Abstract
Background
Syncope is common among children and adolescents. Although it is most commonly caused by vasovagal syncope, it can also be due to undiagnosed, potentially serious, or even life-threatening conditions. We aimed to investigate the distribution of subsequent sinister diagnoses, such as heart disease (HD) and epilepsy, and analyze their demographic characteristics in children presenting with syncope.
Methods
This nationwide, population-based study was conducted using the Korean Health Insurance Review and Assessment Service database. Patients aged <19 years at the time of their first visit between January 2010 and December 2014, who had primary, secondary, or additional diagnostic codes for syncope, were selected and followed up for a minimum of 5 years from the index date to investigate subsequent diagnoses of HD or epilepsy. Patient demographics, diagnostic codes, and prescriptions were retrieved from the database.
Results
A total of 75,839 patients with new-onset syncope were identified, of which 239 (0.3%) and 2,516 (3.3%) were subsequently diagnosed with HD and epilepsy, respectively. In the infant, toddler, and preschool age groups, the proportions of patients with subsequent diagnoses of HD and epilepsy were relatively lower (5/5,353, 0.1%) and higher (206/5,353, 3.8%), respectively, than the proportions in the other age groups. A male preponderance was noted for patients with syncope who were later diagnosed with HD or epilepsy (P<0.001). The proportion of patients experiencing syncope with a subsequent diagnosis of HD was relatively high in the summer.
Conclusions
The subsequent diagnosis of potentially life-threatening diseases in pediatric syncope, including HD and epilepsy, is relatively low in all age groups. In addition to comprehensive history taking and physical examination, demographic data such as age and sex, and season of occurrence, can aid in diagnosing the underlying cause of pediatric syncope by helping to identify patients who may require further investigations.
Keywords: Syncope, children, heart disease (HD), epilepsy, epidemiology
Highlight box.
Key findings
• The proportion of patients with a subsequent diagnosis of heart disease (HD) or epilepsy was significantly low. Characteristic distributions by age, sex, and season of occurrence among patients with syncope depending on the presence of subsequent potentially life-threatening diagnoses were also noted.
What is known and what is new?
• Vasovagal syncope is the most common cause of syncope and accounts for up to 75% of pediatric cases. However, syncope can be a manifestation of undiagnosed, potentially serious, or life-threatening causes.
• This study delineated the demographic characteristics of patients initially presenting with syncope according to the presence of subsequent diagnoses of HD or epilepsy.
What is the implication, and what should change now?
• In clinical practice, along with comprehensive history taking and physical examination, demographic data can aid in diagnosing the underlying cause of pediatric syncope by helping to identify patients who may benefit from further investigations.
Introduction
Syncope is characterized by a sudden and transient loss of consciousness. It is associated with the loss of postural tone due to a transient decrease in cerebral perfusion, followed by spontaneous recovery (1). Syncope is a common condition among children and adolescents and accounts for one in every 2,000 visits to children’s emergency departments (2). Approximately 15% of children experience at least one syncopal episode before the age of 18 years, with a peak in females aged 15–19 years (2-4).
Vasovagal syncope, which is benign, is the most common cause of syncope and accounts for up to 75% of pediatric cases (2,3). However, syncope can be a manifestation of undiagnosed, potentially serious, or life-threatening causes (5). Cardiac syncope is the most dangerous cause of syncope, affecting 1–5% of pediatric patients (6,7). Without intervention, the 6-month mortality rate associated with cardiac causes of syncope exceeds 10% (8). Neurological causes are relatively rare but should be carefully considered when evaluating a patient with syncope.
Although ominous causes of syncope in children are uncommon, extensive diagnostic evaluation is frequently performed in clinical practice because of parental anxiety and clinicians’ uncertainty about the etiology, given the variability in the clinical presentation of pediatric syncope (9). Superfluous testing may lead to unnecessary hospitalization and substantial medical costs for patients with syncope (10). Despite the low diagnostic yield, the number of tests performed on patients presenting to the emergency department with syncope has increased over time (11). Although the etiologies and incidence of pediatric syncope have been demonstrated in previous studies, there is limited data on nationwide population-based epidemiological studies of syncope that can be used by clinicians.
The primary aim of this study was to investigate the distribution of subsequent sinister diagnoses, such as structural heart disease (HD), arrhythmia, and epilepsy, in pediatric patients who initially visited the hospital because of syncope using the Health Insurance Review and Assessment Service (HIRA) of the Korea database. The secondary aim was to analyze the demographic characteristics of patients initially presenting with syncope according to the presence of subsequent diagnoses of HD or epilepsy. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-252/rc).
