Abstract
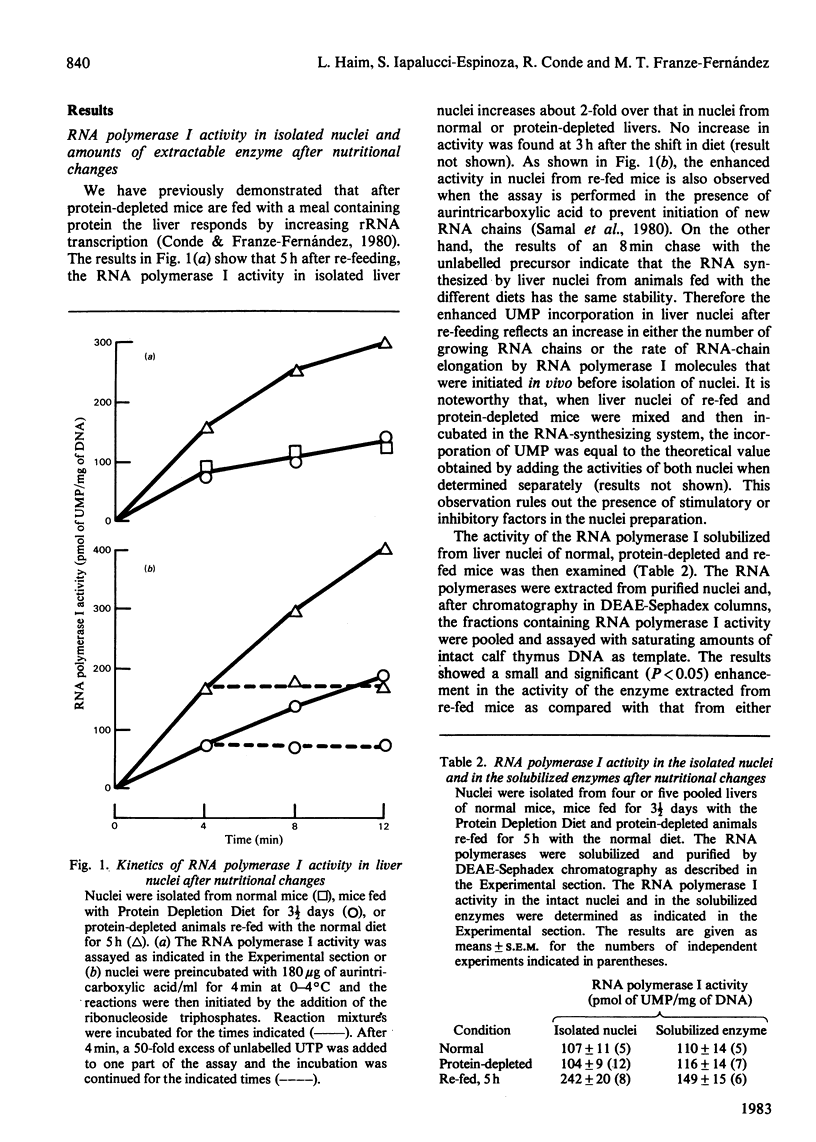
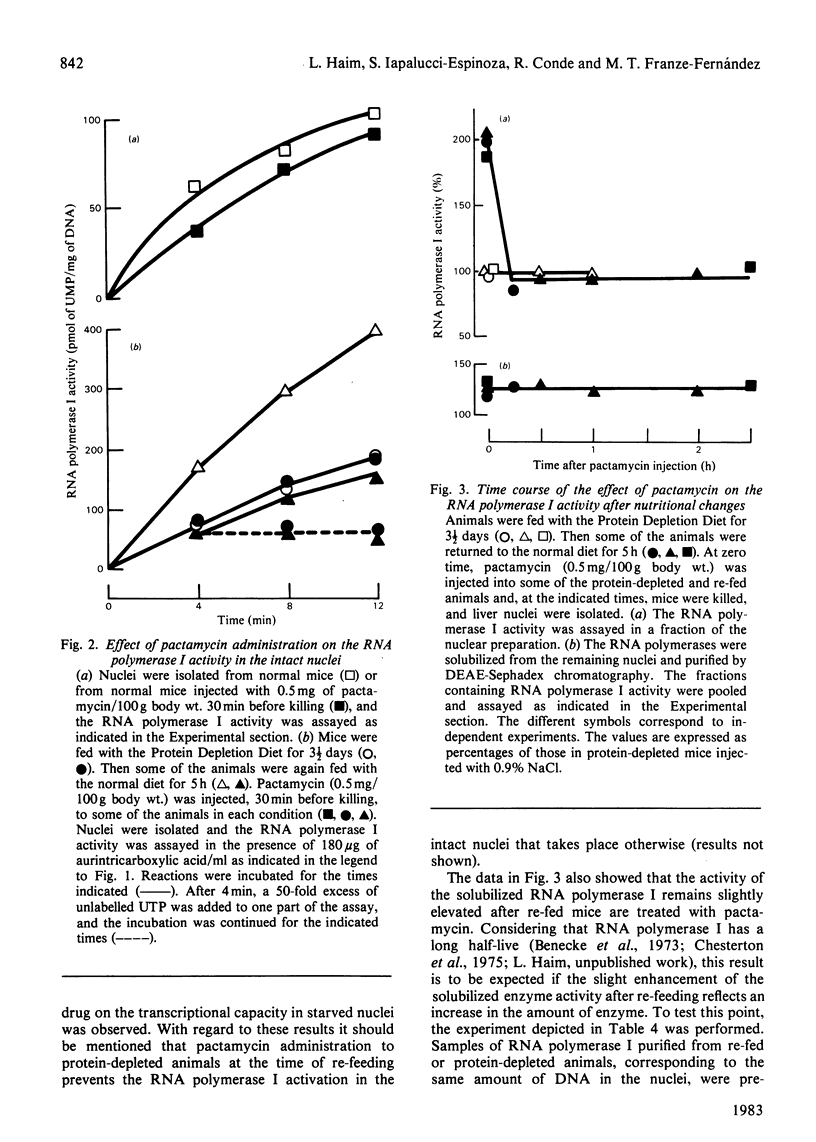
Shortly after feeding protein-depleted mice with a meal containing protein, the RNA polymerase I activity in isolated liver nuclei shows a 2-fold increase over the values in the nuclei of either normal or protein-depleted mice. The activity of the RNA polymerase I solubilized from nuclei of re-fed mice was slightly enhanced, probably reflecting an increase in enzyme amount. However, this increase only accounts for about 30% of the stimulation of transcription in the intact nuclei. Administration of pactamycin, an inhibitor of protein synthesis, to normal or protein-depleted mice has almost no inhibitory effect on the RNA polymerase I activity in the isolated nuclei. On the contrary, within 15 min after treatment with the drug, the stimulated activity in nuclei from re-fed mice declines towards the values in normal or protein-depleted mice and then remains constant. The activity of the solubilized enzyme remains slightly elevated for at least 2 1/2 h after re-fed mice are treated with pactamycin. These observations indicate that the stimulation of the RNA polymerase I activity in the intact nuclei after re-feeding is controlled by mechanisms other than an increase in the enzyme amount and suggest the presence of short-lived proteins required for inducing an activated state of transcription.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benecke B. J., Ferencz A., Seifart K. H. Resistance of hepatic RNA polymerases to compounds effecting RNA and protein synthesis in vivo. FEBS Lett. 1973 Apr 1;31(1):53–58. doi: 10.1016/0014-5793(73)80072-9. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Franze-Fernandez M. T. Purification and properties of RNA polymerase I from Ehrlich ascites cells. Biochim Biophys Acta. 1977 Nov 2;479(1):80–90. doi: 10.1016/0005-2787(77)90127-7. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Franze-Fernández M. T. Ehrlich ascites cells DNA-dependent RNA polymerases: effect of amino acids and protein synthesis inhibition. FEBS Lett. 1974 Apr 15;41(1):161–165. doi: 10.1016/0014-5793(74)80978-6. