Abstract
PURPOSE
HERALD/EPOC1806 was conducted as a multicenter phase II trial assessing trastuzumab deruxtecan (T-DXd) therapy for patients with human epidermal growth factor receptor 2 (HER2)–amplified progressive stage solid tumors detected by cell-free DNA (cfDNA) testing.
PATIENTS AND METHODS
Patients exhibited advanced solid tumors with HER2 amplification that was identified via next-generation sequencing of cfDNA testing, without the requirement for immunohistochemical HER2 testing. The studied group was administered T-DXd at 5.4 mg/kg once every 3 weeks until onset of disease progression or intolerable toxicity.
RESULTS
Overall, 4,734 patients underwent cfDNA testing from December 2019 to January 2022, and 252 demonstrated HER2 amplification. Finally, the study included 62 patients with 16 cancer types with a median baseline plasma HER2 copy number (CN) of 8.55 (range, 2.4-73.9). Confirmed overall response rate (ORR) by investigator assessment was 56.5% (95% CI, 43.3 to 69.0), thus showing a value beyond the 5% threshold. Responses were evaluated for 13 cancer types, including KRAS-mutant colorectal (1/3), PIK3CA-mutant endometrial (5/6), and tissue HER2-negative gastric (1/2) cancers. Plasma HER2 CN above versus below the baseline median value did not differ for impact response; however, clearance of HER2 amplification in cfDNA on cycle 2 day 1 had higher response values compared with persistence. Median progression-free survival and response duration were 7.0 (95% CI, 4.9 to 9.7) and 8.8 (95% CI, 5.8 to 11.2) months, respectively, with the majority of complications being mild to moderate. Interstitial lung diseases were identified in 16 (26%) patients, including 14 patients with grade 1 disease, one patient with grade 2 disease, and one patient with grade 3 disease.
CONCLUSION
T-DXd treatment demonstrated high ORR with durable response in patients with advanced HER2-amplified solid tumors determined with cfDNA testing.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase receptor, the protein structure of which is encoded by the ERBB2 (HER2) gene (chromosome 17q12). Amplification of the HER2 gene is associated with cell proliferation, migration, and differentiation processes in 15%-20% of breast and gastric cancers. HER2-targeted drugs representing HER2 antibodies include trastuzumab and pertuzumab, and tyrosine kinase inhibitors such as lapatinib, neratinib, and tucatinib being a part of common therapeutic regimens.1-3 Moreover, HER2 amplification can be a therapeutic target as observed in 2%-3% of other solid tumors.4-6 However, HER2-targeted therapies for HER2-amplified solid tumors have yet to be standardized for these patients.
CONTEXT
Key Objective
The HERALD/EPOC1806 trial aimed to evaluate the efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients with human epidermal growth factor receptor 2 (HER2)–amplified solid tumors identified via cell-free DNA (cfDNA) testing.
Knowledge Generated
T-DXd treatment was associated with high objective response rate and sustained efficacy in patients with advanced HER2-amplified solid tumors identified by cfDNA testing. The toxicity profile of T-DXd treatment aligned with previous studies investigating T-DXd monotherapy in Japanese patients.
Relevance (G.K. Schwartz)
In a tumor agnostic fashion, cfDNA for amplified HER2 can be successfully used to identify patients with solid tumors who will respond to T-DXd therapy.*
*Relevance section written by JCO Associate Editor Gary K. Schwartz, MD, FASCO.
Cell-free DNA (cfDNA) is routinely used as a marker of genomic alterations that potentially serve as therapeutic targets in advanced solid tumors.7 We initiated a nationwide cfDNA-based screening study, GOZILA, to match patients with progressive stages according to genotyping results and conducted the trials on targeted therapy.8 We have previously reported that cfDNA-based genotyping promoted a higher number of patient enrollments, with efficacy comparable to tissue-based methods.9 Moreover, we found that patients with HER2-amplified metastatic colorectal cancer diagnosed by cfDNA genotyping benefited from trastuzumab plus pertuzumab, similarly to those diagnosed via conventional tissue HER2 assessment.10
Trastuzumab deruxtecan (T-DXd, DS-8201a) is a HER2-targeted antibody-drug conjugate consisting of a humanized anti-HER2 monoclonal antibody, cleavable tetrapeptide-based linker, and cytotoxic topoisomerase I inhibitor.11 T-DXd therapy has improved the overall response and survival rates, leading to its approval in several countries for patients with tissue HER2-positive breast cancer and gastric/gastroesophageal junction adenocarcinoma.12,13 Although a phase I study of T-DXd therapy included several patients with other HER2-expressing solid tumors,14 there has been a shortage of studies concentrating on a large number of patients with HER2-amplified solid tumors. Herein, we present the primary results of an investigator-initiated phase II study evaluating the efficacy and safety of T-DXd in patients with HER2-amplified solid tumors, as identified by cfDNA testing.
PATIENTS AND METHODS
Study Design and Eligibility
HERALD was a multicenter, open-label, single-arm, phase II study conducted across seven institutions in Japan. We enrolled patients age 20 years and older who were histologically confirmed to have advanced or metastatic solid tumors with HER2 amplification in plasma cfDNA obtained within 8 weeks before the study. HER2 amplification was detected by plasma next generation sequencing (NGS) analysis using Guardant360, a 74-gene sequencing circulating tumor DNA panel, at Guardant Health (Redwood City, CA) in the screening GOZILA study.8 Additional inclusion criteria were applied to eligible patients who had an Eastern Cooperative Oncology Group performance status of 0 or 1 and measurable disease according to the RECIST 1.1. Patients confirmed by tumor tissue analysis with HER2-amplified breast and gastric cancers were excluded, while cases of other cancer types with unknown HER2 status in tumor tissue were included in HERALD. Previous HER2-targeted therapy was permissible; however, previous treatment with T-DXd or other antibody-drug conjugates containing an exatecan derivative was not allowed. The study was performed according to the protocol, the Ministerial Ordinance on Good Clinical Practice for Drugs, and the Declaration of Helsinki, after being approved by the institutional review board at each of the involved institutions. All patients signed written informed consent for study participation.
Procedures
Patients were administered T-DXd at 5.4 mg/kg intravenously once every 3 weeks until the manifestation of disease progression, death, unacceptable toxicity, and discontinuation for any other reasons. Computed tomography or magnetic resonance imaging scans were obtained at screening and every 6 weeks (±7 days) during the first 48 weeks of treatment, then every 9 weeks (±14 days) thereafter. Local site investigators and independent central review estimated the response as per RECIST v1.1. The final tumor responses were observed at least 4 weeks after the initial administration. Safety was evaluated as the incidence of treatment-emergent adverse events detected during treatment and 30 days afterward while being graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
The primary end point was confirmed as the overall response rate (ORR), which was defined as the sum of the proportion of patients with complete response (CR) and partial response (PR) lasting for ≥4 weeks, as assessed by site investigators. Key secondary end points included progression-free survival (PFS), duration of response (DoR), time to treatment failure, disease control rate (the proportion of patients who achieved the best response of CR or PR or stable disease [SD]), overall survival (OS), ORR by independent central review, and adverse event incidences.
Biomarker Analysis
Whole-blood samples of 2 × 10 mL were collected into cfDNA BCT (Streck; La Vista, NE) from recruited patients at baseline, 3 weeks after study treatment initiation, and at the end of the protocol treatment. NGS analysis of cfDNA was done using Guardant360 at Guardant Health that identified single-nucleotide variants (SNVs), indels, fusions, and copy number (CN) alterations in 74 genes with a reportable range of ≥0.04%, ≥0.02%, ≥0.04%, and ≥2.12% copies, respectively. Relative clonality was determined by dividing the maximum variant allelic fraction (VAF) of an alteration by the highest somatic VAF observed in the sample, which helped in determining cfDNA clonality for somatic SNVs, indels, and fusions. Mutations with the values of <0.3 fell into the category of subclonal mutations.6 The adjusted plasma CN (pCN) was calculated as follows: adjusted pCN = (observed pCN – 2 × [1 – T%])/T%, where T% = 2 × maximum VAF/100.
