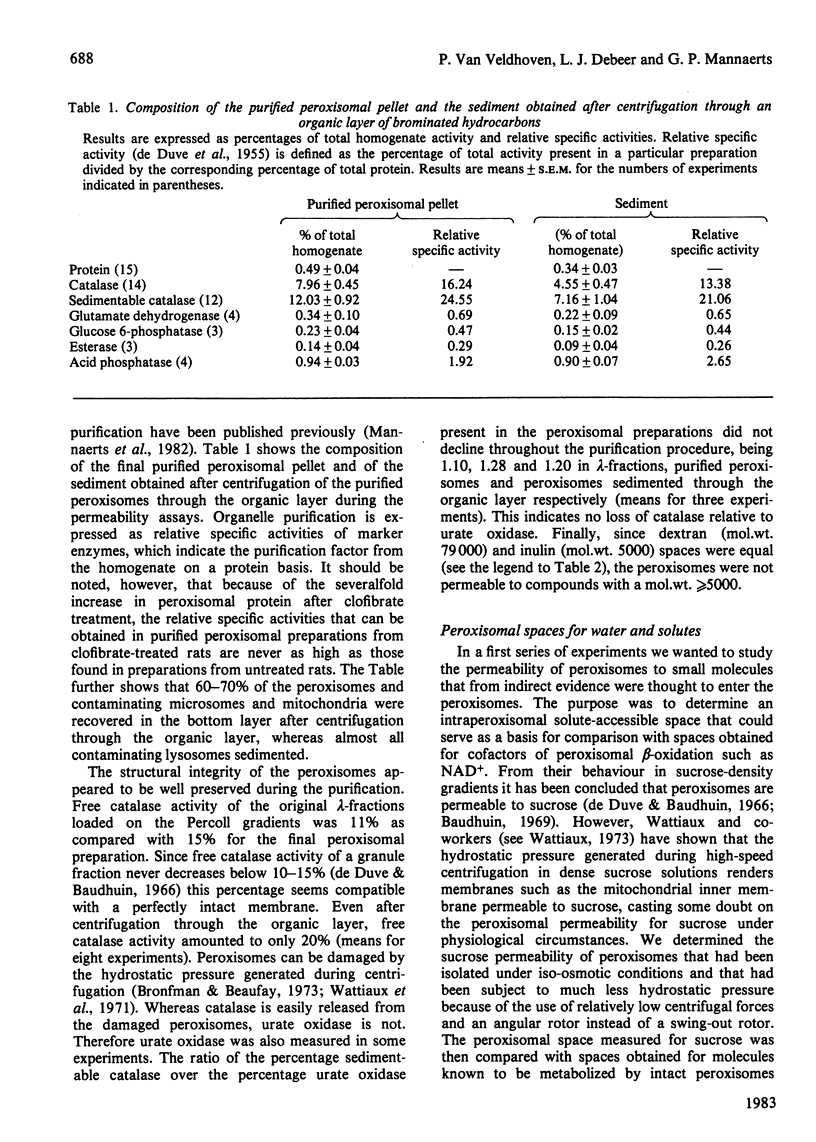
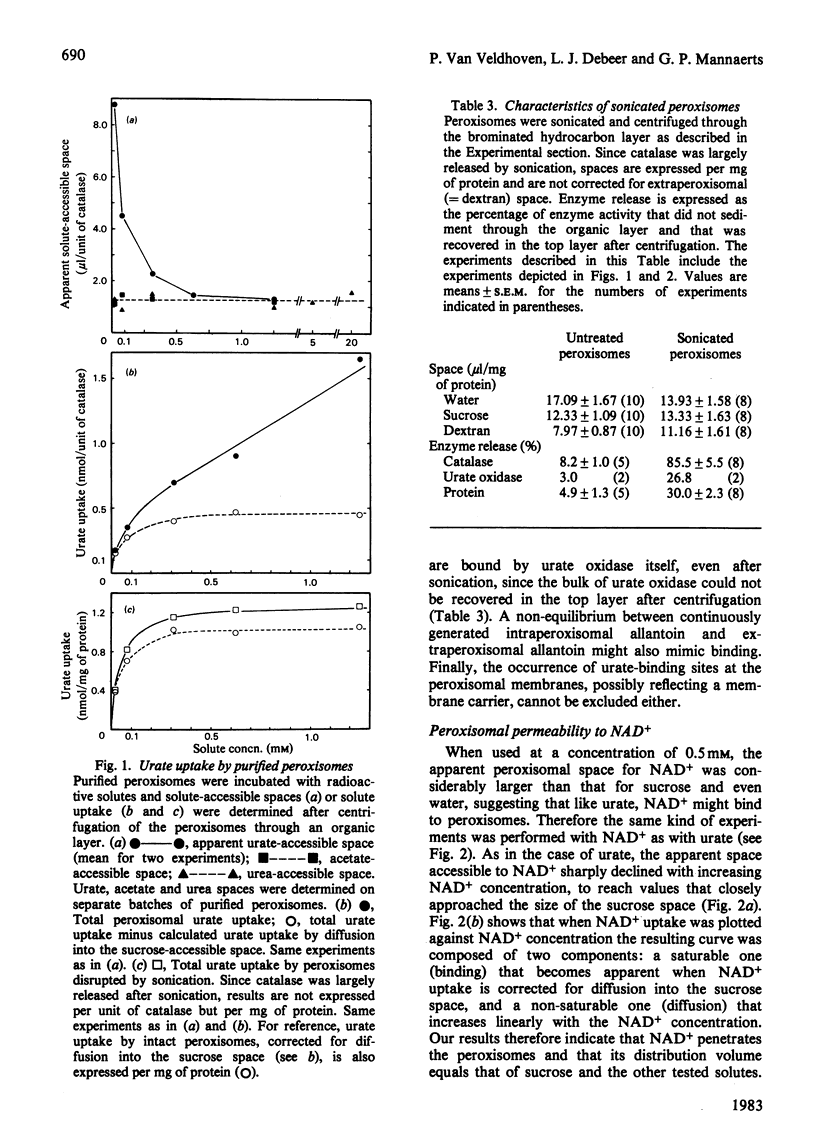
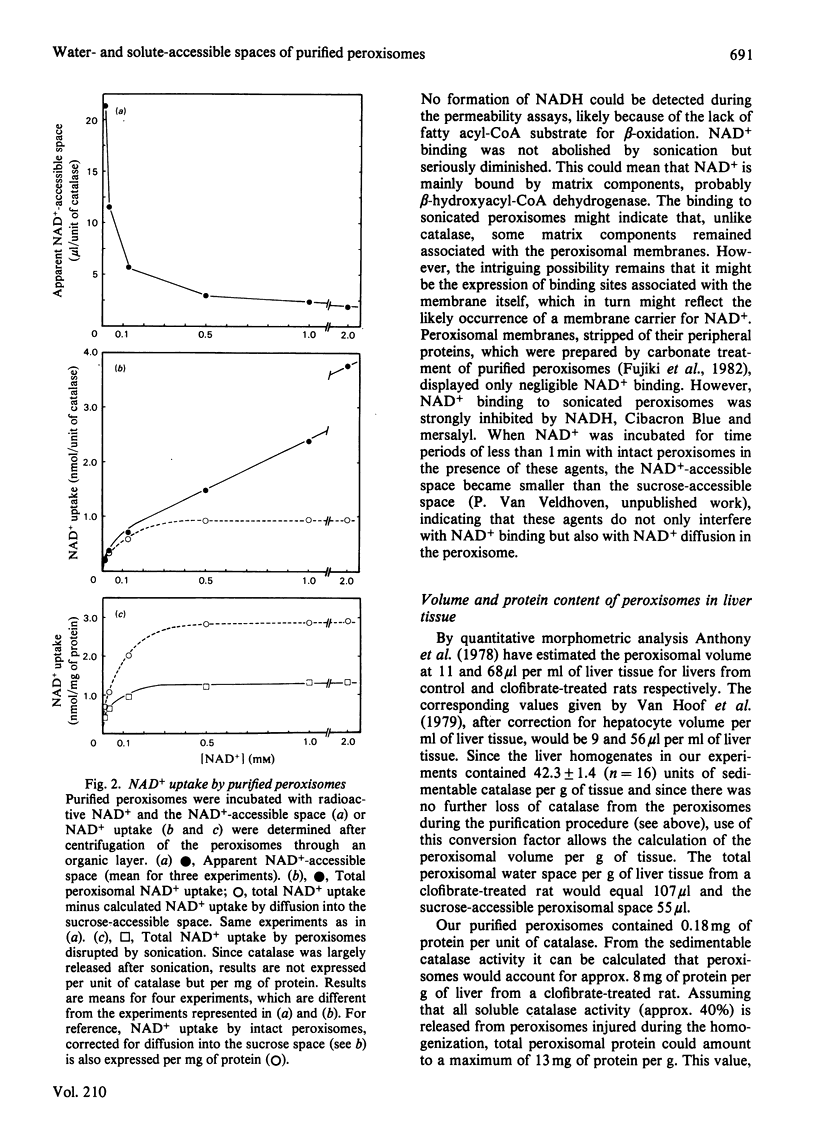
Abstract
Peroxisomes were purified from liver homogenates from rats, treated with the peroxisome proliferator clofibrate, by a combination of differential centrifugation and isopycnic centrifugation in iso-osmotic self-generating Percoll gradients. Structural integrity of the peroxisomes appeared to be preserved as evidenced by a high degree of catalase latency, the absence of catalase release during purification and the exclusion of inulin (mol.wt. +/- 5000). Spaces for water and solutes were measured after incubation of the peroxisomes in iso-osmotic sucrose with radioactive water or solutes and separation of the organelles from their media by centrifugation through an organic layer. Extraperoxisomal water was corrected for by the use of radioactive dextran or inulin. The sucrose, glucose, urea, methanol and acetate-accessible spaces were identical, suggesting that these spaces represent the volume in which molecules that can cross the membrane distribute. This volume equalled 50-65% of the water space. Urate and NAD+, a cofactor of peroxisomal beta-oxidation of fatty acids, also distributed in this volume, but were also partly bound. Urate and NAD+ binding was not abolished by sonication, which released the bulk of matrix catalase activity, but NAD+ binding was seriously diminished. The peroxisomal water and sucrose spaces were estimated to be 107 microliters and 55 microliters per g of liver tissue from a clofibrate-treated rat. From quantitative morphometric data [Anthony, Schmucker, Mooney & Jones (1978) J. Lipid Res. 19, 154-165] and our marker enzyme analyses, as well as from our experimentally determined water spaces of mitochondrial and microsomal