Abstract
Overall, gastric adenocarcinoma (GC) incidence rates have declined in recent years, but racial and ethnic disparities persist. Individuals who identify as Hispanic/Spanish/Latino are diagnosed with GC at younger ages and have poorer outcomes than non-Hispanic individuals. However, our understanding of GC biology across racial/ethnic groups remains limited. We assessed tumor genomic patterns by race/ethnicity among 1019 patients with primary GC in the American Association for Cancer Research (AACR) Project GENIE Consortium. Hispanic individuals presented with significantly higher rates of ERBB2/HER2 amplification vs other racial/ethnic groups (Hispanic: 13.9% vs 9.8% non-Hispanic White, 8.1% non-Hispanic Asian, and 11.0% non-Hispanic Black; P < .001, FDR adjusted q < 0.001). Hispanic patients also had higher odds of an ERBB2 amplification vs non-Hispanic Whites in adjusted models (OR = 2.52, 95%CI = 1.20 to 5.33, P = .015). These findings underscore the important role of genomic factors in GC disparities. Ensuring equitable access to genomic profiling and targeted therapies, such as trastuzumab for HER2-overexpressing GC, is a promising avenue to mitigate GC disparities and improve outcomes.
Despite recent declines in the overall gastric adenocarcinoma incidence, a lack of adequate screening and surveillance modalities have contributed to a disproportionate gastric adenocarcinoma burden across certain population groups, particularly among individuals who identify as Hispanic/Spanish/Latino (1), in addition to higher non-cardia gastric adenocarcinoma incidence rates among racial/ethnic minority populations (2). Hispanic gastric adenocarcinoma patients in the United States also experience higher rates of early-onset disease (age <50 years) disease and have poorer survival outcomes compared with their non-Hispanic counterparts (3,4). Overall, these patterns suggest that genomic features may contribute to racial/ethnic disparities in gastric adenocarcinoma burden, offering an opportunity to identify potential clinically actionable insights that may improve patient outcomes. However, profiling of actionable mutations with current or developing therapies across racial/ethnic groups in gastric adenocarcinoma, particularly across Hispanic/Latino populations, has historically been limited.
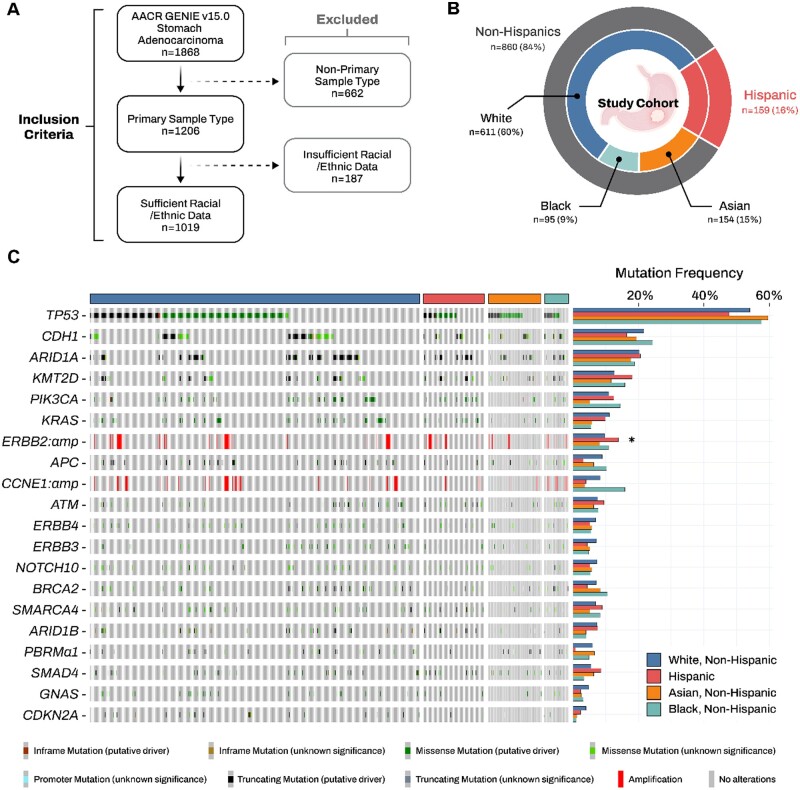
To investigate somatic variant patterns of gastric adenocarcinoma by race/ethnicity, we used the AACR Project GENIE Consortium (v15.0) database and identified 1019 patients with a primary non-gastroesophageal gastric adenocarcinoma and paired demographic, genomic, and clinical data (Figure 1, A) (5,6). Demographic characteristics included patient self-identified race/ethnicity, sex, and age at sequencing. Somatic cancer gene variation data from gastric adenocarcinoma tissues were generated using clinical-grade targeted gene panel sequencing. Detailed information and summaries of sequencing pipelines, genomic information, and profiling at each participating consortium center are comprehensively detailed in the AACR Project GENIE Data Guide 2023 (https://www.aacr.org/wp-content/uploads/2024/02/15.0-public_data_guide-.pdf). To ensure consistent variant calling and minimize artifacts and germline events, GENIE has applied a stringent filtering pipeline to remove putative germline variants. Variants and frequencies were calculated for the 20 most common genetic alterations profiled in at least 80% of the cohort. Our variant-enrichment analysis was restricted to non-silent variants, with ERBB2 and CCNE1 restricted to gene amplification. We compared clinical and variant profiles by self-identified patient race/ethnicity using χ2 and Fisher exact tests, as appropriate. To control for multiple comparisons, we used the Benjamini-Hochberg method for FDR correction. To investigate associations between mutational patterns and race/ethnicity group, we used multivariable logistic regression analyses adjusted for sequencing age, sex, tumor histological subtype, and sequencing center. Data were analyzed using R software, version 4.3.1. All tests were two-sided, and P values <.05 and FDR q-value <0.05 were considered to be statistically significant.