Statistical Analysis
The thresholds for the ORR were set at 5% for the lower limit and 25% for the expected value. According to the original protocol involving Bayesian robust exchangeability that potentially imposes heterogeneity in ORR, 60 patients is sufficient to declare efficacy. The revised protocol from June 2021 modified the primary analysis into a single-stage binomial design because of slow accrual. The revised analysis implies the power of >90% to detect a 15%-20% gain in ORR with a one-sided significance level of 2.5%.
For efficacy analysis, the primary population included all eligible patients who had received at least one dose of T-DXd. This cohort was mirrored in the safety analysis set. The primary analysis criterion for ORR was the evaluation of the lower limit of the 95% CI using the Clopper-Pearson method.
For biomarker analysis, data were summarized descriptively with no formal hypothesis testing. Post hoc analyses were performed to explore the relationship between ctDNA outcomes and tumor response. Baseline HER2 pCN levels were compared between responders and nonresponders using Student's t test. To assess the feasibility of using changes in HER2 pCN levels at specific phases during therapy as an indicator of treatment efficacy, we investigated whether changes in HER2 pCN levels from baseline to week 3 differed according to tumor response. Furthermore, changes were classified as disappearance (clearance group), increase, or decrease (nonclearance group). A P value of <.05 was considered to denote statistical significance. Fisher's exact test was used for data analysis.
Descriptive characteristics of patients, safety data, and antitumor activities were obtained and summarized. Survival curves were generated using the Kaplan-Meier method. All statistical analyses were conducted in SAS (version 9.4) and R (version 4.2.1) programs. The HERALD and GOZILA studies were registered with ClinicalTrials.jp, JapicCTI-194707, and UMIN-CTR, UMIN000029315, respectively.
RESULTS
Patients
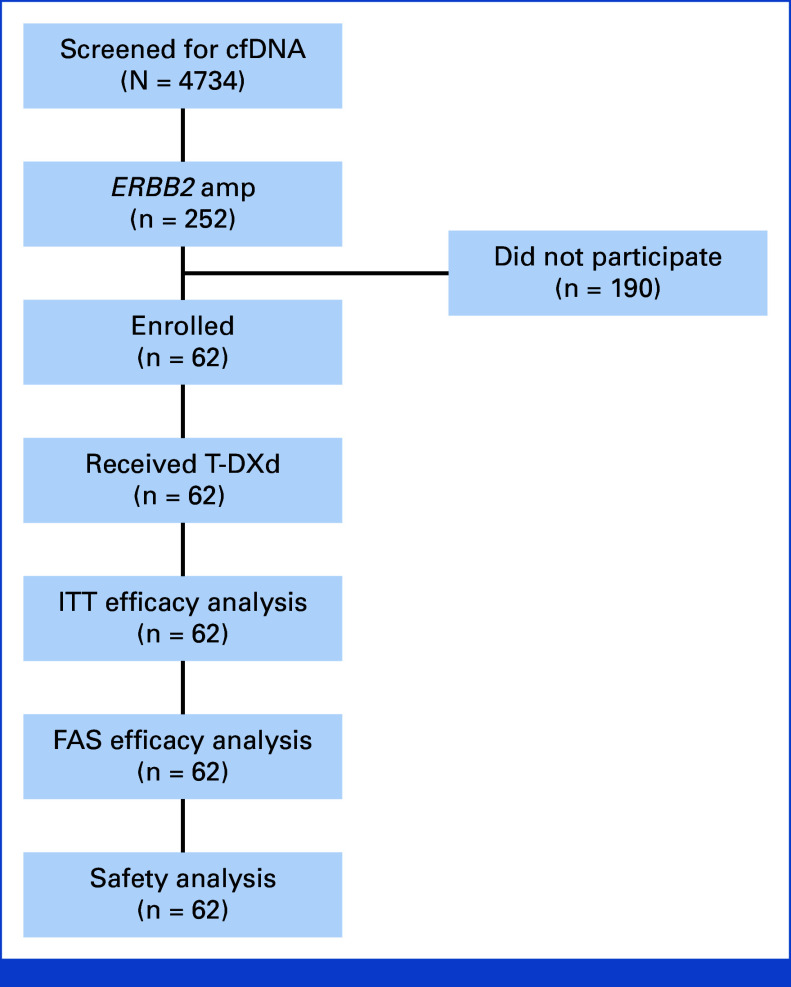
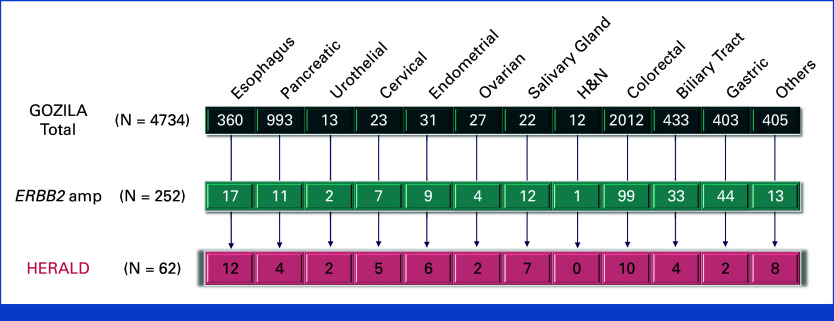
A total of 4,734 patients underwent initial screening by cfDNA in the GOZILA study across 36 sites from December 2019 to January 2022. Among 252 patients (5.3%) with HER2 amplification, 62 patients with 16 cancer types were finally included in HERALD across seven sites in Japan (Appendix Figs A1 and A2, online only). As of the cutoff date on July 17, 2022, treatment was ongoing in 13 patients. The most prominent reasons for therapy discontinuation were disease progression (33 of 49 [67%]) and adverse events (12 of 49 [24%]). The median treatment duration was 6.0 months. All 62 patients were assessed for efficacy and safety analyses.
Table 1 shows the baseline demographics and disease characteristics. The 16 cancer types included esophageal (n = 12), colorectal (n = 10), salivary grand (n = 7), endometrial (n = 6), cervical (n = 5), pancreatic (n = 4), biliary tract (n = 4), urothelial (n = 2), ovarian (n = 2), non–small cell lung (n = 2), gastric (n = 2), small intestinal (n = 2), extramammary Paget's disease (n = 1), melanoma (n = 1), prostate (n = 1), and cancer of unknown primary (n = 1). The histologic esophageal cancer subtypes were squamous cell carcinoma in 10 patients, adenocarcinoma in 1 patient, and another subtype in 1 patient. Except for three patients (all with salivary gland cancer), all had undergone previous anticancer therapy (median, three lines; range, 0-8). Common sites of metastases included lymph nodes (65%), liver (53%), lung (52%), and peritoneum (23%).
TABLE 1.
Patient Characteristics
| Patients at Baseline | Total (N = 62) |
|---|---|
| Age, years, median (range) | 63 (32-80) |
| Sex, No. (%) | |
| Female | 32 (52) |
| Male | 30 (48) |
| ECOG performance status, No. (%) | |
| 0 | 36 (58) |
| 1 | 26 (42) |
| Tumor type, No. (%) | |
| Esophagus | 12 (19) |
| Colorectal | 10 (16) |
| Salivary gland | 7 (11) |
| Endometrium | 6 (10) |
| Cervix | 5 (8) |
| Pancreas | 4 (7) |
| Biliary tract | 4 (7) |
| Gastric | 2 (3) |
| NSCLC | 2 (3) |
| Urothelium | 2 (3) |
| Ovary | 2 (3) |
| Small intestine | 2 (3) |
| Paget's disease | 1 (2) |
| Melanoma | 1 (2) |
| Prostate | 1 (2) |
| Cancer unknown primary | 1 (2) |
| Metastatic site, No. (%) | |
| Lymph node | 40 (65) |
| Liver | 33 (53) |
| Lung | 32 (52) |
| Peritoneum | 14 (23) |
| Bone | 13 (21) |
| Others | 16 (26) |
| Previous therapies, No. (%) | |
| Surgery | 41 (66) |
| Chemotherapy | 59 (95) |
| Radiation | 18 (29) |
| Others | 5 (8) |
| No. of previous treatment regimens | |
| Median (range) | 3 (0-8) |
| Baseline plasma HER2 copy number | |
| Median (range) | 8.55 (2.4-73.9) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; NSCLC, non–small cell lung cancer.