fractions, it could be calculated that the volume contamination by lysosomes, mitochondria and microsomes did not exceed 1, 8 and 6% respectively. Our data indicate that apparently intact peroxisomes are permeable to a number of small molecules, including NAD+. Whether the NAD+-binding sites in sonicated peroxisomes mirror the likely existence of a membrane carrier requires further investigation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony L. E., Schmucker D. L., Mooney J. S., Jones A. L. A quantitative analysis of fine structure and drug metabolism in livers of clofibrate-treated young adult and retired breeder rats. J Lipid Res. 1978 Feb;19(2):154–165. [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P. Liver peroxisomes, cytology and function. Ann N Y Acad Sci. 1969 Dec 19;168(2):214–228. doi: 10.1111/j.1749-6632.1969.tb43111.x. [DOI] [PubMed] [Google Scholar]
- Bronfman M., Beaufay H. Alteration of subcellular organelles induced by compression. FEBS Lett. 1973 Oct 15;36(2):163–168. doi: 10.1016/0014-5793(73)80360-6. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Debeer L. J., Thomas J., De Schepper P. J., Mannaerts G. P. Lysosomal triacylglycerol lipase and lipolysis in isolated rat hepatocytes. J Biol Chem. 1979 Sep 25;254(18):8841–8846. [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R., Stäubli W., Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965 Nov 27;208(5013):856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Coloma L., Koenig C. Structure, composition, physical properties, and turnover of proliferated peroxisomes. A study of the trophic effects of Su-13437 on rat liver. J Cell Biol. 1975 Nov;67(2PT1):281–309. doi: 10.1083/jcb.67.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaerts G. P., Debeer L. J., Thomas J., De Schepper P. J. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats. J Biol Chem. 1979 Jun 10;254(11):4585–4595. [PubMed] [Google Scholar]
