Abstract
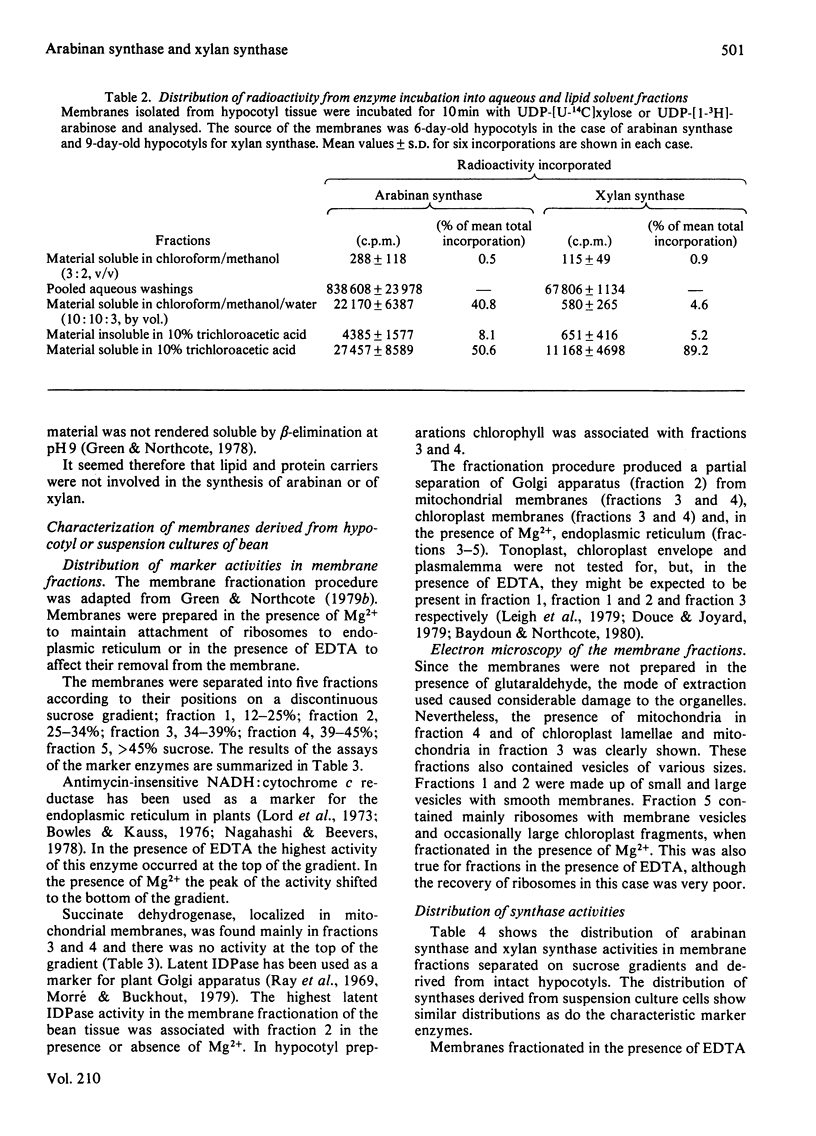
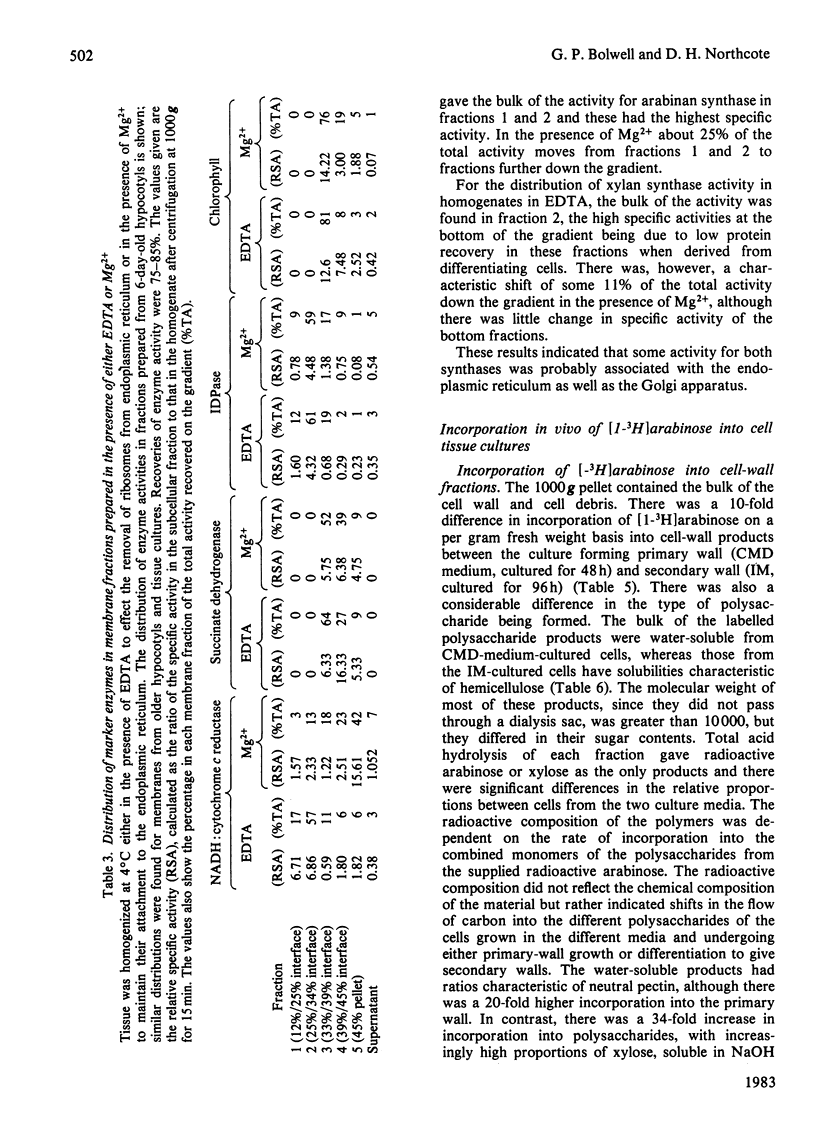
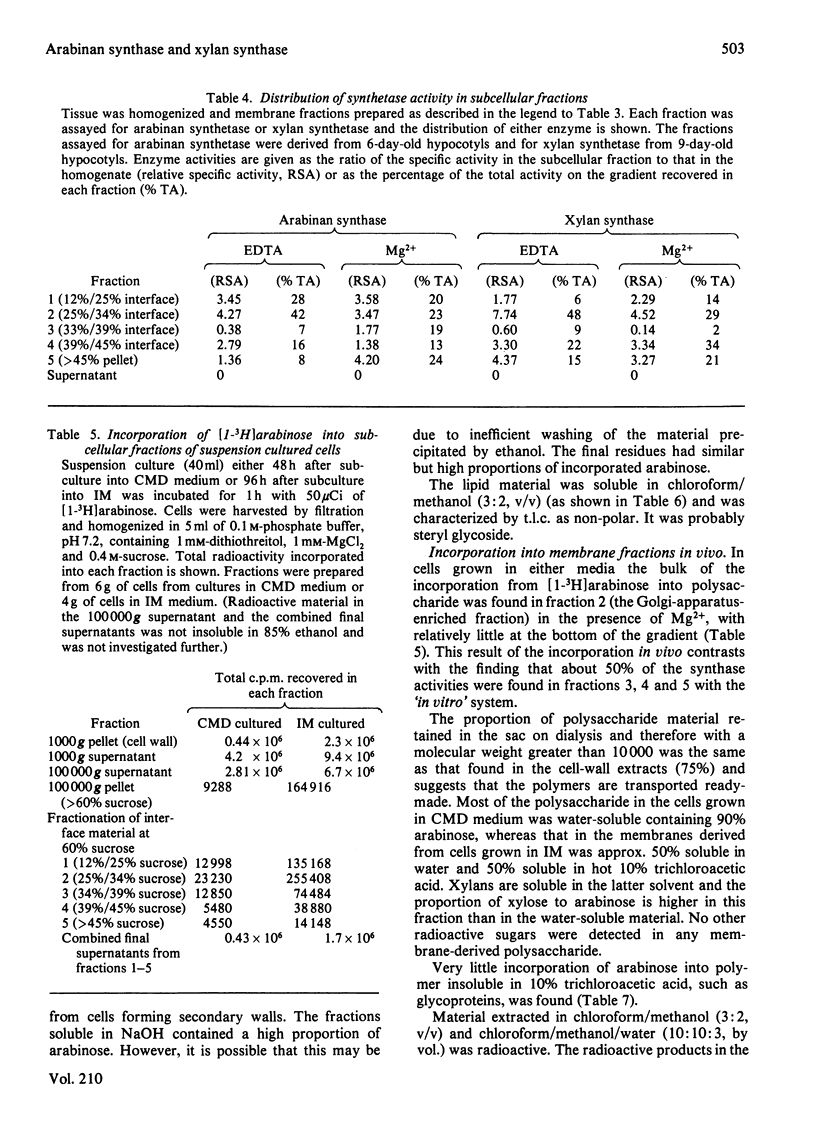
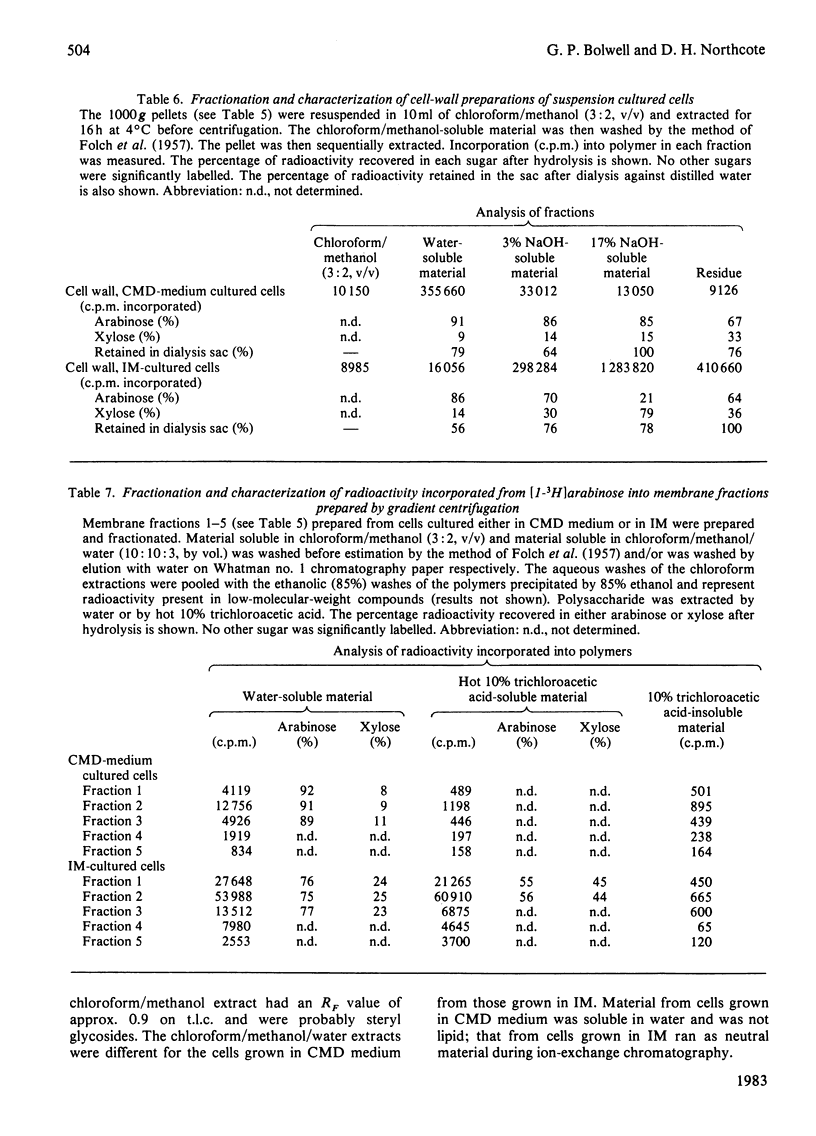
Membrane fractions from bean hypocotyl or suspension cultures incorporated arabinose from UDP-beta-L-arabinose into arabinan and xylose from UDP-alpha-D-xylose in vitro; the level of each activity was dependent on the state of differentiation of the cells. These activities may be due to single transglycosylases, since no lipid or proteinaceous intermediate acceptors were found in either case. Subcellular fractionation studies showed that enzyme activity in vitro was localized in both Golgi-derived membranes and endoplasmic reticulum in similar amounts. However, incorporation into the polymers in vivo in suspension culture cells incubated with [1-3H]arabinose was considerably greater in the Golgi-derived membranes. Thus, although these enzymes may be translated and inserted at the level of the endoplasmic reticulum, their activities are under other levels of control, so that most of the activity in vivo is confined to the Golgi apparatus. Initiation of glycosylation in the endoplasmic activity may, however, occur.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Bailey D. S., Deluca V., Dürr M., Verma D. P., Maclachlan G. A. Involvement of Lipid-linked Oligosaccharides in Synthesis of Storage Glycoproteins in Soybean Seeds. Plant Physiol. 1980 Dec;66(6):1113–1118. doi: 10.1104/pp.66.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D. S., Dürr M., Burke J., Maclachlan G. The assembly of lipid-linked oligosaccharides in plant and animal membranes. J Supramol Struct. 1979;11(2):123–138. doi: 10.1002/jss.400110203. [DOI] [PubMed] [Google Scholar]
