Abstract
Introduction:
Foix–Alajouanine syndrome (FAS) is an uncommon neurological disorder marked by the gradual development of spinal cord congestion. First documented by Foix and Alajouanine in 1926. Although it is infrequent, delayed or misdiagnosis is nonetheless prevalent, resulting in inadequate therapy and unfavorable results.
Methods:
Using the PubMed database, MEDLINE, and EMBASE, we collected data on FAS patients and conducted a pooled analysis. The term ‘FAS’ was used to search for related articles. Our search was restricted to previous clinical case reports or series that were published in English. Non-English articles were excluded. We included the articles in the period from 1974 to 2024. Articles were eligible if the radiographic and clinical findings were indicative of FAS. A thorough research analysis was performed, examining case reports that specifically addressed this issue. This study examines the clinical symptoms, difficulties in diagnosis, methods of treatment, and outcomes related to FAS.
Results:
FAS predominantly impacts the elderly population. A total of 26 patients were diagnosed with FAS. The median age of affected individuals was 53 (SD ±15.96). The ratio of males to females is roughly 5:1. The clinical manifestations encompass gradual muscle weakness and sensory impairments. The diagnosis is dependent on radiological evaluations, specifically MRI and digital subtraction angiography. Possible treatments include endovascular therapy, surgical closure of arteriovenous fistula, or a combination of the two. Significant improvements in neurological impairments can be achieved by early intervention.
Conclusion:
The diagnosis of FAS continues to be difficult due to its infrequency and varied clinical manifestations. Prompt and precise diagnosis is essential for proper intervention, typically utilizing endovascular or surgical methods. Additional research is required to determine prognostic markers and enhance long-term care techniques for this rare neurological condition.
Keywords: arteriovenous fistulas, Foix–Alajouanine syndrome, spinal cord ischemia
Introduction
Highlights
Foix–Alajouanine syndrome (FAS) is an uncommon neurological disorder marked by the gradual development of spinal cord congestion.
This study examines the clinical symptoms, difficulties in diagnosis, methods of treatment, and outcomes related to FAS.
The diagnosis is dependent on radiological evaluations, specifically MRI and digital subtraction angiography. Possible treatments include endovascular therapy, surgical closure of AVF, or a combination of the two. Significant improvements in neurological impairments can be achieved by early intervention.
In 1926, Foix and Alajouanine documented a case of subacute congestive myelopathy, which resulted in the gradual development of paraplegia and ultimately led to the demise of two individuals. An autopsy revealed the presence of spinal cord necrosis and the observation of many convoluted and thickened blood vessels on the surface of the spinal cord. The condition was referred to as necrotizing myelopathy1. After few years, it was discovered that this necrotizing myelopathy was linked to the existence of an arteriovenous fistula (AVF). AVF can result in elevated venous pressure, reduced arteriovenous pressure gradient, and diminished spinal cord perfusion, ultimately causing spinal cord edema. The caudal region of the spinal cord is the most impacted as a result of gravitational forces and the absence of valves in the intraspinal venous system. Foix–Alajouanine syndrome (FAS) remains an uncommon disorder. A neurologist typically encounters a single case approximately every 4–8 years2. FAS occurs frequently in patients aged more than 501. However, most of those patients, when treated promptly with either embolization and/or surgical interruption of the draining veins, can functionally improve, leaving no disability. Furthermore, it is frequently associated with misdiagnosis, underdiagnosis, or delayed diagnosis, leading to inadequate treatment or unfavorable prognosis2,3. The aim of this study is to investigate the details of reported cases and case series4–21 including clinical manifestations, diagnostic challenges, treatment modalities, and outcomes associated with FAS, with the goal of enhancing understanding and improving management strategies for this rare condition.
Charles Foix
Charles Foix was born in Salies-de-Béarn, a town located in close proximity to Bayonne, in the southwestern region of France. He was born to a physician and completed his medical education at the University of Paris, studying under the mentorship of Pierre Marie (1853–1940) in the Salpêtrière. He started as an intern in 1906 and later became a Médecin des hôpitaux in 1919. In 1923, he achieved the degree of agrégé22. Foix was widely recognized as an outstanding instructor and clinician with equal expertise in the fields of general medicine and neurology. Foix primarily employed a comprehensive collection of data from the Salpêtrière and Ivry to establish a connection between arterial thrombosis observed during autopsies and the symptoms and signs exhibited by his patients. Additionally, he authored a book on the blood supply and anatomical structure of the brain22,23. Foix specialized in studying vascular lesions, and he also had a strong fascination with the complex structures of the midbrain and interbrain24.
Théophile Alajouanine
Théophile Alajouanine, born on 12 June 1890, in Verneix, was a distinguished French neurologist whose influence extended far beyond his lifetime. Trained under Joseph Jules Dejerine and collaborating closely with Georges Charles Guillain and Charles Foix, Alajouanine emerged as a leading authority in neurology, particularly in the study of aphasia. His prolific writing encompassed various neurological topics, with a particular emphasis on aphasia, reflecting his deep interest and expertise in the subject. The Laboratoire Théophile-Alajouanine, located at the Centre Hospitalier Côte-des-Neiges in Montréal, Canada, stands as a lasting tribute to his contributions. Alajouanine’s legacy endures through his extensive body of work and the ongoing impact of his research on the field of neurology25,26.