- Mannaerts G. P., Van Veldhoven P., Van Broekhoven A., Vandebroek G., Debeer L. J. Evidence that peroxisomal acyl-CoA synthetase is located at the cytoplasmic side of the peroxisomal membrane. Biochem J. 1982 Apr 15;204(1):17–23. doi: 10.1042/bj2040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., Allen R. Evidence for two types of rat liver microsomes with differing permeability to glucose and other small molecules. J Biol Chem. 1981 Jun 25;256(12):6413–6422. [PubMed] [Google Scholar]
- Nilsson R., Peterson E., Dallner G. Permeability of microsomal membranes isolated from rat liver. J Cell Biol. 1973 Mar;56(3):762–776. doi: 10.1083/jcb.56.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Thomas J., Debeer L. J., De Schepper P. J., Mannaerts G. P. Factors influencing palmitoyl-CoA oxidation by rat liver peroxisomal fractions. Substrate concentration, organelle integrity and ATP. Biochem J. 1980 Sep 15;190(3):485–494. doi: 10.1042/bj1900485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Broekhoven A., Peeters M. C., Debeer L. J., Mannaerts G. P. Subcellular distribution of coenzyme A: evidence for a separate coenzyme A pool in peroxisomes. Biochem Biophys Res Commun. 1981 May 15;100(1):305–312. doi: 10.1016/s0006-291x(81)80097-6. [DOI] [PubMed] [Google Scholar]
- Van Hoof F., Hue L., Sherratt H. S. Protection of rats against hypoglycin and pent-4-enoate toxicity by pretreatment with clofibrate [proceedings]. Biochem Soc Trans. 1979 Feb;7(1):163–165. doi: 10.1042/bst0070163. [DOI] [PubMed] [Google Scholar]
- Wattiaux R. Effets de la centrifugation sur les mitochondries. Arch Int Physiol Biochim. 1973 May;81(2):343–358. [PubMed] [Google Scholar]


