ABSTRACT
Background:
This study aimed to evaluate the effect of new computer-aided design/computer-aided manufacturing all-ceramic materials on the viability and adhesion properties of human gingival fibroblasts (HGFs).
Materials and Methods:
In this experimental study, the proliferation and adhesion potential of the cells were evaluated by seeding the HGF cells on rectangular samples (n = 18 for each group). The studied groups were tetragonal zirconia (TZr), cubic zirconia (CZr), lithium disilicate (LDS), zirconia-reinforced lithium silicate (ZLS), and hybrid ceramic (HyC) (n = 6 for each studied time). The cell viability (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was conducted at determined times (24 h, 72 h, and 168 h) to evaluate the cell proliferation. Subsequently, the cultured cells were processed for scanning electron microscope (SEM) observation at each time interval. The surface roughness and wettability of studied ceramics were assessed using a surface profilometer and water contact angle. Differences in the cellular viability, surface roughness (Ra), and wet ability (wetting angle) of studied groups were compared by one-way analysis of variance and the Tukey multiple comparisons test (á = 0.05).
Results:
The highest percentage of cell viability after 24 h, 72 h, and 168 h cultures was related to ZLS, LDS, and CZr, respectively. The lowest proliferation of fibroblast cells was shown in ZLS compared to other groups. SEM analysis showed that the CZr and LDS groups have better adhesion patterns and morphology. The surface of HyC groups was significantly less rough than other groups. Regarding the water wetting angle (wettability), the TZr and CZr showed significantly larger angles.
Conclusion:
Within the limitation of this study, it can be concluded that CZr and LDS ceramics had better adhesion patterns and typical morphology. On the other hand, zirconia with a larger wetting angle can reduce the chance of bacteria adhesion to the surface.
Keywords: Ceramics, dental materials/toxicity, fibroblasts/drug effects, gingiva/drug effects
INTRODUCTION
Dental ceramics are a group of innovative materials that are used for bridges and crowns for their improved esthetic properties (natural appearance), mechanical characteristics (surface roughness and wear resistance, strength, and low thermal conductivity), optical properties (translucency), chemical stability (chromatic stability), biocompatibility, and low plaque retention and fluids absorption.[1,2,3,4,5] In recent years, the use of computer-aided design/computer-aided manufacturing (CAD/CAM) technologies for fabricating restorations from a variety of materials has become widespread.[4] The CAD/CAM technology improves the properties of ceramic-based restorations and leads to high precision and predictability of the restorative outcomes.[1,6,7,8]
All-ceramic restorations are often in close and prolonged contact with periodontal tissues. Thus, the biocompatibility of these materials is critical.[9] The adverse effects of these materials can jeopardize the tissue surrounding implant-supported restorations and induce an allergic reaction.[9]
Soft-tissue regeneration, soft-tissue integration, osseointegration, and the quality of connective tissue adhesion to the surface of abutments are involved in the long-term success of dental implants by providing a transmucosal biological barrier between the implant and oral environment. This can decrease infections induced by bacteria and peri-implant diseases.[10,11,12,13]
The gingival connective tissue has different cells including fibroblasts, macrophages, mast cells, and osteoblasts. Fibroblasts are the predominant number of gingival connective tissue cells[14,15] which play a major role in the production of extracellular matrix (collagen-rich connective tissue) to accelerate wound healing repair and adhesion to the abutment of the implant.[16,17] Pae et al.[18] and Ferraris et al.[19] showed that gingival fibroblast proliferation is affected by contact guidance and adhesion to the surface of the implant. The fibroblasts are the largest number of cells associated with the surface of the abutment and their attachment to the implant surface is important to generate the mucosal seal.[14,20,21]
Several studies have examined different materials used in dental abutments and evaluated their effects on fibroblast cells in vitro.[2,22,23,24] These studies showed that zirconia has suitable support for soft-tissue integrations and polishing can provide better cell adhesion.[2,24] On the other hand, surface plasma treatments of zirconia can enhance cell adhesion.[22] Treatment of titanium and titanium alloys also can promote cell adhesions.[19,25] Fibroblasts have similar morphology but show different proliferation patterns, adhesion, and gene expression at different surfaces.[19,25,26,27]
Currently, there is little information about the effects of all-ceramic CAD/CAM materials on human gingival fibroblasts (HGFs). The present study aimed to evaluate the in vitro cytotoxicity of CAD/CAM dental ceramics on HGF, it has to be mentioned that no in vivo study was performed on samples. The null hypothesis was that there was no statistical difference between the cytotoxicity of different CAD/CAM dental ceramics on HGF.
MATERIALS AND METHODS
Specimen preparation
In this experimental study, a total of 90 specimens (n = 18 for each group and n = 6 for each time interval) of CAD/CAM ceramic samples (tetragonal zirconia [TZr], cubic zirconia [CZr], lithium disilicate [LDS], zirconia-reinforced lithium silicate, and hybrid ceramic) were cut (CNC Cutting Section Machine; Nemo Fanavaran Pars) with a diamond blade under running water in rectangle shape (12 mm × 10 mm × 1 mm). For zirconia samples, 20% shrinkage of sintering was accounted for.