Methods
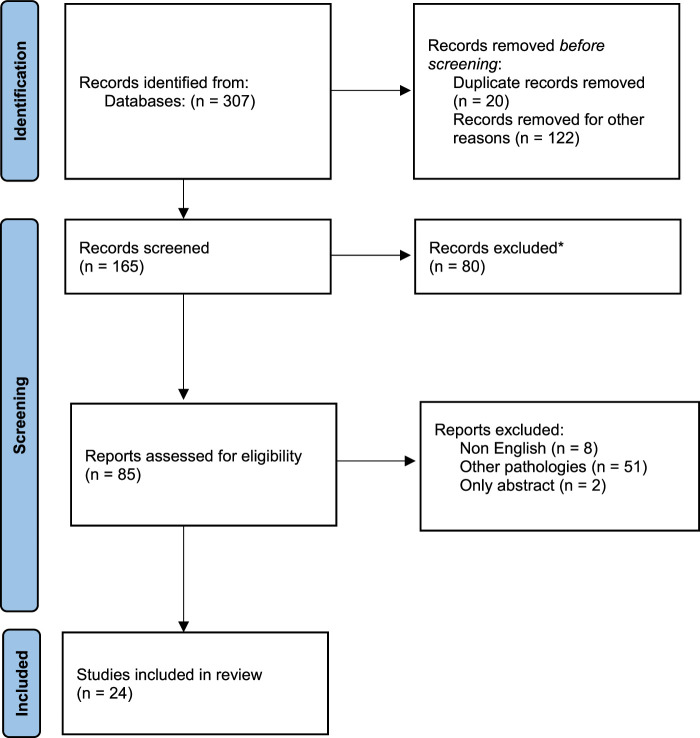
Using the PubMed, MEDLINE, and EMBASE databases, we collected data of FAS patients and conducted a pooled analysis. The term ‘FAS’ was used to search for related articles. Our search was restricted to previous clinical case reports or series that were published in English. Non-English articles were excluded. We included the articles in the period from 1974 to 2024. Articles were eligible if the radiographic and clinical findings are indicative of FAS. We excluded studies evaluating other diseases with similar presentations. Two review authors independently examined the titles and abstracts of all the potential studies to be included, identified by the search strategy. In case of overlaps of the series notified by the same institution or author, only the most recent publication was included in the analysis. Then, the complete text of the relevant primary studies was evaluated and data were extracted. Descriptive statistics are provided. Point-biserial correlations were run to determine the relationship between clinical variables and the overall symptom improvement. The included cases were analyzed in terms of age, sex, location, etiology, clinical presentations, and outcome. The goal of this pooled analysis was to evaluate the factors that could affect the outcome in these cases (Fig. 1).
Figure 1.
Flow diagram of the related articles.
Statistical analysis
Continuous variables were expressed as means or median values. Categorical variables were expressed as numbers and percentages. Continuous variables were compared using the unpaired t-test, non-parametric Mann–Whitney U test, or one-way analysis of variance. However, categorical variables were compared using χ 2 statistics or Fisher’s exact test as appropriate. Univariate regression tests were performed on all variables, and a multivariate logistic regression was performed on statistically significant variables (P<0.05). A P value of <0.05 was considered statistically significant.
Results
Literature search and characteristics of the eligible studies
Three databases were utilized to identify 312 articles. Of the articles, 292 were excluded based on ineligibility determined by having titles and abstracts suggesting apparent ineligibility. Two investigators independently evaluated the entire contents of the remaining 20 articles and ultimately identified these articles as eligible for the study.
A total of 26 patients were diagnosed with FAS. The median age of affected individuals was 53 (SD±15.96). It is more common in males representing about 84% of all our analyzed case cohort. The most common presentation reported is sensory loss, representing about 92%, followed by lower limb weakness, which is about 83.3%. About 19 patients (79.1%) presented with sphincter control problems. The most common location affected was the thoracic region, which was affected by about 54.1% of patients. The most frequently identified pathology was AVF in about 21 patients (84%). Surgical ligation was the method of treatment in 12 patients (50%), while embolization was performed in about 5 patients, and only 4 patients received conservative management. The mean follow-up period was 13.6 months. The outcome is variable, with only 9 patients (37.5) showing improvement, while 3 patients showed progression. Mortality represented only 16.6%. The multivariable logistic regression analysis showed that male gender, early sphincter affection, and lower limb paralysis at presentation were independent risk factors for poor outcomes (P value <0.05, respectively). We noticed that the male factor was an independent risk factor for poor outcome (P<0.05). We did not find any significant effect of symptom duration or treatment method on the outcome (P>0.05) (Table 1).
Table 1.
The multivariable logistic regression analysis shows the correlation of the risk factors
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| Male | 10.857 | 2.325–62.250 | 0.0024 | |||
| Age | 2.737 | 0.6373–14.287 | 0.1778 | |||
| Lower limb paralysis | 7.750 | 1.633–56.660 | 0.0089 | 9.962×107 | 2.194 | 0.0168 |
| Early sphincter affection | 10.75 | 1.504–95.885 | 0.0191 | 6.395 ×1014 | 9.273 | 0.0027 |
Epidemiology
Due to the scarcity of cases and the lack of robust large sample-size case series, epidemiological features of FAS are poorly described. FAS is classified as an uncommon disorder, and precise data regarding its prevalence in the United States is currently unavailable. Nevertheless, it is probable that the condition is not diagnosed as frequently as it should be. An epidemiological study carried out in Germany in 2001 estimated a prevalence rate of 5–10 instances per one million individuals in the general community, indicating that the disease is frequently disregarded27. FAS primarily impacts individuals in the older age group, with the majority of patients being aged 40 or above. Instances in individuals under the age of 30 are infrequently documented (Table 2). The condition exhibits a male-to-female ratio of roughly 5:122,29. Presently, there is a lack of evidence revealing the prevalence of FAS in particular ethnic or racial groups.