Efficacy
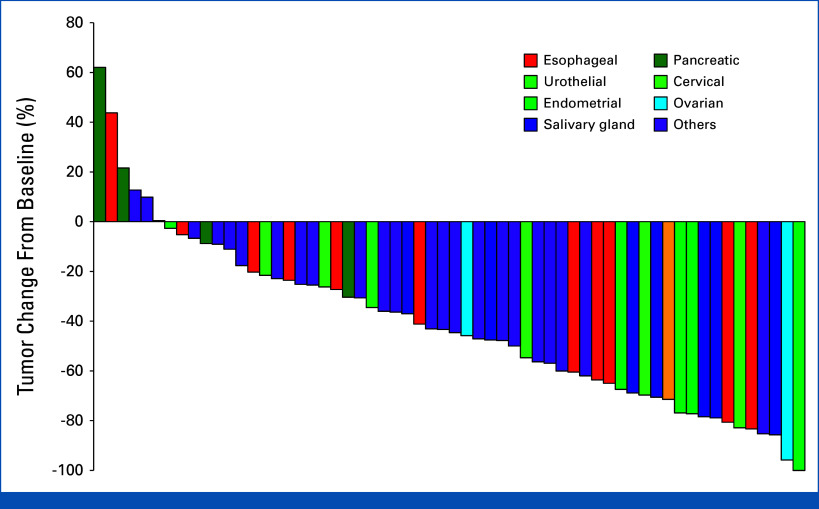
All 62 eligible patients were assigned to the efficacy analysis. The ORR by investigator assessment was 56.5% (95% CI, 43.3 to 69.0), which was statistically higher than the predetermined threshold of 5%. The best overall responses included PR, SD, and progressive disease/not evaluable in 35 (56.5%), 21 (33.9%), and six (9.7%), respectively (Appendix Table A1). Tumor response for each patient is depicted in a waterfall plot (Fig 1) and a spider plot (Fig 2). Responses were observed across 10 of the 12 cancer types with two or more enrolled patients and in three of the four with only one patient recruited (Appendix Table A2). Responses in KRAS-mutant colorectal (1/3), PIK3CA-mutant endometrial (5/6), and tissue HER2-negative gastric (1/2) cancers were also observed. The ORR by independent review was 58.1% (95% CI, 44.8 to 70.5), including one patient with CR, and the disease control rate was 90.3% (95% CI, 80.1 to 96.4; Appendix Fig A3).
FIG 1.
Waterfall plot. Waterfall plot of maximum change in tumor size for the 62 evaluated patients, as per investigator assessment.
FIG 2.
Spider plot of response depth and duration according to cancer type.
Disease progression developed in 44 (71%) patients, and the median PFS was 7.0 months (95% CI, 4.9 to 9.7), per investigator assessment (Fig 3A). Disease progression was found in 43 (69%) patients, with a median PFS of 5.7 months (95% CI, 4.9 to 9.7), per the independent central review. The 6-month PFS rates were 52.7% (95% CI, 39.6 to 64.3) and 43.6% (95% CI, 30.9 to 55.6), respectively. The median OS was 14.6 months (95% CI, 10.8 to 22.3 months; Fig 3B), and 13 (21%) of 62 patients continued T-DXd treatment. At the same time point, the median DoR was 8.8 months (95% CI, 5.8 to 11.2) as per investigator evaluation and 7.3 months (95% CI, 4.3 to 11.3) as per the independent review committee.
FIG 3.
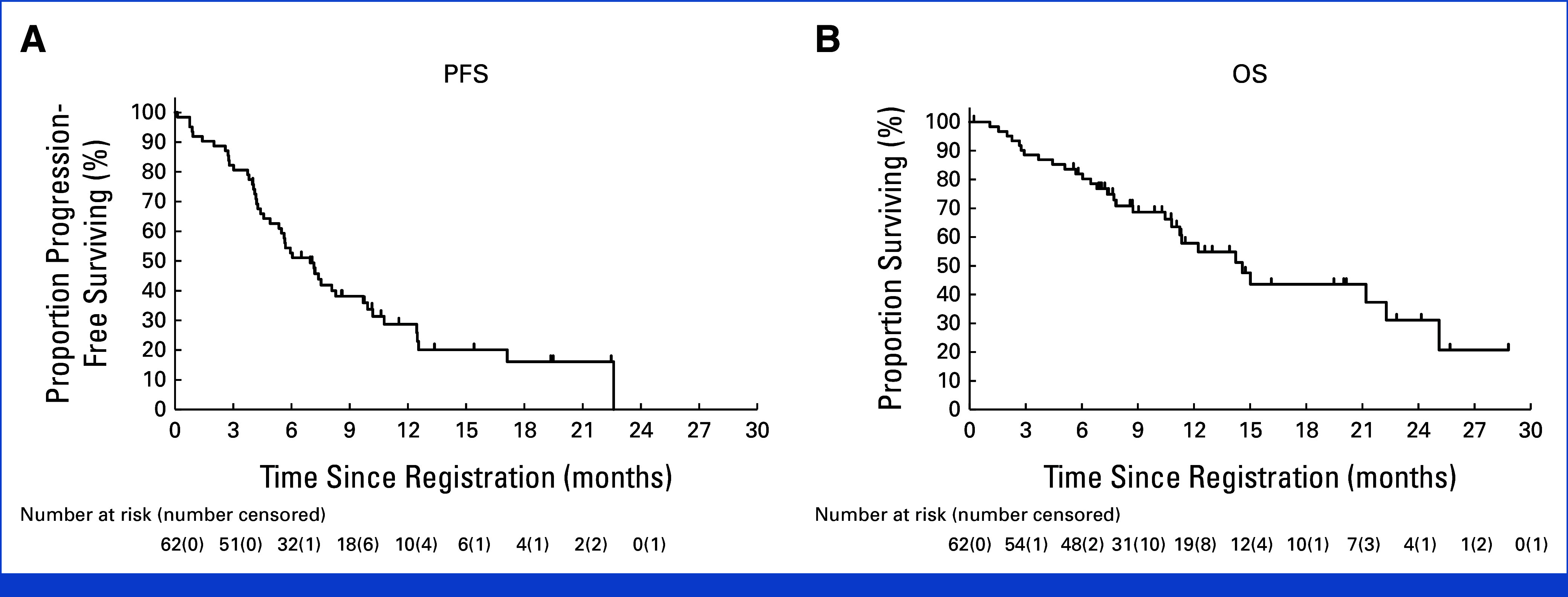
Survival outcomes. (A) PFS per investigator. (B) OS per investigator. OS, overall survival; PFS, progression-free survival.
Safety
In the population assigned to safety analysis, AEs and treatment-related AEs (trAEs) were observed in all 62 and 59 (95%) patients, respectively. The most prominent trAEs included nausea (58%), decreased appetite (53%), malaise (40%), and anemia (39%; Table 2). Severe (≥grade 3) trAEs manifested in 32 (52%) patients with mainly anemia (21%) and decreased neutrophil count (19%). Pneumonitis/interstitial lung disease (ILD) was noted in 16 (26%) patients, including 14 patients (23%) with grade 1 disease, one patient (2%) with grade 2 disease, and one patient (2%) with grade 3 disease. Although three patients with grade 1 pneumonitis/ILD restarted T-DXd treatment, the remaining patients discontinued treatment. One of the three patients who restarted treatment with T-DXd experienced grade 1 pneumonitis/ILD. No pneumonitis/ILD events were reviewed by an independent committee. Treatment-related death occurred in one patient due to sepsis and disseminated intravascular coagulation without decreased neutrophil count.
