Abstract
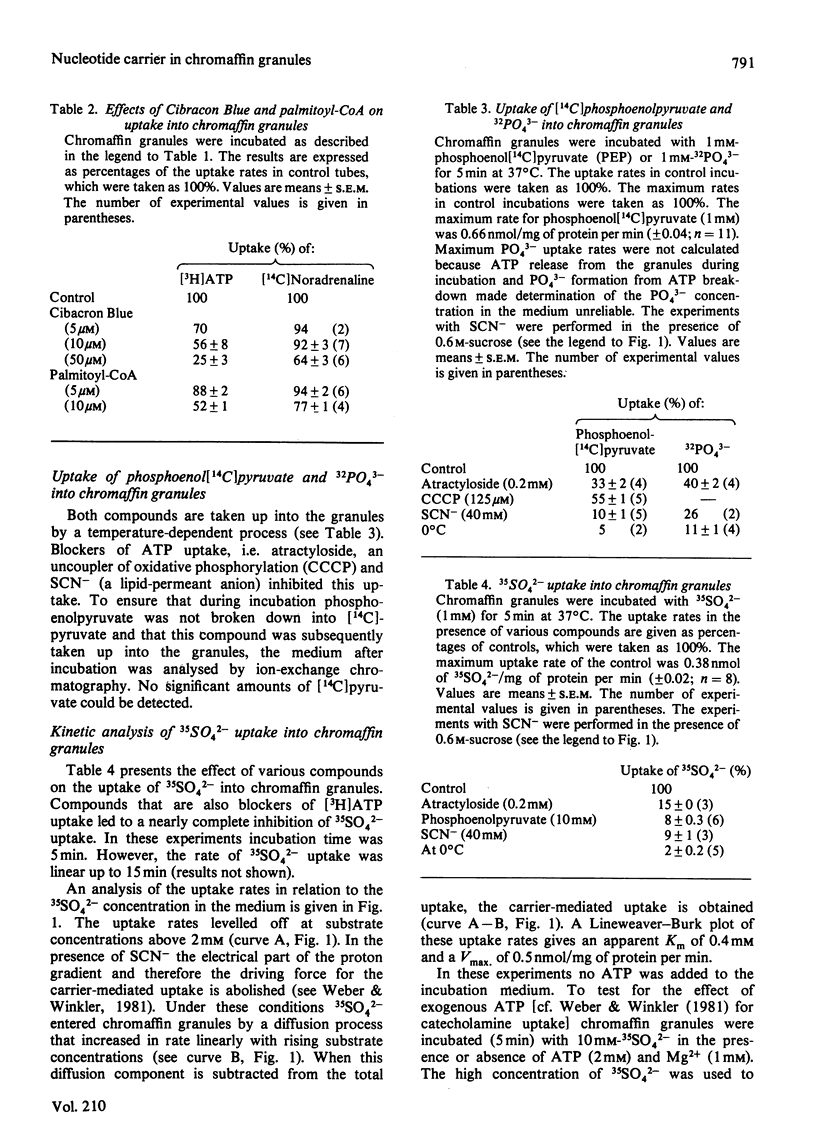
1. The influence of various substances on the uptake of [3H]ATP and [14C]-noradrenaline into isolated bovine chromaffin granules was investigated. The carrier-mediated [3H]ATP uptake is specifically inhibited by SO42-, PO43- and phosphoenolpyruvate. Compounds with carboxylic acid or sulphonic acid groups had no significant inhibitory effects on either uptake. 2. 35SO42-, 32PO43- and phosphoenol[14C]pyruvate are taken up into chromaffin granules by a temperature-dependent process that is inhibited by atractyloside, uncouplers of oxidative phosphorylation and lipid-permeant anions. The apparent Km of 35SO42- uptake is 0.4 mM. 3. These results indicate that the nucleotide carrier in chromaffin granules has a broad specificity, transporting compounds with two strong negative charges. 4. Amino acid probes influence the uptake of ATP and catecholamines differently. Pyridoxal phosphate inhibits both uptake processes, 4,4'-di-isothiocyanostilbene-2,2'-disulphonic acid preferentially blocks ATP uptake, whereas phenylglyoxal blocks only ATP transport. It is suggested that the nucleotide carrier possesses arginine residues in a functionally important position. 5. The significance of these results obtained on isolated granules for the function of chromaffin granules within the cell is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberer W., Kostron H., Huber E., Winkler H. A characterization of the nucleotide uptake of chromaffin granules of bovine adrenal medulla. Biochem J. 1978 Jun 15;172(3):353–360. doi: 10.1042/bj1720353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K., Schatz G. An adenosine triphosphatase isolated from chromaffin-granulate membranes is closely similar to F1-adenosine triphosphatase of mitochondria. Eur J Biochem. 1979 Oct 15;100(2):411–419. doi: 10.1111/j.1432-1033.1979.tb04184.x. [DOI] [PubMed] [Google Scholar]
- BARTELS H., HOHORST H. J. [On the effect of ethionine on the metabolite status of the rat liver]. Biochim Biophys Acta. 1963 Apr 2;71:214–216. doi: 10.1016/0006-3002(63)91011-4. [DOI] [PubMed] [Google Scholar]
- Babel W., Wachter E., Aquila H., Klingenberg M. Amino acid sequence determination of the ADP,ATP carrier from beef heart mitochondria. The sequence of the C-terminal acidolytic fragment. Biochim Biophys Acta. 1981 Sep 29;670(2):176–180. doi: 10.1016/0005-2795(81)90006-4. [DOI] [PubMed] [Google Scholar]
- Block M. R., Lauguin G. J., Vignais P. V. Atractyloside and bongkrekic acid sites in the mitochondrial ADP/ATP carrier protein. An appraisal of their unicity by chemical modifications. FEBS Lett. 1981 Aug 31;131(2):213–218. doi: 10.1016/0014-5793(81)80370-5. [DOI] [PubMed] [Google Scholar]