Figure 1.
Gastric adenocarcinoma mutation patterns by self-identified race and ethnicity: AACR Project GENIE. A) Composition of the study population with inclusion criteria. B) Distribution of self-identified races and ethnicities within the cohort. C) Prevalence and spectrum of somatic alterations across racial/ethnic groups. ERBB2 and CCNE1 are restricted to gene amplification. *P value < .05, and q-value < 0.10. V = version; N = number; AMP = amplification; Δ = non-silent mutation.
Among the 1019 primary gastric adenocarcinoma patients identified in GENIE, 1 in every 6 individuals identified as Hispanic (16%), 9.0% as non-Hispanic Black, 15% as non-Hispanic Asian, and 60% as non-Hispanic White (Figure 1, B). Approximately 1 in every 3 (36%) patients with gastric adenocarcinoma underwent sequencing before age 50 (early-onset gastric adenocarcinoma). Early-onset gastric adenocarcinoma was statistically more frequent among Hispanic and non-Hispanic Asian patients than among non-Hispanic Black and White patients (32.1% and 23.4% vs 17.9% and 16.4%, respectively; P < .001) (Supplementary Table 1, available online). The prevalence and spectrum of somatic variant frequencies for the top 20 altered genes are presented by race and ethnicity in Figure 1, C (Supplementary Table 1, available online). Across individual genes, Hispanic patients presented with statistically higher rates of ERBB2/HER2 amplification compared with other racial/ethnic groups (Hispanic: 13.9% vs 9.8% non-Hispanic White, 8.1% non-Hispanic Asian, and 11.0% non-Hispanic Black; P < .001, FDR adjusted q < 0.001). We further analyzed the frequencies of the top 20 altered genes by race and ethnicity after driver mutation filtering (Supplementary Table 2, available online). Included driver alterations were either classified as oncogenic, likely oncogenic, or conferring resistance in the OncoKB database, or as pathogenic or likely pathogenic in the ClinVar database. ERBB2 amplification remained the only alteration significantly enriched in Hispanic patients. When stratified by sequencing age, the prevalence of ERBB2 amplification remained statistically significantly higher among Hispanic patients with both early-onset and late-onset gastric cancers. Notably, the late-onset group showed stronger statistical significance (Hispanic: 16.9% vs 10.4% non-Hispanic White, 8.7% non-Hispanic Asian, and 11.6% non-Hispanic Black; P < .001; Supplementary Table 3, available online). Overall, Hispanic patients with gastric adenocarcinoma also had statistically significantly higher odds of presenting with an ERBB2 amplification compared with non-Hispanic white patients in adjusted models (OR 2.52, 95% CI 1.20-5.33, P = .015; Supplementary Table 4, available online). Notably, these patterns were not observed across other racial/ethnic groups.
Our findings highlight ethnic patterns in ERBB2 amplification among patients diagnosed with primary gastric adenocarcinoma, as nearly 1 in every 6 individuals who self-identified as Hispanic in our cohort presented with this deleterious copy number alteration. These results raise important questions about the role of genomic factors in gastric adenocarcinoma disparities with potential implications for clinical practice. Given the limited diversity of current cancer genomic studies across racial/ethnic minority populations, these results support the potential expansion of opportunities for diverse patients to yield equitable access to targeted and efficacious cancer treatments (7).