TABLE 2.
Adverse Events
| Event | Adverse Events Regardless of Attribution, No. (%) | Treatment-Related Adverse Events, No. (%) | ||||
|---|---|---|---|---|---|---|
| Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 | |
| Any adverse events | 62 (100) | 33 (53) | 5 (8) | 59 (95) | 26 (42) | 5 (8) |
| Nausea | 36 (58) | 1 (2) | 0 | 35 (56) | 1 (2) | 0 |
| Decreased appetite | 30 (48) | 4 (6) | 0 | 30 (48) | 3 (5) | 0 |
| Malaise | 26 (42) | 0 | 0 | 25 (40) | 0 | 0 |
| Anemia | 11 (18) | 14 (23) | 0 | 11 (18) | 13 (21) | 0 |
| Decreased neutrophil count | 8 (13) | 8 (13) | 4 (6) | 8 (13) | 8 (13) | 4 (6) |
| Decreased white blood cell count | 12 (19) | 6 (10) | 2 (3) | 12 (19) | 6 (10) | 2 (3) |
| Constipation | 17 (27) | 0 | 0 | 8 (13) | 0 | 0 |
| Pneumonitis/ILD | 15 (24) | 1 (2) | 0 | 15 (24) | 1 (2) | 0 |
| Pyrexia | 14 (23) | 1 (2) | 0 | 7 (11) | 0 | 0 |
| Decreased platelet count | 10 (16) | 3 (5) | 2 (3) | 10 (16) | 3 (5) | 2 (3) |
| Stomatitis | 14 (23) | 0 | 0 | 14 (23) | 0 | 0 |
| Diarrhea | 12 (19) | 1 (2) | 0 | 8 (13) | 1 (2) | 0 |
| Vomiting | 11 (18) | 0 | 0 | 10 (16) | 0 | 0 |
Abbreviation: ILD, interstitial lung disease.
Additionally, trAEs causing drug interruption, dose reduction, and drug withdrawal were observed in 24 (39%), 20 (32%), and 13 (21%) patients, respectively. The leading cause of drug discontinuation was pneumonitis/ILD, accounting for 11 (18%) of these cases.
Biomarker Analysis Using cfDNA
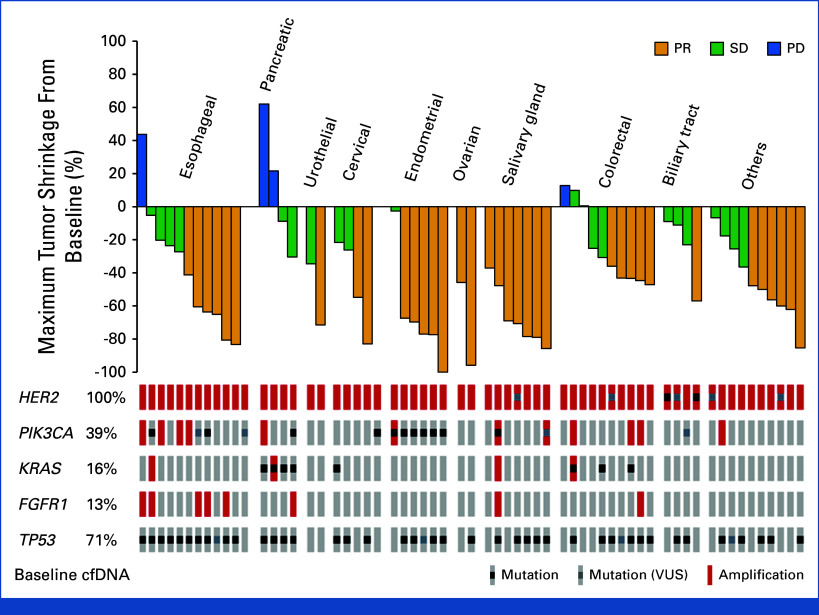
At baseline, the median pCN of HER2 was 8.55 (range, 2.4-73.9; Data Supplement, online only). No difference in baseline pCN was observed between responders and nonresponders (mean [SD], 14.55 [20.221] v 19.87 [16.366]; P = .26; Fig 4). In terms of mutational characteristics, the most frequently observed gene alterations in cfDNA were TP53 (71%) and PIK3CA (39%; Fig 3A).
FIG 4.
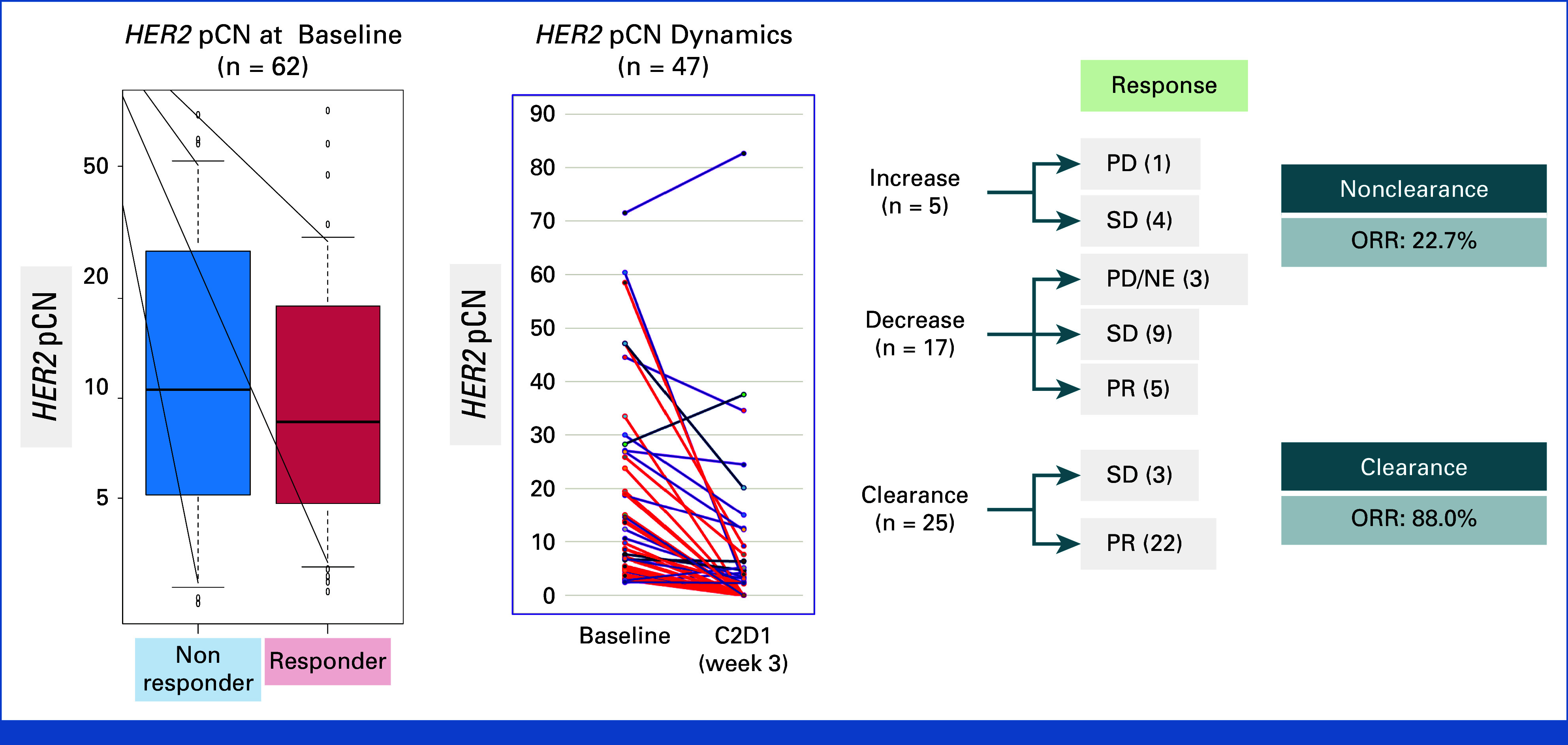
Biomarker analysis. HER2 pCN at baseline and dynamics. ORR was 88.0% in the clearance group and 22.7% in the nonclearance group. C2D1, cycle 2 day 1; HER2, human epidermal growth factor receptor 2; NE, not evaluable; ORR, overall response rate; pCN, plasma copy number; PD, progressive disease; PR, partial response; SD, stable disease.
