on hospital wards remains necessary. It is plausible that early detection of ward hemodynamic disturbances, combined with effective interventions, will reduce serious postoperative complications.
Supplementary Material
HIGH LIGHTS.
Most postoperative vital signs on the general care floor (hospital ward) are monitored every 4–6 hours.
Spot-check of vitals do not allow for early intervention and correction of changes in cardiorespiratory parameters.
We report every 15 seconds data from post-operative hospital ward patients using continuous, wireless, wearable technology.
Heart rate and blood pressure (time spent under thresholds) showed periods of perturbations despite linked alarm systems.
A significant proportion of hemodynamic changes would have been missed had monitoring been intermittent.
Funding
This work was supported by departmental resources. Dr. Segal is supported by grants from the Anesthesia Patient Safety Foundation, the Wake Forest Clinical and Translational Science Institute (UL1TR001420), and the National Institutes of Health (NIBIB; 1 R21 EB029493-01A1). Dr.Khanna, is supported by a grant from the Wake Forest Clinical and Translational Science Institute.
Footnotes
Disclosures
Supported by departmental funds.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclinane.2023.111159.
Declaration of Competing Interest
Dr. Khanna is a consultant for Edwards Lifesciences, Caretaker Medical, Retia Medical, Philips Research North America, GE Healthcare, Baxter, and Medtronic, and is supported by an NIH/NCATS KL2 award for a pilot trial of continuous hemodynamic and oxygenation monitoring on hospital wards. The Department of Anesthesiology at Wake Forest School of Medicine is funded by Edwards Lifesciences, Masimo, and Medtronic. Dr. Sessler is a consultant for Edwards Lifesciences, Sensifree and Perceptive Medical. The Department of OUTCOMES RESEARCH is funded by Edwards Lifesciences, GE Healthcare, and Masimo. None of the other authors have any potential competing interests to report.
References
- [1].Lienhart A, Auroy Y, Pequignot F, Benhamou D, Warszawski J, Bovet M, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105: 1087–97. [DOI] [PubMed] [Google Scholar]
- [2].Li G, Warner M, Lang BH, Huang L, Sun LS. Epidemiology of anesthesia-related mortality in the United States, 1999–2005. Anesthesiology. 2009;110:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I, Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012;307:2295–304. [DOI] [PubMed] [Google Scholar]
- [4].Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017;2:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kristensen SD, Knuuti J, Saraste A, Anker S, Botker HE, De Hert S, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol 2014;31:517–73. [DOI] [PubMed] [Google Scholar]
- [6].Sessler DI, Devereaux PJ. Perioperative troponin screening. Anesth Analg 2016; 123:359–60. [DOI] [PubMed] [Google Scholar]
- [7].Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MTV, Srinathan SK, et al. Association of Postoperative High-Sensitivity Troponin Levels with Myocardial Injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317:1642–51. [DOI] [PubMed] [Google Scholar]
- [8].Andersen LW, Berg KM, Chase M, Cocchi MN, Massaro J, Donnino MW, et al. Acute respiratory compromise on inpatient wards in the United States: incidence, outcomes, and factors associated with in-hospital mortality. Resuscitation. 2016; 105:123–9. [DOI] [PubMed] [Google Scholar]
- [9].Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Vries EN, Ramrattan MA, Smorenburg SM, Gouma DJ, Boermeester MA. The incidence and nature of in-hospital adverse events: a systematic review. Qual Saf Health Care 2008;17:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vascular Events in Noncardiac Surgery Patients Cohort Evaluation Study I, Spence J, LeManach Y, Chan MTV, Wang CY, Sigamani A, et al. Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019;191 (E830-E7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sessler DI, Meyhoff CS, Zimmerman NM, Mao G, Leslie K, Vásquez SM, et al. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE-2 trial. Anesthesiology. 2018;128:317–27. [DOI] [PubMed] [Google Scholar]
- [13].Khanna AK, Shaw AD, Stapelfeldt WH, Boero IJ, Chen Q, Stevens M, et al. Postoperative hypotension and adverse clinical outcomes in patients without intraoperative hypotension, after noncardiac surgery. Anesth Analg 2021;132: 1410–20. [DOI] [PubMed] [Google Scholar]