Table 2.
Details of reported cases and case series
| Symptoms | Location | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| References | Age | Gender | Symptoms duration (months) | Back pain | Loss of sensation | Paralysis of arms | Paralysis of legs | Bladder dysfunction | Bowel dysfunction | Loss of reflexes | Thoracal (T1–12) | Lumbr (L1–L5) | Etiology | Management | Outcome at discharge | Outcome at last follow-up | Follow-up (months) |
| Del Pino-Camposeco,7 | 46 | F | 4 | Yes | Yes | N/A | N/A | Yes | Yes | Yes | T6–11 | AVF type IV | Embolization | N/A | Progressive | 6 | |
| Bordignon,5 | 52 | F | N/A | Yes | Yes | N/A | Yes | Yes | N/A | Yes | L1–L4 | AVF type I | Surgical ligation | N/A | Improvement | 3 | |
| Joswig,9 | 31 | M | 4 | N/A | Yes | N/A | Yes | N/A | N/A | Yes | T9–10 | AVF type I | Surgical ligation | Mild improvement | Improvement | 2 | |
| Krishnan,13 | 54 | M | 36 | N/A | Yes | N/A | N/A | Yes | Yes | Yes | L4 | AVF type IV | Surgical ligation | N/A | Improvement | 12 | |
| Sadighi,18 | 48 | M | 24 | Yes | No | N/A | Yes | N/A | N/A | Yes | N/A | N/A | AVF type IV | Conservative | Improvement | N/A | N/A |
| Siani,19 | 38 | M | 3 | Yes | No | N/A | N/A | Yes | N/A | Yes | L1 | AVF type I | Surgical ligation | N/A | Improvement | 8 | |
| Sood,20 | 47 | M | 5 | No | Yes | N/A | N/A | Yes | N/A | Yes | T10–12 | AVF type IV | Embolization | Unchanged | Improvement | 8 | |
| Menon,14 | 42 | M | <1 | Yes | Yes | N/A | N/A | Yes | Yes | Yes | N/A | N/A | AVF type I | Conservative | Improvement | Improvement | 3 |
| Renowden,17 | 63 | M | 36 | N/A | Yes | N/A | Yes | N/A | N/A | Yes | T1 | L5 | AVF type I | Surgical ligation | N/A | Progressive | N/A |
| Ferrell,8 | 29 | M | 7 | Yes | Yes | N/A | Yes | N/A | Yes | Yes | N/A | N/A | N/A | N/A | Progressive | Died | N/A |
| 27 | M | 3 | N/A | Yes | N/A | N/A | N/A | N/A | Yes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Criscuolo,6 | 61 | M | 1 | N/A | Yes | N/A | Yes | Yes | Yes | N/A | L2 | AVF type I | Embolization | Improvement | Improvement | 14 | |
| 56 | M | 5 | N/A | Yes | N/A | Yes | Yes | N/A | Yes | T9–10 | AVF type I | Surgical ligation | Improvement | Improvement | 3 | ||
| 68 | M | N/A | N/A | Yes | N/A | Yes | N/A | N/A | N/A | T10 | L4 | AVF type I | Surgical ligation | Unchanged | Unchanged | 4 | |
| 60 | M | 2 | N/A | Yes | N/A | Yes | Yes | Yes | N/A | T9–12 | AVF type I | Surgical ligation | Progressive | Unchanged | 42 | ||
| 59 | M | N/A | N/A | Yes | N/A | Yes | Yes | N/A | Yes | T10 | AVF type I | Surgical ligation | Improvement | Improvement | 11 | ||
| Kneisley,11 | 43 | M | 24 | Yes | Yes | N/A | Yes | Yes | N/A | Yes | T11 | L1 | Spinal cord tumor and AVF type I | Embolization and surgical ligation | Progressive | Died | N/A |
| Koeppen,12 | 72 | M | 36 | N/A | Yes | N/A | Yes | Yes | N/A | Yes | N/A | N/A | N/A | N/A | Unchanged | Died | N/A |
| 66 | M | 36 | N/A | Yes | N/A | Yes | Yes | Yes | Yes | T1–12 | AVF type I | Surgical ligation | Improvement | Died | 48 | ||
| Tay,21 | 13 | F | N/A | No | No | N/A | Yes | No | No | Yes | N/A | N/A | Subarachnoid hemorrhage | Conservative | Progressive | Unchanged | 36 |
| Rathnam,16 | 77 | M | 8 | Yes | Yes | N/A | Yes | No | N/A | N/A | T9 | L1 | AVF type I | Surgical ligation | N/A | Improvement | N/A |
| Assadi,4 | 54 | M | <1 | N/A | Yes | N/A | Yes | Yes | Yes | Yes | N/A | N/A | AVF type I | N/A | N/A | Progressive | N/A |
| Patwari,15 | 35 | M | N/A | Yes | Yes | N/A | Yes | Yes | N/A | Yes | N/A | N/A | AVF type I | Conservative | N/A | N/A | N/A |
| Khan,10 | 78 | M | 2 | N/A | Yes | N/A | Yes | Yes | N/A | Yes | L2–4 | AVF type I | Surgical ligation | Improvement | Improvement | 5 | |
| Del Pino-Camposeco J,7 | 46 | F | 4 | Yes | Yes | N/A | Yes | Yes | Yes | Yes | T6–11 | N/A | AVF Type I | Embolization | Unchanged | Unchanged | 6 |
| Hubbard,28 | 68 | F | 12 | Yes | Yes | N/A | Yes | Yes | Yes | Yes | T7–L1 | N/A | AVF type I | Embolization | Unchanged | Unchanged | 3 |
The prognosis of FAS is contingent upon variables such as the duration of symptoms, the pre-treatment level of disability, and the efficacy of the AVF – closure surgery. Usually, signs such as gait disturbances and paresis demonstrate improvement following medical intervention. Nevertheless, symptoms such as urinary dysfunction and pain may not exhibit a favorable response. In this pooled analysis, all patients who died had sphincter – control problems as a clinical presentation8,11,12.