Among the 47 (79%) patients from whom samples were obtained at 3 weeks after T-DXd initiation, HER2 pCN decreased in 42 (89%) with available data, including 25 (60% of the total number) patients who achieved complete clearance of HER2 pCN. Dynamics of HER2 pCN were associated with tumor response: the ORRs were 88.0% (22/25) and 22.7% (5/22) in the clearance and nonclearance groups, respectively (P < .001; Fig 4). No responses were found in five patients with increased HER2 pCN at week 3.
To investigate the genomic mechanisms of acquired T-DXd resistance, cfDNA results were compared between the plasma samples collected after disease progression or discontinuation of study treatment and the samples collected before treatment. Among 39 patients with available data on cfDNA at both baseline and after treatment, 22 patients who responded to T-DXd were included in this analysis. At least one new alteration was identified after treatment in 13 (59%) patients (Appendix Table A3). The acquired alterations were primarily in genes associated with the MAPK, PI3K/AKT, and ATM/p53 pathways. Moreover, HER2 amplification was detected in six (43%) of the 14 patients who experienced disease progression and in none (0%) of the eight patients in whom treatment was interrupted due to AEs.
DISCUSSION
To the best of our knowledge, this is one of the first prospective studies assessing the efficacy and safety of T-DXd in HER2-amplified solid tumors detected by cfDNA testing. Although we recognize that the use of cfDNA to detect HER2 amplification is not novel, as several studies in colorectal and biliary cancers have already contributed to this domain, our study leverages tumor-agnostic screening for T-DXd therapy. This phase II trial answered its primary end point by displaying promising antitumor activity with an ORR of 56.5%, as evaluated by the site investigator. We confirmed a response in 13 different cancer types, which supported the hypothesis that HER2 amplification may have been a target across a broad spectrum of cancers.
This study provided promising evidence for T-DXd efficacy. Previous tumor-agnostic, single-arm studies of trastuzumab emtansine or trastuzumab combined with pertuzumab in patients with HER2 amplification/overexpression reported ORRs ranging from 6% to 25%,15-17 whereas our study revealed encouraging results on T-DXd therapy. Analysis of data from the MyPathway study on trastuzumab plus pertuzumab showed variations in ORR, with rates of 32% (17/57) and 60% (9/15) for colorectal and salivary gland cancers, compared with 50% (5/10) and 100% (7/7), respectively, in HERALD. These disparities highlight potential differences in treatment response. Moreover, the T-DXd regimen showed a marked improvement in OS and ORR compared with trastuzumab emtansine in patients with HER2-positive metastatic breast cancer.18 These findings underscore the robust antitumor activity of T-DXd and suggest its potential as an effective therapeutic option for patients with HER2-amplified solid tumors.
This study included patients with HER2 amplification detected by cfDNA rather than traditional tissue analysis. Recent studies have demonstrated that cfDNA analysis is a reliable method for matching patients with genome-specific clinical trials. However, its use as a selection for patients with solid tumors on the basis of HER2 amplification has been less common. The sensitivity of plasma cfDNA for detecting tissue HER2 amplification/overexpression in metastatic breast, gastric, or colorectal cancer has been reported to range from 56% to 97%.19-21 Although discrepancies may occur in patients who are tissue-positive but cfDNA-negative, possibly because of low tumor shedding, the high concordance in genotyping and rapid turnaround time of cfDNA testing facilitate the inclusion of patients in clinical trials. Our previous work has shown that cfDNA genotyping can lead to a 132% increase in clinical trial enrollment compared with tissue genotyping.8 Furthermore, cfDNA testing may capture genomic abnormalities that represent true therapeutic targets, as evidenced by a responder in this study who was HER2-negative by tissue analysis but HER2-positive by cfDNA. The ORR observed in this study was higher than that of the phase I study of T-DXd in a nonbreast/nongastric cohort with cancer detected by tissue testing.14
Clinicians aim to predict treatment efficacy before its commencement. Previous studies have indicated that baseline HER2-amplification levels may serve as predictive biomarkers when using trastuzumab and pertuzumab treatment regimens. In these studies, patients harboring coalterations, such as KRAS mutation and PIK3CA mutation, exhibited a diminished response to HER2-targeting therapies.17 Conversely, our study found no correlation between HER2 levels and treatment response. Furthermore, T-DXd demonstrated clinically antitumor activity in the presence of KRAS and PIK3CA mutations, which differs from earlier findings with trastuzumab plus pertuzumab. This discrepancy could be attributed to the nature of T-DXd as an antibody-drug conjugate, which has different HER2 inhibition mechanisms of action. Thus, its efficacy may not be directly dependent on the level of HER2 amplification/overexpression or on the resistance mechanisms to HER2-targeted therapies. Notably, our data also suggest that the reduction of HER2 amplification after treatment commencement is indicative of therapeutic efficacy, implying that serial cfDNA analyses could serve as a reliable method for monitoring treatment response.
The obtained toxicity profiles are consistent with those of T-DXd monotherapy from the former reports; however, we noted a relatively high incidence (26%) of pneumonitis/ILD, which has a reported incidence of 15.4%,22 remains among the most serious AEs associated with T-DXd treatment. One contributing factor to this higher incidence could be the inclusion of Japanese participants in this study, as previous research suggests that Japanese individuals may have up to twice the risk of developing pneumonitis/ILD when treated with T-DXd.22 Notably, the pneumonitis/ILD cases in our cohort were predominantly low-grade (1 or 2), with no grade 4 or 5 events reported. This outcome might be attributed to enhanced awareness and the implementation of comprehensive guidelines for the monitoring, diagnosis, and management of pneumonitis/ILD, thus allowing for early detection and intervention that may prevent the progression to more severe grades. Therefore, vigilant management and monitoring of pneumonitis/ILD are crucial for patients under T-DXd therapy, extending beyond the Japanese population.
The limitations of this study include its relatively small sample size and the inclusion of various histologic tumor specimens, which may have caused heterogeneous results on antitumor activity. Nonetheless, the efficacy data observed were encouraging to those from other trials exploring HER2 blockade in solid tumors. Another limitation of this study was the absence of known HER2 status in tumor tissue at the evaluated time point, making it impossible to determine whether tumor and blood can comparably select patients assigned to T-DXd therapy. However, the study was designed around the concept that cfDNA screening would enable the establishment of a single, tissue-agnostic cutoff value, thus potentially overcoming tumor heterogeneity. We are currently gathering tumor specimens and intend to present the findings at future conferences.
In conclusion, to our knowledge, we represent the first prospective study on HER2-amplified solid tumors identified by cfDNA genotyping treated successfully by T-DXd therapy and indicated that comprehensive cfDNA genotyping, including HER2 amplification, enables effective patient selection for this regimen. Although this approach may not be currently suitable for other HER2-targeting agents, establishing eligibility criteria by cfDNA analysis regardless of tissue status may be an optimal method of diagnostic development. Moreover, we recommend both cfDNA genotyping and tissue HER2 testing to be implemented in clinical practice for solid tumors.
ACKNOWLEDGMENT
The authors express their gratitude to participating patients and their families; all clinical and research staff involved at all the study sites; and all the National Cancer, Exploratory Oncology Research & Clinical Trial and National Cancer Centers Hospital East Translational Research Support Section members.
APPENDIX
FIG A1.