The specimen was divided into five groups as follows:
TrZ with 3% yttrium (TZI C, Dentsply Sirona,)
CZr with 9.28% yttrium (3D Multilayer, Aidite)
LDS (e. max CAD, Ivoclare Vicadent)
Zirconia-reinforced lithium silicate (ZLS) (Suprinity, VITA Zahn fabric)
Hybrid ceramic (HyC) (Enamic, VITA Zahn fabric).
Zirconia samples were sintered in an appropriate sintering furnace (Ceramill Therm 3, Amann Girrbach; 1370°C for 7 h). LDS and ZLSs were crystalized in a P-500 furnace (Ivoclare Vicadent; 840°C for 7 min). Each specimen was manually polished using 600-, 1200-, and 2000-grit silicon carbide abrasive papers.
Surface roughness
Measurements of the surface roughness were characterized with an optical profilometer (LPM-D1, Fanavari Kahroba Company, Iran). The surface area of 450 μm × 60 μm was scanned, and the average surface roughness was calculated according to ISO 25178 standard.
Wettability measurement
For wettability measurement, a deionized water droplet (4 μL) was placed on the sample surface of microsyringe of contact angle instrument (contact angle measuring developed in wetting and fluids laboratory at materials and Energy Research Center, Iran), and letting it to be in balance within 10 s. The measurements were repeated 3 times for each group (n = 3).
For sterilizing before cell seeding, the rectangular samples were cleaned in an ultrasonic bath containing isopropyl alcohol for 20 min and were then washed with sterile purified water. The specimens were sterilized using ultraviolet light applied to both sides for 45 min.
Cell culture
Gingival fibroblasts (Gingiva, Human, HGF-PI 1 Fibroblast-like; Pasteur Institute of Iran) were cultured in 24-well cell-culture plates containing sterile samples at concentrations of 20 × 103/cm2 for 24, 72 and 168 h. Two following separate groups were selected as controls. The positive control was the gingival fibroblast cells seeded at the same concentration and time points without samples, and the negative control included only the culture medium without any cell and ceramic samples.
The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to evaluate cell proliferation. 5 mg/mL of MTT solution in phosphate-buffered solution was used (the principle of this assay is based on the reduction activity of mitochondrial succinate dehydrogenase. The previous study[28] reported that this activity could reflect the number and viability of cells around the samples. Viable cells can reduce the MTT reagent (3-[4,5-dimethyl-thiazol- 2-yl]-2,5-diphenyl tetrazolium bromide) into formazan product. The MTT test was performed in the following steps: (1) the culture medium in plates was replaced by a fresh culture medium containing MTT (40 μL) and incubated for 4 h at 37°C (to conversion soluble MTT to insoluble MTT formazan), (2) the contents of the wells were drained, and then 400 μL in dimethyl sulfoxide was added to the wells and incubated for 30 min (to dissolve produced formazan crystals), (3) the color intensity was read using electromagnetic light with the wavelength of 540 nm using a plate reader (Synergy, BioTek). The values obtained from negative control were considered as background.
The cell viability was calculated based on the percentage of viable cells compared to the controls, as follows:
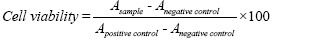
While a refers to absorbance, for example, a sample means the absorbance of the sample.
Scanning electron microscopy analysis
The morphological analyses of HGFS cultured on the discs were confirmed by scanning electron microscope (SEM) (Phillips XL30, Netherlands) after 24, 72, and 168 h. Briefly, the following steps were sequentially conducted to prepare the SEM samples: (1) the discs were fixed in 2% glutaraldehyde in 0.1 molar phosphate buffer pH 7.2; (2) then the discs were washed with 0.15 molar phosphate buffer; (3) then the discs were dehydrated by transferring sequentially to 30%, 50%, 70%, 80%, 90%, 95%, and 100% ethanol alcohol and finally dried in hexamethyldisilazane; (4) finally, samples were metalized by gold in the sputtering device; and (5) prepared samples were analyzed at ×100, ×800, and ×1600 magnification by SEM.
Statistical analysis
All obtained data were statistically analyzed by the SPSS program (SPSS Inc., version 21.0 Chicago, IL, USA). All data were represented as means ± standard deviation. The parametric or nonparametric distributions of data were analyzed by the Kolmogorov–Smirnov test. The statistical significance of differences in the percent of cellular proliferation, surface roughness (Ra), and wet ability (wetting angle) among the different materials were compared using one-way ANOVA followed by the Tukey multiple comparisons test. The level of statistical significance was set at P < 0.05.
RESULTS
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Table 1 shows the cell viability of the studied groups after 24, 72, and 168 h. ANOVA analysis showed that there was no significant difference between the growth of gingival fibroblast cells on different studied ceramics after 24 and 168 h, but a significant difference was found after 72 h [Table 2].