Etiopathology
The cause of FAS is still not well comprehended. The majority of patients exhibit an AVF in the lower thoracic spine18. One hypothesis posits that increased arterial pressure in the dura is transferred to the venous plexus via the intradural venous system, leading to impaired blood flow or thrombosis and causing infarction in the spinal cord tissue. The arterial blood originating from the dural fistula enters the venous system, causing an increase in pressure and impeding the usual drainage from the cord parenchyma30. Thrombosis is a possible occurrence; however, it usually becomes apparent toward the end of the disease’s progression. Infarction may be primarily caused by venous stasis rather than thrombosis. The preference for distal cord involvement is probably caused by orthostasis13,30,31. The predominance of symptoms in middle age indicates that the disease is likely acquired rather than being a congenital abnormality like other vascular malformations. However, the exact reason for its specificity to the spinal cord is still difficult to explain. Over the years, much debate has revolved around the etiology of FAS.
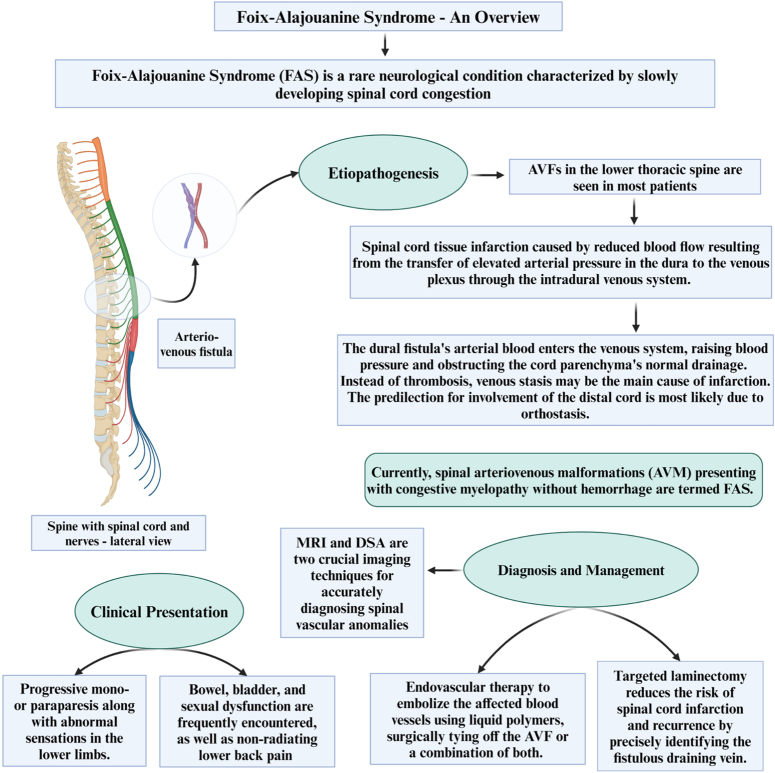
Initially, this designation was linked with spinal artery thrombosis leading to myelopathy, which was thought to carry a poor prognosis6. However, some patients diagnosed with FAS showed improvement, casting doubt on the role of spinal artery thrombosis20. Additionally, in 1989 Criscuolo et al. also contested the association with thrombosis and suggested that the symptoms of this syndrome could be attributed to congestive myelopathy, which is a reversible process6,20. Therefore, nowadays, spinal arteriovenous malformations (AVM) presenting with congestive myelopathy without hemorrhage are termed FAS (Fig. 2)6. The importance of this condition lies in its potential with the acute onset of neurologic dysfunction reminiscent of cauda equina syndrome due to lumbar disk prolapse. This syndrome is not a distinct entity but is rather considered a consequence of spinal AVM resulting from thrombosis within the abnormal vessels of the spinal cord.
Figure 2.
An overview on Foix–Alajouanine syndrome (FAS).
The pathophysiology is described as subacute myelopathy due to venous congestion of the spinal cord in the absence of hemorrhage in a patient with a spinal AVM2,32. The vascular anatomy of the spinal cord has been thoroughly reviewed by Takai and Taniguchi33. The anterior spinal artery is described as communicating with the radiculomedullary artery, and the corresponding veins also have similar anastomosis, with the venous network being far more extensive and connecting to the extradural and extravertebral veins as well14. Anomalous communication between the radiculomedullary artery and vein leads to a significant rise in pressure within the communicating venous network (Fig. 3). The typical pathophysiology of the disease involves venous congestion within the spinal cord, resulting in progressive ischemia and infarction14. Thrombosis often accompanies this condition. To be more specific, the terminology should be reserved for patients experiencing clinically subacute to chronic progressive neurological symptoms due to intradural AVFs leading to congestive myelopathy without hemorrhage6. In summary, the enlarged and irregular veins are associated with dural arteriovenous shunts or fistulas, primarily located within the dura but sometimes outside of it13. As a consequence of these shunts, arterial blood flows back into the spinal cord’s venous drainage, which congests the venous outflow and raises venous pressure in the affected areas, causing possible ischemic damage2,29.