Methods
Data sources
South Korea has a universal health care system with approximately 98% of the overall Korean population being covered by National Health Insurance (NHI). HIRA is a government-affiliated organization that performs claims reviews and quality assessments for NHI. Medical institutions are required to submit medical claims data to HIRA for government reimbursement. The data are then integrated into the claims database. The claims data includes patient demographics, diagnoses, treatments, procedures, surgical histories, and prescribed medications for both inpatient and outpatient settings. Diagnoses are coded according to the International Classification of Diseases, 10th revision (ICD-10). The HIRA database serves as a valuable source of epidemiologic data representative of the national population, thereby enabling comprehensive nationwide studies (12).
Study design and participants
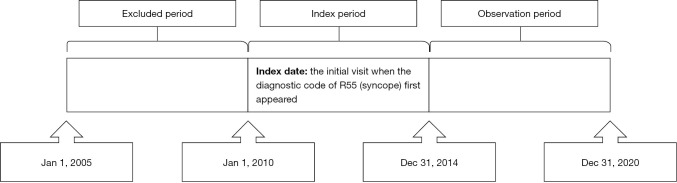
Patients aged <19 years at the time of their first visit between January 2010 and December 2014 with primary, secondary, or additional diagnostic codes for syncope (R55) were identified from the HIRA claims database. The date on which the diagnostic code for syncope first appeared was defined as the index date. Patients diagnosed with HD or epilepsy within at least 5 years preceding the index date were excluded. We regarded patients as having preexisting HD when they had at least one hospital admission with a primary diagnosis of HD, which included heart failure (I50), arrhythmia (I47–I49), ischemic HD (I20–I25), valvular HD (I34–I38), pulmonary HD (I26–I28), hypertensive HD (I11, I13), congenital HD (Q20–Q26), or others (I00–I09, I30–I33, I40–I46, I51) based on ICD-10 (13-15). In addition, we considered patients with preexisting epilepsy when they fulfilled the following requirements: (I) at least one visit with the diagnostic codes for epilepsy or seizure extracted from the code for only the primary diagnosis and (II) the prescription of one or more antiseizure medications for any length of time. According to the ICD-10, G40 (epilepsy), G41 (status epilepticus), F803 (Landau-Kleffner syndrome), and R56 (convulsion) were considered diagnostic codes for epilepsy or seizure. Code R56.0 (febrile convulsions) was excluded. Antiseizure medications included carbamazepine, clobazam, ethosuximide, fosphenytoin, gabapentin, lacosamide, lamotrigine, levetiracetam, oxcarbazepine, perampanel, phenobarbital, phenytoin, pregabalin, primidone, rufinamide, stiripentol, topiramate, valproate, vigabatrin, and zonisamide (16). The included patients were followed up for a minimum of 5 years after the index date to investigate the subsequent diagnosis of HD or epilepsy (Figure 1).
Figure 1.
Illustration of the study design.
Enrolled patients were classified into three groups based on the presence of subsequent diagnoses of HD or epilepsy: patients with a subsequent diagnosis of HD (Group H), those with a subsequent diagnosis of epilepsy (Group E), and those without a subsequent diagnosis of HD or epilepsy (Group S). To determine the distribution of each group at various ages, patients were divided into the following three age groups: the infant, toddler, and preschool group (≤6 years), the school-age group (7–12 years), and the adolescent group (13–18 years). The seasons of the year were defined as follows: spring, March–May; summer, June–August; autumn, September–November; and winter, December–February.
Statistical analysis
All statistical analyses were performed using the SAS Enterprise Guide software version 6.1 (SAS Institute Inc., Cary, NC, USA). Numerical data, including age, were compared using the Mann-Whitney U test. Categorical data, such as demographic characteristics, were analyzed using Fisher’s exact test or the chi-square test. Fisher’s exact test was used when more than 20% of the cells had expected frequencies of <5. Statistical significance was set at P<0.05.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board (IRB) of Catholic Medical Center (IRB approval number: VC21ZASI0230). As all data were collected retrospectively and de-identified, individual consent was not required for this retrospective analysis.