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Coupar B. E., Butterworth P. H., Green M. H. Studies on the control of ribosomal RNA synthesis in HeLa cells. Eur J Biochem. 1975 Sep 1;57(1):79–83. doi: 10.1111/j.1432-1033.1975.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Conde R. D., Franze-Fernández M. T. Increased transcription and decreased degradation control and recovery of liver ribosomes after a period of protein starvation. Biochem J. 1980 Dec 15;192(3):935–940. doi: 10.1042/bj1920935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupar B. E., Davies J. A., Chesterton C. J. Quantification of hepatic transcribing RNA polymerase molecules, polyribonucleotide elongation rates and messenger RNA complexity in fed and fasted rats. Eur J Biochem. 1978 Mar 15;84(2):611–623. doi: 10.1111/j.1432-1033.1978.tb12204.x. [DOI] [PubMed] [Google Scholar]
- Duceman B. W., Jacob S. T. Transcriptionally active RNA polymerases from Morris hepatomas and rat liver. Elucidation of the mechanism for the preferential increase in the tumour RNA polymerase I. Biochem J. 1980 Sep 15;190(3):781–789. doi: 10.1042/bj1900781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Hayashi T. T., MacFarlane K. Comparison of endogenous and exogenous RNA primers of poly(U) polymerase in rat hepatic ribosomes. Biochem J. 1979 Mar 1;177(3):895–902. doi: 10.1042/bj1770895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iapalucci-Espinoza S., Cereghini S., Franze-Fernández M. T. Regulation of ribosomal RNA synthesis in mammalian cells: effect of toyocamycin. Biochemistry. 1977 Jun 28;16(13):2885–2889. doi: 10.1021/bi00632a013. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Leonard T. B., Jacob S. T. Alterations in DNA-dependent RNA polymerase I and II from rat liver by thioacetamide: preferential increase in the level of chromatin-associated nucleolar RNA polymerase IB. Biochemistry. 1977 Oct 4;16(20):4538–4544. doi: 10.1021/bi00639a032. [DOI] [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Shimada N., Higashinakagawa T. Effect of cycloheximide on the nucleolar RNA synthesis in rat liver. J Mol Biol. 1970 Oct 14;53(1):91–106. doi: 10.1016/0022-2836(70)90047-1. [DOI] [PubMed] [Google Scholar]
