Abstract
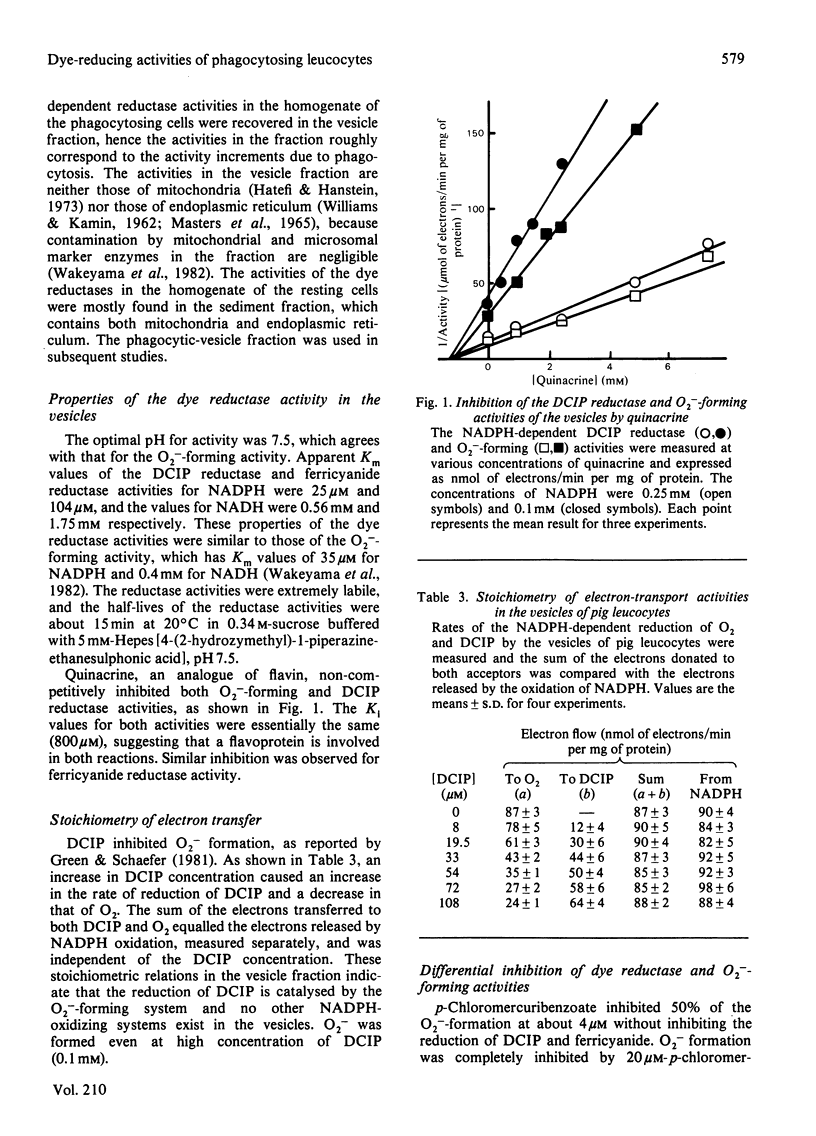
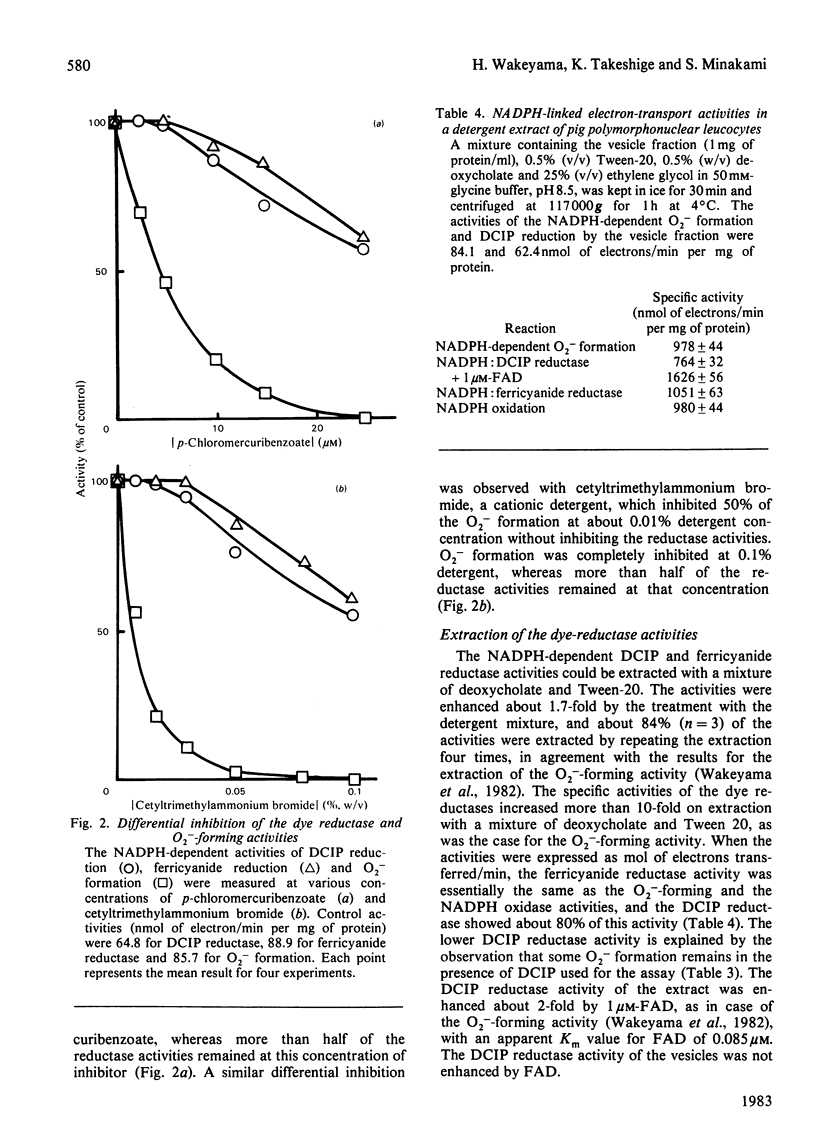
NADPH-dependent 2,6-dichlorophenol-indophenol (DCIP) reductase activity in the homogenate of phagocytosing pig polymorphonuclear leucocytes was twice that of the resting cells and the activity in the phagocytic vesicles corresponded to the activity increment due to phagocytosis. The apparent Km value of the reductase activity in the vesicles for NADPH was 30 microM, which is similar to that of the NADPH-dependent superoxide (O2-) formation. Increasing the DCIP reductase activity by increasing the DCIP concentration caused a decrease in the O2- -forming activity, the NADPH oxidation rate being constant and independent of the dye concentration. p-Chloromercuribenzoate and cetyltrimethylammonium bromide at low concentrations inhibited the O2- -forming activity of the vesicles without inhibiting the DCIP reductase. Quinacrine inhibited both O2- formation and DCIP reduction. The DCIP reductase activity could be extracted with a mixture of deoxycholate and Tween-20, which extracts the O2- -forming activity. The reductase activity in the extract was enhanced 2-fold by the addition of FAD, and its apparent Km was 0.085 microM. These results indicate that the NADPH-dependent DCIP reductase activity of the phagocytic vesicles is catalysed by a flavin-containing component of the O2- -forming system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S. Superoxide-forming enzyme from human neutrophils: evidence for a flavin requirement. Blood. 1977 Sep;50(3):517–524. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Peters W. A. The O2--producing enzyme of human neutrophils. Further properties. J Biol Chem. 1981 Mar 10;256(5):2321–2323. [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. R., Schneider D. L. Identification of ubiquinone-50 in human neutrophils and its