Results
Patient characteristics
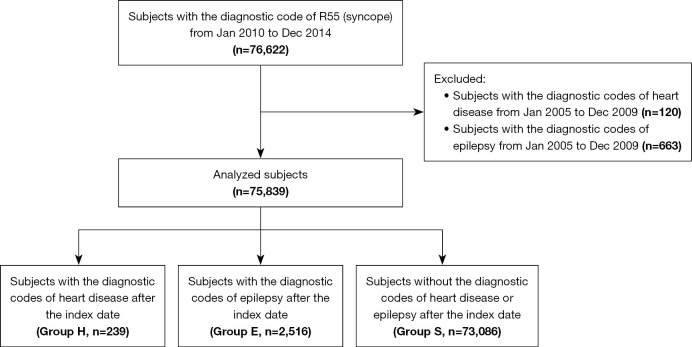
A total of 75,839 patients with new-onset syncope were identified between January 2010 and December 2014. As shown in Figure 2, the patients were grouped by the presence of subsequent diagnoses of HD or epilepsy determined during the 5-year observation period after the index date as follows: Group H (patients with a subsequent diagnosis of HD; n=239, 0.3%), Group E (those with a subsequent diagnosis of epilepsy; n=2,516, 3.3%), and Group S (patients without a subsequent diagnosis of HD or epilepsy; n=73,086, 96.4%). Of them, two patients were finally diagnosed with both HD and epilepsy. Demographic characteristics of the patients are shown in Table 1. The detailed diagnoses of HD in Group H were as follows: heart failure (I50; n=2, 0.8%), atrioventricular and left bundle-branch block (I44; n=10, 4.2%), other conduction disorders (I45; n=33, 13.8%), paroxysmal tachycardia (I47; n=74, 31.0%), atrial fibrillation and flutter (I48; n=15, 6.3%), other cardiac arrhythmias (I49; n=65, 27.2%), non-rheumatic mitral valve disorders (I34; n=3, 1.3%), non-rheumatic aortic valve disorders (I35; n=2, 0.8%), non-rheumatic pulmonary valve disorders (I37; n=3, 1.3%), pulmonary embolism (I26; n=2, 0.8%), other pulmonary HD (I27; n=2, 0.8%), other diseases of pulmonary vessels (I28; n=1, 0.4%), rheumatic tricuspid valve diseases (I07; n=1, 0.4%), acute myocarditis (I40; n=7, 2.9%), cardiomyopathy (I42; n=1, 0.4%), congenital malformations of cardiac septa (Q21; n=13, 5.4%), other congenital malformations of the heart (Q24; n=1, 0.4%), and congenital malformations of great arteries (Q25; n=4, 1.7%).
Figure 2.
Flow diagram of inclusion and exclusion criteria. Group H represents patients who were subsequently diagnosed with heart disease. Group E represents patients who were subsequently diagnosed with epilepsy. Group S refers to patients without a subsequent diagnosis of either heart disease or epilepsy. Two patients were subsequently diagnosed with both heart disease and epilepsy, and thus belong to both Group H and Group E.
Table 1. Demographic characteristics of patients.
| Characteristics | Group H (n=239) | Group H2 (n=156) | Group E (n=2,516) | Group S (n=73,086) | P* | P** | P*** |
|---|---|---|---|---|---|---|---|
| Age (years), mean [range] | 14.6 [1–18] | 14.7 [1–18] | 13.5 [0.2–18] | 13.8 [0–18] | 0.02 | 0.04 | <0.001 |
| Age group | |||||||
| 0–6 years | 5 (2.1) | 2 (1.3) | 206 (8.2) | 5,142 (7.0) | 0.002 | 0.001 | 0.02 |
| 7–12 years | 48 (20.1) | 30 (19.2) | 524 (20.8) | 14,135 (19.3) | 0.77 | 0.97 | 0.06 |
| 13–18 years | 186 (77.8) | 124 (79.5) | 1,786 (71.0) | 53,809 (73.6) | 0.14 | 0.09 | 0.003 |
| Male sex | 142 (59.4) | 96 (61.5) | 1,350 (53.7) | 32,884 (45.0) | <0.001 | <0.001 | <0.001 |
| Urban residence | 105 (43.9) | 70 (44.9) | 1,062 (42.2) | 31,576 (43.2) | 0.82 | 0.67 | 0.32 |
| Season | |||||||
| Spring | 67 (28.0) | 46 (29.5) | 661 (26.3) | 17,948 (24.6) | 0.21 | 0.15 | 0.049 |
| Summer | 75 (31.4) | 47 (30.1) | 678 (26.9) | 22,618 (30.9) | 0.88 | 0.82 | <0.001 |
| Autumn | 43 (18.0) | 26 (16.7) | 588 (23.4) | 15,580 (21.3) | 0.21 | 0.15 | 0.01 |
| Winter | 54 (22.6) | 37 (23.7) | 589 (23.4) | 16,940 (23.2) | 0.83 | 0.87 | 0.78 |
| Hospitalizations | |||||||
| Inpatient | 55 (23.0) | 30 (19.2) | 646 (25.7) | 7,268 (9.9) | <0.001 | <0.001 | <0.001 |
| Outpatient | 184 (77.0) | 126 (80.8) | 1,870 (74.3) | 65,818 (90.1) | <0.001 | <0.001 | <0.001 |
Data are presented as n (%) unless otherwise specified. Group H represents patients who were subsequently diagnosed with heart disease. Group H2 refers to a subset of Group H, consisting of patients whose heart disease was presumed to be a direct cause of syncope. Group E represents patients who were subsequently diagnosed with epilepsy. Group S refers to patients without a subsequent diagnosis of either heart disease or epilepsy. *, comparison between Groups H and S; **, comparison between Groups H2 and S; ***, comparison between Groups E and S.
