Abstract
Background
Follicular lymphoma (FL) is the most common indolent and second most common Non‐Hodgkin`s lymphoma (NHL) in the Western world. Standard treatment usually includes rituximab and chemotherapy. High‐dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) is an option for patients in advanced stages or for second‐line therapy, leading to improved progression‐free survival (PFS) rates. However, the impact of HDT and ASCT remains unclear, as there are hints of an increased risk of second cancers.
Objectives
We performed a systematic review with meta‐analysis of randomised controlled trials (RCTs) comparing HDT plus ASCT with chemotherapy or immuno‐chemotherapy in patients with FL with respect to overall survival (OS), PFS, treatment‐related mortality (TRM), adverse events and secondary malignancies.
Search methods
We searched CENTRAL, MEDLINE, and EMBASE as well as conference proceedings from January 1985 to September 2011 for RCTs. Two review authors independently screened search results.
Selection criteria
Randomised controlled trials comparing chemotherapy or immuno‐chemotherapy with HDT followed by ASCT in adults with previously untreated or relapsed FL.
Data collection and analysis
We used hazard ratios (HR) as effect measures used for OS and PFS as well as relative risks for response rates. Two review authors independently extracted data and assessed the quality of trials.
Main results
Our search strategies led to 3046 potentially relevant references. Of these, five RCTs involving 1093 patients were included; four trials in previously untreated patients and one trial in relapsed patients. Overall, the quality of the five trials is judged to be moderate. All trials were reported as randomised and judged to be open‐label studies, because usually trials evaluating stem cell transplantation are not blinded. Due to the small number of studies in each analysis (four or less), the quantification of heterogeneity was not reliable and not evaluated in further detail. A potential source of bias are uncertainties in the HR calculation. For OS, the HR had to be calculated for three trials from survival curves, for PFS for two trials.
We found a statistically significant increased PFS in previously untreated FL patients in the HDT + ASCT arm (HR = 0.42 (95% confidence interval (CI) 0.33 to 0.54; P < 0.00001). However, this effect is not transferred into a statistically significant OS advantage (HR = 0.97; 95% 0.76 to 1.24; P = 0.81). The subgroup of trials adding rituximab to both intervention arms (one trial) confirms these results and the trial had to be stopped early after an interim analysis due to a statistically significant PFS advantage in the HDT + ASCT arm (PFS: HR = 0.36; 95% CI 0.23 to 0.55; OS: HR = 0.88; 95% CI 0.40 to 1.92). In the four trials in previously untreated patients there are no statistically significant differences between HDT + ASCT and the control‐arm in terms of TRM (RR = 1.28; 95% CI 0.25 to 6.61; P = 0.77), secondary acute myeloid leukaemia/myelodysplastic syndromes (RR = 2.87; 95% CI 0.7 to 11.75; P = 0.14) or solid cancers (RR = 1.20; 95% CI 0.25 to 5.77; P = 0.82). Adverse events were rarely reported and were observed more frequently in patients undergoing HDT + ASCT (mostly infections and haematological toxicity).
For patients with relapsed FL, there is some evidence (one trial, N = 70) that HDT + ASCT is advantageous in terms of PFS and OS (PFS: HR = 0.30; 95% CI 0.15 to 0.61; OS: HR = 0.40; 95% CI 0.18 to 0.89). For this trial, no results were reported for TRM, adverse events or secondary cancers.
Authors' conclusions
In summary, the currently available evidence suggests a strong PFS benefit for HDT + ASCT compared with chemotherapy or immuno‐chemotherapy in previously untreated patients with FL. No statistically significant differences in terms of OS, TRM and secondary cancers were detected. These effects are confirmed in a subgroup analysis (one trial) adding rituximab to both treatment arms. Further trials evaluating this approach are needed to determine this effect more precisely in the era of rituximab. Moreover, longer follow‐up data are necessary to find out whether the PFS advantage will translate into an OS advantage in previously untreated patients with FL.
There is evidence that HDT + ASCT is advantageous in patients with relapsed FL.
Keywords: Female; Humans; Antibodies, Monoclonal, Murine‐Derived; Antibodies, Monoclonal, Murine‐Derived/therapeutic use; Antineoplastic Combined Chemotherapy Protocols; Antineoplastic Combined Chemotherapy Protocols/therapeutic use; Combined Modality Therapy; Combined Modality Therapy/methods; Combined Modality Therapy/mortality; Disease‐Free Survival; Hematopoietic Stem Cell Transplantation; Hematopoietic Stem Cell Transplantation/methods; Immunologic Factors; Immunologic Factors/therapeutic use; Lymphoma, Follicular; Lymphoma, Follicular/mortality; Lymphoma, Follicular/therapy; Neoplasms, Second Primary; Neoplasms, Second Primary/etiology; Randomized Controlled Trials as Topic; Randomized Controlled Trials as Topic/mortality; Recurrence; Rituximab; Transplantation, Autologous; Whole‐Body Irradiation; Whole‐Body Irradiation/methods
Plain language summary
Treatment of follicular lymphoma
Follicular lymphoma is a malignancy of the lymphatic system and a common type of non‐Hodgkin lymphoma. Follicular lymphoma arises from B‐cells, mainly affects older adults and because of its slow growth it is called an indolent lymphoma. Follicular lymphoma grows unnoticed for a long time and is recognised by lymph node enlargement, fever, weight loss, sweating or fatigue. It is called follicular lymphoma because affected lymph nodes show rounded structures called "follicles". Using computer tomography scans, bone marrow biopsy and blood tests, follicular lymphoma is classified into the early Ann Arbor stages I and II or the advanced Ann Arbor stages III or IV, which are diagnosed in the majority of patients. Prognosis and therapy are related to the extent of the disease at initial diagnosis. The small number of patients in stages I or II may be cured by radiotherapy. In advanced stages III or IV, patients are regarded as incurable. Chemotherapy plus the monoclonal antibody rituximab is considered as current treatment strategy for symptomatic patients in advanced stages. Positive effects of high‐dose therapy with transplantation of patients' own stem cells (autologous) are known for patients in advanced stages, especially for the endpoint progression‐free survival. However, this treatment option could have comparatively more treatment‐related late side effects than chemotherapy, including secondary malignancies.
With this assumption, we assessed the role of high‐dose therapy followed by autologous stem cell transplantation in the treatment of follicular lymphoma in adults. We included five trials with 1093 patients in the main analyses. As a result, the meta‐analyses for previously untreated patients (four trials) show no statistical significant differences in terms of survival, treatment‐related mortality or secondary malignancies between the patients treated with high‐dose therapy followed by autologous stem cell transplantation and those treated with chemotherapy only. However, progression‐free survival (tumour control), was significantly improved by the high‐dose chemotherapy and stem cell transplantation. Adverse events are more common in patients treated with high‐dose therapy followed by autologous stem cell transplantation.
There is an advantage of the high‐dose chemotherapy and stem cell transplantation for patients with a relapse of the disease, both in survival and in tumour control (one trial). No data on adverse events are reported in this trial.
Summary of findings
for the main comparison.
| High‐dose chemotherapy plus ASCT versus chemotherapy for adult previously untreated patients with follicular lymphoma | ||||||
|
Patient or population: Adult patients with follicular lymphoma Intervention: High‐dose chemotherapy plus ASCT Comparison: Chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | High‐dose chemotherapy plus ASCT | |||||
|
Overall survival Follow‐up: median 5 years |
Moderate risk population 2 | HR 0.97 (0.76 to 1.22) | 701 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 200 per 1000 | 195 per 1000 (125 to 235) | |||||
| Treatment‐related mortality | Study population | RR 1.28 (0.25 to 6.61) | 941 (4 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 12 per 1000 | 16 per 1000 (3 to 82) | |||||
| Moderate risk population | ||||||
| 11 per 1000 | 14 per 1000 (3 to 73) | |||||
| Progression‐free survival Follow‐up: mean 5 years | Low risk population | HR 0.42 (0.33 to 0.54) | 540 (3 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 560 per 1000 | 297 per 1000 (237 to 538) | |||||
| High risk population | ||||||
| 880 per 1000 | 598 per 1000 (503 to 682) | |||||
|
Secondary malignancies AML/MDS |
Study population | RR 2.87 (0.7 to 11.75) | 1023 (4 studies) | ⊕⊕⊝⊝ low1,3 | As all trials had a median observation time of less than 10 years, long‐term information on secondary malignancies cannot be expected. | |
| 11 per 1000 | 32 per 1000 (8 to 132) | |||||
| Medium risk population | ||||||
| 14 per 1000 | 40 per 1000 (10 to 164) | |||||
|
Secondary malignancies Solid cancer |
Study population | RR 1.2 (0.25 to 5.77) | 701 (3 studies) | ⊕⊕⊝⊝ low1,3 | As all trials had a median observation time of less than 10 years, long‐term information on secondary malignancies cannot be expected. | |
| 37 per 1000 | 44 per 1000 (9 to 211) | |||||
| Medium risk population | ||||||
| 46 per 1000 | 55 per 1000 (11 to 265) | |||||
| Adverse events | see comment | Not estimable | 527 (3 studies) | ⊕⊝⊝⊝ very low1,2 | Acute adverse effects were seldom reported and differ across the three reporting studies. They are higher in the HDT + ASCT arm (haematological, non‐haematological, infection, see Table 2) | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; HR: Hazard Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There might be some heterogeneity between trials.
2 The risk for the low risk population (of patients with follicular lymphoma) was taken from the trial with the highest rates of survival at 5 years (GELF94 trial). The high risk rate is approximately the risk in the CUP‐trial, for relapsed patients.
3 No precise estimation of effect, large confidence interval.
4 Outcome assessors not blinded. This could cause bias.
5Two of the three trials reporting adverse events report a few adverse events only 6 Two of five trials did not report any adverse event
1. Adverse Events.
| CUP trial | GLGSG trial | GITMO/IIL | GOELAMS | GELA/GELF 94 | |
| Number of patients evaluated |
ASCT arm: 46 CT arm: 24 |
ASCT arm: 105 CT arm: 122 |
ASCT arm: 68 CT arm: 66 |
ASCT arm: 86 CT arm: 80 |
ASCT arm: 209 CT arm: 192 |
| Anemia | N.R. | ASCT arm: 44.8% CT arm: 0.8% |
N.R. | N.R. | N.R. |
| Leucocytopenia | N.R. | ASCT arm: 96.2% CT arm: 51.3% |
N.R. | N.R. | N.R. |
| Granulocytopenia | N.R. | ASCT arm: 90.5% CT arm: 37.8% |
N.R. | N.R. | N.R. |
| Thrombocytopenia | N.R. | ASCT arm: 96.2% CT arm: 4.2% |
N.R. | N.R. | N.R. |
| Mucositis | N.R. | ASCT arm: 53.3% CT arm: 0 |
N.R. | N.R. | N.R. |
| Infections | N.R. | ASCT arm: 23.1% CT arm: 1.7% |
ASCT arm: 13.2% CT arm: 6.1% |
ASCT arm: 17.4% CT arm: N.R. |
N.R. |
| Nausea | N.R. | ASCT arm: 32.4% CT arm: 1.7% |
N.R. | N.R. | N.R. |
| diarrhoea | N.R. | ASCT arm: 13.5% CT arm: 1.7% |
N.R. | N.R. | N.R. |
| pulmonary | N.R. | ASCT arm: 4.8% CT arm: 0.9% |
N.R. | ASCT arm: 2.3% interstitial pneumonitis CT arm: N.R. |
N.R. |
| liver | N.R. | ASCT arm: 3.8% CT arm: 0.9% |
N.R. | N.R. | N.R. |
| Renal | N.R. | ASCT arm: 1% CT arm: 0 |
N.R. | ASCT arm: 1.2% haemorrhagic cystitis CT arm: N.R. |
N.R. |
| muscle/bone pain | N.R. | ASCT arm: 2.1% CT arm: 11% |
N.R. | N.R. | N.R. |
| Depression | N.R. | ASCT arm: 1.1% CT arm: 4.9% |
N.R. | N.R. | N.R. |
| Extrahematological toxicities | N.R. | mentioned above | ASCT arm: 38.2% CT arm: 10.6% |
mentioned above | N.R. |
Background
Description of the condition
Follicular lymphoma (FL) is the most common indolent and second most common Non‐Hodgkin`s lymphoma (NHL) in the Western world, accounting for > 20% of the approximately 56,000 new cases of NHL in the U.S. yearly (Armitage 1998; Fisher 2004; Jemal 2005; Higgins 2011a). In contrast to other kinds of cancer, the incidence and mortality of this B‐cell malignancy rises continuously and has doubled in the last three decades, especially in the U.S. and Europe (Anderson 1998; Groves 2000; Clarke 2002 ; Muller 2005).
The World Health Organization (WHO) divides FL into three major grades based on the number of centroblasts per high‐power field (Harris 1999). WHO Grade 1, 2 and 3a correspond to indolent lymphomas. However, the subdivided grade 3b is strictly speaking an aggressive disease and more closely related to diffuse large B‐cell lymphoma, the most common highly malignant lymphoma (Ott 2002). Prognosis and therapy of FL are dependent on the respective Ann Arbor stages. Less than 20 % of patients, situated in Ann Arbor I / II, are treated by curative radiotherapy, applied as extended or involved field irradiation (Hiddemann 2006; Lau 2006; Schulz 2007; Brown 2009). All remaining patients diagnosed at advanced‐stage disease are regarded as incurable (Andreadis 2005; Foster 2009). In up to 25% to 35% of patients, FL transforms to a high‐grade lymphoma with a poor prognosis because of resistance to therapy (Horning 2000). In advanced‐stage, FL relapses in shorter intervals, so that concerned patients die after a median survival time of 8 to 10 years because of progressive disease (Horning 1984; Gallagher 1986; Egger 1997; Moher 1999).