- Carraway K. L. Covalent labeling of membranes. Biochim Biophys Acta. 1975 Dec 29;415(4):379–410. doi: 10.1016/0304-4157(75)90005-2. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Njus D., Radda G. K., Sehr P. A. Adenosine triphosphate-evoked catecholamine release in chromatin granules. Osmotic lysis as a consequence of proton translocation. Biochem J. 1976 Sep 15;158(3):583–588. doi: 10.1042/bj1580583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B. H., Shrago E. Reversible inhibition of adenine nucleotide translocation by long chain acyl-CoA esters in bovine heart mitochondria and inverted submitochondrial particles. Comparison with atractylate and bongkrekic acid. J Biol Chem. 1977 Oct 10;252(19):6711–6714. [PubMed] [Google Scholar]
- Dean P. D., Watson D. H. Protein purification using immobilised triazine dyes. J Chromatogr. 1979 Oct 1;165(3):301–319. doi: 10.1016/s0021-9673(00)88187-x. [DOI] [PubMed] [Google Scholar]
- Edwards R. A., Woody R. W. Spectroscopic studies of Cibacron Blue and Congo Red bound to dehydrogenases and kinases. Evaluation of dyes as probes of the dinucleotide fold. Biochemistry. 1979 Nov 13;18(23):5197–5204. doi: 10.1021/bi00590a026. [DOI] [PubMed] [Google Scholar]
- Goetz U., Da Prada M., Pletscher A. Adenine-, guanine- and uridine-5'-phosphonucleotides in blood platelets and storage organelles of various species. J Pharmacol Exp Ther. 1971 Jul;178(1):210–215. [PubMed] [Google Scholar]
- HILLARP N. A. Adenosinephosphates and inorganic phosphate in the adrenaline and noradrenaline containing granules of the adrenal medulla. Acta Physiol Scand. 1958 Jun 2;42(3-4):321–332. doi: 10.1111/j.1748-1716.1958.tb01566.x. [DOI] [PubMed] [Google Scholar]
- HORTON H. R., KOSHLAND D. E., Jr A HIGHLY REACTIVE COLORED REAGENT WITH SELECTIVITY FOR THE TRYPTOPHAN RESIDUE IN PROTEINS. 2-HYDROXY-5-NITROBENZYL BROMIDE. J Am Chem Soc. 1965 Mar 5;87:1126–1132. doi: 10.1021/ja01083a033. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Protonmotive force and catecholamine transport in isolated chromaffin granules. J Biol Chem. 1979 May 25;254(10):3750–3760. [PubMed] [Google Scholar]
- Klingenberg M., Appel M. Is there a binding center in the ADP, ATP carrier for substrate and inhibitors? Amino acid reagents and the mechanism of the ADP, ATP translocator. FEBS Lett. 1980 Sep 22;119(1):195–199. doi: 10.1016/0014-5793(80)81029-5. [DOI] [PubMed] [Google Scholar]
- Kostron H., Winkler H., Peer L. J., König P. Uptake of adenosine triphosphate by isolated adrenal chromaffin granules: a carrier-mediated transport. Neuroscience. 1977;2(1):159–166. doi: 10.1016/0306-4522(77)90077-x. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H. On the composition and function of large dense cored vesicles in sympathetic nerves. Neuroscience. 1976;1(2):81–92. doi: 10.1016/0306-4522(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Luqmani Y. A. Nucleotide uptake by isolated cholinergic synaptic vesicles: evidence for a carrier of adenosine 5'-triphosphate. Neuroscience. 1981;6(6):1011–1021. doi: 10.1016/0306-4522(81)90067-1. [DOI] [PubMed] [Google Scholar]