Table 1.
Percent of the cell viability of tetragonal zirconia, cubic zirconia, lithium disilicate, zirconia-reinforced lithium silicate, and hybrid-ceramic samples after 24, 72, and 168 h
| Time (h) | Groups | Mean | SD | 95% CI for mean | |
|---|---|---|---|---|---|
|
| |||||
| Lower bound | Upper bound | ||||
| 24 | TZr | 57.90a | 13.93 | 23.30 | 92.49 |
| CZr | 49.13a | 3.00 | 41.67 | 56.59 | |
| LDS | 49.00a | 3.02 | 41.20 | 56.51 | |
| ZLS | 64.90a | 10.97 | 37.63 | 92.16 | |
| HyC | 52.63a | 5.25 | 39.59 | 65.67 | |
| 72 | TZr | 68.60a | 1.73 | 64.29 | 72.91 |
| CZr | 61.30a,b | 11.20 | 33.48 | 89.11 | |
| LDS | 75.87a | 5.02 | 63.38 | 88.35 | |
| ZLS | 45.97b | 11.30 | 17.88 | 74.88 | |
| HyC | 74.33a | 5.46 | 60.76 | 87.90 | |
| 168 | TZr | 51.50a | 3.81 | 42.03 | 60.97 |
| CZr | 64.7a | 3.60 | 55.75 | 73.65 | |
| LDS | 55.67a | 10.43 | 29.76 | 81.57 | |
| ZLS | 55.05a | 9.91 | 29.60 | 80.80 | |
| HyC | 46.50a | 3.34 | 38.20 | 54.80 | |
The groups with different lowercase letters showed significant statistical differences in each studied time (P<0.05; Tukey HSD). TZr: Tetragonal zirconia; CZr: Cubic zirconia; LDS: Lithium disilicate; ZLS: Zirconia-reinforced lithium silicate; HyC: Hybrid ceramic; HSD: Honest significant difference; CI: Confidence interval; SD: Standard deviation
Table 2.
Analysis of variance for cell viability, surface roughness, and wetting of the studied ceramics in studied time intervals
| Sum of squares | df | Mean square | F | Significant | |
|---|---|---|---|---|---|
| Cell viability | |||||
| 24 h | |||||
| Between groups | 541.56 | 4 | 135.39 | 1.88 | 0.19 |
| Within groups | 720.140 | 10 | 72.014 | ||
| Total | 1261.70 | 14 | |||
| 72 h | |||||
| Between groups | 1781.66 | 4 | 445.41 | 7.15 | 0.005 |
| Within groups | 622.580 | 10 | 62.26 | ||
| Total | 2404.24 | 14 | |||
| 168 h | |||||
| Between groups | 537.88 | 4 | 134.47 | 2.63 | 0.098 |
| Within groups | 512.25 | 10 | 51.23 | ||
| Total | 1050.13 | 14 | |||
| Ra | |||||
| Between groups | 4.17 | 4 | 1.043 | 4.97 | 0.018 |
| Within groups | 2.10 | 10 | 0.21 | ||
| Total | 6.27 | 14 | |||
| Wetting angle | |||||
| Between groups | 5602.90 | 4 | 1400.73 | 146.97 | <0.001 |
| Within groups | 95.31 | 10 | 9.53 | ||
| Total | 5698.21 | 14 |
Ra: Surface roughness
For example, the cell viability for TZr, CZr, LDS, ZLS, and HyC samples after 24 h incubation was 57.90, 49.13, 49.00, 64.90, and 52.63%, whereas the cell viability for TZr, CZr, LDS, ZLS, and HyC samples after 168 h incubation was 51.50, 64.70, 55.67, 55.05, and 46.50%.
After 72 h, all groups except ZLS showed increased cell growth, but no significant difference was observed in the growth of gingival cells between them (TZr, CZr, LDS, and HyC groups) (P = 0.21). The lowest cell growth was observed in the ZLS group after 72 h (P < 0.05). The growth of fibroblast cells in the zirconia lithium silicate group showed a nonsignificant decrease at 72 h compared to 24 h (P = 0.072). In this investigation time, only the LDS group showed a significant increase in cell growth compared to 24 h (P < 0.05) [Figure 1].
Figure 1.
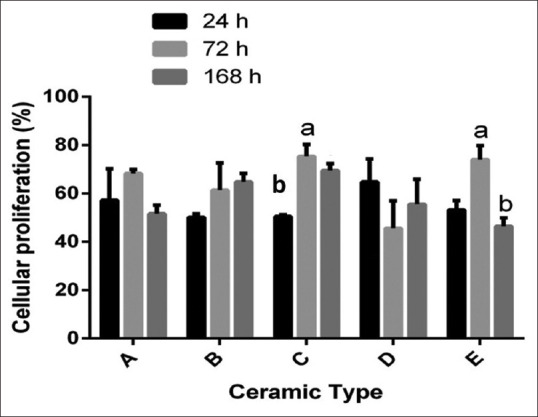
Percent of the cell viability of studied groups after 24, 72, and 168 h. (a) Tetragonal zirconia, (b) Cubic zirconia, (c) Lithium disilicate, (d) Zirconia reinforced lithium silicate, and (e) Hybrid ceramic.