- Barr J., Nordin P. Biosynthesis of glycoproteins by membranes of Acer pseudoplatanus. Incorporation of mannose and N-acetylglucosamine. Biochem J. 1980 Nov 15;192(2):569–577. doi: 10.1042/bj1920569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun E. A., Northcote D. H. Isolation and characterization of membranes from the cells of maize root tips. J Cell Sci. 1980 Oct;45:147–167. doi: 10.1242/jcs.45.1.147. [DOI] [PubMed] [Google Scholar]
- Bevan M., Northcote D. H. The loss of morphogenetic potential and induction of phenylalanine ammonia-lyase in suspension cultures of Phaseolus vulgaris. J Cell Sci. 1979 Oct;39:339–353. doi: 10.1242/jcs.39.1.339. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Benedetti E. L., Bont W. S. Preparation and characterization of free and membrane-bound polysomes. Methods Enzymol. 1974;30:313–327. doi: 10.1016/0076-6879(74)30034-1. [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Northcote D. H. Induction by growth factors of polysaccharide synthases in bean cell suspension cultures. Biochem J. 1983 Feb 15;210(2):509–515. doi: 10.1042/bj2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- Brett C. T., Northcote D. H. The formation of oligoglucans linked to lipid during synthesis of beta-glucan by characterized membrane fractions isolated from peas. Biochem J. 1975 