Abstract
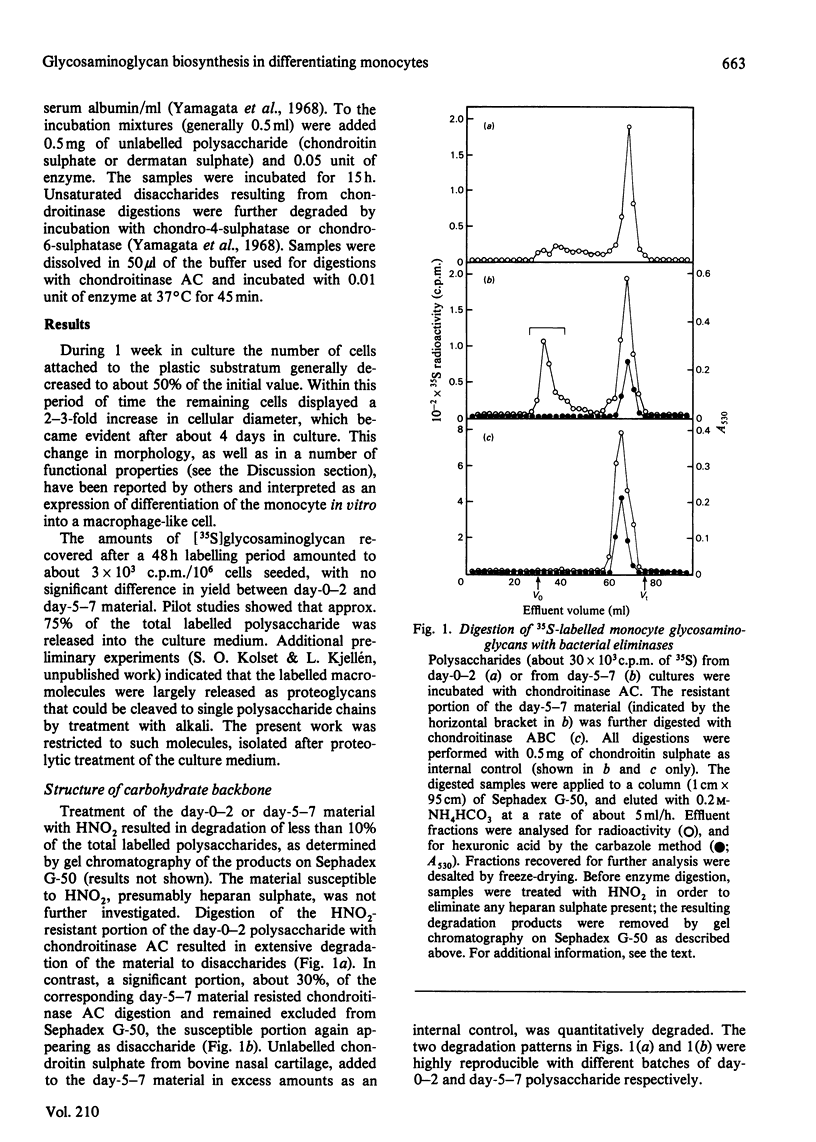
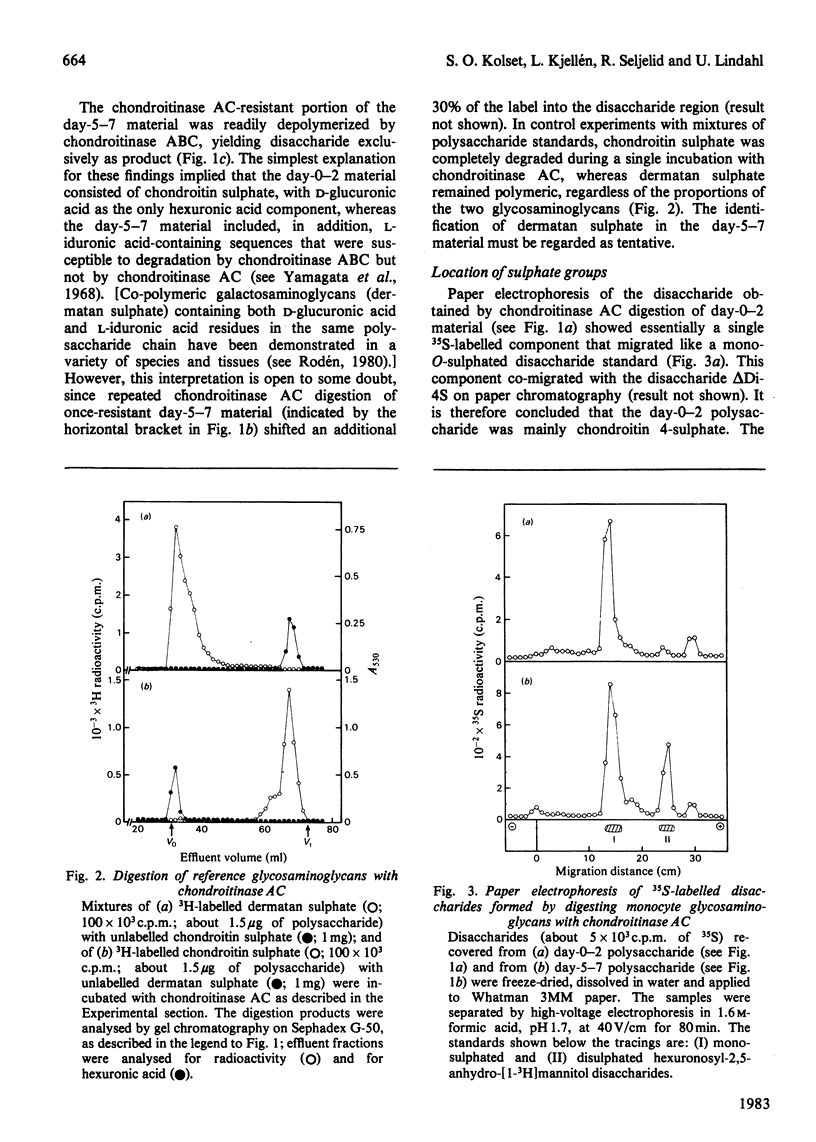
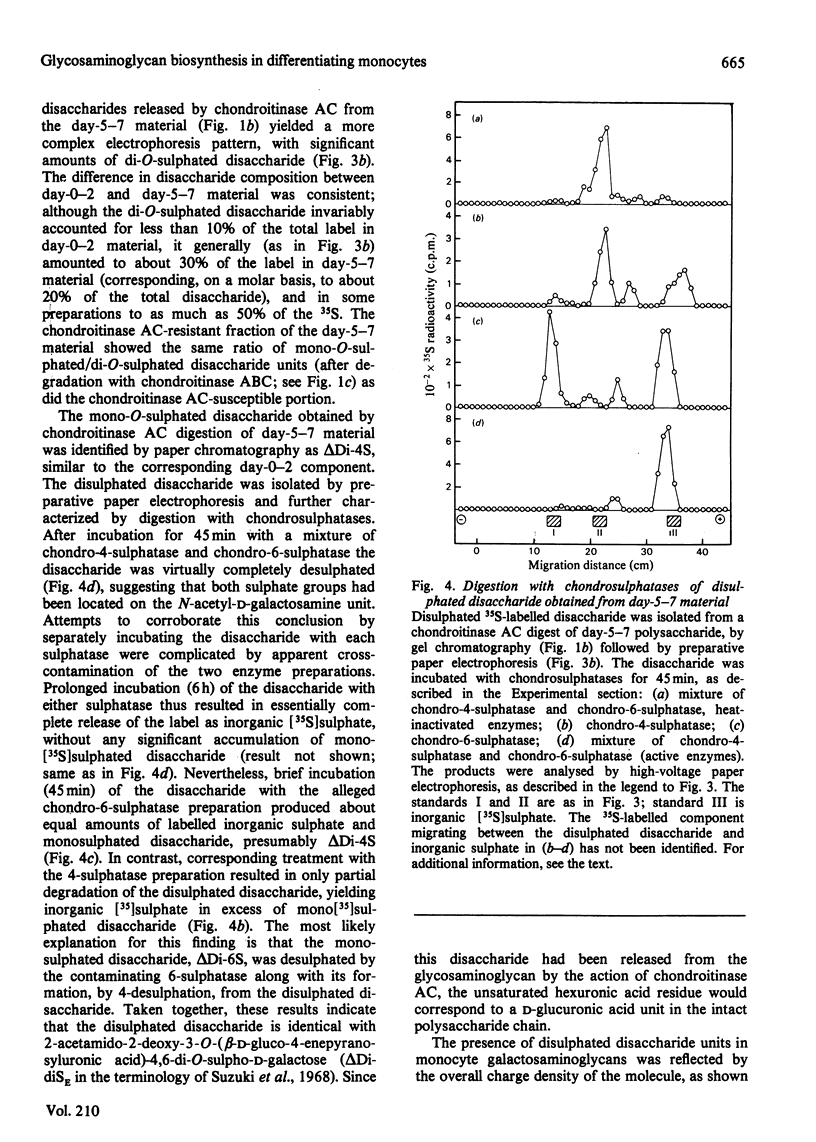
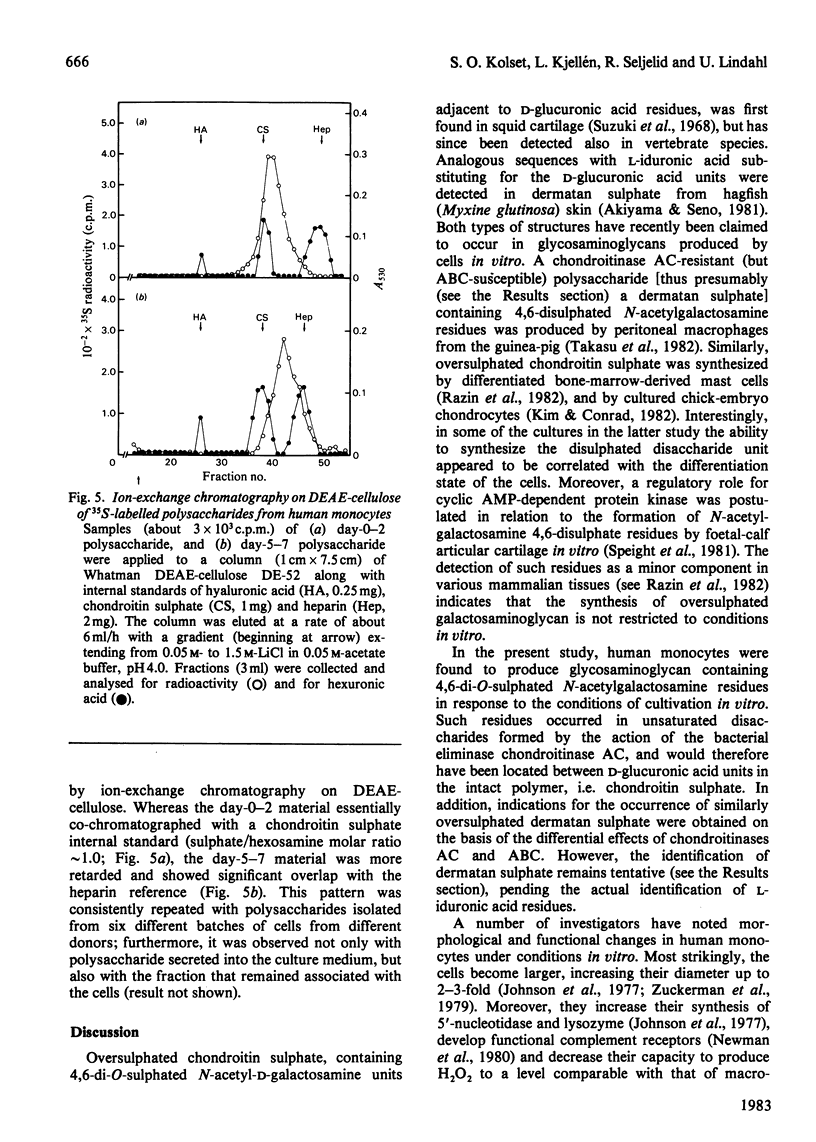
Monocytes isolated from human blood were maintained in vitro on plastic culture dishes. After 3-4 days, adherent cells displayed morphological changes previously attributed to differentiation of the cells into histiocytes. 35S-labelled glycosaminoglycans were isolated after incubation of the cells with inorganic [35S]sulphate. Polysaccharide recovered from the culture medium after labelling from day 0 to day 2 or from day 5 to day 7 in vitro was approximately 90% galactosaminoglycan (resistant to deamination by HNO2), irrespective of labelling period. Whereas day-0-2 material was extensively degraded to disaccharide on incubation with the bacterial eliminase chondroitinase AC, a significant portion, about 30%, of the day-5-7 material resisted degradation under the same conditions. The resistant portion was readily depolymerized by treatment with chondroitinase ABC and may be dermatan sulphate. Paper electrophoresis and paper chromatography of the disaccharides obtained by eliminase digestion identified the day-0-2 labelled galactosaminoglycan as chondroitin 