As shown in Table 1, after 168 h, the viability of gingival fibroblast cells on CZr and LDS was significantly higher than TZr and hybrid ceramic. Figure 1 shows that the growth of fibroblast cells on CZr was higher after 168 h compared with 72 h, but it was not statistically different. There was a significant decrease of viable cells in 168 h compared with 72 h in hybrid ceramic (P < 0.05) [Figure 1].
Figure 2.
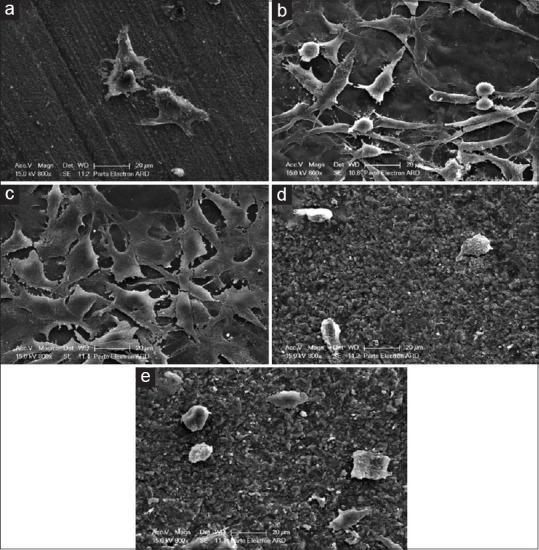
Scanning electron microscope of studied ceramics after 24 h. (a) Tetragonal zirconia, (b) Cubic zirconia, (c) Lithium disilicate, (d) Zirconia-reinforced lithium silicate, (e) Hybrid ceramic.
Scanning electron microscope observation
The adhesion pattern of the cells varied from low, loose, and suitable attachment to the ceramic surface as well as the adhesion morphology depended on ceramic type and time intervals.
SEM observation after 24 h showed the following results [Figure 2a-e].
Zirconia tetragonal
The cells had a little adhesion to the surface of zirconia tetragonal and were round shaped and flattened on the surface with underdeveloped filopodia [Figure 2a].
Cubic zirconia
The cells had a good adhesion pattern to the CZr surface, spindle-shaped, and developed filopodia which had spread to nearby cells [Figure 2b].
Lithium disilicate
The spindle-shaped cells have been transformed into flattened and elongated, and their developed filopodia have spread to nearby the cells [Figure 2c].
Zirconia-reinforced lithium silicate
The cells retained their round and spherical-shaped morphology and had little adhesion to the surface [Figure 2d].
Hybrid ceramic
The cells were scattered and lacked appropriate morphology. The majority of cells were round and spherical, with underdeveloped filopodia [Figure 2e].
After 72 h, the following results were observed [Figure 3a-e].
Figure 3.
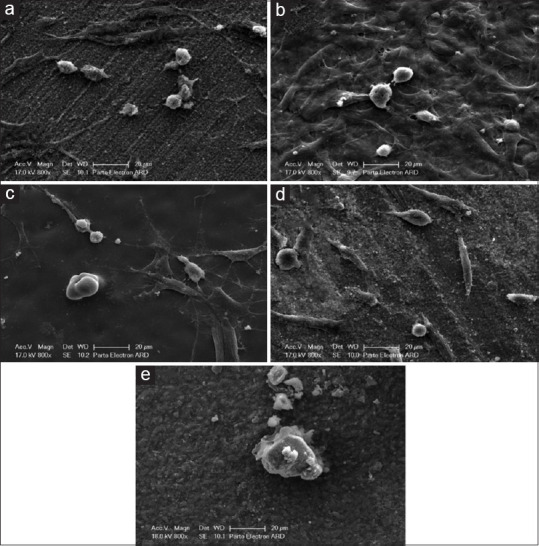
Scanning electron microscope of studied ceramics after 48 h. (a) Tetragonal zirconia, (b) Cubic zirconia, (c) Lithium disilicate, (d) Zirconia-reinforced lithium silicate, (e) Hybrid ceramic.
Zirconia tetragonal
The cells were spindly, their filopodia stretched out, and their adhesion was better than 24 h [Figure 3a].
Cubic zirconia and lithium disilicate
The cell morphology and adhesion pattern of the cells cultured in these groups were very similar; the spindle-shaped cells almost completely attached to the ceramic surface with maximally elongated filopodia [Figure 3b and c].
Zirconia-reinforced lithium silicate
The cells were in an evident spindle form [Figure 3d].
Hybrid ceramic
The cells had no proper morphology and were seen in a round shape [Figure 3e].