Up to 95% of the patients with FL show the translocation t(14;18), which leads to an over‐expression of the BCL‐2 protein (Korsmeyer 1992). This protein, which has an inhibitory effect on apoptosis (programmed cell death), is a diagnostic help in the differentiation between malignant and reactive follicles (Symmans 1995; Diaz‐Alderete 2008). To improve overall survival (OS), different patterns of chemotherapy were prescribed, but these early applied therapies compared with the 'wait and watch' strategy in advanced‐stage did not show advantage concerning OS. That is why only symptomatic patients or those with a high tumour burden are treated (Ardeshna 2003). The natural history of FL includes a high initial rate of response to chemotherapy and radiotherapy. In the past, conventional chemotherapy regimens consisting of single and multiple drug alkylating agent‐based therapies were used in primary treatment for patients with advanced‐stage FL. Despite various chemotherapy combinations in the last decade, no improvement of OS was achieved (Oliansky 2010). Compared with different patterns of chemotherapy such as alkylating agent‐based or anthracycline‐containing regimens, which did not exceed a median response duration of 1.5 to 3 years despite a response rate of 60% to 70%, Rohatiner et al showed, that chemotherapy combined with interferon α were superior with regard to prolonged OS (Brandt 2001; Reiser 2002; Rohatiner 2005). The status of interferon as maintenance therapy for patients with FL remains unclear, although a progression‐free survival (PFS) advantage is reported in a systematic review (Baldo 2010). In the review, interferon was associated with significant toxicities that may have an impact on a patient's quality of life.
In the last few years, progress in the therapy of FL has been recorded (Herold 2007). Monoclonal antibodies, especially rituximab, have shown a proven activity in the therapy of FL and NHL, in salvage therapy as well as in therapy for relapsed FL (Schulz 2007; Vidal 2009).
Description of the intervention
In spite of initially high response rates, FL is usually not curable so that all patients will suffer a relapse (Buske 2007). Non‐myeloablative allogeneic stem cell transplantation is a potentially curative treatment option, but long‐term effectiveness and toxicity of this strategy are unknown, although there are early promising results in a non‐randomised trial (Khouri 2008). Another potentially curative therapy is high‐dose therapy (HDT) with autologous stem cell transplantation (ASCT) (Buske 2005). This regimen has been compared with conventional regimens of chemotherapy, primarily in relapsed FL, later on in first remission. Although randomised trials show promising results concerning prolongation of response duration and relapse rate for patients in first remission as well as relapsed patients, there is still disagreement regarding OS and potential disadvantages due to toxicity and an increased rate of secondary malignancies (Colombat 2001; Forstpointner 2004; Lenz 2004; Lenz 2004a; Deconinck 2005; Hiddemann 2005; Marcus 2005; Hiddemann 2006; Sebban 2006; Van Oers 2006; Weigert 2006; Buske 2007; Herold 2007; Sacchi 2007).
How the intervention might work
Myeloablative doses of chemotherapy or irradiation permits the application of higher doses of anti‐cancer therapy and provides potentially better tumour control. On the other hand, this therapy leads to various adverse events, including damage to the bone marrow and decreased production of leucocytes (white blood cells), thrombocytes (platelets) and erythrocytes (red blood cells). To restore the bone marrow's ability to produce blood cells, the patient receives stem cells from his own body, called autologous stem cell transplantation. These cells are mobilised and collected in advance, frozen and stored and returned to the patient after HDT.
Why it is important to do this review
At this stage, no systematic review or meta‐analysis of ASCT in FL patients are available. Because of the uncertainties of HDT followed by ASCT mentioned above, we undertook this review. We aimed to obtain more evidence regarding the clinical benefit (OS, PFS) and the therapy‐related risks (treatment related mortality (TRM), adverse events), by systematically analysing the reliability and validity of the data and by considering only RCTs for our review.
Objectives
To compare the effectiveness of HDT with ASCT to chemotherapy or immuno‐chemotherapy in patients with newly diagnosed or relapsed FL.
Methods
Criteria for considering studies for this review
Types of studies
Any published (including Internet publication) or unpublished RCTs (including cluster randomised trials) were eligible for inclusion in the review. We did not apply time or language restrictions.
Types of participants
Adult male and female patients (≥ 18 years of age) with a confirmed diagnosis of FL.
Types of interventions
The main intervention was HDT with ASCT compared with chemotherapy and immuno‐chemotherapy. We considered any chemotherapeutic and immunochemotherapeutic regimen for comparison.
Types of outcome measures
Primary outcomes
Overall survival (OS) was evaluated as the primary efficacy endpoint
Secondary outcomes
Progression‐free survival (PFS)
Response rate (RR)
Qualitiy of life (Qol)
Treatment‐related mortality (TRM)
Adverse events
Secondary malignancies
Search methods for identification of studies
Electronic searches
We adopted search strategies from those suggested in Chapter Six of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). To reduce language bias we did not apply language restriction.
The search covered major medical databases from 1985 to September 2011:
the Cochrane Central Register of Controlled Trials (CENTRAL), see Appendix 1 and Appendix 2;
MEDLINE, for search strategy see Appendix 3 and
EMBASE, see Appendix 4.
We searched conference proceedings of annual meetings of the following societies not included in CENTRAL for abstracts:
ASH (American Society of Hematology) 2007 to 2010
ASCO (American Society of Clinical Oncology) 2007 to 2010
EBMT (European Group for Bone and Marrow Transplantation) 2007 to 2010.
We searched the database of ongoing trials: Metaregister of controlled trials:
www.controlled‐trials.com/mrct/
www.eortc.be/
www.ctc.usyd.edu.au/
www.trialscentral.org/index.html
Searching other resources
We handsearched references.
References of all identified trials, relevant review articles and current treatment guidelines
Data collection and analysis
Selection of studies
Two review authors (MS, NS) screened the titles and abstracts of studies identified from the above sources. At the first screening, we discarded studies that were clearly ineligible. If this could not be done satisfactorily based on title and abstract, we obtained the full text version and discussed eligibility. The aim was to be overly inclusive rather than to risk losing relevant studies. We assessed selected studies using an eligibility form to determine whether they met the inclusion criteria; we resolved any disagreement by discussion. If necessary, we sought further information from the authors where articles contained insufficient data to make a decision about eligibility. The eligibility form contained the following questions.
Is the study described as randomised?
Is the diagnosis of FL histologically confirmed?
Were the participants in the experimental group treated by HDT and ASCT?
Were the participants in the control group treated by chemotherapy or immunochemotherapy?
Data extraction and management
Two review authors (MS, NS) independently extracted data concerning details of study population, intervention and outcomes using a standardised data extraction form. This form included the following terms.
General information: author, title, source, publication date, country, language, duplicate publications.
Quality assessments: sequence generation, allocation concealment, blinding (participants, personnel, outcome assessors), incomplete outcome data, selective outcome reporting, other sources of bias.
Study characteristics: trial design, aims, setting and dates, source of participants, inclusion/exclusion criteria, treatment allocation, comparability of groups, subgroup analysis, statistical methods, power calculations, treatment cross‐overs, compliance with assigned treatment, length of follow‐up, time point of randomisation (upfront, after induction).
Participant characteristics: age, gender, ethnicity, number of participants recruited / allocated / evaluated, participants lost to follow‐up, additional diagnoses, percentage actually receiving transplant; prognostic factors
Interventions: setting, type of (multi‐agent) chemotherapy (intensity of induction and conditioning regimen, number of cycles, with or without radiation), stem cell source (bone marrow or peripheral blood); transplantation with or without growth factor support, transplant details, infection prophylaxis, type of maintenance treatment, type of salvage treatment.
Outcomes: OS, PFS, relapse rate, TRM, adverse events, QoL.
Where possible, we sought missing data from the authors.
Assessment of risk of bias in included studies
Two review authors (MS, NS) evaluated independently all included trials using a list of selected quality criteria according to the recommendations in Chapter Eight of the Cochrane Handbook for Systematic Reviews of Interventions for the following criteria (Higgins 2011).
Sequence generation.
Allocation concealment.
Blinding (participants, personnel, outcome assessors).
Incomplete outcome data.
Selective outcome reporting.
Other sources of bias.
The review authors judged each criteria, based on a three‐point scale ("Yes" (low risk of bias), "No" (high risk of bias), "Unclear") and a summary description. We resolved disagreement by consensus. The review authors were not blinded to names of authors, institutions, journals, or the outcomes of the trials.
Measures of treatment effect
For binary outcomes, we calculated risk ratios (RR) with 95% confidence intervals (CI) for each trial. We planned to calculate continuous outcomes as mean difference (MD), but no continuous data were included. For time‐to‐event outcomes we extracted the hazard ratio (HR) from published data according to Parmar 1998 and Tierney 2007.
Dealing with missing data
We planned to follow the general recommendations for dealing with missing data in Cochrane reviews (Higgins 2011b):
Whenever possible, we contacted the original investigators to request missing data.
We would have clearly stated the assumptions of any methods used to cope with missing data (e.g. imputation of missing data and accounting for the fact that these were imputed with uncertainty).
Assessment of heterogeneity
Because of the small number of studies in each analysis (two), the quantification of heterogeneity was not reliable. In meta‐analyses with more trials, we would have assessed heterogeneity of treatment effects between trials using a Chi² test with a significance level at P < 0.1. In that case, we would have used the I² statistic to quantify possible heterogeneity (I² > 30% moderate heterogeneity, I² > 75% considerable heterogeneity) (Deeks 2011). We explored potential causes of heterogeneity by sensitivity and subgroup analyses where possible.
Assessment of reporting biases
We would have explored potential publication bias in meta‐analyses with at least 10 trials by generating a funnel plot and statistically testing by means of a linear regression test. We would have considered a P value < 0.1 as significant for this test (Sterne 2011).
Data synthesis
We performed analyses according to the recommendations of chapter nine of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We used aggregated data for analysis. For statistical analysis, we entered data into the Cochrane statistical package Review Manager 5 (Review Manager (RevMan)). One review author (MS) entered data into the software and a second review author (NS) checked it for accuracy. We performed meta‐analyses using a random‐effects model (for example, the generic inverse variance method for survival data outcomes and Mantel‐Haenszel method for dichotomous data outcomes). We used the random‐effects model in terms of sensitivity analyses.
Subgroup analysis and investigation of heterogeneity
We assessed heterogeneity of treatment effects between trials by using a CHI² test with a significance level at P < 0.1. The I² statistic was used to quantify possible heterogeneity. Wwe performed subgroup analyses on the following characteristics.
Patients treated as front line, refractory or relapse with these treatments.
Subgroup analyses by age, sex or gender were not possible due to the limited amount of data available. Because of the same type of stem cell source in all trials, we did not perform subgroup analysis for this factor. Due to differences of type and intensity of preparative regimen in each trial, we also excluded subgroup analysis in this regard. Subgroup analysis of prognostic factors was left out because they were not reported in any one of the included trials.
Sensitivity analysis
Quality components, including full text publications/abstracts, preliminary results versus mature results.
Fixed‐effect modelling versus random‐effects modelling.
Results
Description of studies
Results of the search
Our literature search produced 3046 potentially relevant references related to treatment of patients with FL. Of these, we excluded 2987 at the initial stage of screening because they did not fulfil our predefined inclusion criteria. The remaining 59 publications were retrieved as full text publications or abstract publications for detailed evaluation. Of these 59 publications, 40 were excluded and finally five trials (19 publications) with 1093 patients were formally included in the main analyses of this review. The overall number of trials screened, identified, selected, excluded and included was documented with reasons according to PRISMA flow diagram (see Figure 1) (Moher 2009).
1.
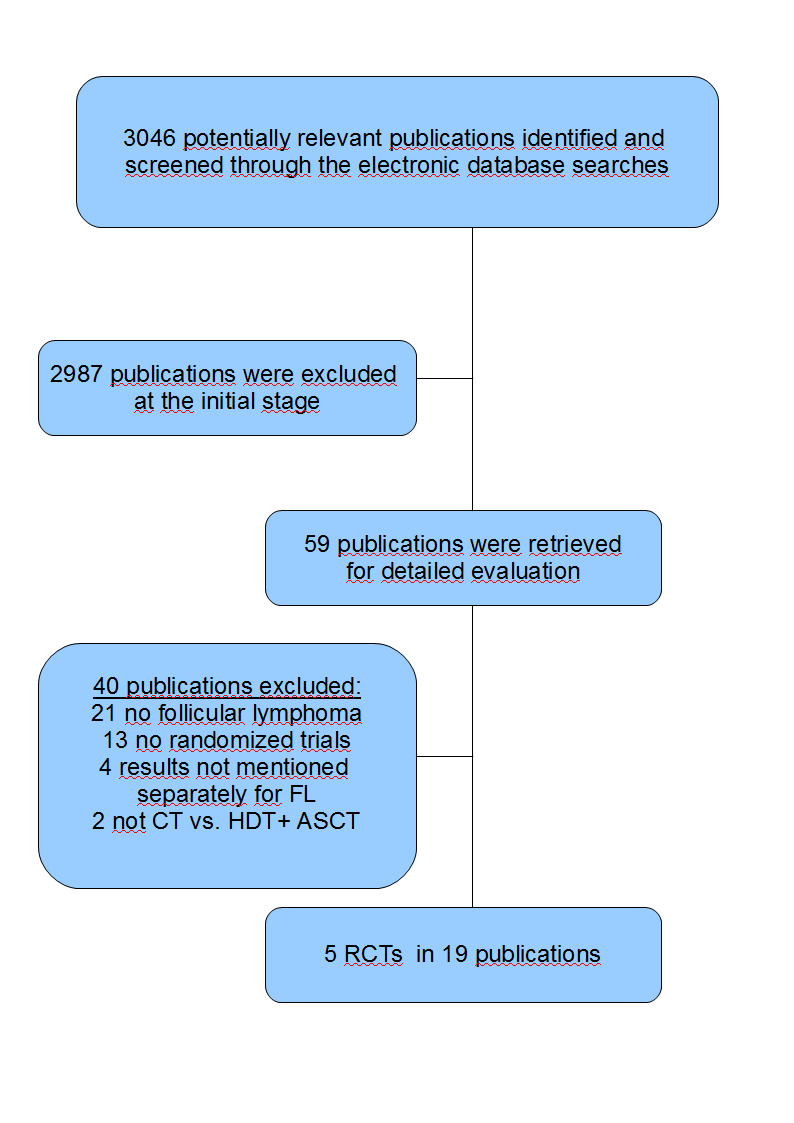
PRISMA flow diagram
Included studies
The characteristics of included trials are also summarised in Characteristics of included studies.
We included five trials (CUP trial; GELA/GELF‐94; GITMO/IIL; GLSG; GOELAMS 064) in the review; four trials in previously untreated patients (GELA/GELF‐94; GITMO/IIL; GLSG; GOELAMS 064) and one trial in patients with relapsed FL (CUP trial). The earliest trial recruited in the time period between 1993 and 1997 and the latest between 2000 and 2005. We extracted the data from full text publications for all trials.