Of the 239 patients in Group H, 156 (65.3%) presumed to have cardiac conditions as a direct cause of syncope were specifically identified (Group H2) (Table 1). The detailed diagnoses of HD in Group H2 were as follows: heart failure (I50; n=2, 1.3%), complete atrioventricular block (I44.2; n=4, 2.6%), pre-excitation syndrome (I45.6; n=25, 16.0%), supraventricular tachycardia (I47.1; n=64, 41.0%), paroxysmal tachycardia (I47.9; n=10, 6.4%), atrial fibrillation and flutter (I48, I48.0, I48.1, I48.9; n=15, 9.6%), ventricular fibrillation and flutter (I49; n=5, 3.2%), sick sinus syndrome (I49.5; n=10, 6.4%), mitral valve insufficiency (I34; n=1, 0.6%), aortic valve insufficiency (I35.1; n=2, 1.3%), pulmonary valve stenosis (I37; n=1, 0.6%), pulmonary valve insufficiency (I37.1; n=2, 1.3%), pulmonary embolism without mention of acute cor pulmonale (I26.9; n=2, 1.3%), other secondary pulmonary hypertension (I27.2; n=2, 1.3%), tricuspid insufficiency (I07.1; n=1, 0.6%), acute myocarditis (I40.8, I40.9; n=7, 4.5%), restrictive cardiomyopathy (I42.5; n=1, 0.6%), and stenosis of pulmonary artery (Q25.6; n=2, 1.3%).
Subgroup analyses according to the demographic factors
Age
As shown in Table 1, patients in Group S experienced an initial episode of syncope at a significantly earlier age than those in Group H (13.8 vs. 14.6 years, P=0.02) and Group H2 (13.8 vs. 14.7 years, P=0.04). Conversely, patients in Group E experienced syncopal episodes at an earlier age than those in Group S (13.5 vs. 13.8 years, P<0.001).
Overall, the proportion of patients in Group S was considerably higher than the proportions in Group H, Group H2, and Group E. Similar results were obtained in the analysis of each age group. However, with a more detailed analysis within each age group, the distinctive distributions of Group H, Group H2, Group E, and Group S were observed. The proportion of Group E patients was relatively higher in the infant, toddler, and preschool group (206/5,353, 3.8%) than in the school-age (524/14,707, 3.6%) and adolescent (1,786/55,781, 3.2%) groups. The proportion of Group E patients was relatively higher in the school-age group than in the adolescent group. Conversely, the proportion of patients in Group H2 was lower in the infant, toddler, and preschool age group (2/5,353, 0.03%) than in the school-age (30/14,707, 0.2%) and adolescent (124/55,781, 0.2%) groups. The proportion of patients in Group H2 was similar in the school-age and adolescent age groups. A similar age pattern was observed in Group H: 0.1% (5/5,353) in the infant, toddler, and preschool group; 0.3% (48/14,707) in the school-age group; and 0.3% (186/55,781) in the adolescent group.