Trial profile. Flow diagram for the HERALD trial. cfDNA, cell-free DNA; ERBB2, Erb-B2 receptor tyrosine kinase 2; FAS, full analysis set; ITT, intention-to-treat; T-DXd, trastuzumab deruxtecan.
FIG A2.
Patient flow. Flow diagram for the HERALD trial by cancer type. ERBB2, Erb-B2 receptor tyrosine kinase 2; H&N, head and neck.
FIG A3.
Tumor shrinkage by baseline cfDNA status. Waterfall plot of maximum change in tumor size for the 62 evaluated patients, as per investigator assessment according to cancer type and baseline cfDNA. cfDNA, cell-free DNA; HER2, human epidermal growth factor receptor 2; PD, progressive disease; PR, partial response; SD, stable disease; VUS, variant of uncertain significance.
TABLE A1.
Overall Response Rate
| Response | Investigator's | Central |
|---|---|---|
| Objective response rate, %, median (95% CI) | 56.5 (43.3 to 69.0) | 58.1 (44.8 to 70.5) |
| Complete response, No. (%) | 0 | 1 (1.6) |
| Partial response, No. (%) | 35 (56.5) | 35 (56.5) |
| Stable disease, No. (%) | 21 (33.9) | 19 (30.6) |
| Progressive disease, No. (%) | 5 (8.1) | 5 (8.1) |
| Not estimable, No. (%) | 1 (1.6) | 2 (3.2) |
TABLE A2.
Tumor Response by Cancer Type
| Cancer Type | ORR, No. (%) |
|---|---|
| Total | 35/62 (56.5) |
| Esophageal (mostly SCC) | 6/12 (50.0) |
| Colorectal | 5/10 (50.0) |
| Salivary gland | 7/7 (100.0) |
| Endometrial | 5/6 (83.3) |
| Cervical | 2/5 (40.0) |
| Biliary tract | 1/4 (25.0) |
| Pancreatic | 0/4 (0.0) |
| Ovarian | 2/2 (100.0) |
| Small intestine | 2/2 (100.0) |
| Urothelial | 1/2 (50.0) |
| Gastric (tissue HER2-neg) | 1/2 (50.0) |
| NSCLC | 0/2 (0.0) |
| Melanoma | 1/1 (100.0) |
| Paget's disease | 1/1 (100.0) |
| Prostate | 1/1 (100.0) |
| Unknown primary | 0/1 (0.0) |
Abbreviations: HER2, human epidermal growth factor receptor 2; NSCLC, non–small cell lung cancer; ORR, objective response rate; SCC, squamous cell carcinoma.
TABLE A3.
Acquired Alterations and Re-Emergence of HER2 Amplification
| ID | Reason for T-Dxd Discontinuation | Plasma HER2 Status During Cycle 2 | Plasma HER2 Status After Treatment | Acquired Gene Alterations |
|---|---|---|---|---|
| 8 | PD | Nonclearance | Present | FGFR1 (SNV) |
| 9 | Adverse event | Clearance | Absent | ATM (CNV) |
| 10 | PD | Nonclearance | Present | CDK12 (Del) |
| 11 | PD | Clearance | Absent | |
| 14 | Adverse event | Clearance | Absent | GNAS (SNV), TP53 (SNV) |
| 17 | Adverse event | Clearance | Absent | |
| 22 | PD | Clearance | Absent | AR (SNV), SMAD4 (SNV), KIT (SNV), ESR1 (SNV) |
| 23 | PD | Clearance | Present | |
| 25 | PD | Clearance | Present | ATM (SNV), ERBB2 (SNV), NRAS (SNV), CDK12 (Del), DDR2 (SNV), KIT (Del) |
| 28 | PD | Clearance | Absent | CDK12 (SNV) |
| 29 | Adverse event | Nonclearance | Absent | |
| 31 | PD | Clearance | Absent | |
| 32 | PD | Clearance | Absent | ATM (SNV), NOTCH1 (SNV) |
| 37 | Adverse event | Clearance | Absent | |
| 38 | PD | Clearance | Present | TP53 (SNV) |
| 41 | PD | Clearance | Absent | TP53 (SNV) |
| 42 | Adverse event | Clearance | Absent | |
| 44 | PD | Clearance | Absent | |
| 46 | Adverse event | Clearance | Absent | TP53 (SNV) |
| 49 | Adverse event | Clearance | Absent | ATM (SNV) |
| 59 | PD | Clearance | Absent | |
| 60 | PD | Nonclearance | Present | ERBB2 (SNV) |
Abbreviations: Del, deletion; HER2, human epidermal growth factor receptor 2; PD, progressive disease; SNV, single-nucleotide variant; T-DXd, trastuzumab deruxtecan.
Masataka Yagisawa
Honoraria: Ono Pharmaceutical, Lilly Japan, Taiho Pharmaceutical, Bristol Myers Squibb K.K, Takeda, Daiichi Sankyo Co, Ltd, Merck
Hiroya Taniguchi
Honoraria: Takeda, Chugai Pharma, Taiho Pharmaceutical, Lilly Japan, Bristol Myers Squibb Japan, MSD K.K, Ono Yakuhin, Amgen, Roche, Merck
Research Funding: Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Takeda (Inst)
Taroh Satoh
Honoraria: Chugai Pharma, Merck Serono, Bristol Myers Squibb, Takeda, Yakult Honsha, Lilly, Bayer Yakuhin, Ono Pharmaceutical, Merck, Astellas Pharma, Taiho Pharmaceutical, Nihonkayaku, Daiichi Sankyo
Consulting or Advisory Role: Bayer Yakuhin, Lilly, Ono Pharmaceutical, Takara Bio, Merck Serono, Nihonkayaku
Research Funding: Yakult Honsha (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Sanofi (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Merck (Inst), Merck Serono (Inst), Gilead Sciences (Inst), Dainippon Sumitomo Pharma (Inst), IQVIA (Inst)
Shigenori Kadowaki
Honoraria: Bayer, Bristol Myers Squibb, Chugai Pharma, Ono Pharmaceutical, Merck KGaA, Daiichi Sankyo, Eisai, MSD, Taiho Pharmaceutical, Otsuka
Research Funding: Ono Pharmaceutical (Inst), Lilly (Inst), MSD (Inst), Chugai Pharma (Inst), Nobelpharma (Inst), Daiichi Sankyo (Inst), Bayer (Inst)
Yu Sunakawa
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol Myers Squibb Japan, Lilly Japan, Merck, Sysmex, MSD K.K, Ono Pharmaceutical, Daiichi Sankyo, Guardant Health, Incyte
Consulting or Advisory Role: Bristol Myers Squibb Japan, MSD K.K, Daiichi Sankyo, Merck
Research Funding: Taiho Pharmaceutical, Chugai Pharma
Tomohiro Nishina
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly, Takeda, Bristol Myers Squibb, Ono Pharmaceutical
Research Funding: Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Dainippon Sumitomo Pharma (Inst), Lilly Japan (Inst), MSD (Inst), Daiichi Sankyo (Inst), Ono Pharmaceutical (Inst), Eisai (Inst)
Yoshito Komatsu
Honoraria: Lilly Japan, Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol Myers Squibb Co, Sanofi/Aventis, Merck, Yakult Honsha, Ono Pharmaceutical, Nipro Corporation, Moroo Co, Asahi Kasei, Mitsubishi Tanabe Pharma, Otsuka, Medical Review Co, Ltd, Daiichi Sankyo
Research Funding: MSD K.K, Taiho Pharmaceutical, Yakult Honsha, Bayer Yakuhin, Daiichi Sankyo Co, Ltd, Ono Pharmaceutical, NanoCarrier, Eisai, Sanofi/Aventis, Sysmex, Shionogi, IQVIA, Parexel International Corporation, Astellas Pharma, Mediscience Planning, Sumitomo Dainippon Pharma Co, Ltd, A2 Healthcare, Incyte, Lilly (Inst), Nipro Corporation (Inst), BeiGene (Inst)
Taito Esaki
Honoraria: Taiho Pharmaceutical, Daiichi Sankyo, Chugai Pharma
Research Funding: Daiichi Sankyo (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Nihonkayaku (Inst), Amgen Astellas BioPharma (Inst), IQVIA (Inst), Quintiles (Inst), Pfizer (Inst), Chugai Pharma (Inst), Syneos Health (Inst), Asahi Kasei (Inst), Amgen (Inst), Jazz Pharmaceuticals (Inst)
Daisuke Sakai
Speakers' Bureau: Chugai Pharma, Daiichi Sankyo/UCB Japan
Research Funding: Chugai Pharma (Inst), Yakult Honsha (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo, Lilly Japan, Astellas Pharma (Inst), Incyte (Inst), Taiho Pharmaceutical (Inst), Eisai (Inst)
Ayako Doi
Honoraria: Taiho Pharmaceutical
Takeshi Kajiwara