Design
Of the five included trials, four trials were two‐armed RCTs and one trial was a three‐armed RCT (CUP trial). The CUP trial randomised patients to chemotherapy only, unpurged stem cells and purged stem cells. We evaluated the two arms (purged and unpurged) together in this review. Of the five multi‐centre trials, three were national (GITMO/IIL; GLSG; GOELAMS 064) and two were international (CUP trial; GELA/GELF‐94). The control arm consisted in one trial of chemotherapy only (CUP trial) and in three trials of chemotherapy plus interferon (GELA/GELF‐94; GLSG; GOELAMS 064). In one trial, the chemotherapy arm was supplemented by the monoclonal antibody rituximab (GITMO/IIL).
Sample size
The smallest trial (CUP trial) randomised 89 patients (70 analysed) and the largest trial 401 patients (GELA/GELF‐94).
Location
The included trials came from a range of research groups from different countries. The trials were conducted in the following countries: one trial in Germany (GLSG); one trial in France and Belgium (GELA/GELF‐94), one trial in different centres of European countries (CUP trial); one trial in France (GOELAMS 064) and one trial in Italy (GITMO/IIL).
Participants
A total of 1.105 male and female patients with histologically proven FL were randomised. For the majority of the patients, histopathologic diagnosis was made according to the Working‐Formulation criteria of the National Cancer Institute and reviewed according to the REAL classification. One thousand and ninety‐three of the randomised patients were evaluated.
Interventions
Patients from included trials were treated either with HDT + ASCT or chemotherapy/immunochemotherapy.
The HDT consisted of CHOP cyclophosphamide and total body irradiation (TBI) in the CUP trial; cyclophosphamide, high‐dose doxorubicin, prednisone, and vincristine (VCAP), ifosamide, methotrexate, and VP‐16 (IMVP‐16) and TBI in the GOELAMS 064 trial; and dexamethasone, cBCNU, melphalan, etoposide, and cytarabine (Dexa‐BEAM), cyclophosphamide and TBI in the GLSG trial. In the GELA/GELF‐94 trial, the HDT arm included TBI, CHOP, cyclophosphamide and etoposide. TBI was not scheduled for the intervention arm of the GITMO/IIL trial. Patients randomised to this arm were treated with doxorubicin, vincristine, and prednisone (APO), Ara‐C, cisplatin, and dexamethasone (DHAP), etoposide, cyclophosphamide, mitoxantrone, melphalan and rituximab.
The chemotherapy regimens of the control arms were as follows: cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in two trials (CUP trial; GITMO/IIL); cyclophosphamide, doxorubicin, teniposide, and prednisolone (CHVP) for two trials (GELA/GELF‐94; GOELAMS 064); and mitoxantrone, chlorambucil, and prednisone (MCP) or CHOP in one trial (GLSG). In three trials, interferon α was given in addition to the therapy of the control‐arm (GELA/GELF‐94; GLSG; GOELAMS 064). In one trial (GITMO/IIL), the chemotherapy arm was supplemented by the monoclonal antibody rituximab. In one trial (CUP trial), the control arm included only chemotherapy.
In all five trials the type of stem cell source was the harvest of peripheral blood stem cells (PBSC).
Outcomes
Primary outcome measure
Overall survival was analysed in four trials (CUP trial; GELA/GELF‐94; GITMO/IIL; GOELAMS 064). The median follow‐up time for OS was as follows: 51 months for the GELA/GELF‐94 trial; 69 months for the CUP trial; 108 months for the GOELAMS 064 trial and 51 months for the GITMO/IIL trial. In one trial, OSoverall‐survival was not reported (GLSG).
Secondary outcome measures
Four trials reported PFS (CUP trial; GITMO/IIL; GLSG; GOELAMS 064). Response rate was analysed in three trials (GELA/GELF‐94; GITMO/IIL; GOELAMS 064). Four trials mentioned treatment‐related mortality and secondary malignancies (GELA/GELF‐94; GITMO/IIL; GLSG; GOELAMS 064). Three trials evaluated adverse events (GITMO/IIL; GLSG; GOELAMS 064) and no trial mentioned quality of life.
Three trials additionally reported event‐free survival (EFS) (GELA/GELF‐94; GITMO/IIL;GOELAMS 064); this endpoint was not analysed in this systematic review. EFS was calculated as the time period form the random assignment to induction failure (stable disease at the end of treatment or progression during treatment), death irrespective of the cause, progression after partial response (PR), relapse after complete response (CR), or last follow‐up. Compared to that, PFS is defined from the end of successful induction therapy until documented progression or death and not directly estimable from EFS.
Funding
Roche supported in part the GITMO/IIL trial, providing rituximab for all the patients. The GOELAMS 064 trial was partly supported by grants from the French Ministry of Health (Paris, France) and Schering‐Plough. Schering‐Plough also supported the GELA/GELF‐94 trial.
Conflict of interest
In one trial, the authors indicated no potential conflict of interest (CUP trial). In all other trials, conflict of interests was not mentioned.
Excluded studies
For information on excluded trials see Characteristics of excluded studies, where reasons for the exclusion are listed.
We excluded a total of 40 articles after detailed evaluation of full text publications. The main reasons for exclusion were:
13 articles: non‐randomised comparisons or reviews;
2 articles: not CT versus HDT + ASCT;
21 articles: no patients with FL;
4 articles: no separated results reported for patients with FL and no reply from the authors for further details.
Risk of bias in included studies
Overall the quality of included trials is moderate. For more details see 'Risk of bias' tables of the included trials in the tables of Characteristics of included studies. The 'Risk of bias' graph illustrates the proportion of studies with each of the judgements "low risk", "high risk" or "unclear risk" of bias for each entry in the tool (see Figure 2). The 'Risk of bias' summary figure presents all of the judgements in a cross‐tabulation of study by entry (see Figure 3).
2.
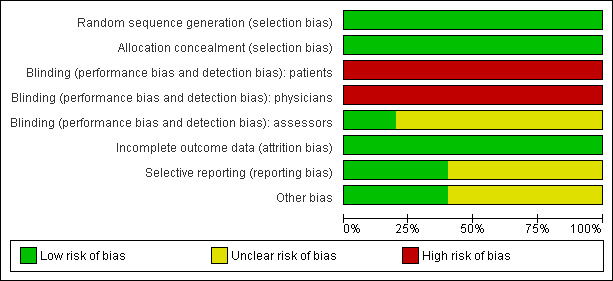
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
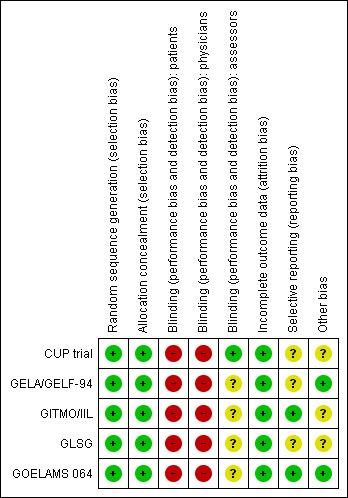
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Treatment allocation of patients was carried out centrally for all trials. In the GITMO/IIL trial, a centralised computer generated a simple randomisation sequence. In the GELA/GELF‐94 trial, treatment allocation of patients was assigned by the study co‐ordinating centre. In the CUP trial, random assignment using the method of minimization was performed at the Medical Research Council Clinical Trials Unit in London by telephone or fax.
In all included trials, sequence generation was judged to be adequate.
Blinding
No trial reported information about blinding of patients and physicians. We judged "high risk of bias" for the question of blinding of patients and physicians, because usually trials evaluating the effect of stem cell transplantation are not blinded, leading to a potential high risk of bias. We judged four of five trials as "unclear" for the question of blinding of the outcome assessors, as this topic was not reported (GELA/GELF‐94; GITMO/IIL; GLSG; GOELAMS 064). One trial reported information about blinding of the outcome assessors and was judged as "low risk of bias" (CUP trial).
Incomplete outcome data
According to the intention‐to‐treat principle most trials (CUP trial; GELA/GELF‐94; GITMO/IIL; GOELAMS 064) included all randomised patients in the analysis. In the GLSG trial, 55 of the 307 randomised patients were not analysed because they did not receive the assigned therapy. This question was judged as "low risk of bias" for all five included trials.
Selective reporting
Two study protocols for the five included trials were available (GITMO/IIL; GOELAMS 064). In both trials, the same outcomes as indicated in the study protocol were reported in the full text publications. Therefore we judged risk of these two trials as low.
Other potential sources of bias
In the CUP trial, the protocol was amended in March 1996 to enable centres that felt uncomfortable treating relapsed patients without HDT and ASCT to provide this regimen to all patients. After March 1996, patients were randomised to purged versus unpurged stem cells only.
The GITMO/IIL trial was stopped early after a planned interim analysis, indicating a significant EFS advantage in patients treated with HDT + ASCT and rituximab compared with CHOP and rituximab. The risk of bias for the premature closure is judged as "unclear".
In the GLSG trial, the risk of bias is judged as "unclear", due to the fact that in July 1998 all patients received CHOP instead of MCP or CHOP. This protocol amendment is based on the publication of a randomised comparison of CHOP with MCP showing that MCP was associated with a significant impairment of haematopoietic stem cell mobilisation.
A potential source of bias are uncertainties in the HR calculation. In three trials the HRs for OS (GELA/GELF‐94; GITMO/IIL; GOELAMS 064) and in two for PFS (GITMO/IIL; GOELAMS 064) had to be based on the survival curves. In all cases, constant censoring was assumed as described by Tierney 2007.
Effects of interventions
See: Table 1
Primary outcome: Overall survival (OS)
Except for the GLSG trial, all trials reported outcomes for OS (four trials, 771 patients).
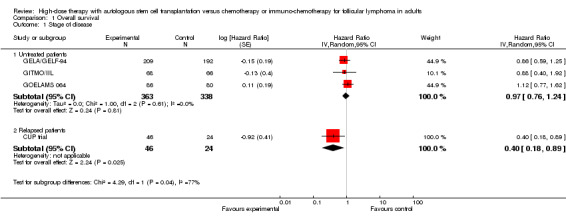
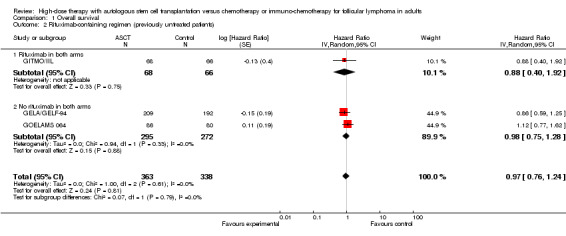
The meta‐analysis of trials evaluating previously untreated patients with FL included 701 patients in three trials. This analysis did not show a statistically significant difference between patients treated with HDT + ASCT compared with chemotherapy only (HR = 0.97; 95% confidence interval (CI) 0.76 to 1.24; P = 0.81) (Figure 4). The subgroup analysis for the rituximab‐containing regimen confirms this finding (HR = 0.88; 95% CI 0.40 to 1.92; P = 0.75). However, only one trial was included in this analysis (GITMO/IIL) and the test for differences across subgroups for this analysis is not statistically significant (P = 0.88).
4.
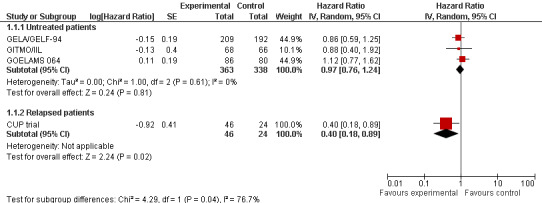
Forest plot of comparison: 1 Overall survival, outcome: 1.1 Stage of disease.
The trial evaluating relapsed patients, the CUP trial is the only trial with a statistically significant benefit for patients in the HDT + ASCT arm (HR = 0.40; 95% CI 0.18 to 0.89; P = 0.002). However, with only 70 patients, it is a small trial (Figure 4).
2.Secondary Outcomes
Progression‐free survival (PFS)
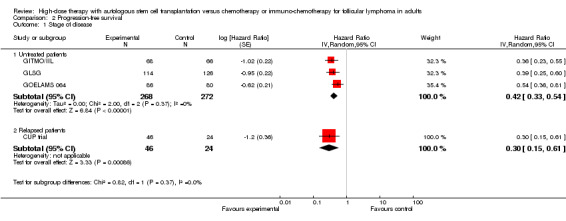
Four trials with a total of 610 patients reported PFS (CUP trial; GITMO/IIL; GLSG; GOELAMS 064).
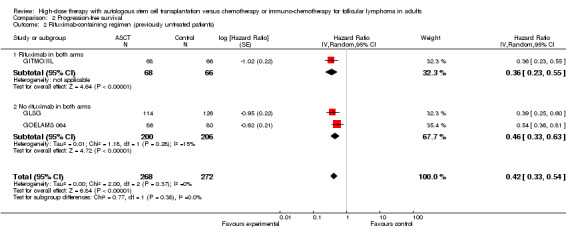
The meta‐analysis in trials with previously untreated patients with FL showed statistically significant improved PFS (HR = 0.42; 95% CI 0.33 to 0.54; P < 0.00001) (three trials, N = 540) (GITMO/IIL; GLSG; GOELAMS 064) (Figure 5). The subgroup analysis for trials adding rituximab in both arms (one trial, GITMO/IIL) confirms this result (HR = 0.36; 95% CI 0.23 to 0.55; P < 0.00001). Again, there is no statistically significant interaction between subgroups, with or without rituximab (P = 0.53).
5.
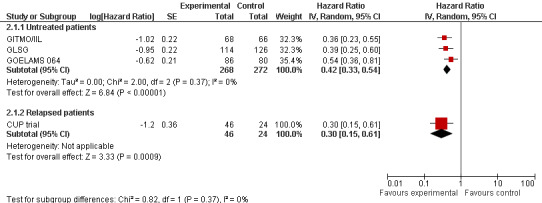
Forest plot of comparison: 2 Progression‐free survival, outcome: 2.1 Stage of disease.
The analysis of the trial randomising patients with relapsed FL (N = 70) showed an statistically significant improvement of PFS (HR = 0.30 (95% CI 0.15 to 0.61; P = 0.0009) (CUP trial) (Figure 5).
Response Rate (RR)
Overall response rate (ORR)
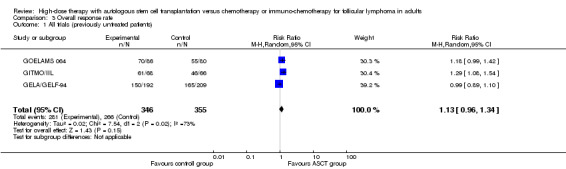
The meta‐analysis included a total of 701 patients from three trials, all in previously untreated patients (GELA/GELF‐94; GITMO/IIL; GOELAMS 064). No evidence of a statistically significant difference between the HDT + ASCT group and the chemotherapy group was found (RR = 1.13; 95% CI 0.96 to 1.34). There is a hint for heterogeneity between trials visible in the forest plot (Figure 6).
