Abstract
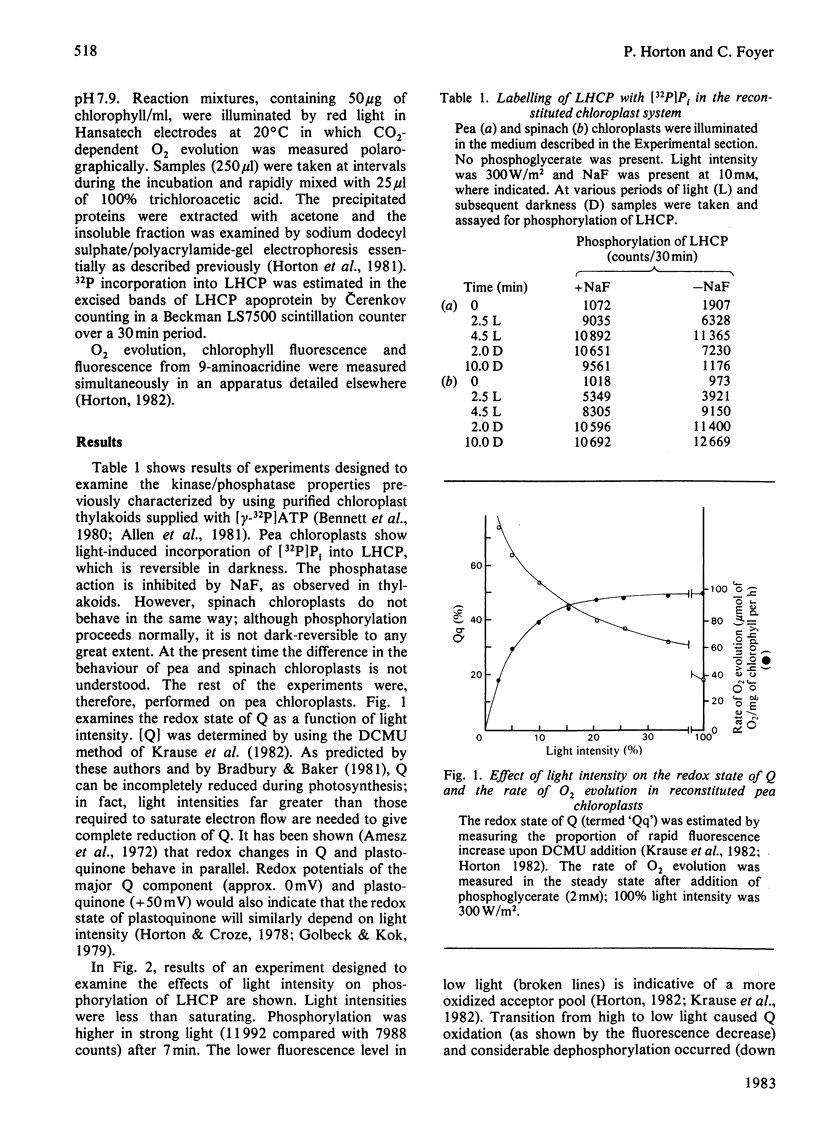
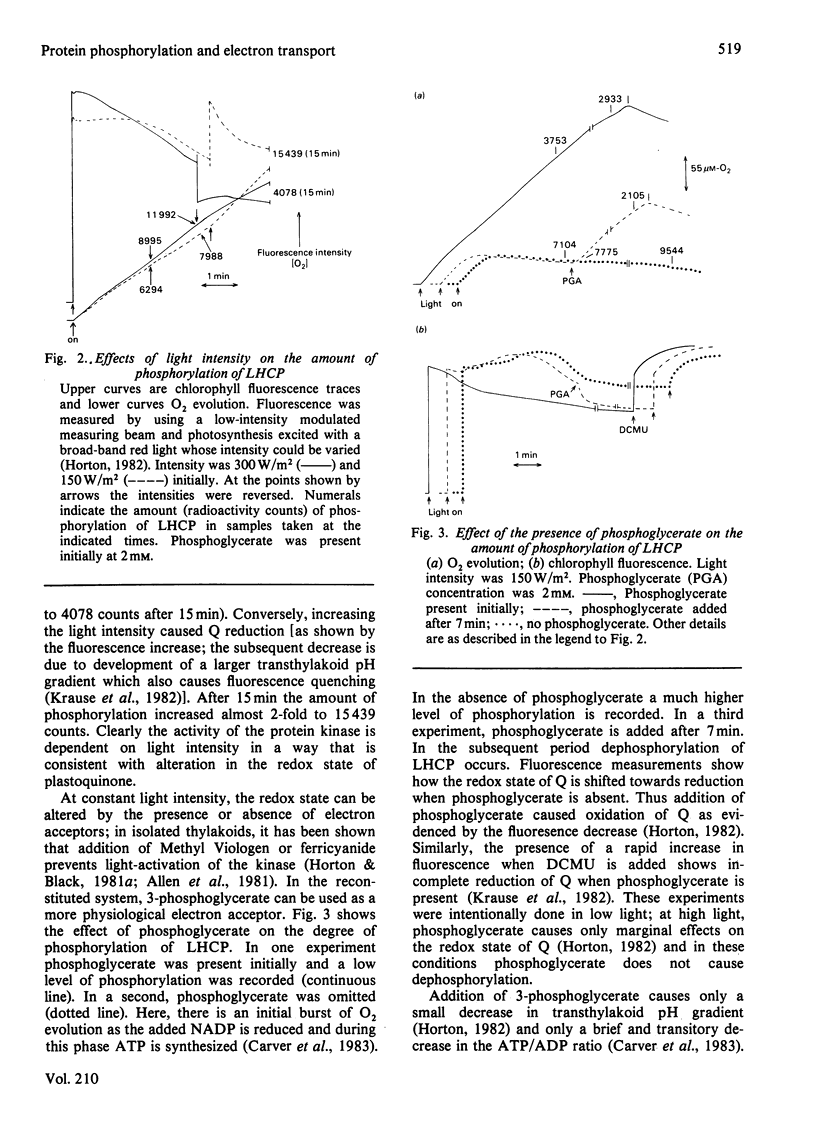
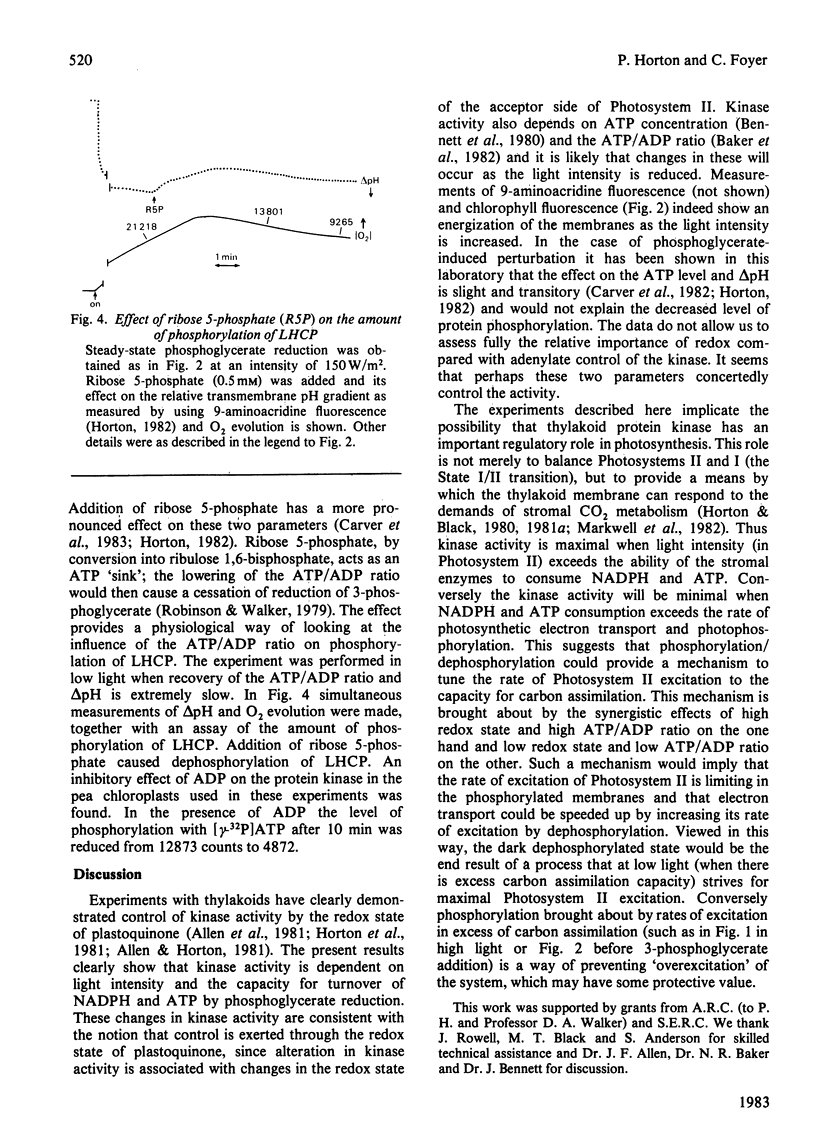
Phosphorylation of the light-harvesting chlorophyll protein (LHCP) by the thylakoid protein kinase has been examined in the reconstituted chloroplast system. The level of phosphorylation by [32P]Pi was maximal at high light intensity and in the absence of 3-phosphoglycerate; dephosphorylation resulted from a subsequent decrease in light intensity or from the addition of 3-phosphoglycerate. Addition of ribose 5-phosphate, which acts as an ATP 'sink', also caused dephosphorylation. It is concluded that the degree of phosphorylation is dependent on the redox state and energy state of the system, thereby providing a mechanism for adapting light harvesting to the demands of carbon assimilation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesz J., Visser J. W., van den Engh G. J., Dirks M. P. Reaction kinetics of intermediates of the photosynthetic chain between the two photosystems. Biochim Biophys Acta. 1972 Feb 28;256(2):370–380. doi: 10.1016/0005-2728(72)90067-9. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. Evidence for a thylakoid-bound phosphoprotein phosphatase. Eur J Biochem. 1980 Feb;104(1):85–89. doi: 10.1111/j.1432-1033.1980.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. Chloroplast phosphoproteins. The protein kinase of thylakoid membranes is light-dependent. FEBS Lett. 1979 Jul 15;103(2):342–344. doi: 10.1016/0014-5793(79)81358-7. [DOI] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M., Baker N. R. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta. 1981 May 13;635(3):542–551. doi: 10.1016/0005-2728(81)90113-4. [DOI] [PubMed] [Google Scholar]
- Carver K. A., Hope A. B., Walker D. A. Adenine nucleotide status, phosphoglycerate reduction and photosynthetic phosphorylation in a reconstituted chloroplast system. Biochem J. 1983 Jan 15;210(1):273–276. doi: 10.1042/bj2100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbeck J. H., Kok B. Redox titration of electron acceptor Q and the plastoquinone pool in photosystem II. Biochim Biophys Acta. 1979 Aug 14;547(2):347–360. doi: 10.1016/0005-2728(79)90016-1. [DOI] [PubMed] [Google Scholar]
- Horton P., Black M. T. Light-dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by adenosine 5'-triphosphate. Biochim Biophys Acta. 1981 Mar 12;635(1):53–62. doi: 10.1016/0005-2728(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Horton P., Croze E. Characterization of two quenchers of chlorophyll fluorescence with different midpoint oxidation-reduction potentials in chloroplasts. Biochim Biophys Acta. 1979 Jan 11;545(1):188–201. doi: 10.1016/0005-2728(79)90125-7. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Walker D. A. The control of 3-phosphoglycerate reduction in isolated chloroplasts by the concentrations of ATP, ADP and 3-phosphoglycerate. Biochim Biophys Acta. 1979 Mar 15;545(3):528–536. doi: 10.1016/0005-2728(79)90161-0. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., Bose S., Kyle D. J. Phosphorylation of the light-harvesting chlorophyll-protein regulates excitation energy distribution between photosystem II and photosystem I. Arch Biochem Biophys. 1982 Jun;216(1):356–361. doi: 10.1016/0003-9861(82)90221-1. [DOI] [PubMed] [Google Scholar]