Figure 3.
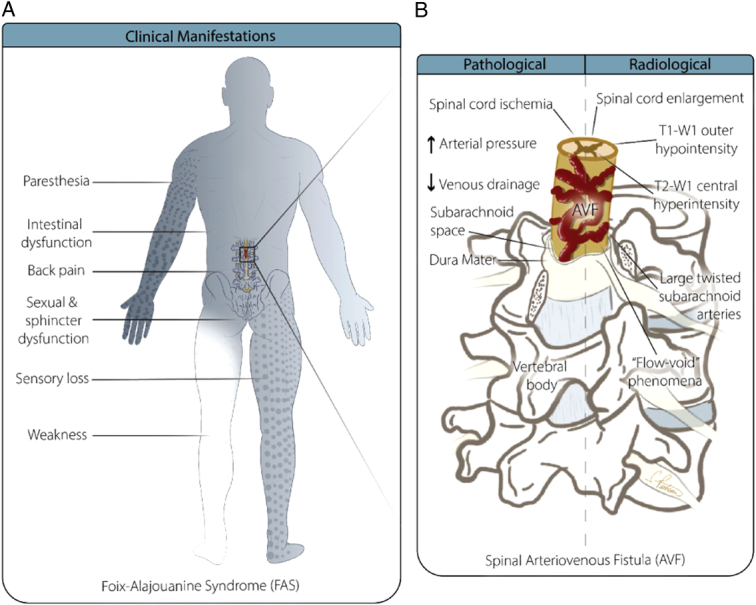
Diagram showing the anomalous communication between the radiculomedullary artery and vein. Panel A illustrates the clinical manifestations of FAS. Panel B provides detailed anatomical and radiological findings of a spinal arteriovenous fistula (AVF), highlighting pathological and radiological characteristics. The diagram on the left side (Pathological) shows the spinal cord with increased arterial pressure and decreased venous drainage, leading to spinal cord ischemia. It also notes the involvement of the subarachnoid space and dura mater surrounding the spinal cord. On the right side (Radiological), the image displays the associated radiological findings, such as spinal cord enlargement, T1-W1 outer hypointensity, T2-W1 central hyperintensity, large twisted subarachnoid arteries, and the “flow-void” phenomena, all of which are characteristic of AVF. The vertebral body is also labeled to provide anatomical orientation.
Clinical presentation
Individuals suffering from FAS may have progressive monoparesis or paraparesis along with abnormal sensations in the lower limbs. These symptoms can occur symmetrically or asymmetrically. Dysfunctions of the intestine, bladder, and sexual organs are frequently encountered. Prevalent are reports of non-radiating lower back pain in the lumbosacral or coccygeal regions, which were initially misidentified as sciatica5,34. The presence of paresis or dysaesthesia may ultimately advance to the upper limbs. However, none of the case reports in our analysis showed any upper limb weakness. The majority of people with FAS experience several years before receiving a diagnosis. However, in a small number of cases, symptoms can also appear suddenly29. In this pooled analysis, the average time of symptoms onset until diagnosis was 13.16 months. Therefore, a high suspicion for the diagnosis of FAS should be considered in patients presenting with sensory or motor symptoms affecting the lower limbs. We found that the duration of symptoms did not correlate with the outcome among those patients. However, Flores et al.35 reported that the delay in diagnosis is the main factor influencing recovery in most patients with spinal vascular malformations. Possible complications of FAS may involve the reappearance of symptoms or fast neurological decline, such as the emergence of paraplegia with total loss of sensation below the affected region29.