6.
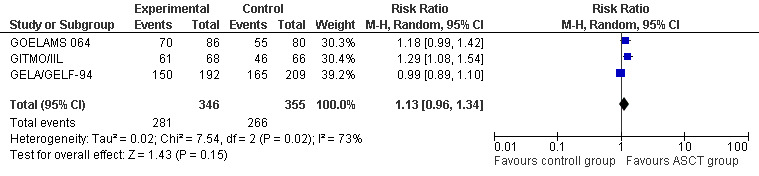
Forest plot of comparison: 3 Overall response rate, outcome: 3.1 All trials (previously untreated patients).
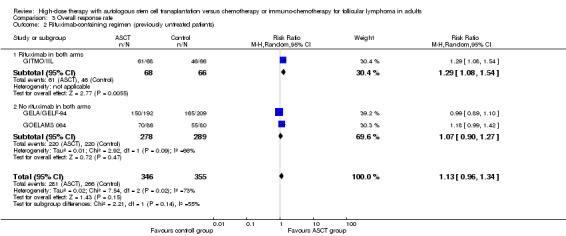
The only trial (GITMO/IIL) adding rituximab in both arms showed a statistically significant advantage for the HDT + ASCT arm (RR = 1.29, 95% CI 1.08 to 1.54; P = 0.006), however, the test for differences between subgroups is not statistically significant (P = 0.14).
Complete response (CR)
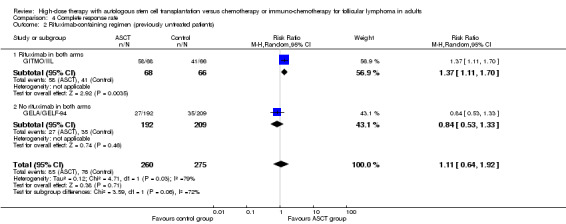
Two trials with 535 patients reported CR (GELA/GELF‐94; GITMO/IIL). No evidence for difference between both arms was found (RR: 1.11, 95% CI 0.64 to 1.92; P = 0.71).
A subgroup analysis was performed for the GITMO/IIL trial, evaluating additional rituximab in both arms. In this trial, CR was statistically significantly improved in the HDT + ASCT‐arm (RR = 1.37, 95% CI, 1.11 to 1.70; P = 0.003), but the test for interaction between subgroups is not statistically significant (P = 0.06).
Treatment‐related mortality (TRM)
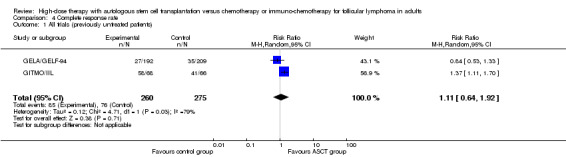
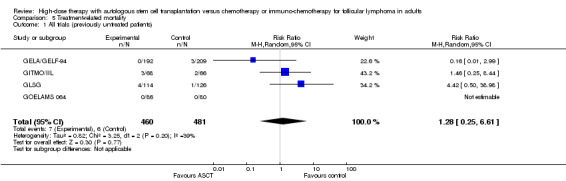
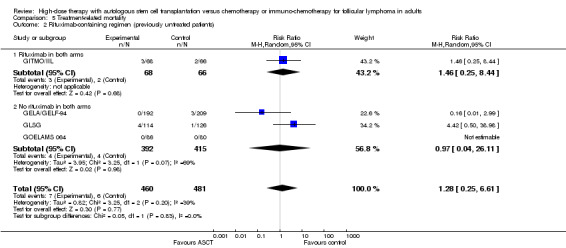
Four trials comprising 941 previously untreated patients reported data of TRM and were meta‐analysed (GELA/GELF‐94; GITMO/IIL; GLSG; GOELAMS 064). Treatment‐related mortality was balanced for both arms and did not show statistically significant differences between both arms (RR = 1.28; 95% CI 0.25 to 6.61; P = 0.77). The same is true for the subgroup analysis including additional rituximab (RR = 1.46; 95% CI 0.25 to 8.44; P = 0.68) and no statistically significant difference across subgroups was found (P = 0.83). However, only one trial (GITMO/IIL) was included in the rituximab‐subgroup.
Secondary malignancies (SM)
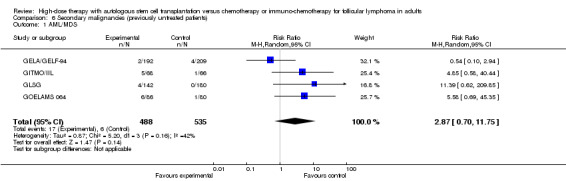
Four trials in previously untreated patients gave details on the development of secondary malignancies, AML (acute myeloid leukaemia) and MDS (myelodysplastic syndrome) as well as on the development of solid cancer (GELA/GELF‐94; GITMO/IIL; GLSG; GOELAMS 064). The occurrence of MDS/AML was reported in four trials including 1023 patients. With a median follow‐up time between 44 and 108 months, there is no statistically significant difference in development of MDS or AML for the compared two interventions (RR = 2.87, 95% CI 0.70, 11.75; P = 0.14), however, the trend is in favour of the control arm consisting of chemotherapy only (Figure 7).
7.
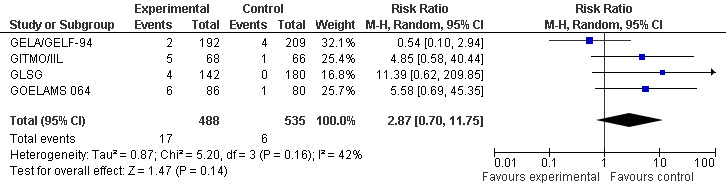
Forest plot of comparison: 4 Secondary Malignancies, outcome: 4.1 AML/MDS.
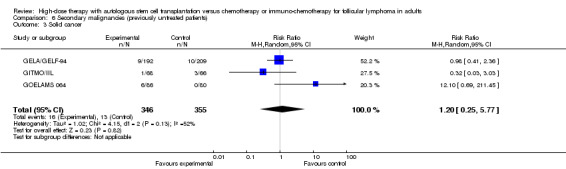
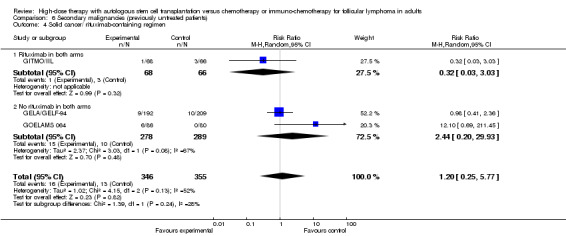
Three trials reported data to the development of solid cancers (GELA/GELF‐94; GITMO/IIL; GOELAMS 064) with a follow‐up time between 51 and 108 months. The meta‐analysis did not show statistical significant differences between the two arms (RR= 1.20; 95% CI 0.25, 5.77; P = 0.82).
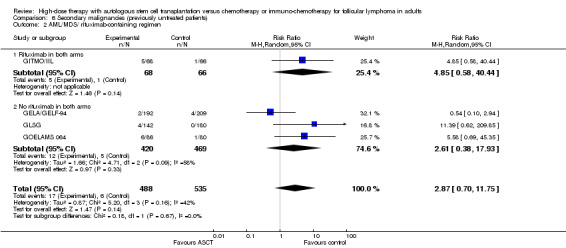
After a median follow‐up time of 51 months, the two subgroup analyses with the trial including additional rituximab did not show statistical significant differences between the arms, neither for the occurrence for AML/MDS (RR = 4.85; 95% CI 0.58 to 40.44; P = 0.14) nor solid cancer (RR = 0.32; 95% CI 0.03 to 3.03; P = 0.32) (GITMO/IIL). Both test for differences across subgroups were not statistically significant (P = 0.67; P = 0.24).
Adverse Events
All adverse events reported in the trials are presented in Table 2.
Three trials reported on some adverse events (GITMO/IIL; GLSG; GOELAMS 064), two trials did not report on any adverse event (CUP trial; GELA/GELF‐94). As expected, more adverse events were observed in the HDT + ASCT arm, especially haematological toxicities. The GLSG trial, in particular, shows a high rate of haematological toxicities such as anaemia, leucocytopenia, granulocytopenia and thrombocytopenia in the HDT + ASCT arm. All three trials describe the higher occurrence of non‐haematological toxicities as well as acute infections in the HDT + ASCT arm.
Quality of life
None of the trials reported quality of life.
Discussion
Summary of main results
The following findings emerge from this meta‐analysis.
Based on currently available research results, HDT followed by ASCT does not lead to an overall survival advantage in comparison with chemotherapy or immuno‐chemotherapy in patients with previously untreated FL. We found a statistically significant advantage for relapsed patients in the HDT + ASCT arm, but this was evaluated in one trial only.
High‐dose therapy and ASCT shows a statistically significant improvement of PFS compared with the chemotherapy or immuno‐chemotherapy, both in previously untreated patients as well as in relapsed patients. This huge effect also is seen in the trial adding rituximab in both treatment arms.
No statistically significant differences were shown between HDT + ASCT and the control intervention in terms of TRM, secondary AML/MDS or solid cancers, or overall or complete response rates. These outcomes were reported for previously untreated patients only.
Adverse events were observed more frequently in the HDT + ASCT arm. Again, these outcomes were reported for previously untreated patients only.
None of the trials reported quality of life.
One reason why the statistically significant PFS did not translate into an OS advantage could be the clinical course of FL. Patients with similar OS may nevertheless have differing lengths of time without symptoms, time to progression or requirement for treatment, depending both on initial treatment and disease characteristics. Follicular lymphoma is an indolent disease, often relapsing after successful first‐line treatment in shorter intervals and requiring salvage therapy. These additional treatment approaches could influence the outcome OS more than the first‐line treatment patients received. Especialy nowadays, in the era of rituximab, salvage therapy is becoming more effective. The influence of salvage therapy is visible in the GITMO/IIL trial: 70% of patients who relapsed after CHOP‐R underwent salvage therapy with rituximab and HDT + ASCT, leading to a complete response rate of 85% and 81% OS at three years. Except for the GITMO/IIL trial, all the included studies started before rituximab was introduced, therefore, most patients will have received rituximab at the time of relapse.
There are no statistically significant differences in terms of secondary malignancies, especially the appearance of AML/MDS or solid cancers. None of the individual trials included in the meta‐analysis described a statistically significant increase of either AML/MDS or solid cancers. Thus, secondary malignancies are probably not the reason for the missing OS benefit. However, long‐term adverse effects such as secondary malignancies are important after HDT + ASCT and can occur later than the reported observation times of the discussed trials.
Overall completeness and applicability of evidence
Five trials, published in nineteen abstracts and full‐texts, compared the use of HDT therapy followed by ASCT chemotherapy or immuno‐chemotherapy for FL in adults. Because of the clinical homogeneity of the included trials, we pooled their outcomes in one meta‐analysis. Apart from the exception of quality of life, which was not reported in any trial, the included trials reported our previously specified protocol outcomes. The randomised trials were heterogeneous in terms of usage of TBI in myeloablative regimen, chemotherapies, interferon and rituximab dosages. The low number of included studies made it difficult to explore the potential impact of these differences in subgroup analyses and to evaluate potential heterogeneity in detail.
Only one of the five trials evaluated the impact of HDT followed by ASCT in patients with relapsed FL. Although the results of this trial are statistically significant in terms of overall and progression‐free survival, the small number of patients evaluated may have overestimated the effects.
Only three of the five included trials reported adverse events, with more haematological and non‐haematological events in the group receiving HDT + ASCT. The non‐publication of adverse events in two trials could have introduced bias.
Quality of life was not reported in any of the trials.
Two trials included patients with FL grade 3b (GITMO/IIL; GOELAMS 064), usually treated as aggressive lymphoma. The inclusion of these patients may have biased the results.
Quality of the evidence
The main analysis according to the inclusion criteria of our protocol included five RCTs with 1093 patients with FL. The overall quality of the five included trials was moderate. The trials were conducted between 1993 and 2005. All the included trials were reported as randomised and as open‐label studies. None of the included trials reported allocation concealment. The open‐label design and unclear allocation concealment could lead to selection, performance or detection biases. In terms of the treatment schedule, the protocol of two trials was amended, indicating a potential risk of reporting bias. In one trial, all patients having received CHOP instead of MCP or CHOP since July 1998. This protocol amendment is based on the publication of a randomised comparison of CHOP with MCP showing that MCP was associated with a significant impairment of haematopoietic stem cell mobilisation. The other protocol was amended in March 1996 to enable centres that felt uncomfortable treating relapsed patients without HDT + ASCT to provide this therapy to all patients. The premature closure of one trial after a planned interim analysis, indicating a significant EFS advantage in patients treated with HDT + ASCT therapy and rituximab compared with CHOP plus rituximab arm could have introduced bias.
A potential source of bias are uncertainties in the HR calculation. In three trials the HRs for OS and in two for PFS were calculated from survival curves with a constant censoring as described by Tierney 2007.
The studies included in this review offered a variety of chemotherapy regimens, such as CHOP, CHVP, APO, DHAP, MCP and VCAP. The low number of included studies made it difficult to explore these regimens in detail in subgroup analyses and to interpret potential underlying heterogeneity.
Potential biases in the review process
We tried to avoid bias by doing all relevant processes in duplicate. We are not aware of any obvious flaws in our review process.
Agreements and disagreements with other studies or reviews
To our knowledge, this comprehensive evaluation is the first meta‐analysis focusing on patients with FL that compares HDT followed by ASCT with chemotherapy or immuno‐chemotherapy.
Therapeutic options for treatment of patients with FL have been investigated in three other Cochrane reviews (Vidal 2009; Schulz 2007; Baldo 2010). The review of Baldo et al. (Baldo 2010) determined the effects of interferon (IFN) in the maintenance therapy of FL. With a total of 1563 patients in eight trials, the review showed that addition of IFN as maintenance therapy for FL improves PFS in contrast to OS. Seven randomised controlled trials involving 1943 patients with FL, mantle cell lymphoma, or other indolent lymphomas were meta‐analysed in the systematic review of Schulz et al. (Schulz 2007), comparing chemotherapy plus rituximab with chemotherapy alone. Schulz could demonstrate that rituximab given in addition to chemotherapy, statistically significantly improves overall survival, overall response rate, complete response rate, and disease control compared with chemotherapy alone. On the subject of maintenance treatment with rituximab in FL patients, Vidal et al. (Vidal 2009) published a Cochrane review with comparable results. The review includes five trials with 1056 adult FL patients. The analysis of OS included 895 patients in four trials. Patients treated with rituximab as maintenance therapy had a significantly better OS compared with observation alone (HR = 0.53; 95% CI 0.38 to 0.73).