Sex
Sex distribution was significantly different among the different groups. A significantly higher number of male patients was subsequently diagnosed with HD (Group H2) or epilepsy (Group E), accounting for 61.5% and 53.7% cases, respectively. On the other hand, the proportion of male patients was significantly lower in Group S (45.0%, P<0.001). The difference between Group H and Group S was also statistically significant (59.4% vs. 45.0%, P<0.001) (Table 1).
Urban residence
The proportions of patients residing in urban areas were similar in Groups H2, E, and S, accounting for 44.9%, 42.2%, and 43.2% of cases, respectively. In addition, the proportion of patients residing in an urban area in Group H was 43.9%, which was comparable to that in the other groups (Table 1).
Seasonality
The seasonal distribution for each group is shown in Table 1. In Groups H and H2, the prevalence of syncope was 18.0% and 16.7% in autumn and 31.4% and 30.1% in summer, respectively. An increase in the prevalence of syncope during summer was also observed in Group S, accounting for approximately one-third of the patients. On the other hand, patients in Group E experienced relatively fewer syncopal episodes in summer (26.9%) than did those in the other groups. The prevalence of syncope was relatively higher in spring (26.3% vs. 24.6%, P=0.049) and autumn (23.4% vs. 21.3%, P=0.01) in Group E than in Group S.
Hospitalizations
A significant difference was observed in the proportion of inpatients among the different groups. A significantly higher number of inpatients was subsequently diagnosed with HD (Group H2) or epilepsy (Group E), accounting for 19.2% and 25.7% of cases, respectively, compared with 9.9% in Group S (P<0.001 for Group H2 vs. Group S, and P<0.001 for Group E vs. Group S). The proportion of inpatients also differed significantly between Group H and Group S (23.0% vs. 9.9%, P<0.001) (Table 1).
Discussion
This was a large-scale study of the etiologic and demographic characteristics of pediatric patients presenting with syncope using a nationwide population-based database. Syncope is a common complaint among pediatric patients seeking medical attention and generates considerable concern among patients, parents, and providers. Despite a detailed history of the event, followed by a comprehensive physical examination to determine the causes of syncope, clinicians usually encounter a diagnostic dilemma owing to its clinical ambiguity. As demonstrated in the present study, the proportion of patients with a subsequent diagnosis of HD or epilepsy was significantly low. Moreover, therapeutic approaches differed according to the diagnosis. In that regard, more focused investigations are required to differentiate syncope caused by serious or life-threatening medical conditions and prevent a waste of time and effort. In this study, we observed the characteristic distributions by age, sex, and season of occurrence among patients with syncope depending on the presence of subsequent potentially life-threatening diagnoses. These demographic details may provide insights that can assist in identifying cases of high-risk pediatric syncope using a targeted approach, although they should not be considered definitive in the diagnostic or treatment process.
Syncope can occur at any age and has several etiologies. Cardiac diseases are the most common cause of syncope in adults. Conversely, most pediatric cases of syncope are usually due to benign causes, such as vasovagal syncope, orthostatic hypotension, hyperventilation, and breath-holding spells, with only a small proportion of cases resulting from serious medical conditions with the potential for sudden death (9,17). Similarly, our data revealed that the proportion of patients with a subsequent diagnosis of HD who required therapeutic changes was extremely low, with 0.3% (239/75,839) in Group H and 0.2% (156/75,839) in Group H2. Additionally, the proportion of patients with a subsequent diagnosis of epilepsy (Group E) was estimated to be 3.3% (2,516/75,839), which was relatively low. A fairly small proportion of patients with a subsequent diagnosis of HD or epilepsy were also noted in each age group. In particular, the proportion of patients with a subsequent diagnosis of HD was significantly lower in the infant, toddler, and preschool group, at 0.1% (5/5,353) in Group H and 0.03% (2/5,353) in Group H2, whereas that of patients with a subsequent diagnosis of epilepsy (Group E) was relatively higher in this age group at 3.8% (206/5,353), compared with the other age groups. These results highlight that multiple, sophisticated investigations without a goal-directed approach for cases of pediatric syncope, often involving hospitalization, can be costly, time-consuming, and frustrating. In this regard, our efforts to delineate the demographic factors among different groups are worthwhile, as they may provide diagnostic clues that could serve as a reference when identifying potential high-risk cases of pediatric syncope for further investigation and treatment adjustments.