- Morris S. J. The structure and stoichiometry of electric ray synaptic vesicles. Neuroscience. 1980;5(9):1509–1516. doi: 10.1016/0306-4522(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Njus D., Radda G. K. Bioenergetic processes in chromaffin granules a new perspective on some old problems. Biochim Biophys Acta. 1978 Mar 10;463(3-4):219–244. doi: 10.1016/0304-4173(78)90001-0. [DOI] [PubMed] [Google Scholar]
- Pazoles C. J., Creutz C. E., Ramu A., Pollard H. B. Permeant anion activation of MgATPase activity in chromaffin granules. Evidence for direct coupling of proton and anion transport. J Biol Chem. 1980 Aug 25;255(16):7863–7869. [PubMed] [Google Scholar]
- Peer L. J., Winkler H., Snider S. R., Gibb J. W., Baumgartner H. Synthesis of nucleotides in adrenal medulla and their uptake into chromaffin granules. Biochem Pharmacol. 1976 Feb 1;25(3):311–315. doi: 10.1016/0006-2952(76)90220-3. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Allison V. P. Proton translocation of the bovine chromaffin-granule membrane. Biochem J. 1978 Mar 15;170(3):661–672. doi: 10.1042/bj1700661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Morton A. G. Adenosine triphosphate in the bovine chromaffin granule. J Physiol (Paris) 1978;74(5):503–508. [PubMed] [Google Scholar]
- Schmidt W., Winkler H., Plattner H. Adrenal chromaffin granules: evidence for an ultrastructural equivalent of the proton-pumping ATPase. Eur J Cell Biol. 1982 Apr;27(1):96–104. [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Robinson R. L., Van Dyke K., Stitzel R. Studies on the synthesis and release of adenosine triphosphate-8- 3 H in the isolated perfused cat adrenal gland. J Pharmacol Exp Ther. 1972 Jun;181(3):463–471. [PubMed] [Google Scholar]
- Ting L. P., Wang J. H. Effect of phosphate and adenine nucleotides on the rate of labeling of functional groups at the catalytic site of F1-ATPase. J Bioenerg Biomembr. 1980 Aug;12(3-4):79–93. doi: 10.1007/BF00744676. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Weber A., Winkler H. Specificity and mechanism of nucleotide uptake by adrenal chromaffin granules. Neuroscience. 1981;6(11):2269–2276. doi: 10.1016/0306-4522(81)90016-6. [DOI] [PubMed] [Google Scholar]
- Winkler H., Hörtnagl H., Smith A. D. Membranes of the adrenal medulla. Behaviour of insoluble proteins of chromaffin granules on gel electrophoresis. Biochem J. 1970 Jun;118(2):303–310. doi: 10.1042/bj1180303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Schöpf J. A., Hörtnagl H. Bovine adrenal medulla: subcellular distribution of newly synthesised catecholamines, nucleotides and chromogranins. Naunyn Schmiedebergs Arch Pharmacol. 1972;273(1):43–61. doi: 10.1007/BF00508079. [DOI] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]