Greb et al. (Greb 2011) investigated the benefit of HDT with ASCT in first‐line treatment for patients with aggressive NHL. The meta‐analysis included 15 randomised controlled trials with 3079 patients and showed that despite higher CR rates, there is no benefit for HDT with ASCT as a first‐linr treatment in patients with aggressive NHL. Thus this review has results in line with our analysis.
Authors' conclusions
Implications for practice.
There is currently no evidence that HDT followed by ASCT improves OS in newly diagnosed patients with FL. However, the increase of PFS is statistically significant and led to a huge effect favouring the HDT + ASCT arm, even in the trial adding rituximab in both intervention arms. We demonstrated no statistically significant differences in terms of treatment related mortality, secondary AML/MDS or solid cancers, however, acute adverse events are observed more frequently in the HDT + ASCT arm, especially haematological toxicities and infections. None of the trials evaluated quality of life.
For patients with relapsed FL, there is evidence (one trial, N = 70) that the addition of HDT followed by ASCT is advantageous regarding overall survival and progression‐free survival.
Implications for research.
Randomised controlled trials with longer follow‐up periods and rituximab‐containing chemotherapy in both arms are needed to determine the potential effect of HDT in the era of rituximab and to evaluate whether the seen PFS‐benefit will translate into a survival advantage.
Acknowledgements
We thank Ms Ina Monsef for developing and reviewing the search strategies.
We are grateful to the following persons for their comments and improving the protocol:
Prof. Lena Specht and Dr. Sue Richards, Editors of the Cochrane Haematological Malignancies Group,
Sabine Kluge, Michaela Rancea and Bettina Schmidtke from the Editorial Base for commenting on the first draft and proof reading and Heather Maxwell for copy editing.
Appendices
Appendix 1. CENTRAL search strategy to November 2010
#1 MeSH descriptor Lymphoma, Follicular explode all trees
#2 (follicul* NEAR/2 lymph*)
#3 (nodular* NEAR/2 lymph*)
#4 ((small* OR large*) NEAR/4 follicul* )
#5 ((small* OR large*) NEAR/4 lymph*)
#6 ((low‐grad* OR low grad*) NEAR/ lymph*)
#7 ((low‐grad* OR low grad*) AND lymph*)
#8 (centro blast* OR zentroblast*)
#9 (follic* NEAR/2 (center* OR centro*) NEAR/ lymph*)
#10 (brill‐symmer* OR brill symmer*)
#11 MeSH descriptor Lymphoma, B‐Cell, this term only
#12 (indolent* NEAR/2 lymph*)
#13 MeSH descriptor Lymphoma, Non‐Hodgkin explode all trees
#14 (non‐hodgkin* OR nonhodgkin* OR non hodgkin*)
#15 (diffus* NEAR/ lymphom*)
#16 (lymphati* sacrom* OR lymphosarcom*)
#17 MeSH descriptor Hematologic Neoplasms explode all trees
#18 (hemato* NEAR/ (malign* OR neoplas*))
#19 (haemato* NEAR/ (malign* OR neoplas*))
#20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) delete
#21 (#17 OR #18 OR #19)
#22 MeSH descriptor Transplantation, Autologous explode all trees
#23 (autolog* NEAR/4 (transplant* OR graft*))
#24 (asct)
#25 (autograft* OR auto‐graft*)
#26 (autotransplant* OR auto‐transplant*)
#27 (#22 OR #23 OR #24 OR #25 OR #26)
#28 MeSH descriptor Transplantation Conditioning explode all trees
#29 (myeloablativ*)
#30 (#28 OR #29)
#31 (#20 AND ( #27 OR #30 )) 351 (295 hits in CENTRAL)
Appendix 2. CENTRAL search strategy from November 2010 to September 2011
#1 MeSH descriptor Lymphoma, Follicular explode all trees
#2 (follicul* NEAR/2 lymph*)
#3 (nodular* NEAR/2 lymph*)
#4 ((small* OR large*) NEAR/4 follicul* )
#5 ((small* OR large*) NEAR/4 lymph*)
#6 ((low‐grad* OR low grad*) NEAR/ lymph*)
#7 ((low‐grad* OR low grad*) AND lymph*)
#8 (centro blast* OR zentroblast*)
#9 (follic* NEAR/2 (center* OR centro*) NEAR/ lymph*)
#10 (brill‐symmer* OR brill symmer*)
#11 MeSH descriptor Lymphoma, B‐Cell, this term only
#12 (indolent* NEAR/2 lymph*)
#13 MeSH descriptor Lymphoma, Non‐Hodgkin explode all trees
#14 (non‐hodgkin* OR nonhodgkin* OR non hodgkin*)
#15 (diffus* NEAR/ lymphom*)
#16 (lymphati* sacrom* OR lymphosarcom*)
#17 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16)
#18 MeSH descriptor Transplantation, Autologous explode all trees
#19 (autolog* NEAR/4 (transplant* OR graft*))
#20 (asct)
#21 (autograft* OR auto‐graft*)
#22 (autotransplant* OR auto‐transplant*)
#23 (#18 OR #19 OR #20 OR #21 OR #22)
#24 MeSH descriptor Transplantation Conditioning explode all trees
#25 (myeloablativ*)
#26 (#24 OR #25)
#27 (#17 AND ( #23 OR #26 ))
#28 (#27), from 2011 to 2011
Appendix 3. MEDLINE search strategy
1. Lymphoma, Follicular/
2. (follicul$ adj2 lymph$).tw,kf,ot.
3. (nodular$ adj2 lymph$).tw,kf,ot.
4. ((small$ or large$) adj4 (follicul$ adj2 lymph$)).tw,kf,ot.
5. ((low‐grad$ or low grad$) adj lymph$).tw,kf,ot.
6. ((centro blast$ or zentroblast$) adj (centrocyst$ or zentrozyt$) adj lymph$).tw,kf,ot.
7. (follic$ adj2 (center$ or centro$) adj lymph$).tw,kf,ot.
8. (brill‐symmer$ or brill symmer$).tw,kf,ot.
9. *Lymphoma, B‐Cell/
10. (indolent$ adj2 lymph$).tw,kf,ot.
11. exp Lymphoma, Non‐Hodgkin/
12. (non‐hodgkin$ or nonhodgkin$ or non hodgkin$).tw,kf,ot.
13. (diffus$ adj lymphom$).tw,kf,ot.
14. (lymphati$ sacrom$ or lymphosarcom$).tw,kf,ot.
15. or/1‐14
16. exp Transplantation, Autologous/
17. (autolog$ adj4 (transplant$ or graft$)).tw,kf,ot.
18. asct.tw.
19. (autograft$ or auto‐graft$).tw,kf,ot.
20. (autotransplant$ or auto‐transplant$).tw,kf,ot.
21. or/16‐20
22. Transplantation Conditioning/
23. myeloablativ$.tw,kf,ot.
24. or/22‐23
25. 21 or 24
26. randomized controlled trial.pt.
27. controlled clinical trial.pt.
28. randomized controlled trial/
29. random allocation/
30. double blind method/
31. single blind method/
32. or/26‐31
33. (ANIMALS not HUMANS).sh.
34. 32 not 33
35. clinical trial.pt.
36. exp clinical trial/
37. (clin$ adj25 trial$).ti,ab.
38. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
39. placebos/
40. placebo$.ti,ab.
41. random$.ti,ab.
42. research design/
43. or/35‐42
44. 43 not 33
45. 44 not 34
46. comparative study/
47. exp evaluation studies/
48. follow up studies/
49. prospective studies/
50. (control$ or prospectiv$ or volunteer$).ti,ab.
51. or/46‐50
52. 51 not 33
53. 52 not (34 or 45)
54. 34 or 45 or 53
55. randomized controlled trial.pt.
56. controlled clinical trial.pt.
57. randomized.ab.
58. drug therapy.fs.
59. randomly.ab.
60. trial.ab.
61. groups.ab.
62. or/55‐61
63. humans.sh.
64. 62 and 63
65. 15 and (21 or 24)
66. 65 and 54 (1212)
67. 65 and 64
68. 66 not 67
69. 67 not 66 (405)
70. 66 or 69 (1617)
(51)
Appendix 4. EMBASE search strategy
1 FOLLICULAR LYMPHOMA/ (3145)
2 (follicul$ adj2 lymph$).tw. (3134)
3 (nodular$ adj2 lymph$).tw. (767)
4 ((small$ or large$) adj4 (follicul$ adj2 lymph$)).tw. (308)
5 ((low‐grad$ or low grad$) adj lymph$).tw. (914)
6 ((centro blast$ or zentroblast$) adj (centrocyst$ or zentrozyt$) adj lymph$).tw. (0)
7 (follic$ adj2 (center$ or centro$) adj lymph$).tw. (154)
8 (brill‐symmer$ or brill symmer$).tw. (12)
9 *B CELL LYMPHOMA/ (6685)
10 (indolent$ adj2 lymph$).tw. (500)
11 exp NONHODGKIN LYMPHOMA/ (53565)
12 (non‐hodgkin$ or nonhodgkin$ or non hodgkin$).tw. (20573)
13 (diffus$ adj lymphom$).tw. (254)
14 (lymphati$ sacrom$ or lymphosarcom$).tw. (850)
15 or/1‐14 (58499)
16 exp AUTOTRANSPLANTATION/ (5600)
17 (autolog$ adj4 (transplant$ or graft$)).tw. (12201)
18 asct.tw. (743)
19 (autograft$ or auto‐graft$).tw. (6980)
20 (autotransplant$ or auto‐transplant$).tw. (3189)
21 or/16‐20 (23169)
22 MYELOABLATIVE CONDITIONING/ (696)
23 myeloablativ$.tw. (2264)
24 or/22‐23 (2625)
25 21 or 24 (25151)
26 Clinical trial/ (505625)
27 Randomized controlled trial/ (158767)
28 RANDOMIZATION/ (25683)
29 SINGLE BLIND PROCEDURE/ (7603)
30 DOUBLE BLIND PROCEDURE/ (69545)
31 CROSSOVER PROCEDURE/ (20372)
32 PLACEBO/ (114333)
33 Randomi?ed controlled trial$.tw. (29147)
34 RCT.tw. (2297)
35 Random allocation.tw. (615)
36 Randomly allocated.tw. (9739)
37 Allocated randomly.tw. (1324)
38 (allocated adj2 random).tw. (553)
39 Single blind$.tw. (7178)
40 Double blind$.tw. (82249)
41 ((treble or triple) adj blind$).tw. (130)
42 Placebo$.tw. (105757)
43 PROSPECTIVE STUDY/ (75226)
44 or/26‐43 (665188)
45 Case study/ (5522)
46 Case report.tw. (112827)
47 Abstract report/ or letter/ (469789)
48 or/45‐47 (586070)
49 44 not 48 (642100)
50 animal/ (18243)
51 human/ (6155100)
52 50 not 51 (14469)
53 49 not 52 (642004)
54 15 and 25 and 53 (648)
Data and analyses
Comparison 1. Overall survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stage of disease | 4 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Untreated patients | 3 | 701 | Hazard Ratio (Random, 95% CI) | 0.97 [0.76, 1.24] |
| 1.2 Relapsed patients | 1 | 70 | Hazard Ratio (Random, 95% CI) | 0.40 [0.18, 0.89] |
| 2 Rituximab‐containing regimen (previously untreated patients) | 3 | 701 | Hazard Ratio (Random, 95% CI) | 0.97 [0.76, 1.24] |
| 2.1 Rituximab in both arms | 1 | 134 | Hazard Ratio (Random, 95% CI) | 0.88 [0.40, 1.92] |
| 2.2 No rituximab in both arms | 2 | 567 | Hazard Ratio (Random, 95% CI) | 0.98 [0.75, 1.28] |
1.1. Analysis.
Comparison 1 Overall survival, Outcome 1 Stage of disease.
1.2. Analysis.
Comparison 1 Overall survival, Outcome 2 Rituximab‐containing regimen (previously untreated patients).
Comparison 2. Progression‐free survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stage of disease | 4 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Untreated patients | 3 | 540 | Hazard Ratio (Random, 95% CI) | 0.42 [0.33, 0.54] |
| 1.2 Relapsed patients | 1 | 70 | Hazard Ratio (Random, 95% CI) | 0.30 [0.15, 0.61] |
| 2 Rituximab‐containing regimen (previously untreated patients) | 3 | 540 | Hazard Ratio (Random, 95% CI) | 0.42 [0.33, 0.54] |
| 2.1 Rituximab in both arms | 1 | 134 | Hazard Ratio (Random, 95% CI) | 0.36 [0.23, 0.55] |
| 2.2 No rituximab in both arms | 2 | 406 | Hazard Ratio (Random, 95% CI) | 0.46 [0.33, 0.63] |
2.1. Analysis.
Comparison 2 Progression‐free survival, Outcome 1 Stage of disease.
2.2. Analysis.
Comparison 2 Progression‐free survival, Outcome 2 Rituximab‐containing regimen (previously untreated patients).
Comparison 3. Overall response rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials (previously untreated patients) | 3 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.96, 1.34] |
| 2 Rituximab‐containing regimen (previously untreated patients) | 3 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.96, 1.34] |
| 2.1 Rituximab in both arms | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.08, 1.54] |
| 2.2 No rituximab in both arms | 2 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.90, 1.27] |
3.1. Analysis.
Comparison 3 Overall response rate, Outcome 1 All trials (previously untreated patients).
3.2. Analysis.
Comparison 3 Overall response rate, Outcome 2 Rituximab‐containing regimen (previously untreated patients).
Comparison 4. Complete response rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials (previously untreated patients) | 2 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.64, 1.92] |
| 2 Rituximab‐containing regimen (previously untreated patients) | 2 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.64, 1.92] |
| 2.1 Rituximab in both arms | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.11, 1.70] |
| 2.2 No rituximab in both arms | 1 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.33] |
4.1. Analysis.
Comparison 4 Complete response rate, Outcome 1 All trials (previously untreated patients).
4.2. Analysis.
Comparison 4 Complete response rate, Outcome 2 Rituximab‐containing regimen (previously untreated patients).