Previous studies have shed light on the potential role of H. pylori infection in increasing ERBB2 signaling, a potential accelerator of gastric adenocarcinoma progression (8). Indeed, recent work by Rammohan and colleagues has identified potential racial/ethnic disparities in H. pylori clearance rates (9). Hispanic patients were found to have the longest infection clearance time compared to other racial/ethnic population groups. Together with our observation that Hispanic patients had the highest rates of ERBB2/HER2 amplification and higher odds of presenting with an ERBB2 amplification after accounting for sequencing age, center, sex, and tumor histological type, these data suggest that 16% of gastric adenocarcinoma cases who identify as Hispanic and presented with an ERBB2 amplification in this cohort could potentially benefit from targeting this genomic alteration with trastuzumab and fam-trastuzumab deruxtecan-nxki, marketed under the brand name Enhertu—current FDA-approved therapies for HER2-overexpressing gastric adenocarcinoma (10).
The Cancer Genome Atlas (TCGA) project has defined 4 consensus subtypes of gastric cancers: EBV-infected tumors (EBV); MSI tumors (MSI); genomically stable tumors (GS); and chromosomally unstable tumors (CIN) (11). Notably, the CIN subtype was found to have the highest prevalence of ERBB2 amplifications and is associated with gastric cancer diagnosed at an older age. As such, the significantly higher frequency of ERBB2 amplification in gastric tumors among Hispanics observed in our study—particularly for 1 in every 5 Hispanic patients who are age 50+ years—may support an enrichment of the CIN subtype of gastric adenocarcinomas within this population. Beyond the CIN subtype, initial studies also suggest that Hispanic/Latino patients often present with GS subtype gastric tumors, particularly among young patients (1,11). Consistent with these reports, our observation of a relatively lower prevalence of ERBB2 amplification among Hispanic patients with early-onset gastric adenocarcinomas supports a potential enrichment of the GS subtype in these cases. However, as TCGA molecular subtype data are unavailable for assessment within GENIE, this precludes our ability to define these subtypes for patients in our study and illustrates the critical need for robust genome-wide sequencing efforts—inclusive of molecular subtypes, in diverse patients with gastric adenocarcinoma. Such efforts will support driving mechanistic insights into gastric cancer disparities and delivering clinically actionable outcomes.
The use of the AACR Project GENIE Consortium is a strength of this study, as it allowed for the inclusion of a diverse cohort of primary gastric adenocarcinoma patients with available paired clinical-grade targeted sequencing and clinical/demographic data. However, we acknowledge the limitations of this work, including the potential that Hispanic individuals with more aggressive gastric adenocarcinomas were likely to receive tumor genomic profiling and to be overrepresented in our cohort. GENIE project, also, does not collect key individual-level factors that could also contribute to these genomic patterns observed among Hispanic patients. This includes germline genetic features, H. pylori infection, lifestyle, familial and environmental factors (eg, socioeconomic status), and detailed clinical characteristics (eg, tumor anatomical location, disease stage, and survival). Although we observed ERBB2 amplification patterns unique to Hispanic patients, we were also unable to assess genomic patterns in Hispanic/Spanish/Latino subpopulations by country of origin to further disentangle genomic patterns within this diverse ethnic group. As race/ethnicity is a social construct, it will be critical to consider the role of genetic ancestry as a biological construct in studying genomic features of gastric adenocarcinoma disparities moving forward. Lastly, our inability to validate these results in an independent cohort due to limited genomic data currently available in multiethnic populations also draws important attention to the timely need for adequate representation of individuals who identify as non-White, including Hispanic/Spanish populations, in translational studies that can filter for ClinVar-annotated variants with known or suspected function changes as well as clinical trials to identify high-risk patients and to optimize gastric adenocarcinoma screening and surveillance strategies. Nevertheless, our findings support a hypothesis that differences in tumor biology may be contributing to a disproportionate gastric adenocarcinoma burden across racial/ethnic groups that warrants further investigation.
In conclusion, our study using data from an international consortium demonstrated ethnic disparities in ERBB2 amplification among patients with primary gastric adenocarcinoma. Further clinical and mechanistic studies should focus on understanding the role of ERBB2 amplification in gastric adenocarcinoma disparities, together with social, biological, clinical, and genetic ancestral factors, in order to develop effective strategies to reduce these disparities. Ensuring equitable access to genomic sequencing and targeted therapies such as trastuzumab also harbors immense potential to improve outcomes for all patients diagnosed with gastric adenocarcinomas.
Supplementary Material
Acknowledgments
The authors acknowledge the American Association for Cancer Research (AACR) and Consortium members for their support in developing the AACR Project GENIE registry. The authors assume sole responsibility for the interpretation presented in this article.
Contributor Information
Muhammad Bilal Mirza, Division of Surgical Oncology and Endocrine Surgery, Department of Surgery, Vanderbilt University Medical Center, Nashville, TN, USA.
Jungyoon Choi, Division of Oncology/Hematology, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Republic of Korea.
Paula Marincola Smith, Division of Surgical Oncology and Endocrine Surgery, Department of Surgery, Vanderbilt University Medical Center, Nashville, TN, USA.
Jordan J Baechle, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, CA, USA.