4-sulphate. In contrast, the corresponding day-5-7 material yielded approximately 20% disulphated disaccharide, both on digestion with chondroitinase AC and on subsequent enzymic degradation of the chondroitinase AC-resistant fraction. Further treatment of the disulphated disaccharide with chondro-4-sulphatase and chondro-6-sulphatase indicated that both sulphate groups were located on the N-acetylgalactosamine residue. In accordance with these findings, the day-5-7 polysaccharide showed a higher negative charge density than the day-0-2 material on ion-exchange chromatography. It is concluded that the novel properties acquired by the monocyte during prolonged culturing on plastic include the ability to synthesize glycosaminoglycan(s) containing 4,6-disulphated N-acetylgalactosamine units.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama F., Seno N. Linkage regions between dermatan polysulfates and peptides. Biochim Biophys Acta. 1981 May 18;674(3):289–296. doi: 10.1016/0304-4165(81)90359-7. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Cappelletti R., Del Rosso M., Vannucchi S., Cella C., Fibbi G., Chiarugi V. P. Involvement of modulation of cell coat glycosaminoglycans in guinea pig peritoneal macrophage activity. J Reticuloendothel Soc. 1980 Apr;27(4):383–391. [PubMed] [Google Scholar]
- Hök M., Riesenfeld J., Lindahl U. N-[3H]Acetyl-labeling, a convenient method for radiolabeling of glycosaminoglycans. Anal Biochem. 1982 Jan 15;119(2):236–245. doi: 10.1016/0003-2697(82)90580-2. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. J., Conrad H. E. Proteochondroitin sulfate synthesis in subcultured chick embryo tibial chondrocytes. J Biol Chem. 1982 Feb 25;257(4):1670–1675. [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Jacobsson I., Hök M., Backström G., Feingold D. S. Biosynthesis of heparin. Loss of C-5 hydrogen during conversion of D-glucuronic to L-iduronic acid residues. Biochem Biophys Res Commun. 1976 May 17;70(2):492–499. doi: 10.1016/0006-291x(76)91073-1. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Musson R. A., Henson P. M. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [PubMed] [Google Scholar]
- Pertoft H., Johnsson A., Wärmegård B., Seljelid R. Separation of human monocytes on density gradients of Percoll. J Immunol Methods. 1980;33(3):221–229. doi: 10.1016/0022-1759(80)90209-4. [DOI] [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Speight G., Handley C. J., Lowther D. A. Effect of dibutyryl cyclic AMP on the sulphation of proteoglycans by chondrocytes. Biochim Biophys Acta. 1981 Jan 7;672(1):89–97. doi: 10.1016/0304-4165(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Saito H., Yamagata T., Anno K., Seno N., Kawai Y., Furuhashi T. Formation of three types of disulfated disaccharides from chondroitin sulfates by chondroitinase digestion. J Biol Chem. 1968 Apr 10;243(7):1543–1550. [PubMed] [Google Scholar]
- Takasu Y., Hasumi F., Mori Y. Biosynthesis of glycosaminoglycans in peritoneal macrophages from the guinea pig. Biochim Biophys Acta. 1982 Jun 16;716(3):316–323. doi: 10.1016/0304-4165(82)90022-8. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982 Mar 1;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Keller J. M. A transformation-dependent difference in the heparan sulfate associated with the cell surface. Biochem Biophys Res Commun. 1975 Mar 17;63(2):448–454. doi: 10.1016/0006-291x(75)90708-1. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- Zuckerman S. H., Ackerman S. K., Douglas S. D. Long-term human peripheral blood monocyte cultures: establishment, metabolism and morphology of primary human monocyte-macrophage cell cultures. Immunology. 1979 Oct;38(2):401–411. [PMC free article] [PubMed] [Google Scholar]