Diagnostic approach
Radiological assessment is pivotal for clinicians, enabling them to distinguish FAS from other causes of progressive myelopathy, monitor patient progress, and devise an appropriate management strategy to halt disease advancement. Magnetic resonance imaging (MRI) and digital subtraction angiography (DSA) are two crucial imaging techniques for accurately diagnosing spinal vascular anomalies, including spinal AVMs. In the early stages of FAS, MRI examinations may show normal findings. However, as the disease advances, T1-W1 scans may indicate enlargement of the spinal cord and hypointensity at the outer edges of the affected levels of the spinal cord. During T2-W1, abnormalities within the spinal cord exhibit increased signal intensity at central regions30,36. Contrast administration frequently results in the formation of serpentine areas of increased intensity and exposes the existence of larger, twisted blood arteries in the subarachnoid space, accompanied by the occurrence of ‘flow void’ phenomena. MR angiograms have the ability to anticipate the precise location and size of the fistula prior to resorting to more invasive catheter angiography. Usually, MRI scans commonly show the presence of fluid in spiraling perimedullary vessels. The key MRI findings of spinal cord AVMs generally include signal voids from high-velocity flow, dilated perimedullary vessels that may indent and/or scallop the cord, and increased cord signal due to cytotoxic edema or myelomalacia8,30,36. Conventional intra-arterial DSA serves as the gold standard for diagnosing, evaluating treatment efficacy, and conducting follow-up examinations for spinal AVMs. Following surgical or endovascular intervention, DSA is typically performed immediately to assess the success of treatment. During the follow-up period, DSA is conducted when patient symptoms deteriorate37–39. Despite angiography’s high sensitivity for diagnosing spinal cord AVMs, in some instances, results may be inconclusive or negative for FAS. Van Dijk et al.40 described two cases with normal angiograms for spinal AV fistula, although the patients had classic clinical signs and symptoms and MRI findings. Criscuolo et al.6 also reported two patients with the diagnosis of FAS and negative spinal arteriography. A high sense of suspicion should be made based on the clinical presentation; therefore, judicious use of contrast-based imaging should be considered to avoid contrast-induced renal injury. The pharmacokinetics of contrast media will determine how long safe waiting intervals between successive contrast-based examinations should be scheduled. Based on the recommendations from the contrast media safety committee, in patients with normal renal functions, the wait time between gadolinium-based imaging should be 12 and 4 h if the condition is urgent41,42. In patients with poor renal functions, the wait time between successive scans should be optimally 7 days and minimally 2.5 days42. Clinicians should also consider non-contrast-based imaging techniques such as arterial spin labeling (ASL) and time of flight MRI (TOF MRI). ASL perfusion maps combined with susceptibility-weighted imaging have been shown to be superior to conventional MRI and equally efficient to DSA in the preoperative evaluation of AVM43. Therefore, clinicians could use several non-contrast imaging techniques that could be helpful in diagnosing FAS. In pediatric cohorts, perfusion maps from ASL have shown high efficacy in identifying nidus and evaluating flow and size of AVM after therapy44.
Management
The purpose of the treatment is to disrupt the direct connection between the arterial and venous channels45. Therefore, the treatment of choice for FAS is either using endovascular therapy to embolize the affected blood vessels, surgically tying off the AVF or a combination of both in certain situations29. Using liquid polymers for embolization is preferred over particles like polyvinyl alcohol (PVA) due to the high recurrence rates of 30–93% associated with particle use.
In contrast, liquid polymers have shown successful occlusion rates ranging from 44 to 100%2. Although spinal dural AVFs can be treated using endovascular methods, there is a risk of recurrence, difficulties in accessing the fistula with a catheter, and the potential for spinal cord infarction46. On the other hand, although targeted laminectomy is a more invasive procedure, it carries minimal risk of complications, enables the precise identification of the fistulous draining vein, and is less prone to recurrence or causing spinal cord infarction9. To conclude, in cases where there is a significant distance between the artery’s origin and the fistula site, it is advisable to opt for clipping as a safer alternative to embolization13. In multiple medical facilities worldwide, interventional radiographic procedures are commonly chosen due to their minimally invasive nature, cost-effectiveness, and favorable outcomes. In Foix–Alajouanine syndrome, there is no evidence of which treatment method is associated with a better outcome. Steinmetz and colleagues performed a microsurgical obliteration of the AVF without failure or complications45. In this pooled analysis, surgical ligation was the method of treatment in 12 patients (50%), while embolization was performed in about 5 patients, and only 4 patients received conservative management. We found that surgical ligation was associated with a higher rate of improvement compared to embolization and conservative management. Lagman et al.47 also reported similar results indicating that surgical clipping or ligation is superior to embolization. Early intervention can lead to significant improvement in neurological deficits. Additionally, diagnostic angiography has been noted to sometimes reverse clinical symptoms on its own14.
Prognosis
Prognosis is supposedly determined by factors such as how long symptoms last, whether the sphincter is involved at presentation, and where the fistula is located2. Temporary deficits following surgical or endovascular treatment are typical and do not impact the overall short-term or long-term results47. Flores et al.35 argue that the primary reason for partial recovery is the delay in diagnosis rather than the extent of neurological damage, as nearly 48% of patients may experience progression of their condition before the diagnosis is made. Patients who experience diminished function due to an acute myelopathic episode may feel some improvement in symptoms following intervention if FAS is accurately identified and treated47.
Limitations
Due to the extremely rare incidence of FAS, the sample size of available case reports is small. Our heterogeneous data and the small sample size challenged the statistical robustness, which further limited the pooled analysis. A larger sample would allow for more detailed analysis. The unavailability of individualized data and reported results of the included literature further limited our review findings. We did not encounter any cases of FAS, which makes our review lack illustrative clinical examples. Neurologists can see one case of FAS once every 8 years2.
Conclusion
In a nutshell, FAS is a neurological illness that is infrequent and difficult to manage. The infrequency of the illness and its many manifestations lead to diagnostic complexities. Prompt identification and care are essential for enhancing neurological impairments, although the hindrance of delayed diagnosis persists as a substantial barrier. To effectively tackle the issues presented by FAS, it is crucial to prioritize increasing awareness, expanding diagnostic tools, and refining treatment options. The cooperation of neurologists, radiologists, and neurosurgeons is essential for the progress in comprehending and treating this uncommon neurological illness.
Ethical approval
Ethics approval was not required for this review.
Consent
Informed consent was not required for this review article.
Source of funding
Not applicable.
Author contribution
All authors have contributed equally in formation of all forms of manuscript.
Conflicts of interest disclosure
The authors declared no conflicts of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Bipin Chaurasia.
Data availability statement
None.
Provenance and peer review
Okay.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 30 September 2024
Contributor Information
Oday Atallah, Email: atallah.oday@mh-hannover.de.