Distinguishing between syncope and epileptic seizures is challenging in clinical practice. Brief myoclonic jerks or tonic-clonic convulsion-like movements can be observed during syncopal events, which may lead to a misdiagnosis of seizures by the physician (1,17,18). Previous studies have reported that 20% of patients admitted with syncope received antiseizure medications, and 31.8% of patients were misdiagnosed with epileptic seizures (19,20). Our data revealed that subsequent diagnoses of epilepsy were more frequently observed in the infant, toddler, and preschool age groups. In younger age groups, it can be challenging to take a detailed history and perform a neurological examination because of inadequate cooperation and comprehension of the questions asked. This limitation may impede the differentiation between seizures and syncope caused by other conditions. Considering that the proportion of patients with a subsequent diagnosis of epilepsy is relatively higher in infants, toddlers, and preschool children, electroencephalography may be beneficial in distinguishing seizures from syncope due to other conditions and avoiding unnecessary investigations. We noted that the proportion of patients experiencing syncope with a subsequent diagnosis of HD was comparably lower in the infant, toddler, and preschool age groups than in other age groups. HD is an important pediatric illness and a major contributor to childhood morbidity and mortality. Thus, it is possible that patients diagnosed with HD at an early age, before the occurrence of syncopal episodes, were not included in this study, which may have influenced the age distribution between patients with HD and those without a subsequent diagnosis of HD or epilepsy.
We found that the proportion of females with a subsequent diagnosis of HD or epilepsy was significantly lower than that of males. This can be partly ascribed to the possibility that females are more prone to experiencing syncopal episodes attributed to benign causes than males. Consistent with our results, a systematic review demonstrated that the percentage of females with vasovagal syncope (58%) was significantly higher than that of males (42%) (21). The reason for this greater involvement of females remains unclear (21). Hormone levels and sympathetic nervous system development may be partly related to sex differences in syncope. Human and animal data demonstrate significant differences between males and females in the basal function of the autonomic nervous system (22,23). Given the possibility that the unequal sex ratio may have biased the results of this study, the findings should be interpreted with caution.
Our study demonstrated that syncope without a subsequent diagnosis of HD or epilepsy was more prevalent in the summer than in those with a subsequent diagnosis of epilepsy. This seasonal prevalence can be attributed to the fact that most syncope in children is vasovagal in nature (2,3) and children are more likely to be exposed to typical triggers for vasovagal syncope, such as a hot environment or dehydration, during summer. In addition, the proportion of patients with a subsequent diagnosis of HD was relatively high in the summer. This may be explained by the fact that exposure to high temperatures is associated with increased strain on the cardiovascular system and a greater risk of heart attacks, arrhythmias, and heart failure. This study revealed no statistically significant differences in urban residence status among the three diagnostic groups: those diagnosed with epilepsy, those diagnosed with HD, and those not diagnosed with either condition. These findings contrast with a previous study, which has shown that factors such as healthcare access, environmental conditions, and socioeconomic status between urban and rural areas can contribute to regional disparities in health outcomes (24). Several factors may account for this lack of observed disparity: first, the differences in healthcare access and other urban-rural factors may not have a significant effect on the diagnosis of epilepsy or HD in the context of syncope; second, urban residence may not be a direct risk factor for the conditions studied, or its impact may be mitigated by other factors.
Identifying the cause of syncope allows clinicians to make disposition decisions for high- and low-risk patients (25). Patients with potentially life-threatening medical conditions, such as cardiac or neurological causes, should be treated promptly, while those with benign causes should be followed up in an outpatient clinic. In most patients, the cause of syncope can be accurately determined through meticulous history taking and physical examination (4). However, conducting a thorough medical history assessment, physical examination, and comprehensive investigation to determine the etiology of syncope remains a diagnostic challenge. Previous studies have demonstrated that the cause of syncope is diagnosed with varying degrees of certainty. In a prospective study of 341 patients with syncope, a cardiac cause was identified in 23% of the cases, a neurally mediated cause in 58%, a neurologic or psychiatric cause in 1%, and in the remaining 18%, the cause of syncope remained unexplained (26). Sarasin et al. (27) conducted a study of 650 patients with syncope and reported a 76% diagnostic yield from a standardized clinical evaluation that included targeted testing. In this regard, our attempt to identify the demographic characteristics of patients who experienced syncope and were later diagnosed with serious illnesses, including HD and epilepsy, would help in selecting those who might benefit from more comprehensive investigation, potentially improving the effectiveness of diagnosis and treatment.