- [14].Smischney NJ, Shaw AD, Stapelfeldt WH, Boero IJ, Chen Q, Stevens M, et al. Postoperative hypotension in patients discharged to the intensive care unit after non-cardiac surgery is associated with adverse clinical outcomes. Crit Care 2020; 24:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gregory A, Stapelfeldt WH, Khanna AK, Smischney NJ, Boero IJ, Chen Q, et al. Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesth Analg 2021;132:1654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126: 47–65. [DOI] [PubMed] [Google Scholar]
- [17].Marik PE, Varon J. Perioperative hypertension: a review of current and emerging therapeutic agents. J Clin Anesth 2009;21:220–9. [DOI] [PubMed] [Google Scholar]
- [18].Sigmund AE, Fang Y, Chin M, Reynolds HR, Horwitz LI, Dweck E, et al. Postoperative tachycardia: clinically meaningful or benign consequence of orthopedic surgery? Mayo Clin Proc 2017;92:98–105. [DOI] [PubMed] [Google Scholar]
- [19].Andersen LW, Kim WY, Chase M, Berg KM, Mortensen SJ, Moskowitz A, et al. The prevalence and significance of abnormal vital signs prior to in-hospital cardiac arrest. Resuscitation. 2016;98:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Turan A, Chang C, Cohen B, Saasouh W, Essber H, Yang D, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology. 2019;130:550–9. [DOI] [PubMed] [Google Scholar]
- [21].Weenk M, Bredie SJ, Koeneman M, Hesselink G, van Goor H, van de Belt TH. Continuous monitoring of vital signs in the general Ward using wearable devices: randomized controlled trial. J Med Internet Res 2020;22:e15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Weenk M, Koeneman M, van de Belt TH, Engelen L, van Goor H, Bredie SJH. Wireless and continuous monitoring of vital signs in patients at the general ward. Resuscitation. 2019;136:47–53. [DOI] [PubMed] [Google Scholar]
- [23].Weenk M, van Goor H, Frietman B, Engelen LJ, van Laarhoven CJ, Smit J, et al. Continuous monitoring of vital signs using wearable devices on the general Ward: pilot study. JMIR Mhealth Uhealth 2017;5:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khanna AK, Ahuja S, Weller RS, Harwood TN. Postoperative ward monitoring - why and what now? Best Pract Res Clin Anaesthesiol 2019;33:229–45. [DOI] [PubMed] [Google Scholar]
- [25].Weller RS, Foard KL, Harwood TN. Evaluation of a wireless, portable, wearable multi-parameter vital signs monitor in hospitalized neurological and neurosurgical patients. J Clin Monit Comput 2018;32:945–51. [DOI] [PubMed] [Google Scholar]
- [26].Liem VGB, Hoeks SE, Mol K, Potters JW, Grüne F, Stolker RJ, et al. Postoperative hypotension after noncardiac surgery and the association with myocardial injury. Anesthesiology. 2020;133:510–22. [DOI] [PubMed] [Google Scholar]
- [27].Eddahchouri Y, Peelen RV, Koeneman M, Touw HRW, van Goor H, Bredie SJH. Effect of continuous wireless vital sign monitoring on unplanned ICU admissions and rapid response team calls: a before-and-after study. Br J Anaesth 2022;128: 857–63. [DOI] [PubMed] [Google Scholar]
- [28].Maheshwari K, Ahuja S, Khanna AK, Mao G, Perez-Protto S, Farag E, et al. Association between perioperative hypotension and delirium in postoperative critically ill patients: a retrospective cohort analysis. Anesth Analg 2020;130: 636–43. [DOI] [PubMed] [Google Scholar]
- [29].Towne JB, Bernhard VM. The relationship of postoperative hypertension to complications following carotid endarterectomy. Surgery. 1980;88:575–80. [PubMed] [Google Scholar]
- [30].Rose DK, Cohen MM, DeBoer DP. Cardiovascular events in the Postanesthesia care unit: contribution of risk factors. Anesthesiology. 1996;84:772–81. [DOI] [PubMed] [Google Scholar]
- [31].Sessler DI, Khan MZ, Maheshwari K, Liu L, Adegboye J, Saugel B, et al. Blood pressure management by anesthesia professionals: evaluating clinician skill from electronic medical records. Anesth Analg 2021;132:946–56. [DOI] [PubMed] [Google Scholar]
- [32].Christensen AL, Jacobs E, Maheshwari K, Xing F, Zhao X, Simon SE, et al. Development and evaluation of a risk-adjusted measure of intraoperative hypotension in patients having nonemergent. Noncardiac Surgery Anesth Analg 2021;133:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.