- Sajdel E. M., Jacob S. T. Mechanism of early effect of hydrocortisone on the transcriptional process: stimulation of the activities of purified rat liver nucleolar RNA polymerases. Biochem Biophys Res Commun. 1971 Nov 5;45(3):707–715. doi: 10.1016/0006-291x(71)90474-8. [DOI] [PubMed] [Google Scholar]
- Samal B., Ballal N. R., Busch H. Initiation of transcription in permeabilized Novikoff hepatoma cells. Cell Biol Int Rep. 1980 Feb;4(2):175–184. doi: 10.1016/0309-1651(80)90072-7. [DOI] [PubMed] [Google Scholar]
- Scornik O. A. In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem. 1974 Jun 25;249(12):3876–3883. [PubMed] [Google Scholar]
- Scornik O. A. In vivo rates of deaggregation of polyribosomes in normal and regenerating liver after the injection of pactamycin. Biochim Biophys Acta. 1974 Nov 20;374(1):76–81. doi: 10.1016/0005-2787(74)90200-7. [DOI] [PubMed] [Google Scholar]
- Stoyanova B. B., Dabeva M. D. Ribosomal RNA precursor transcription in rat liver is not dependent on continuous synthesis of proteins. Biochim Biophys Acta. 1980 Jul 29;608(2):358–367. doi: 10.1016/0005-2787(80)90181-1. [DOI] [PubMed] [Google Scholar]
- Todhunter J. A., Weissbach H., Brot N. Modification of rat liver RNA polymerase I after in vivo stimulation by hydrocortisone or methylisobutylxanthine. J Biol Chem. 1978 Jul 10;253(13):4514–4516. [PubMed] [Google Scholar]
- Weaver R. F., Blatti S. P., Rutter W. J. Molecular structures of DNA-dependent RNA polymerases (II) from calf thymus and rat liver. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2994–2999. doi: 10.1073/pnas.68.12.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Blatti S. P. HeLa cell deoxyribonucleic acid dependent RNA polymerases: function and properties of the class III enzymes. Biochemistry. 1976 Apr 6;15(7):1500–1509. doi: 10.1021/bi00652a022. [DOI] [PubMed] [Google Scholar]
- Yu F. L. An improved method for the quantitative isolation of rat liver nuclear RNA polymerases. Biochim Biophys Acta. 1975 Jul 7;395(3):329–336. doi: 10.1016/0005-2787(75)90204-x. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. The rapid turnover of RNA polymerase of rat liver nucleolus, and of its messenger RNA. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2833–2837. doi: 10.1073/pnas.69.10.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]