This study had certain limitations. First, because the study relied on administrative data, not all data were accessible, which may have hampered the identification of the causal relationships between syncope and HD or epilepsy. In this regard, there is a possibility that etiologies of syncope other than HD or epilepsy, such as reflex anoxic syncope or pallid breath-holding spells, which are common causes of syncope in infants and toddlers, may not have been fully investigated. Consequently, some patients with these conditions may have been included among the syncope cases without a later diagnosis of HD or epilepsy. Although cardiac conditions such as supraventricular tachycardia and valvular HD are potential causes of syncope, hospitalization is relatively uncommon unless accompanied by congenital arrhythmias or structural HD. In this study, children hospitalized with these diagnoses were classified as H2, suggesting that their symptoms were severe and likely contributed to the occurrence of syncope. However, given that this study was based on diagnostic data, a direct causal relationship between these conditions and syncope cannot be definitively established. Although direct causal relationships between certain specific diagnoses and syncope could not be established owing to the nature of nationwide registry data, we endeavored to provide an overview of the distribution of serious causes of syncope by tracking the subsequent diagnoses of patients over a minimum follow-up period of 5 years. This approach offers valuable insights for clinicians. Second, because the study population was selected based on the presence of diagnostic codes for syncope, heterogeneity in the patient population might have influenced the absence of statistical significance. Additionally, considering that some types of seizures occur predominantly in infants and children makes it difficult to generalize this study’s findings to clinical practice, because these results may not apply to other patient populations with different demographic characteristics. Furthermore, factors such as socioeconomic status, educational background, and access to healthcare, which can also affect diagnostic outcomes, were not fully explored in this study. Future studies should consider including socioeconomic and environmental variables to gain a more comprehensive understanding of the factors affecting diagnosis.
Conclusions
The findings of this study demonstrate that the subsequent diagnosis of potentially life-threatening diseases in pediatric cases of syncope, including HD and epilepsy, is considerably low in all age groups. Therefore, an evaluation that fails to approach patients with syncope in a goal-directed manner may be costly, time-consuming, and frustrating. Although the prevalence of HD and epilepsy was very low in this cohort, these conditions should be carefully excluded and evaluated in children with syncope. In the infant, toddler, and preschool age groups, the proportion of patients with a subsequent diagnosis of epilepsy was relatively higher, whereas those with a subsequent diagnosis of HD was comparably lower than in the other age groups. A male predominance among patients with syncope who were later diagnosed with HD or epilepsy was also noted. Further, the proportion of patients experiencing syncope with a subsequent diagnosis of HD was high in the summer. In clinical practice, in addition to comprehensive history taking and physical examination, demographic data can further assist in the diagnosis of pediatric syncope by helping to identify patients who could benefit from further investigation.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by a grant from the Catholic Medical Center Research Foundation for the 2021 program year.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Catholic Medical Center (IRB approval number: VC21ZASI0230). As all data were collected retrospectively and de-identified, individual consent was not required for this retrospective analysis.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-252/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-252/coif). All authors report receiving financial support from the Catholic Medical Center Research Foundation for the 2021 program year. The authors have no other conflicts of interest to declare.
Data Sharing Statement
Available at https://tp.amegroups.com/article/view/10.21037/tp-24-252/dss
References
- 1.Kapoor WN. Syncope. N Engl J Med 2000;343:1856-62. 10.1056/NEJM200012213432507 [DOI] [PubMed] [Google Scholar]
- 2.Kanjwal K, Calkins H. Syncope in Children and Adolescents. Cardiol Clin 2015;33:397-409. 10.1016/j.ccl.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 3.Massin MM, Bourguignont A, Coremans C, et al. Syncope in pediatric patients presenting to an emergency department. J Pediatr 2004;145:223-8. 10.1016/j.jpeds.2004.01.048 [DOI] [PubMed] [Google Scholar]