Comparison 5. Treatment‐related mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All trials (previously untreated patients) | 4 | 941 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.25, 6.61] |
| 2 Rituximab‐containing regimen (previously untreated patients) | 4 | 941 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.25, 6.61] |
| 2.1 Rituximab in both arms | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.25, 8.44] |
| 2.2 No rituximab in both arms | 3 | 807 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.04, 26.11] |
5.1. Analysis.
Comparison 5 Treatment‐related mortality, Outcome 1 All trials (previously untreated patients).
5.2. Analysis.
Comparison 5 Treatment‐related mortality, Outcome 2 Rituximab‐containing regimen (previously untreated patients).
Comparison 6. Secondary malignancies (previously untreated patients).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 AML/MDS | 4 | 1023 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.70, 11.75] |
| 2 AML/MDS/ rituximab‐containing regimen | 4 | 1023 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.70, 11.75] |
| 2.1 Rituximab in both arms | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 4.85 [0.58, 40.44] |
| 2.2 No rituximab in both arms | 3 | 889 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.38, 17.93] |
| 3 Solid cancer | 3 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.25, 5.77] |
| 4 Solid cancer/ rituximab‐containing regimen | 3 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.25, 5.77] |
| 4.1 Rituximab in both arms | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 3.03] |
| 4.2 No rituximab in both arms | 2 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.20, 29.93] |
6.1. Analysis.
Comparison 6 Secondary malignancies (previously untreated patients), Outcome 1 AML/MDS.
6.2. Analysis.
Comparison 6 Secondary malignancies (previously untreated patients), Outcome 2 AML/MDS/ rituximab‐containing regimen.
6.3. Analysis.
Comparison 6 Secondary malignancies (previously untreated patients), Outcome 3 Solid cancer.
6.4. Analysis.
Comparison 6 Secondary malignancies (previously untreated patients), Outcome 4 Solid cancer/ rituximab‐containing regimen.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CUP trial.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Data included for the patients randomly assigned between the three planned arms, before protocol amendment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised controlled phase III trial |
| Allocation concealment (selection bias) | Low risk | "Random assignment, using the method of minimization, was performed at the MRC CTU in London by telephone or fax" |
| Blinding (performance bias and detection bias) patients | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) physicians | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) assessors | Low risk | Assessors: "during the trial the investigators were blinded to the results". This is judged not to be a source of bias for OS and PFS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | The protocol was amended in March 1996 to enable centres, that felt uncomfortable treating relapsed patients without HDT and transplantation. |
GELA/GELF‐94.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised study" |
| Allocation concealment (selection bias) | Low risk | "After stratification according to Center, eligible patients were assigned by the study coordinating Center" |
| Blinding (performance bias and detection bias) patients | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) physicians | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) assessors | Unclear risk | No information about blinding of the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | According to the ITT principle |
| Selective reporting (reporting bias) | Unclear risk | For primary outcome, event‐free survival was chosen. It is unclear why event‐free survival was chosen and not overall survival. No protocol is available. |
| Other bias | Low risk | no indication for other sources of bias |
GITMO/IIL.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prospective multi‐centre randomised trial |
| Allocation concealment (selection bias) | Low risk | "A centralized computer generated a simple randomisation sequence." |
| Blinding (performance bias and detection bias) patients | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) physicians | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) assessors | Unclear risk | No information about blinding of the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT basis |
| Selective reporting (reporting bias) | Low risk | Protocol available at www.controlled‐trials.com/mrct/trial/printfriendly/399237 |
| Other bias | Unclear risk | Trial stopped early "A sample size of 246 patients (123 per arm) over 5 years was required to detect a 20% absolute increase (from 35% to 55%) in 3‐year EFS with an error of .05 and a error of .20, with a median follow‐up of 3 years. A single interim analysis was planned, including the 120 patients who completed the treatment before March 24, 2005. R‐HDS showed a significant EFS improvement (29% absolute increase) compared to CHOP‐R. This result led the steering committee to stop enrolment on May 30." |
GLSG.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Treatment information: Beginning in July 1998, all patients received CHOP instead of MCP or CHOP because a randomised comparison of CHOP with MCP showed that MCP was associated with a significant impairment of hematopoietic stem cell mobilization. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prospective randomised trial |
| Allocation concealment (selection bias) | Low risk | "Randomization was carried out centrally." |
| Blinding (performance bias and detection bias) patients | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) physicians | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) assessors | Unclear risk | No information about blinding of the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis (patients excluded from analysis who did not receive assigned therapy : 55 of 307 patients) |
| Selective reporting (reporting bias) | Unclear risk | No protocol is available. |
| Other bias | Unclear risk | "Beginning in July 1998, all patients received CHOP because a randomised comparison of CHOP with MCP showed that MCP was associated with a significant impairment of hematopoietic stem cell mobilization." |
GOELAMS 064.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | This study was designed by the GOELAMS Lymphoma Committee with the aim of detecting a 25% absolute difference in EFS at 3 years Secondary end points were the response rate at the end of treatment, the OS rate, and the incidence of adverse effects.
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a randomised multicenter study" |
| Allocation concealment (selection bias) | Low risk | "Randomization was carried out centrally, and was stratified according to each center." |
| Blinding (performance bias and detection bias) patients | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) physicians | High risk | Usually trials evaluating stem cell transplantation are not blinded |
| Blinding (performance bias and detection bias) assessors | Unclear risk | No information about blinding of the assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | Protocol available at www.controlled‐trials.com/mrct/trial/printfriendly/450205 |
| Other bias | Low risk | Tthe trial seems free of other bias |
aaPI: age‐adjusted International Prognostic Index; ASCT: stem cell transplantation; CHOP: cyclophosphamide, doxorubicin, vincristine, and prednisone; CHVP: cyclophosphamide, doxorubicin, teniposide, and prednisolone; CNS: central nevous system; CR: complete response; Cy: cyclophosphamide; DFS: Disease‐free survival; ECOG: Eastern Cooperative Oncology Group; EFS: event‐free survival; FL: follicular lymphoma; G‐CSF: Granulocyte colony‐stimulating factor; HBV: hepatitis B virus; HDT: high‐dose therapy; HIV: Human immunodeficiency virus; IFN: interferon; ITT: intention‐to‐treat; LDH: i.v. intravenous; Lactate dehydrogenase; NHL: non‐Hodgkin`s lymphoma; OS: overall survival; PBSC: peripheral blood stem cells; PFS: progression‐free survival; p.o.: orally; PR: partial response; PS: performance status; RR: response rate; s.c.: subcutaneous; TBI: total body irradiation; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baldissera 2006 | Patients with aggressive high‐risk NHL, and not FL, were randomised. |
| Brice 2000 | Not a randomised controlled trial. Patients were analysed retrospectively. |
| Cao 2001 | Not a randomised controlled trial. Patients were analysed retrospectively. |
| De Souza 2003 | Patients with aggressive high‐risk NHL, and not FL, were randomised |
| De Souza 2004 | Patients with aggressive high‐risk NHL, and not FL, were randomised |
| Gianni 1997 | Patients with diffuse large‐cell lymphoma, and not FL, were randomised. |
| Guglielmi 1993 | Patients with aggressive NHL, and not FL, were randomised. |
| Hagenbeek 1991 | Patients with aggressive NHL, and not FL were randomised. |
| Haioun 1993 | Patients with aggressive NHL, and not FL, were randomised. |
| Haioun 1994 | Patients with aggressive high‐risk NHL, and not FL, were randomised. |
| Haioun 1997 | Patients with aggressive NHL, and not FL, were randomised. |
| Intragumtornchai 2000 | Patients with aggressive NHL, and not FL, were randomised. |
| Intragumtornchai 2006 | Patients with aggressive NHL, and not FL, were randomised. |
| Kaiser 2002 | Patients with aggressive NHL, and not FL, were randomised. |
| Kluin‐Nelemans 2001 | Patients with aggressive NHL, and not FL, were randomised. |
| Leppa 2006 | Patients with FL were randomised to rituximab maintenance treatment or to observation. |
| Marin 2001 | No randomised controlled trial. |
| Martelli 1996 | Patients with aggressive NHL, and not FL, were randomised. |
| Martelli 1999 | Patients with aggressive NHL, and not FL, were randomised. |
| Martelli 2003 | Not a randomised controlled trial. |
| Martinez 1995 | Not a randomised controlled trial. |
| McBride 1996 | Not a randomised controlled trial. |
| Metzner 2002 | Not a randomised controlled trial. |
| Moser 2005 | Patients with aggressive NHL were analysed. |
| Mounier 2000 | Patients with aggressive NHL, and not FL, were randomised. |
| Olivieri 2005 | Patients with aggressive NHL, and not FL, were randomised |
| Pettengell 1996 | No randomised controlled trial. Patients with FL not mentioned separately. |
| Philip 1991 | Patients with FL not mentioned separately. |
| Philip 1995 | Patients with FL not mentioned separately. |
| Philip 1995a | Patients with FL not mentioned separately. |
| Proctor 2003 | Patients with aggressive NHL, and not FL, were randomised. |
| Rabinowe 1990 | All randomised patients were treated with autologous bone marrow transplantation. |
| Rohatiner 1991 | Not a randomised controlled trial. |
| Rohatiner 1994 | Not a randomised controlled trial. |
| Rohatiner 1994a | Not a randomised controlled trial. |
| Santini 1991 | Not a randomised controlled trial. |
| Santini 1997 | Patients with follicular lymphoma not mentioned separately. |
| Santini 1998 | Patients with aggressive NHL, and not follicular lymphoma, were randomised. |
| Sonnen 1998 | Not a randomised controlled trial. |
| Sweetenham 2001 | Patients with lymphoblastic lymphoma, and not FL, were randomised. |
| Winter 2005 | Not a randomised controlled trial. |
FL: follicular lymphoma; NHL: non‐Hodgkin`s lymphoma.
Contributions of authors
Schaaf M: Abstract screening, data extraction, quality assessment (RoB), data analysis and interpretation, drafting of the review
Skoetz N: Drafting of the protocol, abstract screening, data extraction, data entry into RevMan, drafting of the review, data checking, communication between authors, proofreading, update screening
Reiser M: Clinical expertise, advice for the protocol
Borchmann P: Clinical expertise, content input and revising the final draft
Engert A: Clinical expertise, content input
Sources of support
Internal sources
University Hospital of Cologne, Cologne, Germany.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
CUP trial {published data only}
- Schouten HC, Kvaloy S, Sydes M, Qian W, Fayers PM. The CUP trial: a randomized study analyzing the efficacy of high dose therapy and purging in low‐grade non‐Hodgkin's lymphoma (NHL). Annals of Oncology 2000;11 Suppl 1:91‐4. [PubMed] [Google Scholar]
- Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE, et al. High‐dose therapy improves progression‐free survival and survival in relapsed follicular non‐Hodgkin's lymphoma: results from the randomized European CUP trial. Journal of Clinical Oncology : The American Society of Clinical Oncology 2003;21(21):3918‐27. [DOI] [PubMed] [Google Scholar]
- Stade BG, Koll R, Drexler K, Spaethe R, Gibson D, Schouten H. European cup: a randomised trial comparing the efficacy of chemotherapy versus purged or unpurged autologous bone marrow transplantation (ABMT) in patients with relapsed follicular non‐hodgkin's lymphoma (NHL). Onkologie 1994;17(Suppl 2):143. [Google Scholar]
GELA/GELF‐94 {published data only}
- Sebban C, Brice P, Delarue R, Haioun C, Souleau B, Mounier N, et al. Impact of rituximab and/or high‐dose therapy with autotransplant at time of relapse in patients with follicular lymphoma: a GELA study. Journal of Clinical Oncology 2008;26(21):3614‐20. [DOI] [PubMed] [Google Scholar]
- Sebban C, Mounier N, Brousse N, Belanger C, Brice P, Haioun C, et al. Standard chemotherapy with interferon compared with CHOP followed by high‐dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF‐94 randomized study from the Groupe d'Etude des Lymphomes de l'Adulte (GELA). Blood 2006;108(8):2540‐4. [DOI] [PubMed] [Google Scholar]
- Sebban C, et al. A randomized trial in follicular lymphoma comparing a standard chemotherapy regimen with 4 courses of CHOP followed by autologous stem cell transplant with TBI: the GELF94 trial from GELA. European Hematology Association Website http://www.ehaweb.org. 2003.