After 168 h, the following results were observed [Figure 4a-e].
Figure 4.
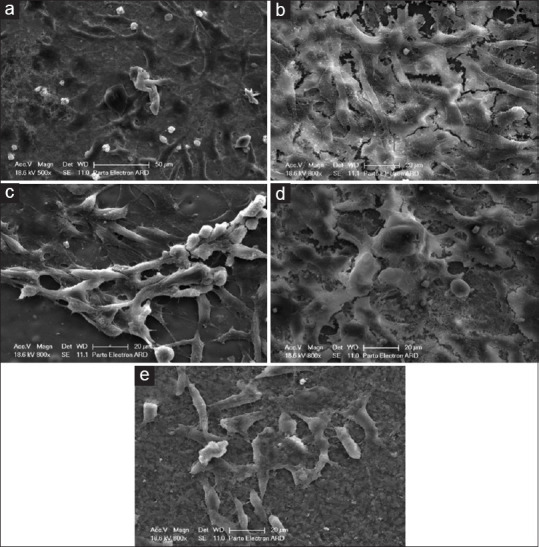
Scanning electron microscope of studied ceramics after 168 h. (a) Tetragonal zirconia, (b) Cubic zirconia, (c) Lithium disilicate, (d) Zirconia-reinforced lithium silicate, (e) Hybrid ceramic.
Zirconia tetragonal
the cell morphology was worse than 72 h, several cells remained in spindle form, and some of them flattened with filopodia elongation [Figure 4a].
Cubic zirconia
The cells were flattened and covered the surface of CZr ceramic with fine-developed filopodia which had spread and reached nearby cells [Figure 4b].
Lithium disilicate
The cells were spindly shaped and had well-characterized and extensive filopodia elongation [Figure 4c].
Zirconia-reinforced lithium silicate
The morphology of the cells varied from round-to-spindle and flattened shape [Figure 4d].
Hybrid ceramic
The cells were spindly shaped and lacking specific cytoplasmic elongations. Generally, CZr and LDS have better adhesion patterns and morphology [Figure 4e].
The mean values of water contact angle were 75, 73, 32, 37, and 35 degrees for TZr, CZr, LDS, ZLS, and HyC samples, respectively. It is evident that the lowest water contact angle is related to the LDS sample, and the highest water contact angle is related to the TZr sample. Using the water contact angle measurement, the hydrophilicity or hydrophobicity of the surface of the sample can be determined in Figure 5.
Figure 5.
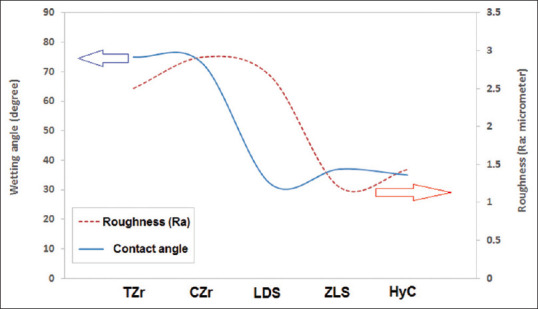
The water contact angle and the surface roughness of tetragonal zirconia, cubic zirconia, lithium disilicate, zirconia-reinforced lithium silicate, and hybrid-ceramic samples. TZr: Tetragonal zirconia, CZr: Cubic zirconia, LDS: Lithium disilicate, ZLS: Zirconia-reinforced lithium silicate, HyC: hybrid ceramic.
Furthermore, the surface roughness of samples was characterized using an optical profilometer. The mean values of Ra were 2.5 ± 0.59, 2.91 ± 0.27, 2.66 ± 0.47, 1.20 ± 0.89, and 1.43 ± 0.26 μm for TZr, CZr, LDS, ZLS, and HyC samples, respectively. It is evident that the smallest Ra is related to the ZLS sample, and the largest Ra is related to the CZr sample [Figure 5].
DISCUSSION
The null hypothesis was considered to be, no statistical difference between the cytotoxicity of CAD/CAM dental ceramic on HGFs on different samples. This hypothesis is rejected.
Hybrid ceramics have become one of the most popular materials for using customized abutments for dental implants. The topography of ceramic surfaces has important effects on cell morphology, proliferation, and adhesion on the other hand, the cytotoxic effects of various ceramics have been assessed by HGF cells.[29,30] The cytotoxicity of dental ceramics was evaluated by the millipore filter method, the agar overlay method, and the MTT assay in previous studies.[31] To achieve the goal, the MTT test was used to estimate the cell proliferation and viability percentage.
Kilic et al.[28] reported that low-fusing ceramic and yttria-stabilized TZr ceramic have no toxic effects on L929 mouse skin fibroblast cells. The present study showed that the viability of HGF cells cultured on the surface of ceramics was significantly lower than the positive control. This result suggests that ceramics hurt the proliferation and viability of HGFs. Although these results differ from some published studies conducted by Josset et al.[32] and Sjögren et al.[31] which observed no cytotoxicity for the ceramics. Messer et al.[33] reported that five different ceramics significantly inhibited mouse fibroblast Balb/c 3T3 proliferation and caused only mild in vitro suppression of cell function.