Apr;148(1):107–117. doi: 10.1042/bj1480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalessandro G., Northcote D. H. Changes in enzymic activities of nucleoside diphosphate sugar interconversions during differentiation of cambium to xylem in pine and fir. Biochem J. 1977 Feb 15;162(2):281–288. doi: 10.1042/bj1620281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalessandro G., Northcote D. H. Changes in enzymic activities of nucleoside diphosphate sugar interconversions during differentiation of cambium to xylem in sycamore and poplar. Biochem J. 1977 Feb 15;162(2):267–279. doi: 10.1042/bj1620267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Biosynthesis of a cell wall glucomannan in mung bean seedlings. J Biol Chem. 1969 Mar 25;244(6):1608–1616. [PubMed] [Google Scholar]
- Ericson M. C., Gafford J. T., Elbein A. D. Tunicamycin inhibits GlcNAc-lipid formation in plants. J Biol Chem. 1977 Nov 10;252(21):7431–7433. [PubMed] [Google Scholar]
- Ericson M. C., Gafford J., Elbein A. D. Bacitracin Inhibits the Synthesis of Lipid-linked Saccharides and Glycoproteins in Plants. Plant Physiol. 1978 Sep;62(3):373–376. doi: 10.1104/pp.62.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Green J. R., Northcote D. H. Location of fucosyl transferases in the membrane system of maize root cells. J Cell Sci. 1979 Dec;40:235–244. doi: 10.1242/jcs.40.1.235. [DOI] [PubMed] [Google Scholar]