- Sebban C, et al. Comparison of CHVP + interferon with CHOP followed by autologous stem cell transplantation with TBI conditioning regimen in untreated patients with high tumor burden follicular lymphoma: a result of the randomized GELF94 trial (G.E.L.A. Study Group). Blood 2003;102.11 Pt 1:104a. [Google Scholar]
GITMO/IIL {published data only}
- Ladetto M, Corradini P, Vallet S, Benedetti F, Vitolo U, Martelli M, et al. High rate of clinical and molecular remissions in follicular lymphoma patients receiving high‐dose sequential chemotherapy and autografting at diagnosis: a multicenter, prospective study by the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Blood 2002;100(5):1559‐65. [DOI] [PubMed] [Google Scholar]
- Ladetto M, Marco F, Benedetti F, Vitolo U, Patti C, Rambaldi A, et al. Prospective, multicenter randomized GITMO/IIL trial comparing intensive (R‐HDS) versus conventional (CHOP‐R) chemoimmunotherapy in high‐risk follicular lymphoma at diagnosis: the superior disease control of R‐HDS does not translate into an overall survival advantage. Blood 2008;111(8):4004‐13. [DOI] [PubMed] [Google Scholar]
- Ladetto M, Ricca I, Benedetti F, Liberati M, Vitolo U, Musso M, et al. Multicenter prospective randomized GITMO trial comparing high dose sequential chemotherapy with autografting and CHOP both supplemented with rituximab in poor‐risk follicular lymphoma: early evaluation on treatment feasibility and response rate [abstract]. Bone Marrow Transplantation 2003;31(Suppl 1):7‐8. [Google Scholar]
- Ladetto M, Vallet S, Benedetti F, Vitolo U, Martelli M, Callea V, et al. Prolonged survival and low incidence of late toxic sequelae in advanced follicular lymphoma treated with a TBI‐free autografting program: updated results of the multicenter consecutive GITMO trial. Leukemia 2006;20(10):1840‐7. [DOI] [PubMed] [Google Scholar]
GLSG {published data only}
- Lenz G, Dreyling M, Schiegnitz E, Forstpointner R, Wandt H, Freund M, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression‐free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low‐Grade Lymphoma Study Group. Blood 2004;104(9):2667‐74. [DOI] [PubMed] [Google Scholar]
- Lenz G, Dreyling M, Schiegnitz E, Haferlach T, Hasford J, Unterhalt M, et al. Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem‐cell transplantation in patients with indolent lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. Journal of Clinical Oncology 2004; Vol. 22, issue 24:4926‐33. [DOI] [PubMed]
- Nickenig C, Dreyling M, Hoster E, Ludwig WD, Dorken B, Freund M, et al. Initial chemotherapy with mitoxantrone, chlorambucil, prednisone impairs the collection of stem cells in patients with indolent lymphomas‐results of a randomized comparison by the German Low‐Grade Lymphoma Study Group. Annals of Oncology 2007;18(1):136‐42. [DOI] [PubMed] [Google Scholar]
- Nickenig C, Dreyling M, Hoster E, Pfreundschuh M, Trumper L, Reiser M, et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low‐Grade Lymphoma Study Group. Cancer 2006;107(5):1014‐22. [DOI] [PubMed] [Google Scholar]
GOELAMS 064 {published data only}
- Deconinck E, Colombat P, Foussard C, Milpied N. High dose therapy with autologous purged stem cell transplantation and doxorubicin based immunochemotherapy in patients with advanced follicular lymphoma: A GOELAMS study [Abstract]. Annals of Oncology 2005;16 Suppl 5(12):109. [Google Scholar]
- Deconinck E, Foussard C, Bertrand P, et al. Value of autologous stem cell transplantation in first line therapy of follicular lymphoma with high tumor burden: final results of the randomized GOELAMS 064 trial. Blood 2003;102.11 Pt 1:246a. [Google Scholar]
- Deconinck E, Foussard C, Milpied N, Bertrand P, Michenet P, Cornillet‐LeFebvre P, et al. High‐dose therapy followed by autologous purged stem‐cell transplantation and doxorubicin‐based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by GOELAMS. Blood 2005;105(10):3817‐23. [DOI] [PubMed] [Google Scholar]
- Gyan E, Foussard C, Bertrand P, Michenet P, Gouill S, Berthou C, et al. High‐dose therapy followed by autologous purged stem cell transplantation and doxorubicin‐based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by the GOELAMS with final results after a median follow‐up of 9 years. Blood 2009;113(5):995‐1001. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baldissera 2006 {published data only}
- Baldissera RC, Nucci M, Vigorito AC, Maiolino A, Simoes BP, Lorand‐Metze I, et al. Frontline therapy with early intensification and autologous stem cell transplantation versus conventional chemotherapy in unselected high‐risk, aggressive non‐Hodgkin's lymphoma patients: a prospective randomized GEMOH report. Acta Haematologica 2006;115(1‐2):15‐21. [DOI] [PubMed] [Google Scholar]
Brice 2000 {published data only}
- Brice P, Simon D, Bouabdallah R, Belanger C, Haioun C, Thieblemont C, et al. High‐dose therapy with autologous stem‐cell transplantation (ASCT) after first progression prolonged survival of follicular lymphoma patients included in the prospective GELF 86 protocol. Annals of Oncology 2000;11(12):1585‐90. [DOI] [PubMed] [Google Scholar]
Cao 2001 {published data only}
- Cao TM, Horning S, Negrin RS, Hu WW, Johnston LJ, Taylor TL, et al. High‐dose therapy and autologous hematopoietic‐cell transplantation for follicular lymphoma beyond first remission: the Stanford University experience. Biology of Blood & Marrow Transplantation 2001;7(5):294‐301. [DOI] [PubMed] [Google Scholar]
De Souza 2003 {published data only}
- Souza C, Cardoso RB, Simoes BP, Maiolino A, Moraes AA, Ruiz MA. Prospective randomized study comparing the third generation VACOP‐B regimens versus 6 week VACOP‐B Plus high‐dose sequential followed by autologous bone marrow transplantation in unselected high‐risk IPI non Hodgkin's lymphoma. A GEMOH report. Blood 2003;102(11 Pt 2):268b. [Google Scholar]
De Souza 2004 {published data only}
- Souza CA, Cardoso RB, Simoes BP, Maiolino A, Moraes AJ, Ruiz MA, et al. Prospective randomised study comparing 12‐week VACOP‐B regimen versus 6‐week VACOP‐B plus high‐dose sequential followed by autologous bone marrow transplantation in high‐risk IPI aggressive non‐Hodgkin lymphoma: a Brazilian GEMOH report [abstract]. Bone Marrow Transplantation 2004;33(Suppl 1):349. [Google Scholar]
Gianni 1997 {published data only}
- Gianni AM, Bregni M, Siena S, Brambilla C, Nicola M, Lombardi F, et al. High‐dose chemotherapy and autologous bone marrow transplantation compared with MACOP‐B in aggressive B‐cell lymphoma.[see comment]. New England Journal of Medicine 1997;336(18):1290‐7. [DOI] [PubMed] [Google Scholar]
Guglielmi 1993 {published data only}
- Guglielmi C, Chauvin F, Hagenbeek A, Somers R, LJ, Coiffier B, et al. The PARMA International randomized prospective study in relapsed non‐Hodgkin's lymphoma: second interim analysis of 172 patients and update (as of 20/11/92: 200 patients) [abstract]. 5th International Conference on Malignant Lymphoma, Lugano, Switzerland, June. 1993.
Hagenbeek 1991 {published data only}
- Hagenbeek A, Philip T, Bron D, Guglielmi C, Coiffier B, Gisselbrecht C, et al. The Parma international randomized study in relapsed non Hodgkin lymphoma: 1st interim analysis of 128 patients (as 15 January 1991: 153 patients). Bone Marrow Transplantation 1991;7 Suppl 2:142. [PubMed] [Google Scholar]
Haioun 1993 {published data only}
- Haioun C, Lepage E, Gisselbrecht C, Coiffier B, Bosly A, Tilly H, et al. Autologous bone marrow transplantation (ABMT) versus sequential chemotherapy in first complete remission aggressive non‐Hodgkin's lymphoma (NHL): a study on 469 patients (LNH87 protocol) [abstract]. 5th International Conference on Malignant Lymphoma, Lugano, Switzerland, June. 1993.
Haioun 1994 {published data only}
- Haioun C, Lepage E, Gisselbrecht C, Coiffier B, Bosly A, Tilly H, et al. Comparison of autologous bone marrow transplantation with sequential chemotherapy for intermediate‐grade and high‐grade non‐Hodgkin's lymphoma in first complete remission: a study of 464 patients. Groupe d'Etude des Lymphomes de l'Adulte.[see comment]. Journal of Clinical Oncology 1994;12(12):2543‐51. [DOI] [PubMed] [Google Scholar]
Haioun 1997 {published data only}
- Haioun C, Lepage E, Gisselbrecht C, Bastion Y, Coiffier B, Brice P, et al. Benefit of autologous bone marrow transplantation over sequential chemotherapy in poor‐risk aggressive non‐Hodgkin's lymphoma: updated results of the prospective study LNH87‐2. Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology 1997;15(3):1131‐7. [DOI] [PubMed] [Google Scholar]
Intragumtornchai 2000 {published data only}
- Intragumtornchai T, Prayoonwiwat W, Numbenjapon T, Assawametha N, O'Charoen R, Swasdikul D. CHOP versus CHOP plus ESHAP and high‐dose therapy with autologous peripheral blood progenitor cell transplantation for high‐intermediate‐risk and high‐risk aggressive non‐Hodgkin's lymphoma. Clinical Lymphoma 2000;1(3):219‐25. [DOI] [PubMed] [Google Scholar]
Intragumtornchai 2006 {published data only}
- Intragumtornchai T, Bunworasate U, Nakorn TN, Rojnuckarin P. Rituximab‐CHOP‐ESHAP vs CHOP‐ESHAP‐high‐dose therapy vs conventional CHOP chemotherapy in high‐intermediate and high‐risk aggressive non‐Hodgkin's lymphoma. Leukemia & Lymphoma 2006;47(7):1306‐14. [DOI] [PubMed] [Google Scholar]
Kaiser 2002 {published data only}
- Kaiser U, Uebelacker I, Abel U, Birkmann J, Trumper L, Schmalenberg H, et al. Randomized study to evaluate the use of high‐dose therapy as part of primary treatment for "aggressive" lymphoma.[see comment]. Journal of Clinical Oncology 2002;20(22):4413‐9. [DOI] [PubMed] [Google Scholar]
Kluin‐Nelemans 2001 {published data only}
- Kluin‐Nelemans HC, Zagonel V, Anastasopoulou A, Bron D, Roozendaal KJ, Noordijk EM, et al. Standard chemotherapy with or without high‐dose chemotherapy for aggressive non‐Hodgkin's lymphoma: randomized phase III EORTC study.[see comment]. Journal of the National Cancer Institute 2001;93(1):22‐30. [DOI] [PubMed] [Google Scholar]
Leppa 2006 {published data only}
- Leppa S, Linna M, Nyman H, Taimela E. Cost‐effectiveness of rituximab maintenance treatment versus autologous stem cell transplantation (ASCT) in patients with relapsed follicular lymphoma (FL). Blood. 2006; Vol. 108, issue 11.
Marin 2001 {published data only}
- Marin GH, Menna ME, Bergna MI, Malacalza J, Martin C, Mendez MC, et al. Induction of anti‐tumor activity following autologous stem cell transplantation: immunotherapeutic implications. Transplantation Proceedings 2001;33(1‐2):2004‐7. [DOI] [PubMed] [Google Scholar]
Martelli 1996 {published data only}
- Martelli M, Vignetti M, Zinzani PL, Gherlinzoni F, Meloni G, Fiacchini M, et al. High‐dose chemotherapy followed by autologous bone marrow transplantation versus dexamethasone, cisplatin, and cytarabine in aggressive non‐Hodgkin's lymphoma with partial response to front‐line chemotherapy: a prospective randomized italian multicenter study. Journal of Clinical Oncology 1996;14(2):534‐42. [DOI] [PubMed] [Google Scholar]
Martelli 1999 {published data only}
- Martelli M, Gherlinzoni F, Zinzani PL, Meloni G, Cantonetti M, Storti S. Early autologous stem cell transplantation as first‐line therapy in poor prognosis non Hodgkin's lymphoma (NHL): An Italian randomized trial. Annals of Oncology 1999;10(Suppl 3):78. [Google Scholar]
Martelli 2003 {published data only}
- Martelli M, Gherlinzoni F, Renzo A, Zinzani PL, Vivo A, Cantonetti M, et al. Early autologous stem‐cell transplantation versus conventional chemotherapy as front‐line therapy in high‐risk, aggressive non‐Hodgkin's lymphoma: an Italian multicenter randomized trial. Journal of Clinical Oncology 2003;21(7):1255‐62. [DOI] [PubMed] [Google Scholar]
Martinez 1995 {published data only}
- Martinez C, Mateu R, Sureda A, Brunet S, Amill B, Madoz P, et al. Peripheral blood stem cell mobilization following salvage chemotherapy (IAPVP‐16) plus granulocyte colony‐stimulating factor and autografting for non‐Hodgkin's lymphoma. Transplantation Proceedings 1995;27(4):2355‐6. [PubMed] [Google Scholar]
McBride 1996 {published data only}
- McBride JA, Pugh WC. Autologous bone marrow transplantation in relapsed non‐Hodgkin's lymphoma.[comment]. New England Journal of Medicine 1996;334(15):991‐2. [PubMed] [Google Scholar]
Metzner 2002 {published data only}
- Metzner B. High‐dose therapy and autologous bone marrow transplantation for follicular lymphoma in first remission. Strahlentherapie und Onkologie 2002;178(6):353, 2002. [Google Scholar]
Moser 2005 {published data only}
- Moser EC, Noordijk EM, Carde P, Tirelli U, Baars JW, Thomas J, et al. Late non‐neoplastic events in patients with aggressive non‐Hodgkin's lymphoma in four randomized European Organisation for Research and Treatment of Cancer trials. Clinical Lymphoma & Myeloma 2005;6(2):122‐30. [DOI] [PubMed] [Google Scholar]
Mounier 2000 {published data only}
- Mounier N, Haioun C, Cole BF, Gisselbrecht C, Sebban C, Morel P, et al. Quality of life‐adjusted survival analysis of high‐dose therapy with autologous bone marrow transplantation versus sequential chemotherapy for patients with aggressive lymphoma in first complete remission. Groupe d'Etude les Lymphomes de l'Adulte (GELA). Blood 2000;95(12):3687‐92. [PubMed] [Google Scholar]
Olivieri 2005 {published data only}
- Olivieri A, Santini G, Patti C, Chisesi T, Souza C, Rubagotti A, et al. Upfront high‐dose sequential therapy (HDS) versus VACOP‐B with or without HDS in aggressive non‐Hodgkin's lymphoma: long‐term results by the NHLCSG. Annals of Oncology 2005;16(12):1941‐8. [DOI] [PubMed] [Google Scholar]
Pettengell 1996 {published data only}
- Pettengell R, Radford JA, Morgenstern GR, Scarffe JH, Harris M, Woll PJ, et al. Survival benefit from high‐dose therapy with autologous blood progenitor‐cell transplantation in poor‐prognosis non‐Hodgkin's lymphoma. Journal of Clinical Oncology 1996;14(2):586‐92. [DOI] [PubMed] [Google Scholar]
Philip 1991 {published data only}
- Philip T, Chauvin F, Bron D, Guglielmi C, Hagenbeek A, Coiffier B, et al. PARMA international protocol: pilot study on 50 patients and preliminary analysis of the ongoing randomized study (62 patients). Annals of Oncology 1991;2 Suppl 1:57‐64. [DOI] [PubMed] [Google Scholar]
Philip 1995 {published data only}
- Philip T, Guglielmi C, Hagenbeek A, Somers R, Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy‐sensitive non‐Hodgkin's lymphoma.[see comment]. New England Journal of Medicine 1995;333(23):1540‐5. [DOI] [PubMed] [Google Scholar]
Philip 1995a {published data only}
- Philip T, Guglielmi C, Chauvin F, Hagenbeek A, Lely J, Bron D, et al. Autologous bone marrow transplantation (ABMT) vs conventional chemotherapy (DHAP) in relapsed non‐Hodgkin's lymphoma (NHL): final analysis of the Parma randomized study (216 patients) [abstract]. Journal of Clinical Oncology 1995;14:390. [Google Scholar]
Proctor 2003 {published data only}
- Proctor SJ. Phase III Randomized Study of High‐Dose Chemotherapy and Radiotherapy Plus Autologous Bone Marrow Transplantation in Patients With Aggressive Non‐Hodgkin's Lymphoma. National Institutes of Health, ClinicalTrials Gov [http://www.clinicaltrials.gov]. 2003.