Yasser F. Almealawy, Email: almealawyyasser@gmail.com.
Roua Arian, Email: Rouaarian2001@gmail.com.
Assma Dwebi, Email: assma.dwebi@gmail.com.
Amr Badary, Email: amr.badary@hotmail.com.
Abbas F. Abdul Hussein, Email: abbasfadhil3785868@gmail.com.
Vivek Sanker, Email: viveksanker@gmail.com.
Saber Zafarshamspour, Email: saberzsp@gmail.com.
Bipin Chaurasia, Email: trozexa@gmail.com.
Amit Agrawal, Email: dramitagrawal@hotmail.com.
Santiago Pastrana Brandes, Email: spastranab88@gmail.com.
Mohammed A. Azab, Email: mohammed.azab@kasralainy.edu.eg.
References
- 1. Foix CH. La myelite necrotique subaique. Rev Neurol 1926;2:1–42. [Google Scholar]
- 2. Jellema K, Tijssen CC, Gijn JV. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain 2006;129:3150–3164. [DOI] [PubMed] [Google Scholar]
- 3. Nasr DM, Brinjikji W, Rabinstein AA, et al. Clinical outcomes following corticosteroid administration in patients with delayed diagnosis of spinal arteriovenous fistulas. J NeuroInterv Surg 2017;9:607–610. [DOI] [PubMed] [Google Scholar]
- 4. Demir CF, Yildiz M, Özdemir H, et al. Paraplegia in pregnancy: A case of spinal vascular malformation with Klippel–Trenaunay syndrome. Spine. 2012;37:E1218–20. [DOI] [PubMed] [Google Scholar]
- 5. Bordignon KC, Montú MB, Ramina R, et al. Foix–Alajouanine syndrome: case report. Arq Neuro-Psiquiatr 2005;63:527–529. [DOI] [PubMed] [Google Scholar]
- 6. Criscuolo GR, Oldfield EH, Doppman JL. Reversible acute and subacute myelopathy in patients with dural arteriovenous fistulas: Foix–Alajouanine syndrome reconsidered. J Neurosurg 1989;70:354–359. [DOI] [PubMed] [Google Scholar]
- 7. del Pino-Camposeco J, Villanueva-Castro E, Ponce-Gómez JA, et al. Foix–Alajouanine syndrome: a case report. Cureus 2023;15:e36696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrell AS, Tubbs RS, Acakpo-Satchivi L, et al. Legacy and current understanding of the often-misunderstood Foix–Alajouanine syndrome: historical vignette. J Neurosurg 2009;111:902–906. [DOI] [PubMed] [Google Scholar]
- 9. Joswig H, Haji FA, Martinez-Perez R, et al. Rapid recovery from paraplegia in a patient with Foix–Alajouanine syndrome. World Neurosurg 2017;97:750–e1. [DOI] [PubMed] [Google Scholar]
- 10. Khan UA, Koumellis P, Almahfoudh R, et al. Bilateral mirror image lumbar spinal dural arterial venous fistula: a rare case and systematic review of the literature. Br J Neurosurg 2023;37:982–985. [DOI] [PubMed] [Google Scholar]
- 11. Kneisley LW, Dominguez MR, Bignami A, et al. Paraplegia following surgery in Foix and Alajouanine syndrome. Spinal Cord 1980;18:33–41. [DOI] [PubMed] [Google Scholar]
- 12. Koeppen AH, Barron KD, Cox JF. Foix–Alajouanine syndrome. Acta Neuropathol 1974;29:187–197. [DOI] [PubMed] [Google Scholar]
- 13. Krishnan P, Banerjee TK, Saha M. Congestive myelopathy (Foix–Alajouanine syndrome) due to intradural arteriovenous fistula of the filum terminale fed by anterior spinal artery: case report and review of literature. Ann Indian Acad Neurol 2013;16:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menon KV, Sorour TM, Raniga SB. Foix–Alajouanine syndrome presenting as acute cauda equina syndrome: a case report. Global Spine J 2014;4:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patwari S, Verma A, Srivastava A, et al. Thoracic intradural extramedullary AV malformation presenting as Foix–Alajouanine syndrome: a case report. Nepalese J Radiol 2011;1:27–29. [Google Scholar]
- 16. Rathnam AS, Memon AB. Foix–Alajouanine syndrome mimicking longitudinally extensive transverse myelitis. Eur J Case Rep Intern Med 2020;7:002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renowden SA, Molyneux AJ. Case report: spontaneous thrombosis of a spinal dural AVM (Foix–Alajouanine syndrome) – magnetic resonance appearance. Clin Radiol 1993;47:134–136. [DOI] [PubMed] [Google Scholar]
- 18. Sadighi N, Tajmalzai A, Salahshour F. Spinal arteriovenous malformations causing Foix–Alajouanine syndrome, a case report and review of the literature. Radiol Case Rep 2021;16:2187–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siani A, Garrett A, Thomas N. Case report: Differential diagnosis of lower extremity weakness in a young male-consider Foix Alajouanine syndrome. Clin Pract Cases Emerg Med 2022;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sood D, Mistry KA, Khatri GD, et al. Congestive myelopathy due to intradural spinal AVM supplied by artery of Adamkiewicz: case report with brief literature review and analysis of the Foix–Alajouanine syndrome definition. Pol J Radiol 2015;80:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tay CH. The Foix–Alajouanine syndrome. Med J Malaya 1971;25:298–300. [PubMed] [Google Scholar]
- 22. Caplan LR. Charles Foix--the first modern stroke neurologist. Stroke 1990;21:348–356. [DOI] [PubMed] [Google Scholar]
- 23. Foix C. Les lésions anatomiques de la maladie de Parkinson. Rev Neurol (Paris) 1921;28:593–600. [Google Scholar]
- 24. Nicolesco J. Anatomie cérébrale: les noyaux gris centraux et la région mésencéphalo-sous-optique, suivi d’un appendice sur l’anatomie pathologique de la maladie de Parkinson, par Ch. Foix. J Nicolesco Masson; 1925.