Our results 24 h after culture were similar to the Kilic et al.’s[28] results. It is interesting to note that in all ceramic groups in the current study, there were no statistically significant differences in proliferation and percentage of viable cells after 24 h of culture. Kilic et al.[28] stated that each ceramic has its specific effects on cells. Our research shows that the viability of cells cultured on the surfaces of all groups increased after 72 h compared with 24 h (except the ZLS group), but it was only significant in LDS. Our results suggested that ceramics have time-dependent in vitro biological effects on HGFs. Messer et al.[33] showed a time-dependent toxic effect of LDS-based ceramic using the MTT test. Sabaliauskas et al.[34] evaluated the cytotoxicity of permanent prosthetic materials. They showed decreasing cytotoxicity levels of materials with the extension of the incubation time (reverse time dependent).
On the other hand, our results confirmed that the effects of all ceramics on proliferation are not equal. Messer et al.[33] found that the most toxic effects of ceramics belong to LDS-based ceramic.
As noted in the introduction section, the attachment of gingival fibroblast cells to the ceramic adhesive surface plays an essential role in the clinical outcome. Previous studies have shown that the roughness of the ceramic surface increases the proliferation and adhesion of the cells.[35]
In the present study, SEM evaluation showed that cell culture on the surface of CZr and LDS ceramics had the best morphology, proliferation, and adhesion to the surface of samples. It should be noted that the morphology and adhesion were checked in the best views and can be different from the results of the MTT assay [Table 1]. These results are consistent with those of other studies.[35,36] Tetè et al.[35] demonstrated that the proliferation of fibroblasts cultured on the LDS and polished zirconia discs was significantly more than those cultured on the feldspathic ceramics. Raffaelli et al.[36] attribute this result to the biocompatibility of these ceramics and their ability to induce fibroblast attachment. Tetè et al.,[35] however, showed that LDS ceramics can also have cytotoxic effects, so this ceramic disc is not completely biologically inert.
Zizzari et al.[2] evaluated HGF growth onto CAD/CAM zirconia and veneering ceramic for zirconia by SEM they demonstrated that the zirconia surface is rougher than the veneering ceramic, so it triggers the proliferation and adhesion of fibroblasts. Fibroblast cells tend to have flat morphology at rough surfaces such as zirconia.[2] In the present study, the best adhesion pattern and typical morphology were observed at 72 h after culture in fibroblast cells cultured onto the surface of CZr and LDS ceramics.
According to the results of surface roughness measurement, the smallest Ra is related to the ZLS sample, and the largest Ra is related to the CZr sample. The small Ra value implies to smoother surface, and the large Ra value presents a rough surface. The ZLS sample had the smoothest surface after polishing. This sample is resistant to abbreviation and scratching, whereas the CZr sample had the roughest surface, implying poor resistance to scratching. The polishing conditions for all samples were the same, but the ZLS sample was so hard to scratch deeply. It seems the ZLS sample will have greater wearing resistance than that of the others. Of course, the wear test can present more comprehensive data about the wear behavior of samples, and it will be used in our future studies.
By comparing the water contact angle and surface roughness (Ra) of samples, it is evident that the water contact angle was reduced by reducing the surface roughness.
CONCLUSION
Within the limitation of this study, the following conclusion can be drawn: All studied ceramics results showed significantly reduced viability of HGF cells than positive control and have time-dependent in vitro biological effects on HGFs. The effects of all ceramics on proliferation are not equal and have different behavior, with CZr and LDS ceramics having the least adverse effect on morphology, proliferation, and adhesion. Furthermore, the most hydrophile sample (lowest water contact angle) was the LDS sample, and the most hydrophobe sample (highest water contact angle) was the TZr sample. The hydrophilicity of the surface is affected by the material type significantly.
Authors contribution
Omid Savabi: Concept and design, data collection, and data interpretation
Farahnaz Nejatidanesh: Critical appraisal and final approval
Morteza Sharifi: Concept and design, data collection, and data interpretation
Mohammadjavad Shirani: Critical appraisal and final approval
Alireza Valanezhad: Critical revision and data interpretation
Kuya Watanabe: Critical revision and data interpretation
Batool Hashemi Beni: Data interpretation and drafting article
Mohammad Khodaei: Critical revision and data interpretation.
Ethical approval
This study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1398.065).
Financial support and sponsorship
This research was supported by the Dental Materials Research Center of Isfahan University of Medical Sciences (Grant#397743).
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
Acknowledgment
This study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.REC.1398.065). This research was supported by the Dental Materials Research Center of Isfahan University of Medical Sciences (Grant#397743).