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly, Bristol Myers Squibb Japan
Justin Odegaard
Employment: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Shogo Nomura
Employment: Asahi Kasei
Honoraria: AstraZeneca, Chugai Pharma, Asahi Kasei, MSD
Research Funding: AstraZeneca (Inst), Chugai Pharma (Inst)
Hideaki Bando
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Ono Pharmaceutical
Akihiro Sato
Honoraria: Dainippon Sumitomo Pharma, AstraZeneca, Astellas Pharma
Research Funding: Taiho Pharmaceutical (Inst), Boehringer Ingelheim (Inst), Bayer (Inst), Chugai Pharma (Inst), Eisai (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Takeda (Inst), Dainippon Sumitomo Pharma (Inst), Oncolys BioPharma (Inst), Aspyerian Therapeutics (Inst), Pentax Medical Devices (Inst), Daiichi Sankyo/UCB Japan (Inst)
Takayuki Yoshino
Honoraria: Chugai Pharma, MSD K.K, Takeda, Merck
Consulting or Advisory Role: Sumitomo Corp
Research Funding: MSD (Inst), Daiichi Sankyo Company, Limited (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst), Pfizer (Inst), Sysmex (Inst), Chugai Pharma (Inst), Eisai (Inst), Molecular Health (Inst), Roche (Inst), FALCO Biosystems Ltd (Inst), Merus (Inst), Bristol Myers Squibb Japan (Inst), Medical & Biological Laboratories Co, Ltd (Inst), Takeda (Inst)
Yoshiaki Nakamura
Consulting or Advisory Role: Natera, Inc, Roche Ltd/, Seagen, Inc, Premo Partners, Inc, Daiichi Sankyo Co, Ltd, Takeda, Exact Sciences, Gilead Sciences, Guardant Health Pte Ltd
Speakers' Bureau: MSD K.K, Eisai, Zeria Pharmaceutical, Miyarisan Pharmaceutical, Merck, CareNet, Inc, Hisamitsu Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo Co, Ltd, Chugai Pharma, Becton Dickinson, Guardant Health Japan Corp, Guardant Health Pte Ltd
Research Funding: Seagen, Inc (Inst), Genomedia (Inst), Guardant Health AMEA, Inc (Inst), Guardant Health (Inst), Tempus (Inst), Roche Diagnostics K.K (Inst), Daiichi Sankyo Co, Ltd (Inst), Chugai Pharma (Inst)
No other potential conflicts of interest were reported.
DISCLAIMER
The National Cancer Center Hospital East, Japan, had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
PRIOR PRESENTATION
Presented at the ASCO 2023 Annual Meeting, Chicago, IL, June 2-6, 2023.
SUPPORT
Supported by grants from the Japan Agency for Medical Research and Development (AMED) under No. JP22lk0201084h0005 and Daiichi Sankyo Co, Ltd. Trastuzumab deruxtecan was provided by Daiichi Sankyo and had the opportunity to review the final manuscript without the right to interfere with the publication.
CLINICAL TRIAL INFORMATION
JapicCTI-194707, and UMIN-CTR, UMIN000029315
M.Y. and H.T. contributed equally as first author.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02626.
AUTHOR CONTRIBUTIONS
Conception and design: Masataka Yagisawa, Hiroya Taniguchi, Taroh Satoh, Tomohiro Nishina, Shogo Nomura, Takayuki Yoshino
Administrative support: Hiromi Ono, Masatoshi Asano, Satoshi Fujii, Hideaki Bando, Yoshiaki Nakamura
Provision of study materials or patients: Yu Sunakawa, Tomohiro Nishina, Yoshito Komatsu, Daisuke Sakai, Takeshi Kajiwara, Satoshi Fujii, Hideaki Bando
Collection and assembly of data: Masataka Yagisawa, Hiroya Taniguchi, Taroh Satoh, Shigenori Kadowaki, Yu Sunakawa, Tomohiro Nishina, Yoshito Komatsu, Taito Esaki, Daisuke Sakai, Ayako Doi, Takeshi Kajiwara, Hiromi Ono, Masatoshi Asano, Nami Hirano, Shogo Nomura, Hideaki Bando, Akihiro Sato, Takayuki Yoshino, Yoshiaki Nakamura
Data analysis and interpretation: Masataka Yagisawa, Hiroya Taniguchi, Shigenori Kadowaki, Tomohiro Nishina, Yoshito Komatsu, Justin Odegaard, Satoshi Fujii, Shogo Nomura, Hideaki Bando, Takayuki Yoshino
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Trastuzumab Deruxtecan in Advanced Solid Tumors With Human Epidermal Growth Factor Receptor 2 Amplification Identified by Plasma Cell-Free DNA Testing: A Multicenter, Single-Arm, Phase II Basket Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Masataka Yagisawa
Honoraria: Ono Pharmaceutical, Lilly Japan, Taiho Pharmaceutical, Bristol Myers Squibb K.K, Takeda, Daiichi Sankyo Co, Ltd, Merck
Hiroya Taniguchi
Honoraria: Takeda, Chugai Pharma, Taiho Pharmaceutical, Lilly Japan, Bristol Myers Squibb Japan, MSD K.K, Ono Yakuhin, Amgen, Roche, Merck
Research Funding: Ono Pharmaceutical (Inst), Daiichi Sankyo (Inst), Takeda (Inst)
Taroh Satoh
Honoraria: Chugai Pharma, Merck Serono, Bristol Myers Squibb, Takeda, Yakult Honsha, Lilly, Bayer Yakuhin, Ono Pharmaceutical, Merck, Astellas Pharma, Taiho Pharmaceutical, Nihonkayaku, Daiichi Sankyo
Consulting or Advisory Role: Bayer Yakuhin, Lilly, Ono Pharmaceutical, Takara Bio, Merck Serono, Nihonkayaku
Research Funding: Yakult Honsha (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Sanofi (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Merck (Inst), Merck Serono (Inst), Gilead Sciences (Inst), Dainippon Sumitomo Pharma (Inst), IQVIA (Inst)
Shigenori Kadowaki
Honoraria: Bayer, Bristol Myers Squibb, Chugai Pharma, Ono Pharmaceutical, Merck KGaA, Daiichi Sankyo, Eisai, MSD, Taiho Pharmaceutical, Otsuka
Research Funding: Ono Pharmaceutical (Inst), Lilly (Inst), MSD (Inst), Chugai Pharma (Inst), Nobelpharma (Inst), Daiichi Sankyo (Inst), Bayer (Inst)
Yu Sunakawa
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol Myers Squibb Japan, Lilly Japan, Merck, Sysmex, MSD K.K, Ono Pharmaceutical, Daiichi Sankyo, Guardant Health, Incyte
Consulting or Advisory Role: Bristol Myers Squibb Japan, MSD K.K, Daiichi Sankyo, Merck
Research Funding: Taiho Pharmaceutical, Chugai Pharma
Tomohiro Nishina
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly, Takeda, Bristol Myers Squibb, Ono Pharmaceutical
Research Funding: Taiho Pharmaceutical (Inst), Chugai Pharma (Inst), Dainippon Sumitomo Pharma (Inst), Lilly Japan (Inst), MSD (Inst), Daiichi Sankyo (Inst), Ono Pharmaceutical (Inst), Eisai (Inst)
Yoshito Komatsu
Honoraria: Lilly Japan, Taiho Pharmaceutical, Chugai Pharma, Takeda, Bayer Yakuhin, Bristol Myers Squibb Co, Sanofi/Aventis, Merck, Yakult Honsha, Ono Pharmaceutical, Nipro Corporation, Moroo Co, Asahi Kasei, Mitsubishi Tanabe Pharma, Otsuka, Medical Review Co, Ltd, Daiichi Sankyo
Research Funding: MSD K.K, Taiho Pharmaceutical, Yakult Honsha, Bayer Yakuhin, Daiichi Sankyo Co, Ltd, Ono Pharmaceutical, NanoCarrier, Eisai, Sanofi/Aventis, Sysmex, Shionogi, IQVIA, Parexel International Corporation, Astellas Pharma, Mediscience Planning, Sumitomo Dainippon Pharma Co, Ltd, A2 Healthcare, Incyte, Lilly (Inst), Nipro Corporation (Inst), BeiGene (Inst)
Taito Esaki
Honoraria: Taiho Pharmaceutical, Daiichi Sankyo, Chugai Pharma