- Green J. R., Northcote D. H. Polyprenyl phosphate sugars synthesized during slime-polysaccharide production by membranes of the root-cap cells of maize (Zea mays). Biochem J. 1979 Mar 15;178(3):661–671. doi: 10.1042/bj1780661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. R., Northcote D. H. The structure and function of glycoproteins synthesized during slime-polysaccharide production by membranes of the root-cap cells of maize (Zea mays). Biochem J. 1978 Mar 15;170(3):599–608. doi: 10.1042/bj1700599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. J., Northcote D. H. Patterns of polysaccharide biosynthesis in differentiating cells of maize root-tips. Biochem J. 1970 Dec;120(3):479–491. doi: 10.1042/bj1200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselbeck A., Tanner W. Dolichyl phosphate-mediated mannosyl transfer through liposomal membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1520–1524. doi: 10.1073/pnas.79.5.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., James D. W., Jr, Elbein A. D. Isolation and characterization of the Glc3Man9GlcNAc2 from lipid-linked oligosaccharides of plants. Arch Biochem Biophys. 1982 Apr 15;215(1):12–21. doi: 10.1016/0003-9861(82)90273-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Brew K. Glycosyltransferases in the Golgi membranes of onion stem. Biochem J. 1974 Aug;142(2):203–209. doi: 10.1042/bj1420203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Cooperative action of beta-glucan synthetase and UDP-xylose xylosyl transferase of Golgi membranes in the synthesis of xyloglucan-like polysaccharide. Biochim Biophys Acta. 1980 May 22;629(3):431–444. doi: 10.1016/0304-4165(80)90149-x. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Regulation of beta-Glucan Synthetase Activity by Auxin in Pea Stem Tissue: I. Kinetic Aspects. Plant Physiol. 1973 Apr;51(4):601–608. doi: 10.1104/pp.51.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Mellor R. B., Lord J. M. Glycoprotein fucosyl transferase in the endoplasmic reticulum of castor bean endosperm cells. FEBS Lett. 1980 Apr 21;113(1):90–94. doi: 10.1016/0014-5793(80)80502-3. [DOI] [PubMed] [Google Scholar]
- Schachter H., Narasimhan S., Gleeson P., Vella G. J., Brockhausen I. Oligosaccharide branching of glycoproteins: biosynthetic mechanisms and possible biological functions. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1099):145–159. doi: 10.1098/rstb.1982.0162. [DOI] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Leloir L. F. Transfer of oligosaccharide to protein from a lipid intermediate in plants. Plant Physiol. 1981 Nov;68(5):1175–1179. doi: 10.1104/pp.68.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Ugalde R. A., Leloir L. F. Presence in a plant of a compound similar to the dolichyl diphosphate oligosaccharide of animal tissue. Biochem J. 1980 Oct 1;191(1):257–260. doi: 10.1042/bj1910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneloni R. J., Ugalde R. A., Leloir L. F. Addition of glucose to dolichyl diphosphate oligosaccharide and transfer to protein. Eur J Biochem. 1980 Apr;105(2):275–278. doi: 10.1111/j.1432-1033.1980.tb04498.x. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]