REFERENCES
- 1.Zarone F, Russo S, Sorrentino R. From porcelain-fused-to-metal to zirconia: Clinical and experimental considerations. Dent Mater. 2011;27:83–96. doi: 10.1016/j.dental.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Zizzari V, Borelli B, De Colli M, Tumedei M, Di Iorio D, Zara S, et al. SEM evaluation of human gingival fibroblasts growth onto CAD/CAM zirconia and veneering ceramic for zirconia. Ann Stomatol (Roma) 2013;4:244–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Xie C, Zhang JF, Li S. Polymer infiltrated ceramic hybrid composites as dental materials. OHDS. 2018;1:2. [Google Scholar]
- 4.Goujat A, Abouelleil H, Colon P, Jeannin C, Pradelle N, Seux D, et al. Mechanical properties and internal fit of 4 CAD-CAM block materials. J Prosthet Dent. 2018;119:384–9. doi: 10.1016/j.prosdent.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Park JH, Park S, Lee K, Yun KD, Lim HP. Antagonist wear of three CAD/CAM anatomic contour zirconia ceramics. J Prosthet Dent. 2014;111:20–9. doi: 10.1016/j.prosdent.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Nejatidanesh F, Savabi G, Amjadi M, Abbasi M, Savabi O. Five year clinical outcomes and survival of chairside CAD/CAM ceramic laminate veneers –A retrospective study. J Prosthodont Res. 2018;62:462–7. doi: 10.1016/j.jpor.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Nejatidanesh F, Amjadi M, Akouchekian M, Savabi O. Clinical performance of CEREC AC Bluecam conservative ceramic restorations after five years –A retrospective study. J Dent. 2015;43:1076–82. doi: 10.1016/j.jdent.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Nejatidanesh F, Moradpoor H, Savabi O. Clinical outcomes of zirconia-based implant- and tooth-supported single crowns. Clin Oral Investig. 2016;20:169–78. doi: 10.1007/s00784-015-1479-3. [DOI] [PubMed] [Google Scholar]
- 9.Wataha JC. Biocompatibility of dental casting alloys: A review. J Prosthet Dent. 2000;83:223–34. doi: 10.1016/s0022-3913(00)80016-5. [DOI] [PubMed] [Google Scholar]
- 10.Abrahamsson I, Berglundh T, Glantz PO, Lindhe J. The mucosal attachment at different abutments. An experimental study in dogs. J Clin Periodontol. 1998;25:721–7. doi: 10.1111/j.1600-051x.1998.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 11.Heitz-Mayfield LJ. Peri-implant diseases: Diagnosis and risk indicators. J Clin Periodontol. 2008;35:292–304. doi: 10.1111/j.1600-051X.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- 12.Rompen E, Domken O, Degidi M, Pontes AE, Piattelli A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin Oral Implants Res. 2006;17(Suppl 2):55–67. doi: 10.1111/j.1600-0501.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 13.Chehroudi B, Gould TR, Brunette DM. The role of connective tissue in inhibiting epithelial downgrowth on titanium-coated percutaneous implants. J Biomed Mater Res. 1992;26:493–515. doi: 10.1002/jbm.820260407. [DOI] [PubMed] [Google Scholar]
- 14.Buser D, Weber HP, Donath K, Fiorellini JP, Paquette DW, Williams RC. Soft tissue reactions to non-submerged unloaded titanium implants in beagle dogs. J Periodontol. 1992;63:225–35. doi: 10.1902/jop.1992.63.3.225. [DOI] [PubMed] [Google Scholar]
- 15.Pabst AM, Walter C, Grassmann L, Weyhrauch M, Brüllmann DD, Ziebart T, et al. Influence of CAD/CAM all-ceramic materials on cell viability, migration ability and adenylate kinase release of human gingival fibroblasts and oral keratinocytes. Clin Oral Investig. 2014;18:1111–8. doi: 10.1007/s00784-013-1098-9. [DOI] [PubMed] [Google Scholar]
- 16.Berglundh T, Lindhe J, Ericsson I, Marinello CP, Liljenberg B, Thomsen P. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2:81–90. doi: 10.1034/j.1600-0501.1991.020206.x. [DOI] [PubMed] [Google Scholar]
- 17.Palaiologou AA, Yukna RA, Moses R, Lallier TE. Gingival, dermal, and periodontal ligament fibroblasts express different extracellular matrix receptors. J Periodontol. 2001;72:798–807. doi: 10.1902/jop.2001.72.6.798. [DOI] [PubMed] [Google Scholar]
- 18.Pae A, Lee H, Kim HS, Kwon YD, Woo YH. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed Mater. 2009;4:025005. doi: 10.1088/1748-6041/4/2/025005. [DOI] [PubMed] [Google Scholar]
- 19.Ferraris S, Giachet FT, Miola M, Bertone E, Varesano A, Vineis C, et al. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater Sci Eng C. 2017;76:1–12. doi: 10.1016/j.msec.2017.02.152. [DOI] [PubMed] [Google Scholar]