Research Funding: Daiichi Sankyo (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Astellas Pharma (Inst), Nihonkayaku (Inst), Amgen Astellas BioPharma (Inst), IQVIA (Inst), Quintiles (Inst), Pfizer (Inst), Chugai Pharma (Inst), Syneos Health (Inst), Asahi Kasei (Inst), Amgen (Inst), Jazz Pharmaceuticals (Inst)
Daisuke Sakai
Speakers' Bureau: Chugai Pharma, Daiichi Sankyo/UCB Japan
Research Funding: Chugai Pharma (Inst), Yakult Honsha (Inst), Ono Pharmaceutical (Inst), Daiichi Sankyo, Lilly Japan, Astellas Pharma (Inst), Incyte (Inst), Taiho Pharmaceutical (Inst), Eisai (Inst)
Ayako Doi
Honoraria: Taiho Pharmaceutical
Takeshi Kajiwara
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Lilly, Bristol Myers Squibb Japan
Justin Odegaard
Employment: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Shogo Nomura
Employment: Asahi Kasei
Honoraria: AstraZeneca, Chugai Pharma, Asahi Kasei, MSD
Research Funding: AstraZeneca (Inst), Chugai Pharma (Inst)
Hideaki Bando
Honoraria: Taiho Pharmaceutical, Chugai Pharma, Ono Pharmaceutical
Akihiro Sato
Honoraria: Dainippon Sumitomo Pharma, AstraZeneca, Astellas Pharma
Research Funding: Taiho Pharmaceutical (Inst), Boehringer Ingelheim (Inst), Bayer (Inst), Chugai Pharma (Inst), Eisai (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Takeda (Inst), Dainippon Sumitomo Pharma (Inst), Oncolys BioPharma (Inst), Aspyerian Therapeutics (Inst), Pentax Medical Devices (Inst), Daiichi Sankyo/UCB Japan (Inst)
Takayuki Yoshino
Honoraria: Chugai Pharma, MSD K.K, Takeda, Merck
Consulting or Advisory Role: Sumitomo Corp
Research Funding: MSD (Inst), Daiichi Sankyo Company, Limited (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Amgen (Inst), Sanofi (Inst), Pfizer (Inst), Sysmex (Inst), Chugai Pharma (Inst), Eisai (Inst), Molecular Health (Inst), Roche (Inst), FALCO Biosystems Ltd (Inst), Merus (Inst), Bristol Myers Squibb Japan (Inst), Medical & Biological Laboratories Co, Ltd (Inst), Takeda (Inst)
Yoshiaki Nakamura
Consulting or Advisory Role: Natera, Inc, Roche Ltd/, Seagen, Inc, Premo Partners, Inc, Daiichi Sankyo Co, Ltd, Takeda, Exact Sciences, Gilead Sciences, Guardant Health Pte Ltd
Speakers' Bureau: MSD K.K, Eisai, Zeria Pharmaceutical, Miyarisan Pharmaceutical, Merck, CareNet, Inc, Hisamitsu Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo Co, Ltd, Chugai Pharma, Becton Dickinson, Guardant Health Japan Corp, Guardant Health Pte Ltd
Research Funding: Seagen, Inc (Inst), Genomedia (Inst), Guardant Health AMEA, Inc (Inst), Guardant Health (Inst), Tempus (Inst), Roche Diagnostics K.K (Inst), Daiichi Sankyo Co, Ltd (Inst), Chugai Pharma (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Swain SM, Shastry M, Hamilton E: Targeting HER2-positive breast cancer: Advances and future directions. Nat Rev Drug Discov 22:101-126, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W, Zhang X, Du Y, et al. : HER2-targeted advanced metastatic gastric/gastroesophageal junction adenocarcinoma: Treatment landscape and future perspectives. Biomark Res 10:71, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strickler JH, Cercek A, Siena S, et al. : Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol 24:496-508, 2023 [DOI] [PubMed] [Google Scholar]
- 4.Oh DY, Bang YJ: HER2-targeted therapies—A role beyond breast cancer. Nat Rev Clin Oncol 17:33-48, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Dumbrava EEI, Balaji K, Raghav K, et al. : Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCO Precis Oncol 10.1200/PO.18.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan M, Schwaederle M, Arguello D, et al. : HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev 34:157-164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odegaard JI, Vincent JJ, Mortimer S, et al. : Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res 24:3539-3549, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Fujisawa T, Taniguchi H, et al. : SCRUM-Japan GI-SCREEN and MONSTAR-SCREEN: Path to the realization of biomarker-guided precision oncology in advanced solid tumors. Cancer Sci 112:4425-4432, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura Y, Taniguchi H, Ikeda M, et al. : Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med 26:1859-1864, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Okamoto W, Kato T, et al. : Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nat Med 27:1899-1903, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogitani Y, Hagihara K, Oitate M, et al. : Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 107:1039-1046, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi S, Saura C, Yamashita T, et al. : Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 382:610-621, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shitara K, Bang YJ, Iwasa S, et al. : Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 382:2419-2430, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Tsurutani J, Iwata H, Krop I, et al. : Targeting HER2 with trastuzumab deruxtecan: A dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov 10:688-701, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda S, Kudo R, Yamashita Y, et al. : Primary results from JUPITER, a phase 2 basket trial of combination therapy with trastuzumab and pertuzumab in patients with HER2-amplified solid tumors. J Clin Oncol 40, 2022. (suppl 16; abstr 3131) [Google Scholar]
- 16.Jhaveri KL, Wang XV, Makker V, et al. : Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: Results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol 30:1821-1830, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meric-Bernstam F, Hainsworth JD, Bose R, et al. : MyPathway HER2 basket study: Pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J Clin Oncol 39, 2021. (suppl 15; abstr 3004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurvitz SA, Hegg R, Chung WP, et al. : Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401:105-117, 2023 [DOI] [PubMed] [Google Scholar]
- 19.Catenacci DVT, Kang YK, Park H, et al. : Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): A single-arm, phase 1b-2 trial. Lancet Oncol 21:1066-1076, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Modi S, Andre F, Krop IE, et al. : Trastuzumab deruxtecan for HER2-positive metastatic breast cancer: DESTINY-Breast01 subgroup analysis. J Clin Oncol 38, 2020. (suppl 15; abstr 1036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siravegna G, Sartore-Bianchi A, Nagy RJ, et al. : Plasma HER2 (ERBB2) copy number predicts response to HER2-targeted therapy in metastatic colorectal cancer. Clin Cancer Res 25:3046-3053, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Powell CA, Modi S, Iwata H, et al. : Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 7:100554, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.02626.