- 4.Strickberger SA, Benson DW, Biaggioni I, et al. AHA/ACCF Scientific Statement on the evaluation of syncope: from the American Heart Association Councils on Clinical Cardiology, Cardiovascular Nursing, Cardiovascular Disease in the Young, and Stroke, and the Quality of Care and Outcomes Research Interdisciplinary Working Group; and the American College of Cardiology Foundation: in collaboration with the Heart Rhythm Society: endorsed by the American Autonomic Society. Circulation 2006;113:316-27. 10.1161/CIRCULATIONAHA.105.170274 [DOI] [PubMed] [Google Scholar]
- 5.Villafane J, Miller JR, Glickstein J, et al. Loss of Consciousness in the Young Child. Pediatr Cardiol 2021;42:234-54. 10.1007/s00246-020-02498-6 [DOI] [PubMed] [Google Scholar]
- 6.Geggel RL. Conditions leading to pediatric cardiology consultation in a tertiary academic hospital. Pediatrics 2004;114:e409-17. 10.1542/peds.2003-0898-L [DOI] [PubMed] [Google Scholar]
- 7.Massin MM, Malekzadeh-Milani S, Benatar A. Cardiac syncope in pediatric patients. Clin Cardiol 2007;30:81-5. 10.1002/clc.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002;347:878-85. 10.1056/NEJMoa012407 [DOI] [PubMed] [Google Scholar]
- 9.Zavala R, Metais B, Tuckfield L, et al. Pediatric Syncope: A Systematic Review. Pediatr Emerg Care 2020;36:442-5. 10.1097/PEC.0000000000002149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg LA, Knilans TK. Syncope in children: diagnostic tests have a high cost and low yield. J Pediatr 2005;146:355-8. 10.1016/j.jpeds.2004.10.039 [DOI] [PubMed] [Google Scholar]
- 11.Driscoll DJ, Jacobsen SJ, Porter CJ, et al. Syncope in children and adolescents. J Am Coll Cardiol 1997;29:1039-45. 10.1016/s0735-1097(97)00020-x [DOI] [PubMed] [Google Scholar]
- 12.Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health 2014;36:e2014008. 10.4178/epih/e2014008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56-e528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 14.Ritchey MD, Loustalot F, Bowman BA, et al. Trends in mortality rates by subtypes of heart disease in the United States, 2000-2010. JAMA 2014;312:2037-9. 10.1001/jama.2014.11344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidney S, Quesenberry CP, Jr, Jaffe MG, et al. Heterogeneity in national U.S. mortality trends within heart disease subgroups, 2000-2015. BMC Cardiovasc Disord 2017;17:192. 10.1186/s12872-017-0630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon JY, Lee H, Shin JY, et al. Increasing Trends in the Incidence and Prevalence of Epilepsy in Korea. J Clin Neurol 2021;17:393-9. 10.3988/jcn.2021.17.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayram AK, Pamukcu O, Per H. Current approaches to the clinical assessment of syncope in pediatric population. Childs Nerv Syst 2016;32:427-36. 10.1007/s00381-015-2988-8 [DOI] [PubMed] [Google Scholar]
- 18.MacNeill EC, Vashist S. Approach to syncope and altered mental status. Pediatr Clin North Am 2013;60:1083-106. 10.1016/j.pcl.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 19.Ferrie CD. Preventing misdiagnosis of epilepsy. Arch Dis Child 2006;91:206-9. 10.1136/adc.2005.088906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidi A, Clough P, Cooper P, et al. Misdiagnosis of epilepsy: many seizure-like attacks have a cardiovascular cause. J Am Coll Cardiol 2000;36:181-4. 10.1016/s0735-1097(00)00700-2 [DOI] [PubMed] [Google Scholar]
- 21.Alboni P, Messop AC, Lauri A, et al. Are women really more affected by vasovagal syncope than men? J Cardiovasc Med (Hagerstown) 2021;22:69-78. 10.2459/JCM.0000000000001009 [DOI] [PubMed] [Google Scholar]
- 22.Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res 2002;53:678-87. 10.1016/s0008-6363(01)00508-9 [DOI] [PubMed] [Google Scholar]
- 23.Hu E, Liu X, Chen Q, et al. Investigation on the Incidence of Syncope in Children and Adolescents Aged 2-18 Years in Changsha. Front Pediatr 2021;9:638394. 10.3389/fped.2021.638394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med 2014;46:e19-29. 10.1016/j.amepre.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 25.Ouyang H, Quinn J. Diagnosis and evaluation of syncope in the emergency department. Emerg Med Clin North Am 2010;28:471-85. 10.1016/j.emc.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 26.Alboni P, Brignole M, Menozzi C, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol 2001;37:1921-8. 10.1016/s0735-1097(01)01241-4 [DOI] [PubMed] [Google Scholar]
- 27.Sarasin FP, Louis-Simonet M, Carballo D, et al. Prospective evaluation of patients with syncope: a population-based study. Am J Med 2001;111:177-84. 10.1016/s0002-9343(01)00797-5 [DOI] [PubMed] [Google Scholar]