- 20.Lee SW, Kim SY, Rhyu IC, Chung WY, Leesungbok R, Lee KW. Influence of microgroove dimension on cell behavior of human gingival fibroblasts cultured on titanium substrata. Clin Oral Implants Res. 2009;20:56–66. doi: 10.1111/j.1600-0501.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 21.Dalby MJ, Giannaras D, Riehle MO, Gadegaard N, Affrossman S, Curtis AS. Rapid fibroblast adhesion to 27nm high polymer demixed nano-topography. Biomaterials. 2004;25:77–83. doi: 10.1016/s0142-9612(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 22.Zheng M, Yang Y, Liu XQ, Liu MY, Zhang XF, Wang X, et al. Enhanced biological behavior of in vitro human gingival fibroblasts on cold plasma-treated zirconia. PLoS One. 2015;10:e0140278. doi: 10.1371/journal.pone.0140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gómez-Florit M, Ramis JM, Xing R, Taxt-Lamolle S, Haugen HJ, Lyngstadaas SP, et al. Differential response of human gingival fibroblasts to titanium- and titanium-zirconium-modified surfaces. J Periodontal Res. 2014;49:425–36. doi: 10.1111/jre.12121. [DOI] [PubMed] [Google Scholar]
- 24.Fischer NG, Wong J, Baruth A, Cerutis DR. Effect of clinically relevant CAD/CAM zirconia polishing on gingival fibroblast proliferation and focal adhesions. Materials (Basel) 2017;10:1358. doi: 10.3390/ma10121358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Könönen M, Hormia M, Kivilahti J, Hautaniemi J, Thesleff I. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J Biomed Mater Res. 1992;26:1325–41. doi: 10.1002/jbm.820261006. [DOI] [PubMed] [Google Scholar]
- 26.Lampin M, Warocquier-Clérout R, Legris C, Degrange M, Sigot-Luizard MF. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J Biomed Mater Res. 1997;36:99–108. doi: 10.1002/(sici)1097-4636(199707)36:1<99::aid-jbm12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Kunzler TP, Drobek T, Schuler M, Spencer ND. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials. 2007;28:2175–82. doi: 10.1016/j.biomaterials.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Kilic K, Kesim B, Sumer Z, Polat Z, Kesim S. In vitro cytotoxicity of all-ceramic substructural materials after aging. J Dent Sci. 2013;8:231–8. [Google Scholar]
- 29.Eldeniz AU, Mustafa K, Ørstavik D, Dahl JE. Cytotoxicity of new resin-, calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J. 2007;40:329–37. doi: 10.1111/j.1365-2591.2007.01211.x. [DOI] [PubMed] [Google Scholar]
- 30.Messer RL, Lucas LC. Evaluations of metabolic activities as biocompatibility tools: A study of individual ions'effects on fibroblasts. Dent Mater. 1999;15:1–6. doi: 10.1016/s0109-5641(99)90023-4. [DOI] [PubMed] [Google Scholar]
- 31.Sjögren G, Sletten G, Dahl JE. Cytotoxicity of dental alloys, metals, and ceramics assessed by millipore filter, agar overlay, and MTT tests. J Prosthet Dent. 2000;84:229–36. doi: 10.1067/mpr.2000.107227. [DOI] [PubMed] [Google Scholar]
- 32.Josset Y, Oum’Hamed Z, Zarrinpour A, Lorenzato M, Adnet JJ, Laurent-Maquin D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J Biomed Mater Res. 1999;47:481–93. doi: 10.1002/(sici)1097-4636(19991215)47:4<481::aid-jbm4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Messer RL, Lockwood PE, Wataha JC, Lewis JB, Norris S, Bouillaguet S. In vitro cytotoxicity of traditional versus contemporary dental ceramics. J Prosthet Dent. 2003;90:452–8. doi: 10.1016/s0022-3913(03)00533-x. [DOI] [PubMed] [Google Scholar]
- 34.Sabaliauskas V, Juciute R, Bukelskiene V, Rutkunas V, Trumpaite-Vanagiene R, Puriene A. In vitro evaluation of cytotoxicity of permanent prosthetic materials. Stomatologija. 2011;13:75–80. [PubMed] [Google Scholar]
- 35.Tetè S, Zizzari VL, Borelli B, De Colli M, Zara S, Sorrentino R, et al. Proliferation and adhesion capability of human gingival fibroblasts onto zirconia, lithium disilicate and feldspathic veneering ceramic in vitro . Dent Mater J. 2014;33:7–15. doi: 10.4012/dmj.2013-185. [DOI] [PubMed] [Google Scholar]
- 36.Raffaelli L, Rossi Iommetti P, Piccioni E, Toesca A, Serini S, Resci F, et al. Growth, viability, adhesion potential, and fibronectin expression in fibroblasts cultured on zirconia or feldspatic ceramics in vitro . J Biomed Mater Res A. 2008;86:959–68. doi: 10.1002/jbm.a.31693. [DOI] [PubMed] [Google Scholar]