- 25. Boudin G, Nick J. Eulogy of Théophile Alajouanine (1890–1980). Bull Acad Natl Med 1982;166:313–323. [PubMed] [Google Scholar]
- 26. Lhermitte F, Lecours A, Signoret JL. Obituary: Théophile Alajouanine (1890–1980). Brain Language 1981;13:191–196. [DOI] [PubMed] [Google Scholar]
- 27. Thron A. Spinale durale arteriovense Fisteln. Der Radiol 2001;41:955–960. [DOI] [PubMed] [Google Scholar]
- 28. Hubbard ZS, Cunningham CM, Spiotta AM. A case of extremely rapid progression in Foix–Alajouanine syndrome. World Neurosurg 2024;185:1–2. [DOI] [PubMed] [Google Scholar]
- 29. Mishra R, Kaw R. Foix–Alajouanine syndrome: an uncommon cause of myelopathy from an anatomic variant circulation. South Med J 2005;98:567–570. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez FJ, Crum BA, Krauss WE, et al. Venous congestive myelopathy: a mimic of neoplasia. Mod Pathol 2005;18:710–718. [DOI] [PubMed] [Google Scholar]
- 31. Di Chiro G. Foix–Alajouanine syndrome. Am J Neuroradiol 1990;11:1286. [PMC free article] [PubMed] [Google Scholar]
- 32. Hurst RW, Kenyon LC, Lavi E, et al. Spinal dural arteriovenous fistula: the pathology of venous hypertensive myelopathy. Neurology 1995;45:1309–1313. [DOI] [PubMed] [Google Scholar]
- 33. TaKai K, Taniguchi M. Comparative analysis of spinal extradural arteriovenous fistulas with or without intradural venous drainage: a systematic literature review. Neurosurg Focus 2012;32:E8. [DOI] [PubMed] [Google Scholar]
- 34. Iovtchev I, Hiller N, Ofran Y, et al. Late diagnosis of spinal dural arteriovenous fistulas resulting in severe lower-extremity weakness: a case series. Spine J 2015;15:e39–e44. [DOI] [PubMed] [Google Scholar]
- 35. Flores BC, Klinger DR, White JA, et al. Spinal vascular malformations: treatment strategies and outcome. Neurosurg Rev 2017;40:15–28. [DOI] [PubMed] [Google Scholar]
- 36. Minami S, Sagoh T, Nishimura K, et al. Spinal arteriovenous malformation: MR imaging. Radiology 1988;169:109–115. [DOI] [PubMed] [Google Scholar]
- 37. Gonzalez LF, Spetzler RF. Spinal dural arteriovenous fistulae: clinical features and long-term results-commentary. Neurosurgery 2008;62:166. [DOI] [PubMed] [Google Scholar]
- 38. Rodesch G, Lasjaunias P. Spinal cord arteriovenous shunts: from imaging to management. European journal of radiology 2003;46:221–232. [DOI] [PubMed] [Google Scholar]
- 39. Veznedaroglu E, Nelson PK, Jabbour PM, et al. Endovascular treatment of spinal cord arteriovenous malformations. Neurosurgery 2006;59:S3–202. [DOI] [PubMed] [Google Scholar]
- 40. Van Dijk JM, TerBrugge KG, Willinsky RA, et al. Multidisciplinary management of spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke 2002;33:1578–1583. [DOI] [PubMed] [Google Scholar]
- 41. van der Molen AJ, Dekkers IA, Geenen RWF, et al. Waiting times between examinations with intravascularly administered contrast media: a review of contrast media pharmacokinetics and updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2024;34:2512–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stacul F, van der Molen AJ, Reimer P, et al. Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011;21:2527–2541. [DOI] [PubMed] [Google Scholar]
- 43. Hodel J, Leclerc X, Kalsoum E, et al. Intracranial arteriovenous shunting: detection with arterial spin-labeling and susceptibility-weighted imaging combined. AJNR Am J Neuroradiol 2017;38:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nabavizadeh SA, Edgar JC, Vossough A. Utility of susceptibility-weighted imaging and arterial spin perfusion imaging in pediatric brain arteriovenous shunting. Neuroradiology 2014;56:877–884. [DOI] [PubMed] [Google Scholar]
- 45. Steinmetz MP, Chow MM, Krishnaney AA, et al. Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery 2004;55:77–88. [DOI] [PubMed] [Google Scholar]
- 46. Gokhale S, Khan S, McDonagh D, et al. Comparison of surgical and endovascular approach in management of spinal dural arteriovenous fistulas: a single center experience of 27 patients. Surg Neurol Int 2014;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lagman C, Chung LK, Chitale RV, et al. Dural arteriovenous fistula and Foix–Alajouanine syndrome: assessment of functional scores with review of pathogenesis. World Neurosurg 2017;106:206–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.