role in microbicidal events. J Biol Chem. 1982 Jun 25;257(12):6662–6668. [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabig T. G., Schervish E. W., Santinga J. T. Functional relationship of the cytochrome b to the superoxide-generating oxidase of human neutrophils. J Biol Chem. 1982 Apr 25;257(8):4114–4119. [PubMed] [Google Scholar]
- Green T. R., Schaefer R. E. Intrinsic dichlorophenolindophenol reductase activity associated with the superoxide-generating oxidoreductase of human granulocytes. Biochemistry. 1981 Dec 22;20(26):7483–7487. doi: 10.1021/bi00529a024. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Interactions of reduced and oxidized triphosphopyridine nucleotides with the electron-transport system of bovine heart mitochondria. Biochemistry. 1973 Aug 28;12(18):3515–3522. doi: 10.1021/bi00742a026. [DOI] [PubMed] [Google Scholar]
- Masters B. S., Bilimoria M. H., Kamin H., Gibson Q. H. The mechanism of 1- and 2-electron transfers catalyzed by reduced triphosphopyridine nucleotide-cytochrome c reductase. J Biol Chem. 1965 Oct;240(10):4081–4088. [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Cohen H. J. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980 Jan;55(1):85–92. [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. The subcellular distribution and some properties of the cytochrome b component of the microbicidal oxidase system of human neutrophils. Biochem J. 1979 Jul 15;182(1):181–188. doi: 10.1042/bj1820181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]
- Wakeyama H., Takeshige K., Takayanagi R., Minakami S. Superoxide-forming NADPH oxidase preparation of pig polymorphonuclear leucocyte. Biochem J. 1982 Sep 1;205(3):593–601. doi: 10.1042/bj2050593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost F. J., Jr, Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):4905–4908. [PubMed] [Google Scholar]