Rabinowe 1990 {published data only}
- Rabinowe S, Freedman A, Demetri G, Soiffer R, Ritz J, Blake K. Randomized double blinded trial of rh‐GM‐CSF in patients with B‐cell non‐Hodgkin's lymphoma (B‐NHL) undergoing high dose chemoradiotherapy and monoclonal antibody purged autologous bone marrow transplantation (ABMT). Blood 1990;76(10 Suppl 1):161a. [Google Scholar]
Rohatiner 1991 {published data only}
- Rohatiner AZ, Price CG, Arnott S, Norton A, Evans ML, Cotter F, et al. Myeloablative therapy with autologous bone marrow transplantation as consolidation of remission in patients with follicular lymphoma. Annals of Oncology 1991;2 Suppl 2:147‐50. [DOI] [PubMed] [Google Scholar]
Rohatiner 1994 {published data only}
- Rohatiner AZ, Johnson PW, Price CG, Arnott SJ, Amess JA, Norton AJ, et al. Myeloablative therapy with autologous bone marrow transplantation as consolidation therapy for recurrent follicular lymphoma. Journal of Clinical Oncology 1994;12(6):1177‐84. [DOI] [PubMed] [Google Scholar]
Rohatiner 1994a {published data only}
- Rohatiner AZ, Freedman A, Nadler L, Lim J, Lister TA. Myeloablative therapy with autologous bone marrow transplantation as consolidation therapy for follicular lymphoma. Annals of Oncology 1994;5 Suppl 2:143‐6. [DOI] [PubMed] [Google Scholar]
Santini 1991 {published data only}
- Santini G, Coser P, Chisesi T, Porcellini A, Sertoli R, Contu A, et al. Autologous bone marrow transplantation for advanced stage adult lymphoblastic lymphoma in first complete remission. Report of the Non‐Hodgkin's Lymphoma Cooperative Study Group (NHLCSG). Annals of Oncology 1991;2 Suppl 2:181‐5. [DOI] [PubMed] [Google Scholar]
Santini 1997 {published data only}
- Santini G, Salvagno L. VACOP‐B vs VACOP‐B + autologous BM transplantation (ABMT) for aggressive non‐Hodgkin's lymphoma. A study of the Non‐Hodgkin's Lymphoma Cooperative Study Group (NHLCSG) [abstract]. Bone Marrow Transplant 1997;19 Suppl:S154. [PubMed] [Google Scholar]
Santini 1998 {published data only}
- Santini G, Salvagno L, Leoni P, Chisesi T, Souza C, Sertoli MR, et al. VACOP‐B versus VACOP‐B plus autologous bone marrow transplantation for advanced diffuse non‐Hodgkin's lymphoma: results of a prospective randomized trial by the non‐Hodgkin's Lymphoma Cooperative Study Group. Journal of Clinical Oncology 1998;16(8):2796‐802. [DOI] [PubMed] [Google Scholar]
Sonnen 1998 {published data only}
- Sonnen R, Schmitz N, Dreger P, Lietz B, Kuse R. High dose chemotherapy followed by autologous stem cell‐transplantation in B‐high‐grade non‐hodgkin's lymphoma patients with unfavourable risk factors; a pilot study. Annals of Hematology 1998;77 Suppl 2:S65. [Google Scholar]
Sweetenham 2001 {published data only}
- Sweetenham JW, Santini G, Qian W, Guelfi M, Schmitz N, Simnett S, et al. High‐dose therapy and autologous stem‐cell transplantation versus conventional‐dose consolidation/maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: Results of a randomized trial of the european group for blood and marrow transplantation and the united kingdom lymphoma group. Journal of Clinical Oncology 2001;19(11):2927‐36. [DOI] [PubMed] [Google Scholar]
Winter 2005 {published data only}
- Winter JN. The role of high‐dose therapy followed by autologous purged stem cell transplantation in previously untreated patients with advanced follicular lymphoma. Current Hematology Reports 2005;4(4):249‐51. [PubMed] [Google Scholar]
Additional references
Anderson 1998
- Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non‐Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non‐Hodgkin's Lymphoma Classification Project. Annals of Oncology 1998;9(7):717‐20. [DOI] [PubMed] [Google Scholar]
Andreadis 2005
- Andreadis C, Schuster SJ, Chong EA, Svoboda J, Luger SM, Porter DL, et al. Long‐term event‐free survivors after high‐dose therapy and autologous stem‐cell transplantation for low‐grade follicular lymphoma. Bone Marrow Transplantation 2005;36(11):955‐61. [DOI] [PubMed] [Google Scholar]
Ardeshna 2003
- Ardeshna KM, Smith P, Norton A, Hancock BW, Hoskin PJ, MacLennan KA, et al. Long‐term effect of a watch and wait policy versus immediate systemic treatment for asymptomatic advanced‐stage non‐Hodgkin lymphoma: a randomised controlled trial. Lancet 2003;362(9383):516‐22. [DOI] [PubMed] [Google Scholar]
Armitage 1998
- Armitage JO, Weisenburger DD. New approach to classifying non‐Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non‐Hodgkin's Lymphoma Classification Project. Journal of Clinical Oncology 1998;16(8):2780‐95. [DOI] [PubMed] [Google Scholar]
Baldo 2010
- Baldo P, Rupolo M, Compagnoni A, Lazzarini R, Bearz A, Cannizzaro R, et al. Interferon‐alpha for maintenance of follicular lymphoma. Cochrane Database of Systematic Reviews 20 January 2010, Issue 1:CD004629. [DOI: 10.1002/14651858.CD004629] [DOI] [PubMed] [Google Scholar]
Brandt 2001
- Brandt L, Kimby E, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in indolent non‐Hodgkin's lymphoma. Acta Oncologica 2001;40(2‐3):213‐23. [DOI] [PubMed] [Google Scholar]
Brown 2009
Buske 2005
- Buske C, Dreyling M, Unterhalt M, Hiddemann W. Transplantation strategies for patients with follicular lymphoma. Current Opinion Hematology 2005;12(4):266‐72. [DOI] [PubMed] [Google Scholar]
Buske 2007
- Buske C, Unterhalt M, Hiddeman W. Therapy of follicular lymphoma [Therapie des follikularen Lymphoms.]. Internist (Berl) 2007;48(4):372‐81. [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke CA, Glaser SL. Changing incidence of non‐Hodgkin lymphomas in the United States. Cancer 2002;94(7):2015‐23. [DOI] [PubMed] [Google Scholar]
Colombat 2001
- Colombat P, Salles G, Brousse N, Eftekhari P, Soubeyran P, Delwail V, et al. Rituximab (anti‐CD20 monoclonal antibody) as single first‐line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood 2001;97(1):101‐6. [DOI] [PubMed] [Google Scholar]
Deconinck 2005
- Deconinck E, Foussard C, Milpied N, Bertrand P, Michenet P, Cornillet‐LeFebvre P, et al. High‐dose therapy followed by autologous purged stem‐cell transplantation and doxorubicin‐based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by GOELAMS. Blood 2005;105(10):3817‐23. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available from www.cochrane‐handbook.org. [Google Scholar]
Diaz‐Alderete 2008
- Diaz‐Alderete A, Doval A, Camacho F, Verde L, Sabin P, Arranz‐Saez R, et al. Frequency of BCL2 and BCL6 translocations in follicular lymphoma: relation with histological and clinical features. Leukemia & Lymphoma 2008;49(1):95‐101. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2004
- Fisher SG, Fisher RI. The epidemiology of non‐Hodgkin's lymphoma. Oncogene 2004;23(38):6524‐34. [DOI] [PubMed] [Google Scholar]
Forstpointner 2004
- Forstpointner R, Dreyling M, Repp R, Hermann S, Hanel A, Metzner B, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood 2004;104(10):3064‐71. [DOI] [PubMed] [Google Scholar]
Foster 2009
- Foster M, Gabriel DA, Shea T. Role of hematopoietic stem cell transplant in the management of follicular lymphoma. The Oncologist 2009;14(7):726‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 1986
- Gallagher CJ, Gregory WM, Jones AE, Stansfeld AG, Richards MA, Dhaliwal HS, et al. Follicular lymphoma: prognostic factors for response and survival. Journal of Clinical Oncology 1986;4(10):1470‐80. [DOI] [PubMed] [Google Scholar]
Greb 2011
- Greb A, Bohlius J, Schiefer D, Schwarzer G, Schulz H, Engert A. High‐dose chemotherapy with autologous stem cell transplantation in the first line treatment of aggressive non‐Hodgkin lymphoma (NHL) in adults. Cochrane Database of Systematic Reviews 2011;4:CD004024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groves 2000
- Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non‐Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. Journal of the National Cancer Institute 2000;92(15):1240‐51. [DOI] [PubMed] [Google Scholar]
Harris 1999
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller‐Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Annals of Oncology 1999;10(12):1419‐32. [DOI] [PubMed] [Google Scholar]
Herold 2007
- Herold M, Haas A, Srock S, Neser S, Al‐Ali KH, Neubauer A, et al. Rituximab added to first‐line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. Journal of Clinical Oncology 2007;25(15):1986‐92. [DOI] [PubMed] [Google Scholar]
Hiddemann 2005
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced‐stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood 2005;106(12):3725‐32. [DOI] [PubMed] [Google Scholar]
Hiddemann 2006
- Hiddemann W, Unterhalt M. Current treatment strategies in follicular lymphomas. Annals of Oncology 2006;17 Suppl 10:x155‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Horning 1984
- Horning SJ, Rosenberg SA. The natural history of initially untreated low‐grade non‐Hodgkin's lymphomas. New England Journal of Medicine 1984;311(23):1471‐5. [DOI] [PubMed] [Google Scholar]
Horning 2000
- Horning SJ. Follicular lymphoma: have we made any progress?. Annals of Oncology 2000;11 Suppl 1:23‐7. [PubMed] [Google Scholar]
Jemal 2005
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA: A Cancer Journal for Clinicians 2005;55(1):10‐30. [DOI] [PubMed] [Google Scholar]
Khouri 2008
- Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight‐year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood 2008;111(12):5530‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korsmeyer 1992
- Korsmeyer SJ. Bcl‐2 initiates a new category of oncogenes: regulators of cell death. Blood 1992;80(4):879‐86. [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333(7568):597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
Lenz 2004
- Lenz G, Dreyling M, Schiegnitz E, Forstpointner R, Wandt H, Freund M, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression‐free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low‐Grade Lymphoma Study Group. Blood 2004;104(9):2667‐74. [DOI] [PubMed] [Google Scholar]
Lenz 2004a
- Lenz G, Dreyling M, Schiegnitz E, Haferlach T, Hasford J, Unterhalt M, et al. Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem‐cell transplantation in patients with indolent lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. Journal of Clinical Oncology 2004; Vol. 22, issue 24:4926‐33. [DOI] [PubMed]
Marcus 2005
- Marcus R, Imrie K, Belch A, Cunningham D, Flores E, Catalano J, et al. CVP chemotherapy plus rituximab compared with CVP as first‐line treatment for advanced follicular lymphoma. Blood 2005;105(4):1417‐23. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The Prisma Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
Muller 2005
- Muller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non‐Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Annals of Hematology 2005;84(1):1‐12. [DOI] [PubMed] [Google Scholar]
Oliansky 2010
- Oliansky DM, Gordon LI, King J, Laport G, Leonard JP, McLaughlin P, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of follicular lymphoma: an evidence‐based review. Biology of blood and marrow transplantation : American Society for Blood and Marrow Transplantation 2010;16(4):443‐68. [DOI] [PubMed] [Google Scholar]
Ott 2002
- Ott G, Katzenberger T, Lohr A, Kindelberger S, Rudiger T, Wilhelm M, et al. Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood 2002;99(10):3806‐12. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Reiser 2002
- Reiser M, Diehl V. Current treatment of follicular non‐Hodgkin's lymphoma. European Journal of Cancer 2002;38(9):1167‐72. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rohatiner 2005
- Rohatiner AZ, Gregory WM, Peterson B, Borden E, Solal‐Celigny P, Hagenbeek A, et al. Meta‐analysis to evaluate the role of interferon in follicular lymphoma. Journal of Clinical Oncology 2005;23(10):2215‐23. [DOI] [PubMed] [Google Scholar]
Sacchi 2007
- Sacchi S, Pozzi S, Marcheselli L, Bari A, Luminari S, Angrilli F, et al. Introduction of rituximab in front‐line and salvage therapies has improved outcome of advanced‐stage follicular lymphoma patients. Cancer 2007;109(10):2077‐82. [DOI] [PubMed] [Google Scholar]
Schulz 2007
- Schulz H, Bohlius J, Skoetz N, Trelle S, Kober T, Reiser M, et al. Chemotherapy plus Rituximab versus chemotherapy alone for B‐cell non‐Hodgkin's lymphoma. Cochrane Database of Systematic Reviews 2007;CD003805(4):CD003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sebban 2006
- Sebban C, Mounier N, Brousse N, Belanger C, Brice P, Haioun C, et al. Standard chemotherapy with interferon compared with CHOP followed by high‐dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF‐94 randomized study from the Groupe d'Etude des Lymphomes de l'Adulte (GELA). Blood 2006;108(8):2540‐4. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Symmans 1995
- Symmans WF, Katz RL, Ordonez NG, Dalton H, Romaguera JE, Cabanillas F. Transformation of follicular lymphoma. Expression of p53 and bcl‐2 oncoprotein, apoptosis and cell proliferation. Acta Cytologica 1995;39(4):673‐82. [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Oers 2006
- Oers MH, Klasa R, Marcus RE, Wolf M, Kimby E, Gascoyne RD, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non‐Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood 2006;108(10):3295‐301. [DOI] [PubMed] [Google Scholar]
Vidal 2009
- Vidal L, Gafter‐Gvili A, Leibovici L, Shpilberg O. Rituximab as maintenance therapy for patients with follicular lymphoma. Cochrane Database of Systematic Reviews 2009;Issue 2:Art. No.: CD006552. DOI: 10.1002/14651858.CD006552. [DOI] [PubMed] [Google Scholar]
Weigert 2006
- Weigert O, Dreyling M, Unterhalt M, Hiddemann W, Buske C. Investigational strategies in autologous stem cell transplantation for follicular lymphoma. Current Oncology Reports 2006;8(5):368‐75. [DOI] [PubMed] [Google Scholar]