Chandrasekhar Padmanabhan, Division of Surgical Oncology and Endocrine Surgery, Department of Surgery, Vanderbilt University Medical Center, Nashville, TN, USA.
Andreana N Holowatyj, Department of Medicine, Vanderbilt University Medical Center, Vanderbilt-Ingram Cancer Center, Nashville, TN, USA.
Shailja C Shah, GI Section, VA San Diego Health System, La Jolla, CA, USA; Division of Gastroenterology, Department of Medicine, University of California, San Diego, CA, USA.
Xingyi Guo, Department of Biomedical Informatics, Department of Medicine, Vanderbilt Epidemiology Center, and Vanderbilt-Ingram Cancer Center, Nashville, TN, USA.
Kamran Idrees, Division of Surgical Oncology and Endocrine Surgery, Department of Surgery, Vanderbilt University Medical Center, Nashville, TN, USA.
Data availability
The data and analytics tools used in this study are publicly available at: (The AACR Project GENIE Consortium et al., 2024 - https://www.aacr.org/professionals/research/aacr-project-genie/aacr-project-genie-data/).
Author contributions
Muhammad Bilal Mirza, MD (Conceptualization; Investigation; Writing—original draft; Writing—review & editing), Jungyoon Choi, MD, PhD (Formal analysis; Investigation; Methodology; Software; Validation; Writing—review & editing), Paula Marincola Smith, MD, PhD (Validation; Visualization; Writing—original draft; Writing—review & editing), Jordan J. Baechle, MD (Data curation; Formal analysis; Methodology), Chandrasekhar Padmanabhan, MD (Investigation; Writing—original draft; Writing—review & editing), Andreana N. Holowatyj, PhD, MS (Writing—original draft; Writing—review & editing), Shailja C. Shah, MD (Writing—original draft; Writing—review & editing), Xingyi Guo, PhD (Writing—original draft; Writing—review & editing), Kamran Idrees, MD, MSCI, MMHC, FACS (Conceptualization; Writing—original draft; Writing—review & editing).
Funding
This work was, in part, supported by National Institutes of Health (NIH)/NICHD grant K12 HD043483 to A.N.H. and by NIH/National Cancer Institute grant P50 CA236733. The contents of this article are solely the responsibility of the authors and do not necessarily represent official views of the NIH.
Conflicts of interest
A.N.H. is Chair of the Scientific Advisory Board for the Appendix Cancer Pseudomyxoma Peritonei (ACPMP) Research Foundation and reports receiving grants from the NIH for the conduct of this study; receiving grants from the NIH, ACPMP Research Foundation, American Cancer Society, Pfizer, Inc, and the Dalton Family Foundation; and personal fees from MJH Life Sciences outside the submitted work.
References
- 1. Toal TW, Estrada-Florez AP, Polanco-Echeverry GM, et al. Multiregional sequencing analysis reveals extensive genetic heterogeneity in gastric tumors from Latinos. Cancer Res. Commun. 2022;2(11):1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah SC, McKinley M, Gupta S, Peek RM, Martinez ME, Gomez SL.. Population-based analysis of differences in gastric cancer incidence among races and ethnicities in individuals age 50 years and older. Gastroenterology. 2020;159(5):1705-1714.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merchant SJ, Kim J, Choi AH, Sun V, Chao J, Nelson R.. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer. 2017;20(2):226-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holowatyj AN, Ulrich CM, Lewis MA.. Racial/ethnic patterns of young-onset noncardia gastric cancer. Cancer Prev Res (Phila). 2019;12(11):771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. André F, Arnedos M, Baras AS, Baselga J, et al. ; The AACR Project GENIE Consortium. AACR Project GENIE: powering precision medicine through an International Consortium. Cancer Discov. 2017;7(8):818-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pugh TJ, Bell JL, Bruce JP, et al. ; AACR Project GENIE Consortium, Genomics and Analysis Working Group. AACR Project GENIE: 100,000 cases and beyond. Cancer Discov. 2022;212(9):2044-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landry LG, Ali N, Williams DR, Rehm HL, Bonham VL.. Lack of diversity in genomic databases is a barrier to translating precision medicine research into practice. Health Aff (Millwood). 2018;37(5):780-785. [DOI] [PubMed] [Google Scholar]
- 8. Chaturvedi R, Asim M, Piazuelo MB, et al. Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology. 2014;146(7):1739-1751.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rammohan R, Magam SG, Joy M, et al. Unpacking the racial gap: helicobacter pylori infection clearance among different racial groups. Cureus. 2023;15(8):e43080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bang Y-J, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. [DOI] [PubMed] [Google Scholar]
- 11. Bass AJ, Thorsson V, Shmulevich I, et al. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and analytics tools used in this study are publicly available at: (The AACR Project GENIE Consortium et al., 2024 - https://www.aacr.org/professionals/research/aacr-project-genie/aacr-project-genie-data/).